Abstract
Purpose
To determine the effect of intravitreal ranibizumab and a dexamethasone implant on aqueous humour cytokine, protein and enzyme levels and to correlate findings to morphologic and functional changes.
Methods
In a prospective, randomized, controlled, double‐blind study, patients with clinically significant diabetic macular oedema (CSME) were randomly allocated to receive either monthly intravitreal injections of ranibizumab (Lucentis, Novartis Pharma) or a single dexamethasone implant (Ozurdex, Pharm‐Allergan) at baseline (BL). Aqueous humour samples were collected at BL and weeks 2, 8 and 20.
Results
The study included 18 eyes of 18 patients. In the dexamethasone implant group, soluble intercellular adhesion molecule 1 (sICAM‐1) (weeks 2 and 8), CXCL9/monokine induced by gamma interferon (MIG) (weeks 2 and 8), soluble vascular cell adhesion protein 1 (sVCAM‐1) (weeks 2 and 8) and monocyte chemo‐attractant protein 1 (MCP‐1) (week 2) levels were significantly decreased compared with baseline. In the ranibizumab group, placental growth factor (PIGF) (week 2) and vascular endothelial growth factor (VEGF) (week 2 and 8) levels were significantly decreased compared with baseline. No significant changes in central retinal thickness (CRT) or Early Treatment Diabetic Retinopathy Study (ETDRS) best corrected visual acuity (BCVA) were observed in the Ozurdex group at any time‐points. ETDRS scores significantly increased at week 20 (84.88 ± 8.88 letters) compared with baseline (74.78 ± 14.85 letters), and the CRT decreased significantly at week 4 (381.00 ± 114.64 μm) compared with baseline (440 ± 144 μm) in the Lucentis group.
Conclusion
The dexamethasone implant affected the aqueous cytokines and proteins MCP‐1, sICAM‐1, sVCAM‐1 and MIG, whereas ranibizumab treatments reduced VEGF and PIGF levels. Morphological changes may diverge from cytokine changes. Results may indicate a rationale for a combination therapy for CSME using both agents, the dexamethasone implant and repeatedly administered ranibizumab injections.
Keywords: aqueous humour cytokines, dexamethasone implant, diabetic macular oedema, ranibizumab
Introduction
Diabetic macular oedema (DME) is the most common cause of visual impairment in patients with diabetes. This condition results from a breakdown of the blood–retinal barrier, leading to vascular leakage, fluid accumulation and thickening of the macula (Schmidt‐Erfurth et al. 2017). The treatment of DME has undergone a paradigm shift recently. Traditionally, photocoagulation represented the standard treatment, but pharmacologic treatments are nowadays being used as first‐line therapy. All currently available drug therapies for DME are either anti‐vascular endothelial growth factor (VEGF) agents or corticosteroids (Schwartz et al. 2014).
The anti‐VEGF agent ranibizumab (Lucentis; Novartis Pharma, Basel, Switzerland) is approved for the treatment of visual impairment associated with DME. The dexamethasone implant (Ozurdex; Pharm‐Allergan, Dublin, Ireland) achieved similar rates of visual acuity improvement compared with anti‐VEGF agents and is also approved for this indication. Both treatments were associated with improvement in vision‐related quality of life (Gillies et al. 2014). Recently, a combination therapy was able to provide superiority in means of retinal morphology, but not in means of visual acuity in a phase 2 study. The researchers concluded that it may be beneficial for some patients, but further analysis would be required (Maturi et al. 2018).
Therefore, there was considerable interest in recent research to clarify the mechanisms of DME development and define possible treatment targets (Vujosevic & Simo 2017). As cytokines play a role in inflammation and neo‐angiogenesis, multiple studies were performed to assess cytokine levels in patients with diabetic retinopathy and DME (Hang et al. 2014; Sonoda et al. 2014; Dong et al. 2015; Hillier et al. 2018; Yu et al. 2018). Aqueous and serum cytokine levels seem to be elevated in patients with DME and decrease under treatment with intravitreal bevacizumab or triamcinolone in a short follow‐up time of one month (Sohn et al. 2011). Although both triamcinolone and dexamethasone are steroids, their structures, availability and half‐lives differ and therefore cannot be compared.
To the best of our knowledge, this is the first study investigating differences in cytokine levels during and after treatment with an intravitreal anti‐VEGF (ranibizumab) and a dexamethasone implant over a period of 6 months.
The purpose of this pilot study was to provide new insights into changes in aqueous humour cytokines following anti‐VEGF and corticosteroid intravitreal injections in DME and their correlation to retinal morphology and function. These findings may lead to further understanding of the disease process and aid future treatment strategies.
Patients and Methods
This prospective, randomized, controlled, double‐blind pilot study included Caucasian patients with clinically significant diabetic macular oedema (CSME). Patients were randomized into two groups and received either monthly intravitreal injections of ranibizumab (Lucentis, Novartis Pharma) or one dexamethasone implant (Ozurdex, Pharm‐Allergan) at baseline. All subjects underwent a comprehensive screening examination, including a slit‐lamp examination with indirect funduscopy and measurement of intraocular pressure using Goldmann applanation tonometry.
Patients were examined at baseline, visit 3 (week 2), visit 4 (week 4), visit 5 (week 8), visit 6 (week 12), visit 7 (week 16) and visit 8 (week 20). A slit‐lamp and fundus examination and colour fundus imaging were performed. Best corrected visual acuity (BCVA) was assessed using the standard ETDRS visual acuity chart. Central retinal thickness (CRT) measurements were performed at each monthly visit. Optical coherence tomography (OCT) imaging was performed with spectral‐domain OCT by Spectralis OCT® (Heidelberg Engineering, Dossenheim, Germany). The main outcome variables were the changes in cytokine, protein and enzyme levels over time, while changes in BCVA and CRT represented secondary outcome variables.
