Abstract
Both phototropins (phot1 and phot2) and cryptochromes (cry1 and cry2) were proven as the Arabidopsis thaliana blue light receptors. Phototropins predominately function in photomovement, and cryptochromes play a role in photomorphogenesis. Although cryptochromes have been proposed to serve as positive modulators of phototropic responses, the underlying mechanism remains unknown. Here, we report that depleting sucrose from the medium or adding gibberellic acids (GAs) can partially restore the defects in phototropic curvature of the phot1 phot2 double mutants under high‐intensity blue light; this restoration does not occur in phot1 phot2 cry1 cry2 quadruple mutants and nph3 (nonphototropic hypocotyl 3) mutants which were impaired phototropic response in sucrose‐containing medium. These results indicate that GAs and sucrose antagonistically regulate hypocotyl phototropism in a cryptochromes dependent manner, but it showed a crosstalk with phototropin signaling on NPH3. Furthermore, cryptochromes activation by blue light inhibit GAs synthesis, thus stabilizing DELLAs to block hypocotyl growth, which result in the higher GAs content in the shade side than the lit side of hypocotyl to support the asymmetric growth of hypocotyl. Through modulation of the abundance of DELLAs by sucrose depletion or added GAs, it revealed that cryptochromes have a function in mediating phototropic curvature.
The concluded function of cryptochromes in hypocotyls phototropism is controversial. By phenotypic analysis of mutant lines grown on sucrose free medium or medium with added gibberellic acids, we demonstrated that cryptochromes‐mediated hypocotyl phototropism was regulated antagonistically by gibberellin and sugar. This process showed a crosstalk with phototropin signaling on NPH3.

Edited by: Hongtao Liu, Institute of Plant Physiology and Ecology, CAS, China
INTRODUCTION
Phototropism is a growth response in which plants orient their photosynthetic organs to maximize light perception (Goyal et al. 2013; Hohm et al. 2013; Goyal et al. 2016), and achieved by the asymmetric distribution of the phytohormone auxin, which causes asymmetric growth and consequent bending of plant organs (Whippo and Hangarter 2006; Ding et al. 2011; Christie and Murphy 2013; Liscum et al. 2014). Recent studies have identified several genes involved in blue light (BL)‐induced hypocotyl phototropism in Arabidopsis. The BL receptors phototropin1 (phot1) and phot2 localize at the inner surface of the plasma membrane, and are essential for detecting the direction of light and inducing the asymmetric distribution of auxin (Christie et al. 1998; Kagawa et al. 2001; Esmon et al. 2006; Fankhauser and Christie 2015). Phot1 regulates the pulse light‐induced first positive phototropic response and the continuous light‐induced second positive phototropic response to the broad range of wavelengths of BL (Sakai et al. 2001; Haga et al. 2015). By contrast, phot2 only mediates hypocotyl phototropism in response to high‐intensity blue light (HBL) (Sakai et al. 2001; Inada et al. 2004; Zhao et al. 2013).
Ongoing work has identified additional protein components involved in phototropin signaling. For example, both NONPHOTOTROPIC HYPOCOTYL 3 (NPH3) and ROOT PHOTOTROPISM 2 (RPT2) belonging to the NPH3/RPT2‐like (NRL) family of BTB (Broad complex, Tramtrack, and Bric‐à‐brac) domain proteins are essential for the early steps of phototropin signal transduction (Motchoulski and Liscum 1999; Lariguet et al. 2006; Pedmale and Liscum 2007; Demarsy et al. 2012; Haga et al. 2015; Zhao et al. 2018b). The dephosphorylation of NPH3, which requires not only the kinase activity of phot1 but also a light stimulus is essential for phototropism (Petersen et al. 2017). Other proteins involved in hypocotyl phototropism include PHYTOCHROME KINASE SUBSTRATE (PKS) protein families (PKS1 to PKS4) and ATP‐BINDING CASSETTE B 19 (ABCB19) (Lariguet et al. 2006; Nagashima et al. 2008; Schepens et al. 2008; de Carbonnel et al. 2010). In Arabidopsis seedlings, a lateral auxin gradient forms across the hypocotyl and promotes asymmetric growth, which in turn causes phototropic bending (Stowe‐Evans et al. 2001; Esmon et al. 2006; Nagashima et al. 2008). The DR5rev::GFP auxin reporter gene and fluorescent auxin analog, NBD‐NAA are commonly used for determination of auxin flow during phototropic responses (Sakai 2019). However, the mechanisms that link phototropin activity to the changes in auxin mobilization remain largely unknown.
Cryptochromes (cry1 and cry2) blue‐light photoreceptors, mediate the suppression of hypocotyl elongation and the promotion of cotyledon expansion and root growth (Ma et al. 2016; He et al. 2019; Sakaguchi et al. 2019). Cry2 is predominantly nuclear protein (Lin and Todo 2005; Stone et al. 2005; Mazur et al. 2019) and cry1 locates both in the nucleus (Mazur et al. 2019) and cytoplasm (Wu and Spalding 2007). Blue light‐dependent CRY signal transduction via CRY‐CIBs modulation of transcription, and the CRY‐SPA1/COP1 suppression of proteolysis (Liu et al. 2011). In addition, a E3 ubiquitin ligase—ZEITLUPE (ZTL)/LOVKELCH PROTEIN 2 (LKP2)/FLAVIN‐BINDING KELCHREPEAT F‐BOX 1 (FKF1) has been identified as blue‐light photoreceptors, which mediates ubiquitin‐dependent protein degradation under blue‐light induction, and the protein turnover mediated by FKF1/ZTL/LKP2 is crucial for the regulation of the circadian clock and flowering time (Zoltowski and Imaizumi 2014; Lee et al. 2018). Plants lacking cryptochromes show attenuation of the first‐positive curvature and plants with overexpression of the cryptochrome genes show hypersensitivity to BL in first‐positive phototropism (Ahmad et al. 1998; Lascève et al. 1999), indicating that cryptochromes may have a modifying or indirect effect on phototropism, as phyA does in Arabidopsis (Janoudi et al. 1997; Wan et al. 2008). Interestingly, cryptochromes are not required for low or high‐light induction of second‐positive phototropism (Ahmad et al. 1998; Lascève et al. 1999; Ohgishi et al. 2004). However, Whippo and Hangarter (2003) found by kinetic and mechanical analysis of the second‐positive phototropism that HBL (100 μmol · m−2 s−1) attenuates phototropism and low‐intensity BL (< 0.1 μmol · m−2 s−1) enhances phototropism, depending on phototropins and cryptochromes respectively. These results indicate that cryptochromes function in phototropin‐mediated phototropism, which is controversial to previous publications. The previous published study of Arabidopsis phototropism indicates that the activation of cryptochromes induces the expression of ROOT PHOTOTROPISM PROTEIN 2 (RPT2), which encodes a signal transduction protein that functions in phototropin‐mediated phototropism. Meantime, the activation of cryptochromes suppresses the expression of ABCB19 and causes a reduction in the levels of gibberellic acids (GAs), and thus affecting the hypocotyl phototropic response (Sakai et al. 2000; Whippo and Hangarter 2006; Zhao et al. 2007a; Nagashima et al. 2008; Tsuchida‐Mayama et al. 2010), but the molecular mechanisms underlying this process remains scant.
Phototropic curvature is the consequence of the cell elongation on the shaded side of the hypocotyl (Fankhauser and Christie 2015). Light and GAs antagonistically control hypocotyl elongation in etiolated seedlings (Zhao et al. 2007a; Tsuchida‐Mayama et al. 2010; Hedden and Sponsel 2015; Li et al. 2016b; Wang et al. 2018; Schrager‐Lavelle et al. 2019). CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) a key negative regulator in light signaling that physically interacts with cryptochromes and phytochromes to regulate light‐suppression of hypocotyl elongation (Jia et al. 2014). Whereas, in darkness, COP1 directly targets WAVE‐DAMPENED 2‐LIKE 3 (WDL3) to promote hypocotyl cell elongation (Lian et al. 2017). DELLA proteins are a group of master transcriptional regulators in GAs signaling pathway (Li et al. 2016a,b). GAs promote hypocotyl elongation through the formation of the GA‐GID1‐DELLA complex and the induction of the rapid degradation of DELLAs releasing the PHYTOCHROME INTERACTING FACTOR (PIF)‐family basic helix‐loop‐helix transcription factors PIF3 and PIF4. Surprisingly, a GA biosynthesis inhibitor (paclobutrazol, PAC) partially recovered the hypocotyl phototropic defect in the phyA phyB cry1 cry2 plants, and GAs specifically repressed hypocotyl phototropism in the phyA cry1 cry2 mutant (Tsuchida‐Mayama et al. 2010). However, both PAC and GAs are not showing any suppression effects in the WT, nor in cry1 cry2 or phot1 phot2 mutants. These results suggest that phyA is required for the actions of GAs during hypocotyl phototropism. Analysis of GA contents further showed that the accumulation of GAs is reduced by the activities of the cryptochromes (Zhao et al. 2007a; Tsuchida‐Mayama et al. 2010; Hirose et al. 2012). Therefore, it is possible that cryptochromes act by controlling the level of GAs to promote hypocotyl elongation and release the suppression of hypocotyl phototropism in the phot1 phot2 mutant, although genetic and photobiological evidence has indicated that light‐mediated hypocotyl phototropism and elongation are two distinct processes (Cosgrove 1985; Liscum and Briggs 1995; Zhao et al. 2018a).
Here, we determine whether cryptochromes modulate hypocotyl phototropism by controlling the level of GAs in Arabidopsis, and uncover the molecular action of cryptochromes in phototropism. We found that the phototropic defects of the phot1 phot2 mutant were partly restored when the plants were grown on sucrose free medium or GAs containing medium. Given that GAs and sugar control the balance of DELLAs (Li et al. 2014), we measured the changes of the DELLAs abundances and confirmed that GAs and sucrose act to balance the level of DELLAs to control hypocotyl phototropism. This regulation is dependent on cryptochromes and independent of phototropins; moreover, it indicates the existence of crosstalk between cryptochrome and phototropin signaling on NPH3. Our results indicated that cryptochromes play dual roles in regulating hypocotyl phototropism by controlling the abundance of DELLA proteins, which negatively regulate hypocotyl curvature.