The study protocol was reviewed and approved by the local ethics committee and followed the guidelines set forth in the Declaration of Helsinki. Written informed consent was obtained from patients before inclusion in the study.
Subjects
Patients from the outpatient clinic presenting CSME secondary to diabetes were included. The exclusion criteria were therapeutic intravitreal treatment within the last three months or other retinal diseases such as epiretinal membrane, vitreomacular traction syndrome, retinal atrophy or glaucoma. Patients were screened for recent cardiovascular events (3 months prior to treatment) and active infectious disease by medical records.
Treatment
The ranibizumab group received a loading dose of four monthly injections (ranibizumab 0.5 mg/0.05 ml); a further two injections were administered in a pro re nata regimen. The dexamethasone group received an intravitreal dexamethasone implant at baseline.
All injections and implantations were performed under sterile conditions after preparation of the conjunctiva using 5% povidone–iodine solution, topical anaesthetic and positioning of the lid speculum. All injections were performed in a surgical setting.
Aqueous humour sample collection
Aqueous humour samples were collected at baseline, week 2, week 8 and week 20 before the intravitreal injection of the agent, if an intravitreal injection was performed. After topical anaesthesia application, sterile covering and insertion of an eye speculum, aqueous humour (0.1 to 0.3 ml) was drawn into a conventional tuberculin syringe and samples were stored at ‐20 degrees Celsius until testing. Aqueous humour cytokine, protein and enzyme levels were analysed with a Luminex 100 multiplex array (Luminex Corporation, Austin, TX, USA) (Maier et al. 2008).
The following cytokines, proteins and enzymes were investigated: interleukin 6 (IL‐6), interleukin 8 (IL‐8), monocyte chemo‐attractant protein 1 (MCP‐1), platelet‐derived growth factor (PDGF), VEGF, soluble intercellular adhesion molecule 1 (sICAM‐1), macrophage migration inhibitory factor (MIF), monokine induced by gamma interferon (MIG), matrix metallopeptidase 9 (MMP‐9), plasminogen activator inhibitor 1 (PAI‐1), placental growth factor (PIGF), transforming growth factor beta 1, 2 and 3 (TGF‐beta1,2,3) and soluble vascular cell adhesion protein (sVCAM). Cytokine, protein and enzyme concentrations were determined from standard curves expressed as picogram per millilitre (pg/ml).
Statistics
Statistical analyses were conducted using SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA). Descriptive data are presented as mean and standard deviation. Parameters were checked for normality (Shapiro‐Wilk, p > 0.05; Q‐Q‐Plot). As normality was not present, the non‐parametric Mann–Whitney U test and Wilcoxon signed‐rank test were used to check for significant differences within each group; the Wilcoxon signed‐rank test was used between time‐points. The significance level was set to p = 0.05 for significant correlations and p = 0.01 for highly significant correlations. Bonferroni's correction was applied to compensate for multiple testing.
Results
Eighteen eyes of 18 patients with DME were included in this study. The mean age was 66.89 ± 8.80 years in the Lucentis group and 64.56 ± 9.0 years in the Ozurdex group (p > 0.05). Central retinal thickness (CRT) was similar between the groups at baseline (Lucentis: 440 ± 144 μm; Ozurdex: 471.33 ± 122.60 μm; p > 0.05). None of the patients showed active systemic or ocular infectious disease or recent cardiovascular events.
In the Lucentis group, the ETDRS scores significantly increased at week 20 (84.88 ± 8.88 letters) compared with baseline (74.78 ± 14.85 letters). The CRT decreased significantly at week 4 (381.00 ± 114.64 μm) compared with baseline (440 ± 144 μm). No significant changes in CRT or ETDRS BCVA were observed in the Ozurdex group at any time. In the Ozurdex group, one patient was excluded at week 12 and three patients at week 16, due to increased oedema. Table 1 displays BCVA and CRT results.
Table 1.
BCVA and CRT for ranibizumab and dexamethasone implant, respectively. P values present comparisons to baseline for each group.
| Ranibizumab | p Value | Dexamethasone implant | p Value | |
|---|---|---|---|---|
| BCVA (in letters) | ||||
| Baseline (mean ± SD) | 74.78 ± 14.85 (n = 9) | 67.22 ± 10.52 (n = 9) | ||
| Week 2 (mean ± SD) | 81.78 ± 9.56 (n = 9) | 0.021 | 71.00 ± 13.08 (n = 9) | 0.160 |
| Week 4 (mean ± SD) | 80.33 ± 7.84 (n = 9) | 0.160 | 69.38 ± 15.16 (n = 9) | 0.610 |
| Week 8 (mean ± SD) | 83.33 ± 6.33 (n = 9) | 0.058 | 77.13 ± 5.59 (n = 8) | 0.035 |
| Week 12 (mean ± SD) | 83.33 ± 7.52 (n = 9) | 0.038 | 74.38 ± 6.84 (n = 8) | 0.260 |
| Week 16 (mean ± SD) | 82.44 ± 8.88 (n = 9) | 0.018 | 77.00 ± 4.79 (n = 5) | 0.279 |
| Week 20 (mean ± SD) | 84.88 ± 8.88 (n = 9) | 0.008 | 74.60 ± 11.99 (n = 5) | 1.00 |
| CRT (μm) | ||||
| Baseline (mean ± SD) | 440.89 ± 144.47 (n = 9) | 471.33 ± 122.60 (n = 9) | ||
| Week 2 (mean ± SD) | 384.67 ± 106.08 (n = 9) | 0.011 | 363.78 ± 77.53 (n = 9) | 0.011 |
| Week 4 (mean ± SD) | 381.00 ± 114.64 (n = 9) | 0.008 | 373.75 ± 85.67 (n = 9) | 0.017 |
| Week 8 (mean ± SD) | 376.44 ± 119.24 (n = 9) | 0.011 | 353.75 ± 97.13 (n = 8) | 0.012 |
| Week 12 (mean ± SD) | 365.56 ± 106.21 (n = 9) | 0.011 | 422.13 ± 142.46 (n = 8) | 0.092 |
| Week 16 (mean ± SD) | 379.33 ± 119.59 (n = 9) | 0.051 | 350.20 ± 94.57 (n = 5) | 0.043 |
| Week 20 (mean ± SD) | 363.56 ± 108.54 (n = 9) | 0.028 | 370.80 ± 126.90 (n = 5) | 0.500 |
p values are displayed for each group. Bonferri correction was used for adjustment for multiple testing. Statistically significant p values after correction are displayed in bold.