RESULTS
Cryptochromes positively regulate phototropism independent of phototropins
Phototropic curvature of the hypocotyl is mainly mediated by phototropins (phot1 and phot2) (Lascève et al. 1999; Sakai et al. 2001; Ohgishi et al. 2004). However, hypocotyl curvature is attenuated in the cry1 cry2 mutant plants in the first positive phototropic curvature (Ahmad et al. 1998; Lascève et al. 1999). This inspired us to pursue the role of cryptochromes during the process of the phototropic response. To test if cryptochromes act synergistically with phototropins to regulate phototropism, we examined hypocotyl curvature in seedlings expressing cryptochromes and phototropins and in the various mutants. The 3‐d‐old etiolated seedlings were transferred to medium with or without 3% sucrose and irradiated with continuous lateral blue light of 0.01, 0.1, 1, 10, and 100 µmol · m−2 s−1 respectively for 12 h. We found that wild‐type and phot2 mutant seedlings showed similar BL‐dependent hypocotyl bending on sucrose‐containing medium and sucrose‐free medium (Figure 1A, B). Similar to other reports (Liscum and Briggs 1995; Sakai et al. 2001), we also did not detect any phototropism under low light conditions in phot1 single mutants, phot1 phot2 double mutants and phot1 phot2 cry1 cry2 quadruple mutants (Figure 1A, B). However, in contrast to previous publication (Sakai et al. 2001), we found that hypocotyl curvature was enhanced in phot1 mutants at 100 µmol · m−2 s−1, indicating that phot1 may partially inhibit phototropism under HBL (Zhao et al. 2013), but curvature was not affected by added sucrose (Figure 1).
Figure 1.

Functions of cryptochromes and phototropins in hypocotyl curvature induced by different intensities of blue light in the presence or absence of sucrose (A, C) Hypocotyl phototropism in 3‐d‐old etiolated seedlings of the wild type (WT) and phot1 phot2, cry1 cry2, and phot1 phot2 cry1 cry2 Arabidopsis mutants grown on the same vertical plates and exposed to blue light (BL) illumination at different intensities (from 0.01 to 100 μmol · m−2 s−1) for 12 h in medium with sucrose (A) or without sucrose (C). (B) Hypocotyl curvature measured as the change in hypocotyl angle determined from an analysis of etiolated seedlings of A. The values are the means ± SD of three independent experiments (28–31 seedlings each). (D) Hypocotyl curvature measured as the change in hypocotyl angle determined from an analysis of etiolated seedlings of (C). The values are the means ± SD of three independent experiments (34–36 seedlings each). Scale bar = 5 mm.
Interestingly, the phototropic defects of the phot1 phot2 mutants were partially restored as the plants were grown on sucrose‐free medium, only under HBL (>1 μmol · m−2s−1) (Figure 1C, D). By contrast, under these blue light condition, phototropism was not observed in phot1 phot2 double mutants in sucrose‐containing medium (Figure 1A, B) (Sakai et al. 2001; Zhao et al. 2013). These results showed that cryptochromes function as positive modulators of phototropic responses and act independent of phototropins because the impaired phototropic response of the phot1 phot2 cry1 cry2 quadruple mutant seedlings could not be recovered by depleting sucrose from the medium, compared to the phot1 phot2 double mutants (Figure 1C, D). Consistently, the differences are not apparent in cry1 cry2 mutants under sucrose‐containing medium compared to sucrose‐free medium. Therefore, we concluded that the function of cryptochromes in mediating hypocotyl phototropism is masked by sucrose. A further detailed understanding of how sucrose conducts its function during this process will, therefore, be the key to unlocking the molecular mechanism of cryptochrome mediated phototropic growth.
NPH3 contributes to cryptochrome‐mediated hypocotyl phototropism
Collectively, the above‐described results demonstrate a clear negative correlation between sucrose and crys‐mediated hypocotyl phototropic curvature. There are studies showing that NPH3 (NON‐PHOTOTROPIC HYPOCOTYL 3) and RPT2 (ROOT PHOTOTROPISM 2) are playing a central role during hypocotyl phototropism, based on the fact that mutant nph3‐6 and rpt2‐2 seedlings exhibit defects in hypocotyl phototropic curvature (Motchoulski and Liscum 1999; Sakai et al. 2000; Inada et al. 2004; Tsuchida‐Mayama et al. 2008; Haga et al. 2015). Furthermore, the PHYTOCHROME KINASE SUBSTRATE (PKS) proteins interact with phototropins, and mediate HBL‐induced hypocotyl bending (Lariguet et al. 2006; de Carbonnel et al. 2010; Zhao et al. 2013). To explore whether NPH3, RPT2 and PKSs also function in cryptochrome‐mediated phototropism, the experimental sucrose‐free assay with nph3‐6, rpt2‐2, and pks1 pks2 pks4 mutant seedlings were conducted. The nph3‐6, rpt2‐2, and pks1 pks2 pks4 seedlings were grown alongside with their corresponding wild‐type seedlings on sucrose‐free medium under dark conditions for 3 d, after which they were transferred to medium with or without 3% sucrose and irradiated with continuous lateral BL of 0.01, 0.1, 1, 10, and 100 µmol · m−2 s−1 for 12 h. The results indicated that the rpt2‐2 mutant seedlings showed phototropism only under weak BL intensities (< 0.01 μmol · m−2 s−1) (Figure 2A, B). However, in the absence of sucrose, hypocotyl curvature of the rpt2‐2 mutant was induced by a much broader range of BL fluence rates from 0.01 to 10 μmol m−2 s−1 or higher (Figure 2C, D).
Figure 2.

Effects of sucrose on hypocotyl curvature in wild‐type and different mutant seedlings (A, C) Hypocotyl phototropism of 3‐d‐old etiolated seedlings of the wild type (WT) and rpt2‐2, nph3‐6, phot1 phot2, and pks1 pks2 pks4 Arabidopsis mutants grown on the same vertical plates and exposed to BL illumination at different intensities (from 0.01 to 100 μmol · m−2 s−1) for 12 h in medium with sucrose (A) or without sucrose (C). (B) Hypocotyl curvature measured as the change in hypocotyl angle determined from an analysis of etiolated seedlings in (A). The values are the means ± SD of three independent experiments (25–28 seedlings each). (D) Hypocotyl curvature measured as the change in hypocotyl angle determined from an analysis of etiolated seedlings in (C). The values are the means ± SD of three independent experiments (32–35 seedlings each). Scale bar = 5 mm.
Interestingly, the hypocotyl phototropic curvature of the pks1 pks2 pks4 triple mutant was also enhanced when plants were grown on sucrose‐free medium (Figure 2), which were consistent with that phot1 phot2 mutant. By contrast, the nph3‐6 mutant showed phototropic defects, regardless of the presence or absence of sucrose (Figure 2). These results implied that NPH3 may regulate cryptochrome‐mediated phototropism, while RPT2 and PKSs are not involved in the cryptochrome‐mediated phototropic response. However, there is no efficient evidence to explain why rpt2‐2 was sensitive to a broad range of BL intensity which is three times that of others.
GA3 regulates cryptochrome‐mediated hypocotyl phototropism
Previous studies have indicated that BL irradiation caused the levels of auxin and GAs to decrease in etiolated Arabidopsis seedlings (Zhao et al. 2007a; Nagashima et al. 2008). Therefore, we speculated that cryptochromes may regulate phototropism by modulating the synthesis or accumulation of these hormones. It is possible that inhibition of GA signaling by cryptochromes may inhibit hypocotyl phototropism in the phot1 phot2 mutant. After analyzing the effect of GAs on phototropism in the phot1 phot2, cry1 cry2, and phot1 phot2 cry1 cry2 mutants under low‐ and high‐intensity of BL, we found that the phot1 phot2 mutant showed a slight recovery of the phototropic response when GA3 was added into sucrose‐containing medium (Figure 3A, B). By contrast, GA3 treatment (1 or 10 µM) did not cause hypocotyl curvature in phot1 phot2 seedlings under low‐fluence BL (Figure 3C, D). The phot1 phot2 cry1 cry2 mutant seedlings exhibited phototropic defects in all of the above conditions, at the presence or absence of GA3, and under low‐ and high‐intensity BL (Figure 3). Thus, these results revealed that cryptochromes play a specific role in regulating HBL‐induced phototropism mediated by GAs.
Figure 3.

Effects of sucrose and GA3 on hypocotyl phototropism in wild‐type seedlings and the blue light photoreceptor mutants (A, C) Hypocotyl phototropism in 3‐d‐old etiolated seedlings of the wild type (WT) and phot1 phot2, cry1 cry2, and phot1 phot2 cry1 cry2 Arabidopsis mutants grown on the same vertical plates and exposed to BL illumination at a fluence rate of 0.01 or 100 μmol · m−2 s−1 for 12 h in medium with sucrose (MS), without sucrose (MS‐S), or MS plus 1 (MS + 1 μM GA3) or 10 μmol · L−1 GA3 (MS + 10 μM GA3). (B) Hypocotyl curvature was measured as the change in hypocotyl angle determined from an analysis of etiolated seedlings in (A). The values are the means ± SD of three independent experiments (31–33 seedlings each). (D) Hypocotyl curvature was measured as the change in hypocotyl angle determined from an analysis of etiolated seedlings in (C). The values are the means ± SD of three independent experiments (29–32 seedlings each). Scale bar = 5 mm.
Effects of sucrose and GAs on hypocotyl phototropism is mediated by NPH3
Mutation of NPH3 or RPT2 leads to defects in hypocotyl phototropism induced by HBL. NPH3 and RPT2 bind to both PHOT1 and PHOT2, and their mutants showed similar phototropism defects as the phot1 phot2 double mutant under HBL (Motchoulski and Liscum 1999; Inada et al. 2004; Haga et al. 2015). To further elucidate the detailed molecular mechanism of NPH3 and RPT2 in hypocotyl phototropism, we examined the effects of GAs and sucrose on the phototropic response of the phot1 nph3‐6, phot2 nph3‐6, phot1 rpt2‐2, and phot2 rpt2‐2 mutants. The phototropic response of the rpt2‐2 and phot1 rpt2‐2 mutants was enhanced on medium without sucrose or with added GA3. However, the hypocotyl phototropism of the phot2 rpt2‐2 mutant could not be rescued obviously by depletion of sucrose or adding GA3 into the medium (Figure 4).
Figure 4.

Effects of sucrose and GA on hypocotyl phototropism mediated by NPH3 and RPT2 (A) Hypocotyl phototropism of 3‐d‐old etiolated seedlings of the wild type (WT) and nph3‐6, phot1 nph3‐6, and phot2 nph3‐6 Arabidopsis mutants grown on the same vertical plates and exposed to BL illumination at a fluence rate of 100 μmol · m−2 s−1 for 12 h in the presence of sucrose (MS), without sucrose (MS‐S), or MS plus GA3 (MS +GA3). (B) Hypocotyl curvature measured as the change in hypocotyl angle determined from an analysis of etiolated seedlings in (A). (C) Hypocotyl curvature of 3‐d‐old etiolated seedlings of the wild type (WT) and rpt2‐2, phot1 rpt2‐2, phot2 rpt2‐2, and phot1 phot2 Arabidopsis mutants grown on the same vertical plates and exposed to BL illumination at a fluence rate of 100 μmol · m−2 s−1 for 12 h in the presence of sucrose (MS), without sucrose (MS‐S), or MS plus GA3 (MS+GA3). (D) Hypocotyl curvature measured as the change in hypocotyl angle determined from an analysis of etiolated seedlings in (C). Scale bar = 5 mm.