BCVA = Best corrected visual acuity, CRT = central retinal thickness, SD = standard deviation.
There were no statistically significant changes in MIF, IL‐6, IL‐8, TGFb1, TGFb2 or TGFb3 concentrations over time in either group. No significant differences were exhibited at any visit between the groups.
The levels of sICAM‐1, CXCL9/MIG, sVCAM‐1, MMP‐9, PDGF‐AA and MCP‐1 were significantly altered in the Ozurdex group. After correction for multiple testing, levels of sICAM‐1 (weeks 2 and 8), CXCL9/MIG (weeks 2 and 8), sVCAM‐1 (weeks 2 and 8) and MCP‐1 (week 2) were significantly decreased compared with baseline.
The change in sICAM‐1 levels in the Ozurdex group was most prominent at week 2 (173.49 ± 58.84 pg/ml compared with 313.63 ± 192.25 pg/ml at baseline). The CXCL9/MIG level was significantly decreased at week 2 (92.33 ± 77.85 pg/ml) and week 8 (184.69 ± 316.84 pg/ml) compared with baseline (321.96 ± 471.71 pg/ml), respectively. The sVCAM‐1 level was significantly decreased at week 2 (1790.88 ± 1659.45 pg/ml) and week 8 (2037.75 ± 2736.62 pg/ml) compared with baseline (9363.86 ± 14 298.90 pg/ml). The MCP‐1 level was significantly decreased at week 2 (764.04 ± 224.55 pg/ml) compared with baseline (2696.86 ± 2685.72 pg/ml). As Table 2 illustrates, sICAM‐1, PIGF and VEGF levels decreased in the Lucentis group. After correction for multiple testing, the PIGF (week 2) and VEGF (weeks 2 and 8) levels were significantly decreased compared with baseline. The PIGF level was significantly decreased from a baseline value of 6.72 ± 6.42 pg/ml to 1.50 ± 1.41 pg/ml at week 2 (Fig. 1). The VEGF level was significantly reduced from 135.26 ± 117.99 pg/ml at baseline to values of 4.80 ± 5.81 pg/ml at week 2 and 8.73 ± 7.09 pg/ml at week 8.
Table 2.
Groupwise comparison of inflammatory markers by dexamethasone and ranibizumab groups to baseline. P values are displayed for each group. Bonferri correction was used for adjustment for multiple testing
| Baseline | Week 2 | Week 8 | Week 20 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ranibizumab | Dexamethasone implant | Ranibizumab | p Value | Dexamethasone implant | p Value | Ranibizumab | p Value | Dexamethasone implant | p value | Ranibizumab | p Value | Dexamethasone implant | p Value | |
| MIF (mean ± SD) | 154.10 ± 209.10 | 113.20 ± 123.20 | 152.13 ± 218.23 | 0.767 | 102.37 ± 63.54 | 0.859 | 85.51 ± 102.49 | 0.173 | 75.78 ± 68.30 | 0.362 | 89.52 ± 93.02 | 0.594 | 157.78 ± 98.03 | 0.345 |
| sICAM‐1 (mean ± SD) | 408.17 ± 317.88 | 313.63 ± 192.25 | 284.53 ± 256.19 | 0.021 | 173.49 ± 58.84 | 0.011 | 237.69 ± 150.01 | 0.086 | 238.28 ± 181.16 | 0.012 | 306.33 ± 159.24 | 0.327 | 457.38 ± 372.28 | 0.249 |
| CXCL9/MIG (mean ± SD) | 286.62 ± 377.52 | 321.96 ± 471.71 | 198.18 ± 307.95 | 0.161 | 92.33 ± 77.85 | 0.008 | 132.26 ± 67.03 | 0.441 | 184.69 ± 316.84 | 0.012 | 346.55 ± 328.66 | 0.515 | 365.50 ± 527.83 | 0.345 |
| PIGF (mean ± SD) | 6.72 ± 6.42 | 6.12 ± 4.96 | 1.50 ± 1.41 | 0.008 | 4.63 ± 2.37 | 0.374 | 1.79 ± 0.81 | 0.028 | 6.88 ± 3.48 | 0.674 | 2.91 ± 2.63 | 0.058 | 6.33 ± 5.77 | 0.345 |
| sVCAM‐1 (mean ± SD) | 4907.63 ± 8130.80 | 9363.86 ± 14 298.90 | 4557.44 ± 8745.29 | 0.515 | 1790.88 ± 1659.45 | 0.008 | 2020.24 ± 1113.85 | 0.086 | 2037.75 ± 2736.62 | 0.012 | 5821.29 ± 9038.60 | 0.208 | 16 909.40 ± 26 985.52 | 0.345 |
| PAI‐1 (mean ± SD) | 441.43 ± 330.61 | 638.37 ± 1065.12 | 273.36 ± 266.28 | 0.015 | 547.49 ± 462.08 | 0.314 | 228.37 ± 164.45 | 0.038 | 699.29 ± 1036.13 | 1 | 301.98 ± 215.42 | 0.161 | 847.60 ± 1289.51 | 0.345 |
| MMP‐9 (mean ± SD) | 110.52 ± 123.67 | 80.30 ± 66.88 | 104.77 ± 99.41 | 0.624 | 30.11 ± 20.82 | 0.025 | 119.0 ± 114.43 | 0.374 | 29.73 ± 22.54 | 0.068 | 121.58 ± 116.03 | 0.249 | 94.73 ± 60.95 | 0.753 |
| PDGF (mean ± SD) | 28.71 ± 16.96 | 25.84 ± 14.89 | 15.06 ± 10.59 | 0.011 | 16.05 ± 5.97 | 0.093 | 19.86 ± 10.34 | 0.441 | 15.65 ± 5.24 | 0.063 | 27.95 ± 7.31 | 0.917 | 27.37 ± 9.48 | 0.043 |
| IL‐6 (mean ± SD) | 22.34 ± 29.10 | 29.26 ± 52.73 | 17.37 ± 23.98 | 0.767 | 5.99 ± 2.57 | 0.025 | 8.54 ± 6.96 | 0.038 | 9.00 ± 8.42 | 0.237 | 12.10 ± 10.44 | 0.249 | 39.17 ± 72.76 | 0.686 |
| IL‐8 (mean ± SD) | 15.61 ± 9.95 | 19.26 ± 16.57 | 17.40 ± 15.11 | 0.575 | 14.44 ± 8.02 | 0.327 | 15.08 ± 14.00 | 0.767 | 19.26 ± 15.28 | 0.933 | 24.83 ± 12.35 | 0.115 | 23.52 ± 21.20 | 0.345 |
| MCP‐1 (mean ± SD) | 1706.26 ± 1076.98 | 2696.86 ± 2685.72 | 1323.29 ± 1176.57 | 0.374 | 764.04 ± 224.55 | 0.012 | 1261.11 ± 761.87 | 0.26 | 932.95 ± 371.60 | 0.028 | 2186.18 ± 556.22 | 0.345 | 3903.00 ± 4809.97 | 0.345 |
| VEGF (mean ± SD) | 135.26 ± 117.99 | 149.38 ± 162.01 | 4.80 ± 5.81 | 0.008 | 70.60 ± 61.23 | 0.028 | 8.73 ± 7.09 | 0.012 | 75.69 ± 47.33 | 0.917 | 5.33 ± 4.55 | 0.028 | 84.73 ± 59.02 | 0.138 |
| TGFb1 (mean ± SD) | 49.70 ± 27.87 | 48.89 ± 47.60 | 47.57 ± 36.16 | 0.465 | 27.30 ± 14.30 | 0.599 | 55.08 ± 27.49 | 0.109 | 26.18 ± 20.08 | 0.285 | 54.98 ± 26.70 | 1 | 77.33 ± 108.83 | 0.08 |
| TGFb3 (mean ± SD) | 31.28 ± 11.10 | 29.53 ± 8.93 | 28.45 ± 11.28 | 0.714 | 21.14 ± 7.01 | 0.075 | 29.75 ± 8.62 | 0.715 | 18.10 ± 7.87 | 0.144 | 31.10 ± 8.40 | 1 | 30.44 ± 20.16 | 0.5 |
| TGFb2 (mean ± SD) | 2650.15 ± 1984.40 | 2135.33 ± 510.61 | 2410.80 ± 1718.25 | 0.273 | 1082.91 ± 659.89 | 0.046 | 2710.43 ± 1641.25 | 0.715 | 955.38 ± 354.16 | 0.068 | 3581.32 ± 1811.68 | 0.273 | 2189.87 ± 1138.48 | 0.893 |
All results are displayed in pg/mL.
p values are displayed for each group. Bonferri correction was used for adjustment for multiple testing. Statistically significant p values after correction are displayed in bold.
IL‐6 = iInterleukin 6, IL‐8 = interleukin 8, MCP‐1 = monocyte chemo‐attractant protein 1, MIF = Macrophage migration inhibitory factor, MIG/CXCL 9 = monokine induced by gamma interferon, MMP‐9 = matrix metallopeptidase 9, PAI‐1 = plasminogen activator inhibitor‐1, PDGF = platelet‐derived growth factor, PIGF = placental growth factor, sICAM‐1 = soluble intercellular adhesion molecule 1, sVCAM = soluble vascular cell adhesion protein, TGF‐beta1,2,3 = transforming growth factor beta 1,2 and 3, VEGF = vascular endothelial growth factor.
Figure 1.