Interestingly, the hypocotyl curvature of the phot1 nph3‐6 mutant was rescued by adding GA3 to the medium, but it was not recovered on medium without sucrose (Figure 4), suggesting that additional pathway mediated by phototropins and cryptochromes may exist for phototropism, and was inhibited by PHOT1. The phototropism response of the rpt2 mutant was similar to that of the phot1 phot2 double mutant in response to HBL, regardless of the existence of exogenous sucrose and GA3 (Figure 4C, D), which indicated that RPT2 may function downstream of phototropins instead of cryptochromes.
Sucrose and GA3 antagonistically regulate the abundance of DELLA proteins
Hypocotyl phototropism requires the promotion at the same time the inhibition of growth of the shaded and lit sides of the hypocotyl (Whippo and Hangarter 2003). Given that DELLA proteins act as hypocotyl growth repressors (Gallego‐Bartolome et al. 2011), we propose that sucrose and GA3 antagonistically regulate cryptochrome‐mediated hypocotyl phototropic response, possibly by controlling the abundance of DELLA proteins, which function as key regulators in sucrose‐specific signaling and as a point of integration for diverse metabolic and hormonal signals (Li et al. 2014). To test the hypothesis that sucrose and GA3 antagonistically regulate cryptochrome‐mediated hypocotyl phototropism indeed through regulation of the abundance of DELLA proteins, we analyzed the effect of sucrose on the stability of DELLAs. Remarkably, sucrose significantly inhibited the degradation of REPRESSOR OF GA1‐3, especially in the phot1 phot2 mutant compared to a sucrose‐free control (Figure 5B), indicating that phototropins may control the stability of DELLAs in the absence of sucrose. Thus, the phototropic defect of phot1 phot2 mutants may be due to the increase of DELLAs when exogenous sucrose is present.
Figure 5.
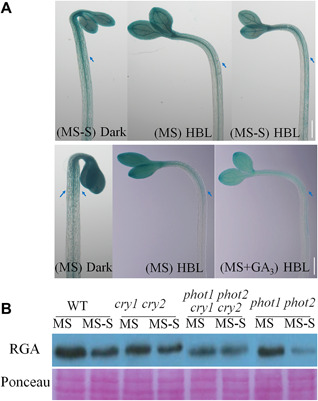
Auxin distribution of wild type in response to GA3 or Suc and the expression of DELLAs in the hypocotyls (A) GUS staining patterns in the DR5:GUS wild‐type (Col) seedlings. Three‐d‐old WT seedlings harboring DR5:GUS. From left to right: etiolated dark‐grown seedling (Dark), seedling grown in continuous 100 µmol · m−2s−1 blue light (HBL), HBL‐grown seedling in MS or MS‐S, HBL‐grown seedling with 10 µmol · L−1 GA3 (HBL+GA3). Arrowheads indicate the asymmetric expression of GUS in the hypocotyls. Scale bars = 5 mm. (B) RGA stability after addition of 90 mmol/L sucrose (MS+S) or without sucrose (MS‐S) in the WT and phot1 phot2, cry1 cry2, and phot1 phot2 cry1 cry2 mutant seedlings irradiated by high‐intensity blue light for 3 h. Ponceau staining and specific bands serve as equal loading controls. Scale bar = 1 mm.
Cryptochromes Mediate HBL‐induced phototropism via phytohormone signaling
To further document the role of the interaction of GAs and auxin in regulating the phototropic response of Arabidopsis, we tested the distribution of auxin between the shaded and lit sides of the hypocotyl by observing GUS staining in the hypocotyls of seedlings expressing the auxin reporter DR5::GUS (Ulmasov et al. 1997). In dark conditions, the wild‐type hypocotyls showed equal staining in a lateral direction, suggesting a symmetric distribution of auxin. Upon BL irradiation, the hypocotyls showed staining on the shaded side of the hypocotyl (Figure 5A), suggesting that the BL induced an asymmetric distribution of auxin. Furthermore, the phytohormones GA3 significantly increased the auxin transport from the cotyledons to the hypocotyl and promoted the accumulation of auxin on the shaded side of the hypocotyl (Figure 5A). Consistently, the accumulation of auxin was significantly higher on the shaded side of the hypocotyl by deleting sucrose from the medium (Figure 5A).