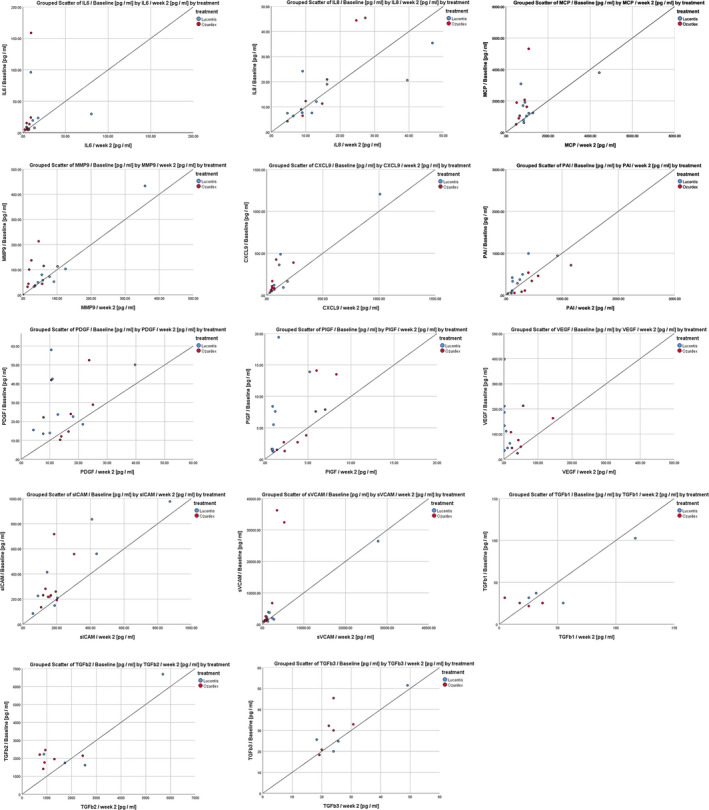
Scatter plots demonstrating the correlation between inflammatory markers at baseline and week 2 (visit 3) for ranibizumab (blue) and the dexamethasone implant (red), respectively. First line left: IL‐6 (interleukin‐6) levels baseline compared with week 2; first line middle: IL‐8 (interleukin 8); first line right: MCP‐1 (monocyte chemo‐attractant protein 1); second line left: MMP‐9 (matrix metallopeptidase 9); second line middle: MIG/CXCL9 (monokine induced by gamma interferon); second line right: PAI‐1 (plasminogen activator inhibitor‐1); third line left: PDGF (platelet‐derived growth factor); third line middle: PIGF (placental growth factor); third line right: VEGF (vascular endothelial growth factor); fourth line left: sICAM‐1 (soluble intercellular adhesion molecule 1); fourth line middle: sVCAM (soluble vascular cell adhesion protein); fourth line right: TGFb1 (transforming growth factor beta 1); fifth line left: TGFb2 (transforming growth factor beta 2); and fifth line middle: TGFb3 (transforming growth factor beta 3).
Discussion
The aim of this study was to identify and compare changes in cytokine, protein and enzyme levels in patients with DME treated with ranibizumab injections or a dexamethasone implant over a period, of the so‐called loading dose, of 6 months. Results indicate that intravitreal ranibizumab and dexamethasone induce detectable changes in pro‐inflammatory cytokine cascades. There were high intraindividual differences in cytokine concentrations in both treatment groups. The dexamethasone implant exhibited an earlier effect than intravitreal ranibizumab regarding the cytokine and adhesion molecules concentration and affected several additional markers as opposed to intravitreal ranibizumab. In contrast, ranibizumab injected monthly displayed a longer lasting effect. Even if both agents reduced CRT (not statistically significant in the dexamethasone implant group); it was achieved by influencing different regulatory pathways and pro‐inflammatory markers.
Previous studies presented different results regarding the changes in levels of cytokines and proteins, such as VEGF, ICAM‐1, IL‐6 or IL‐8, in patients with macular oedema treated with anti‐VEGF or intravitreal triamcinolone (Sohn et al. 2011). In this study, MIG/CXCL9 levels decreased significantly in the dexamethasone group compared with baseline. As a member of the CXC chemokine family, MIG/CXCL9 is a chemokine, which is a T‐cell chemo‐attractant induced by tumour necrosis factor‐alpha. It was recently investigated in mouse models to play a role in the development of diabetic nephropathy (Zychowska et al. 2015). Levels of both IL‐8 and IP‐10 (Interferon gamma‐induced protein 10), members of CXC chemokine family, were demonstrated to be higher in patients with DME compared with control groups in previous studies (Sohn et al. 2011). A recent study associated higher level of IL‐8 with therapy responsiveness, with higher levels of IL‐8 in the therapy refractory group under anti‐VEGF treatment (Kwon & Jee 2018). Furthermore, the CXC chemokine family is known for its role in angiogenesis (Romagnani et al. 2004). We did not observe a change in IL‐8 levels during treatment in either group.
The MCP‐1 is a member of the CC chemokine family and is one of the key chemokines that regulate migration and infiltration of monocytes and macrophages (Deshmane et al. 2009). Upregulation or a polymorphism of the MCP‐1 in patients with DME and proliferative diabetic retinopathy (PDR) has been demonstrated in previous studies (Funatsu et al. 2009; Roh et al. 2009; Oh et al. 2010; Dong et al. 2014). Results of the current study indicate that MCP‐1 was affected by the dexamethasone implant at the first follow‐up for aqueous samples but was not statistically significantly reduced later. That may be explained by the complex pathway of MCP‐1 activation and newly investigated polymorphisms, which are not fully understood in various diseases and may not be fully affected by the application of dexamethasone in the vitreous (Jo et al. 2003; Deshmane et al. 2009).
Intercellular adhesion molecule 1 and VCAM‐1 are adhesion molecules, which can be induced by interleukin‐1 (IL‐1) and tumour necrosis factor, and are expressed by the vascular endothelium, macrophages and lymphocytes. They facilitate leucocyte‐endothelial transmigration and play an essential role in diabetic vascular leakage and capillary non‐perfusion and can be pro‐angiogenetic factors (Jo et al. 2003; Tang & Kern 2011). Current data suggest that VCAM‐1 and ICAM‐1 levels are elevated in diabetic patients with microalbuminuria and diabetic retinopathy (Meleth et al. 2005; Karimi et al. 2018). Therefore, it may be useful as a prognostic factor; however, a study by Ugurlu et al (2013) did not reveal a difference in ICAM‐1 and VCAM‐1 levels in diabetic patients with and without diabetic retinopathy. It must be considered that the levels of these adhesion molecules may vary among patients; therefore, statistical significance may not be evident in small cohorts. In our study, we demonstrated the anti‐inflammatory influence of dexamethasone on sICAM‐1 and sVCAM‐1 levels. It can be argued that these factors may be useful for treatment monitoring in patients with DME. These results on sICAM‐1 match the results of a study where ICAM‐1 was found to be asscociated with disease severity of macular oedema (Hillier et al. 2017).
A homologue of VEGF and PIGF plays a role as an angiogenesis mediating factor in diabetic retinopathy. PlGF can affect the growth, migration and survival of endothelial cells via different mechanisms, such as binding directly to the vascular endothelial growth factor receptor‐1 (VEGFR‐1), displacing VEGF‐A from the transmembrane or soluble VEGFR‐1, activating more VEGFR‐2 via VEGF‐A and by the formation of a PlGF/VEGF‐A complex (Van Bergen et al. 2019). Knockout mice models suggest that the deletion of PIGF prevents diabetic retinopathy (Huang et al. 2015). Furthermore, PIGF seems to be associated with diabetes‐activated hypoxia‐inducible factor‐1α‐VEGF pathway inhibition. In anti‐VEGF treatment, arm levels of VEGF and PIGF dropped after injections; therefore, a relation of the two factors is conclusive, as mentioned above (Fig. 2). Conversely, a study analysing patients with PDR receiving anti‐VEGF treatment did not demonstrate any changes in PIGF levels under treatment, although PIGF levels were elevated in patients with active PDR compared with non‐active PDR (Al Kahtani et al. 2017). It can be hypothesized that these differences may be caused by different mechanisms in DME and PDR. A combination therapy of VEGF and PIGF inhibition may provide superior outcomes in certain patients (Nguyen et al. 2018).
Figure 2.
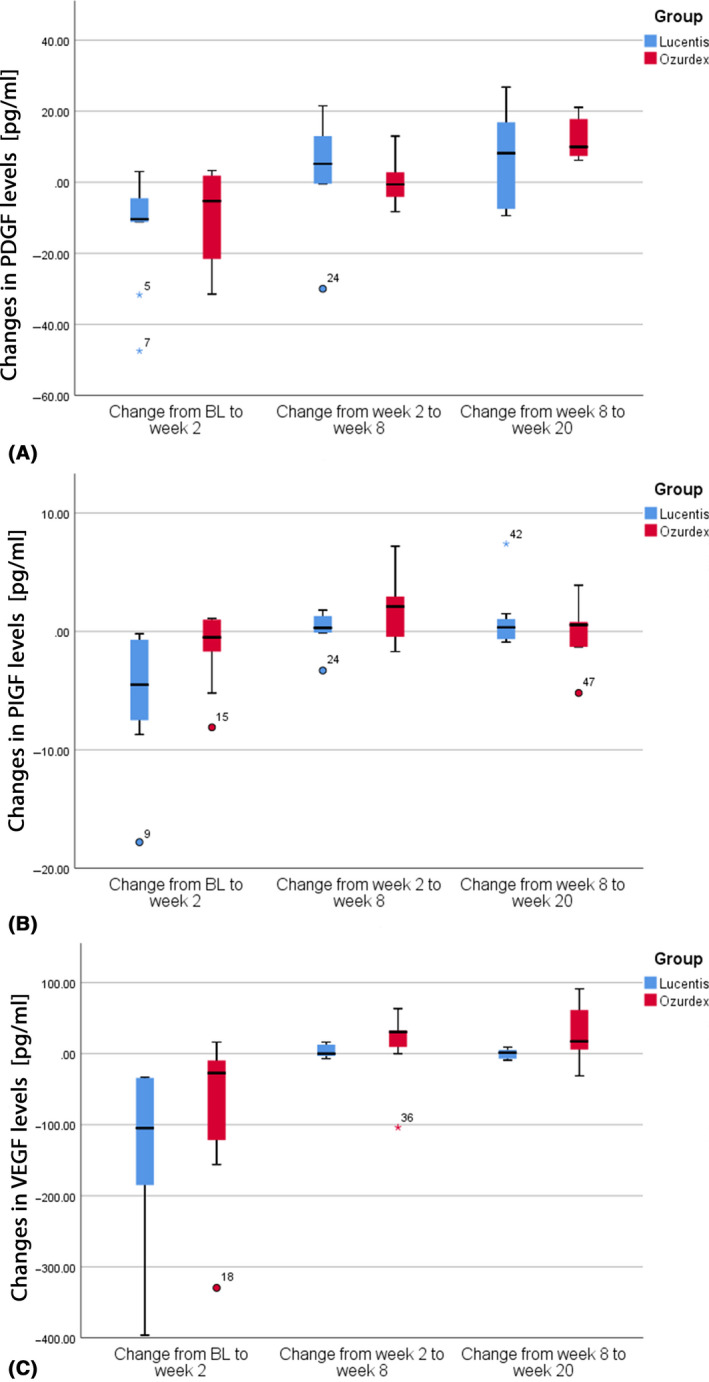
Box plots demonstrating changes in PIGF (placenta growth factor), PDGF (platelet‐derived growth factor) and VEGF (vascular endothelial growth factor) for ranibizumab (blue) and dexamethasone implant (red), respectively.
In a previous study, triamcinolone showed an effect on VEGF by decreasing its levels after injections (Sohn et al. 2011). In experimental settings, corticosteroids inhibited vascular permeability via ICAM‐1 or VEGF downregulation (Wang et al. 2008). We did not see this effect on VEGF but on sICAM‐1. It can be hypothesized that this is due to using dexamethasone, not triamcinolone as in previous studies, or the sample size was too small to detect this change.
Recently, a study has demonstrated a correlation between serous cytokine levels and the morphological response during anti‐VEGF treatment in patients with DME (Brito et al. 2018). We observed some trends in our study. However, there were individual changes in several cytokine levels over time. In the ranibizumab group, two patients were identified, showing an initial reduction of anti‐VEGF levels, an increase near week 2 and a recurrence of macular oedema, which responded well to the next ranibizumab injection. Furthermore, interleukin 6 and 8 levels were not observed to decrease as opposed to patients without repeated macular oedema.