Involvement of the cryptochromes in the reduction of GA levels
To confirm that the impaired phototropism of the phot1 phot2 mutant seedlings was related to the accumulation of GAs, especially GA4, the major active form of GA in Arabidopsis (Yamaguchi 2008; Zhao et al. 2007b), we measured the levels of GAs in 3‐d‐old etiolated seedlings in response to HBL on sucrose‐containing and sucrose‐free medium. Consistent with previous reports (Tsuchida‐Mayama et al. 2010), we did find that HBL irradiation notably inhibited the accumulation of GA4 in the wild‐type and phot1 phot2 mutant seedlings. By contrast, we did not detect the change of GA4 by HBL irradiation in the cry1 cry2 or phot1 phot2 cry1 cry2 mutants, regardless the presence or absence of sucrose (Figure 6A). However, the effects of sucrose were less obvious. These results confirmed that the inhibition of GA4 accumulation was mediated by cryptochromes in response to HBL(Zhao et al. 2007a; Tsuchida‐Mayama et al. 2010). Therefore, it is possible that GAs partly restored the hypocotyl curvature of the phot1 phot2 mutant via reverse inhibitory effect of cryptochromes upon GA4 accumulation in response to HBL.
Figure 6.

GA and IAA contents in etiolated seedlings exposed to blue light in sucrose‐free and sucrose‐containing medium (A) GA4 content in 3‐d‐old etiolated seedlings exposed to 100 µmol · m−2s−1 of blue light for 6 h or no in sucrose‐free medium and 3% sucrose‐containing medium. (B) IAA content in 3‐d‐old etiolated seedlings of different mutants exposed to100 µmol · m−2s−1 of blue light for 6 h in sucrose‐free medium and 3% sucrose‐containing medium. (C) GA8, GA12, GA19, GA24 and GA34 content in 3‐d‐old etiolated seedlings exposed to 100 µmol · m−2s−1 of blue light for 6 h in sucrose‐free medium and 3% sucrose‐containing medium. Values are the means ± SD of three replicates. phot1 phot2 (P1P2), cry1 cry2 (C1C2), and phot1 phot2 cry1 cry2 (P1P2C1C2).
In addition, we characterized the levels of the other forms of GA and found that exogenous sucrose up‐regulated the contents of GA8 and GA12 independent of cryptochromes. We also found that the accumulation of GA24 and GA34 were induced in the cry1 cry2 and phot1 phot2 cry1 cry2 mutants in the presence or absence of exogenous sucrose, which indicated that the accumulation of GA24 and GA34 was not dependent on exogenous sucrose. This implied that the GAs might play an important role in regulating cryptochrome‐induced phototropism in response to high‐intensity blue light.
Following the activation of phototropins, an auxin gradient forms in the hypocotyl that precedes asymmetric growth (Esmon et al. 2006). The importance of auxin redistribution suggests that spatial auxin transport is a likely mechanism controlling phototropism (Rakusová et al. 2015). Young leaves (and cotyledons) are considered a major source of auxin production, which is then transported down towards to the lower parts of the plant, including stems (hypocotyls) and the roots (Tao et al. 2008). Consistent with this, our results showed that the contents of indole‐3‐acetic acid (IAA) were significantly induced by exogenous sucrose (Figure 6B).
Role of cryptochromes in the regulation of gene expression under blue light
To analyze the expression of genes related to hypocotyl elongation, which are downregulated by cryptochromes but upregulated by GA and auxin (Wang et al. 2016), we performed reverse transcription quantitative PCR (RT‐qPCR) and determined whether these genes might be regulated by sucrose‐mediated BL signaling. Indeed, some genes were expressed at significantly higher levels in sucrose‐containing medium than in sucrose‐free medium (Figure 7), including PRE6, SAUR9, and ABCB19. Moreover, upon irradiation with BL, these genes were expressed at much higher levels in the cry1 cry2 and phot1 phot2 cry1 cry2 mutants than in the WT or phot1 phot2 mutant (Figure 7).
Figure 7.

RT‐qPCR analysis of the expression of PRE6, SAUR9, IAA19, RPT2, ABCB19, and PIF4 in the hypocotyl of etiolated Arabidopsis seedlings under the different treatments (A–F) RT‐qPCR analysis of the expression of PRE6, SAUR9, IAA19, RPT2, ABCB19, and PIF4 in the hypocotyl of the WT and phot1 phot2, cry1 cry2, and phot1 phot2 cry1 cry2 mutant seedlings irradiated by high‐intensity blue light for 1 h or 3 h in the presence of sucrose (MS), without sucrose (MS‐S), and MS plus GA3 (MS+GA3). The values are the average of three independent experiments with SD.
We examined the role of GA3 in sucrose‐mediated regulation of these genes through RT‐qPCR and found that the genes specifically regulated by BL were expressed at a lower level in the cry1 cry2 and phot1 phot2 cry1 cry2 mutant seedlings exposed to BL in GA3‐containing medium than in sucrose‐containing medium; these genes included PRE6, ABCB19, and PIF4. Similarly, the BL‐inhibited genes were expressed at a lower level in the WT and the phot1 phot2 mutant than in the cry1 cry2 and phot1 phot2 cry1 cry2 seedlings exposed to blue light, and were not affected by GA3, such as SAUR9 and IAA19 (Figure 7). These results further support the involvement of sucrose and GAs in mediating the regulation by cryptochromes of the expression of phytohormone‐responsive genes.