In the Ozurdex group, we noticed two patients with decreasing cytokines levels, but without morphological or functional changes. Hyperreflective foci (HRF) in the macular cysts in these patients were revealed by optical coherence tomography (OCT). These well‐demarcated morphologic changes characteristic for DME have been described in previous studies and were suggested to represent extravasated lipoproteins, which are an early subclinical barrier breakdown sign in DME (Bolz et al. 2009). The HRF were found in several patients in both groups, but the patients without functional response showed HRF in the cysts and cyst borders (Fig. 3). It can be hypothesized HRF in retinal cysts seem to represent an advanced morphologic alteration that does not respond to dexamethasone (Jonas et al. 2012).
Figure 3.
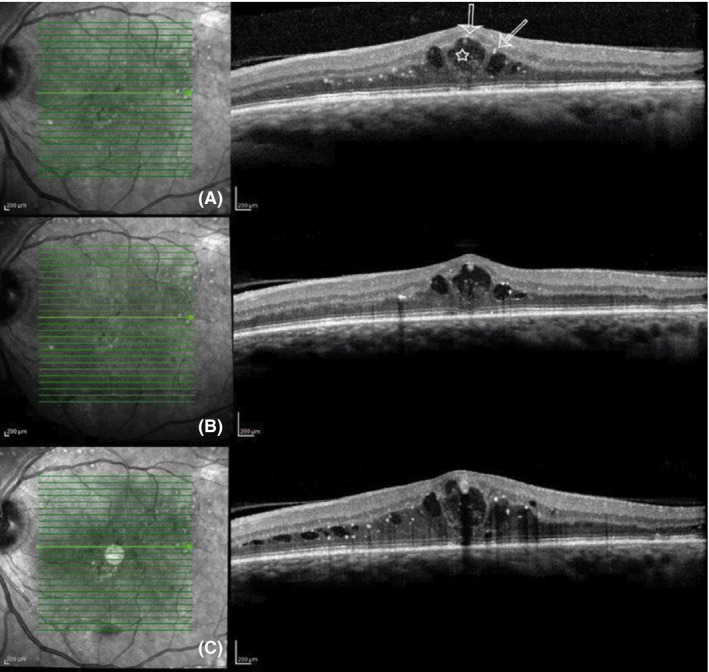
Example of a patient treated with a dexamethasone implant with cytokine response at week 2 and missing morphological improvement. (A) Baseline optical coherence tomography (OCT) scan. The arrows mark the hyperreflective foci at the cyst borders. The star marks the intraretinal partly fibrotic tissue. (B) OCT at week 2. Cytokine levels decreased, but morphological and functional outcomes remain similar. (C) OCT at week 20. Cytokine levels increased after three months. Increased clinically significant macular oedema.
In our study, a statistically significant difference in BCVA and CRT was not observed for the Ozurdex group, probably due to the small sample size. Previous studies, as described in a meta‐analysis, showed statistically significant effect on the CRT (He et al. 2018). A control group of subjects without DME was not included in the study, which could limit further interpretation. Previous studies show, that the concentrations of various cytokines were significantly different between healthy patients and patients with DME (Jonas et al. 2012; Kwon & Jee 2018). As we focused on the changes in cytokine levels during two different treatments regimens in DME, no control group was used.
Furthermore, only one dexamethasone treatment was performed in this study as the effects of the loading phase of both agents were observed. However, a significant impact of the therapeutic agent on retinal morphology and cytokine levels was indicated.
In a recently published study comparing the morphological changes and visual acuity of patients with persistent DME having an intravitreal injection of ranibizumab alone and combined with a dexamethasone implant, the results revealed that the combined therapy does not lead to a significantly higher increase in visual acuity than the ranibizumab monotherapy (Maturi et al. 2018). However, it must be considered that dexamethasone was administered after a loading dose with ranibizumab – as opposed to this study. Even in the cited trial with a follow‐up of only six months, there was a significantly higher and faster decrease in CRT in the combination group, which could have an impact on retreatment frequency and visual outcomes in the long term. Above all in chronic disease, such as DME, morphologic parameters detectable by OCT can be considered as more reliable end‐points than function, which depends on the individual duration and level of intraretinal and intracecullar alteration.
In conclusion, this proof of principle study identified cytokines and proteins that are sensitive to treatment with anti‐VEGF or dexamethasone and monitored the levels during the loading phase of 20 weeks. Ranibizumab has a significant, long‐acting impact on VEGF and PIGF levels. Dexamethasone has a fast‐acting impact on levels of additional cytokines and proteins, such as sICAM‐1, CXCL9/MIG, sVCAM‐1 and MCP‐1. Our findings underline several cytokines and proteins involved in the complex and diverse molecular pathway of the emergence of DME. Both agents, ranibizumab and dexamethasone, seem to address different pro‐inflammatory markers to a very individual degree that indicates a rationale for a combination therapy in cases of CSME. Further studies will be necessary to validate the results of our proof of principle study.
This study was supported with a research grant from Allergan.
MB reports consultancy for Allergan. All other authors have no financial interest to disclose.