DISCUSSION
The role of cryptochromes in regulating phototropism
Phototropism is an adaptive strategy that enables plants to maximize photosynthetic light capture under fluctuating light conditions (Stowe‐Evans et al. 2001; Hohm et al. 2013; Goyal et al. 2016). This is crucial during early‐stage seedling establishment and gap filling in dense canopy (Galen et al. 2007; Goyal et al. 2013). Phototropism is mainly induced by blue light signaling mediated by the phototropin receptors (Briggs et al. 2007), with PHOT1 functioning at wide range of light intensities and PHOT2 functioning at high light intensities. Blue light is also perceived by the cryptochromes, and previous studies have implied cryptochromes play a role during phototropism process, as phototropism is attenuated in the cry1 cry2 mutants, especially in the first positive phototropic curvature (Ahmad et al. 1998; Lascève et al. 1999). It was reported that expression of RPT2 and ABCB19 are altered in the cry mutants, and the hypocotyl phototropism of phyA cry1 cry2 and phyA phyB cry1 cry2 mutants was disrupted, mainly due to elevation of endogenous GA levels, even though exogenous GA application did not abolish hypocotyl phototropism of WT or the phyA and cry1 cry2 mutants (Nagashima et al. 2008; Tsuchida‐Mayama et al. 2008), only appears to partially suppress. Besides, a significant degree of curvature was observed in the phot1 phot2 double mutant at a fluence of 10 μmol · m−2 s−1 (Ohgishi et al. 2004), implying that cryptochromes either indirectly control the process by modulating blue light‐regulated growth or directly regulate phototropism in Arabidopsis. However, the exact module of how cryptochromes modulate phototropism and the underlying mechanisms remain elusive. In the present study, we analyzed the hypocotyl curvature of the phot1 phot2 double mutants under different growth conditions. Under lower intensity blue light (<1 μmol · m−2 s−1), we did not detect influence of sucrose on its phototropism (Figure 1). However, the phototropic defects of the phot1 phot2 double mutants were partly restored under high‐intensive blue light when exogenous sucrose was not present in the medium (Figure 1A, B). Moreover, the phot1 phot2 cry1 cry2 quadruple mutants showed a minimal phototropic response at all fluence rates of blue light examined, even in the absence of sucrose (Figure 1A, B), indicating that cryptochromes are required for Arabidopsis phototropism, and this response is dependent on the growth conditions, but independent of (and masked by) the phototropins. Given that CRY2 degradation is triggered by light (Guo et al. 1999; Whippo and Hangarter 2003), we propose that CRY1 may play a vital role in mediating the phototropic curvature in high‐light conditions.
It was previously established that phototropism is the balance between the differential growth response and the hypocotyl growth inhibition in a fluence rate‐dependent fashion (Whippo and Hangarter 2003). While GAs relieve the growth inhibition by promoting DELLAs degradation via the ubiquitin‐proteasome pathway in a dose‐dependent manner (Sasaki et al. 2003; Fu et al. 2004; Hauvermale et al. 2012; Gomez et al. 2019; Sanchez‐Montesino et al. 2019), sucrose stabilize DELLA proteins and inhibits growth (Li et al. 2014). We analyzed the effect of GAs on phototropism and found that the phototropic response of the phot1 phot2 mutant was slightly rescued by GA3 (Figure 3A, B) and, in contrast, the impaired phototropic response phenotype of the phot1 phot2 cry1 cry2 quadruple mutant was not affected by GAs (Figure 3A, B), similar to the effects of depleting sucrose from the medium (Figures 1, 2). We also found that sucrose induces anthocyanin biosynthesis in the cotyledons of the wild type, and the action of sucrose was antagonized by GA3 (data not shown). In sharp contrast, neither the sucrose nor GA3 treatment caused anthocyanin accumulation in the cry1 cry2 or phot1 phot2 cry1 cry2 mutants, which are consistent with the previous study (Li et al. 2014). Our results, thus, indicate that cry1 and cry2 are required for phototropic curvature in high‐light conditions, which is independent of phototropins, but is masked by the hypocotyl inhibition effects of sucrose. In the wild, this mechanism may help the plant optimize its growth for highest photosynthetic light quality.
Possible mechanisms by which cryptochromes regulate phototropism
We quantified the concentration of GAs and found that the major bioactive gibberellin GA4 was elevated in cry1 cry2 and phot1 phot2 cry1 cry2, in accordance with the previous hypothesis that CRY protein negatively regulate the cellular level of GAs (Zhao et al. 2007a; Tsuchida‐Mayama et al. 2010). However, no significant difference was found for the GA4 level in phot1 phot2 mutants on medium with and without sucrose (Figure 6A), indicating that the effect of depleting sucrose on restoration of phototropism was not related to GA. As demonstrated by previous study, GAs and sucrose control the balance of DELLAs (Li et al. 2014), which negatively regulate hypocotyl growth (Zhao et al. 2007b; Zheng et al. 2018), and this mediations appear to play a positive role in hypocotyl gravitropism in Arabidopsis. However, the function of the DELLA proteins in hypocotyl phototropism is not known. We suspect that the changes of DELLAs control hypocotyl phototropism and this process is induced by cryptochromes. Consistent with this hypothesis, sucrose significantly inhibited the degradation of RGA (REPRESSOR OF GA1‐3, one member of DELLAs), especially when the phot1 phot2 mutants were grown on the sucrose containing medium (Figure 5B), indicating that phototropins may contribute to the stability of DELLAs in the absence of sucrose. Compared to wild type plant, the abundance of RGA in cry1 cry2 and phot1 phot2 cry1 cry2 was slightly lower, regardless of the presence and absence of sucrose (Figure 5B). Thus, the phototropic defect of phot1 phot2 mutants is likely caused by the increased level of DELLAs when the etiolated seedlings were cultivated with exogenous sucrose. However, the hypocotyl curvature of phot1 phot2 cry1 cry2 was attenuated, even though hypocotyl growth inhibition is released by sucrose depletion and DELLA degradation. This indicated that blue light perceived by cryptochromes is necessary for Arabidopsis phototropism establishment and cryptochromes may act as the primary phototropic receptors upon relieving growth inhibition by sucrose depletion or GA3, possibly via regulating the abundance of DELLAs to influence on hypocotyl elongation (Whippo and Hangarter 2003; Wang et al. 2018), and the phototropic response of Arabidopsis hypocotyls under high light conditions, which is masked by phototropins.