References
- Al Kahtani E, Xu Z, Al RS et al. (2017): Vitreous levels of placental growth factor correlate with activity of proliferative diabetic retinopathy and are not influenced by bevacizumab treatment. Eye (Lond) 31: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz M, Schmidt‐Erfurth U, Deak G, Mylonas G, Kriechbaum K & Scholda C (2009): Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 116: 914–920. [DOI] [PubMed] [Google Scholar]
- Brito P, Costa J, Gomes N, Costa S, Correia‐Pinto J & Silva R (2018): Serological inflammatory factors as biomarkers for anatomic response in diabetic macular edema treated with anti‐VEGF. J Diabetes Complications 32: 643–649. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S & Sawaya BE (2009): Monocyte chemoattractant protein‐1 (MCP‐1): an overview. J Interferon Cytokine Res 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Lv XY, Wang BJ, Wang YQ, Mu H, Feng ZL & Liu P (2014): Association of monocyte chemoattractant protein‐1 (MCP‐1)2518A/G polymorphism with proliferative diabetic retinopathy in northern Chinese type 2 diabetes. Graefes Arch Clin Exp Ophthalmol 252: 1921–1926. [DOI] [PubMed] [Google Scholar]
- Dong N, Xu B, Chu L & Tang X (2015): Study of 27 aqueous humor cytokines in type 2 diabetic patients with or without macular edema. PLoS ONE 10: e0125329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu H, Noma H, Mimura T, Eguchi S & Hori S (2009): Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 116: 73–79. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Lim LL, Campain A et al. (2014): A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 121: 2473–2481. [DOI] [PubMed] [Google Scholar]
- Hang H, Yuan S, Yang Q, Yuan D & Liu Q (2014): Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol Vis 20: 1137–1145. [PMC free article] [PubMed] [Google Scholar]
- He Y, Ren XJ, Hu BJ, Lam WC & Li XR (2018): A meta‐analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti‐vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol 18: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier RJ, Ojaimi E, Wong DT et al. (2017): Aqueous humor cytokine levels as biomarkers of disease severity in diabetic macular edema. Retina (Philadelphia, Pa.) 37: 761–769. [DOI] [PubMed] [Google Scholar]
- Hillier RJ, Ojaimi E, Wong DT et al. (2018): Aqueous humor cytokine levels and anatomic response to intravitreal ranibizumab in diabetic macular edema. JAMA Ophthalmol 136: 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, He J, Johnson D et al. (2015): Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1alpha‐VEGF pathway inhibition. Diabetes 64: 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo N, Wu GS & Rao NA (2003): Upregulation of chemokine expression in the retinal vasculature in ischemia‐reperfusion injury. Invest Ophthalmol Vis Sci 44: 4054–4060. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Jonas RA, Neumaier M & Findeisen P (2012): Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina (Philadelphia, Pa.) 32: 2150–2157. [DOI] [PubMed] [Google Scholar]
- Karimi Z, Kahe F, Jamil A et al. (2018): Intercellular adhesion molecule‐1 in diabetic patients with and without microalbuminuria. Diabetes Metab Syndr 12: 365–368. [DOI] [PubMed] [Google Scholar]
- Kwon JW & Jee D (2018): Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti‐VEGF treatment. PLoS ONE 13: e0203408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R, Weger M, Haller‐Schober EM et al. (2008): Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis 14: 637–643. [PMC free article] [PubMed] [Google Scholar]
- Maturi RK, Glassman AR, Liu D et al. (2018): Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR Network Phase 2 Randomized Clinical Trial. JAMA Ophthalmol 136: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meleth AD, Agron E, Chan CC et al. (2005): Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci 46: 4295–4301. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, De Falco S, Behar‐Cohen F et al. (2018): Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol 96: e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh IK, Kim SW, Oh J, Lee TS & Huh K (2010): Inflammatory and angiogenic factors in the aqueous humor and the relationship to diabetic retinopathy. Curr Eye Res 35: 1116–1127. [DOI] [PubMed] [Google Scholar]
- Roh MI, Kim HS, Song JH, Lim JB & Kwon OW (2009): Effect of intravitreal bevacizumab injection on aqueous humor cytokine levels in clinically significant macular edema. Ophthalmology 116: 80–86. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Lasagni L, Annunziato F, Serio M & Romagnani S (2004): CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol 25: 201–209. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Garcia‐Arumi J, Bandello F et al. (2017): Guidelines for the management of diabetic macular edema by the european society of retina specialists (EURETINA). Ophthalmologica 237: 185–222. [DOI] [PubMed] [Google Scholar]
- Schwartz SG, Flynn HW Jr & Scott IU (2014): Emerging drugs for diabetic macular edema. Expert Opin Emerg Drugs 19: 397–405. [DOI] [PubMed] [Google Scholar]
- Sohn HJ, Han DH, Kim IT, Oh IK, Kim KH, Lee DY & Nam DH (2011): Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol 152: 686–694. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Otsuka H & Sonoda Y (2014): Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina (Philadelphia, Pa.) 34: 741–748. [DOI] [PubMed] [Google Scholar]
- Tang J & Kern TS (2011): Inflammation in diabetic retinopathy. Prog Retin Eye Res 30: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurlu N, Gerceker S, Yulek F, Ugurlu B, Sari C, Baran P & Cagil N (2013): The levels of the circulating cellular adhesion molecules ICAM‐1, VCAM‐1 and endothelin‐1 and the flow‐mediated vasodilatation values in patients with type 1 diabetes mellitus with early‐stage diabetic retinopathy. Intern Med 52: 2173–2178. [DOI] [PubMed] [Google Scholar]
- Van Bergen T, Etienne I, Cunningham F, Moons L, Schlingemann RO, Feyen JHM & Stitt AW (2019): The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases. Prog Retin Eye Res 69: 116–136. [DOI] [PubMed] [Google Scholar]
- Vujosevic S & Simo R (2017): Local and systemic inflammatory biomarkers of diabetic retinopathy: an integrative approach. Invest Ophthalmol Vis Sci 58: Bio68–bio75. [DOI] [PubMed] [Google Scholar]
- Wang K, Wang Y, Gao L, Li X, Li M & Guo J (2008): Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin‐induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule‐1 expression. Biol Pharm Bull 31: 1541–1546. [DOI] [PubMed] [Google Scholar]
- Yu SY, Nam DH & Lee DY (2018): Changes in aqueous concentrations of various cytokines after intravitreal bevacizumab and subtenon triamcinolone injection for diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 256: 39–47. [DOI] [PubMed] [Google Scholar]
- Zychowska M, Rojewska E, Pilat D & Mika J (2015): The role of some chemokines from the CXC subfamily in a mouse model of diabetic neuropathy. J Diabetes Res 2015: 750182. [DOI] [PMC free article] [PubMed] [Google Scholar]