Collective evidence has showed that the phototropin signaling pathway plays a pivotal role in regulating the asymmetric distribution of auxin and phototropism. It is possible that CRY1 and CRY2 interact primarily with the phototropin‐dependent signaling pathway responsible for asymmetric auxin distribution or through controlling the net growth rate of the illuminated and shaded sides of the hypocotyl (Figure 5A). Indeed, cryptochromes were reported to exert a negative effect on PHOT1 transcript and protein accumulation in light‐grown seedlings (Kang et al. 2008), indicating the integration of the two pathways. Meanwhile, CRY1 and CRY2 also promote the expression of RPT2 and PKS1 gene under high‐intensity blue light (Figure 7D; Tsuchida‐Mayama et al. 2010; Kami et al. 2012), while suppressing the accumulation of ABCB19 transcript and protein level (Figure 7E; Nagashima et al. 2008).
Cryptochrome‐mediated hypocotyl phototropism is regulated by NPH3
The nph3‐6, rpt2‐2, and pks mutants showed phototropic defects at high fluence rates of blue light in sucrose‐containing medium, which is similar to the phot1 phot2 double mutant (Motchoulski and Liscum 1999; Lariguet et al. 2006; Pedmale and Liscum 2007; Demarsy et al. 2012; Haga et al. 2015; Zhao et al. 2018b). We examined the contribution of RPT2, PKSs, and NPH3 to cryptochrome‐mediated phototropism. When sucrose was omitted from the medium, phototropism was induced in the rpt2‐2 mutant by fluence rates from 0.1 to 10 μmol · m−2 s−1 or higher (Figure 2), spanning two orders of magnitude, which was also observed in the phot1 phot2 double mutant. Interestingly, phototropism was also enhanced in the pks1 pks2 pks4 triple mutant when sucrose was removed. These results implied that while cryptochromes modulate hypocotyl phototropism via regulation of RPT2 and PKSs expression, which is in accordance with previous reports that CRY1 and CRY2 promote the expression of RPT2 and PKS1 under high‐intensity blue light (Figure 6B; Tsuchida‐Mayama et al. 2010; Kami et al. 2012). In contrast, the nph3‐6 mutant showed phototropic defects under all conditions tested (Figure 2). Similarly, the phototropic response of the phot2 rpt2‐2 mutant was not rescued by depletion of sucrose or adding GA3 into the medium (Figure 4). Given that phot2 and RPT2 regulate the stabilization and relocation of NPH3 to the plasma membrane to mediate hypocotyl phototropism (Haga et al. 2015; Zhao et al. 2018b). It's reasonable that the phenotype of phot2 rpt2‐2 mutants were similar with that of nph3‐6 mutant, the hypocotyl phototropism was not restored by depletion of sucrose or adding GA3.These results suggested that NPH3 mediate a crosstalk between cryptochrome and phototropin signaling to regulate hypocotyl phototropism. Interestingly, phototropism of the phot1 nph3‐6 mutant was rescued by added GA3, but not by depleting sucrose from the medium (Figure 4), which indicated that GAs may act downstream of NPH3 or in a parallel pathway to restore the phototropic response. However, the hypothesis needs to be further verified, and the regulatory machinery between cryptochromes and NPH3 remain to be elucidated in the future.
Taken together, our results indicate that cryptochrome activation by HBL to inhibit GA synthesis, thus stabilize DELLAs to inhibit hypocotyl growth. By contrast, the DELLAs were degraded by depleting sucrose from the medium to release the inhibitory of hypocotyl growth. So, unilateral HBL irradiation results in higher GAs content in the shade side than the lit side of hypocotyl to support the asymmetric growth of hypocotyl, especially in sucrose‐free medium. That is, releasing hypocotyl growth inhibition by depleting sucrose from the medium or adding GAs, cryptochromes mediate the phototropic curvature may function in sensing the direction of blue light (Figure 8). In addition, we found that GAs and sucrose antagonistically regulated cryptochrome‐mediated hypocotyl phototropism, which showed a crosstalk with phototropin signaling on NPH3.
Figure 8.

Model for phototropic adaptation mechanisms Continuous irradiation with high‐intensity blue light activates PHOT1, which interacts with NPH3‐RPT2 complexes, whereas PHOT2 regulates the reconstruction of the phot1‐NPH3 complex for its re‐localization back to the plasma membrane. The phot signal is transduced through the RPT2‐NPH3 complexes to induce the asymmetric distribution of auxin and asymmetric hypocotyl growth. On the other hand, upon HBL irradiation, cryptochromes inhibit the GA synthesis to stabilize DELLAs to control the PIFs transcription module in the regulation of hypocotyl growth. By contrast, sucrose blocks the GA‐mediated degradation of DELLAs to inhibit hypocotyl growth. So, it is possible that the release of hypocotyl growth inhibited via reducing the DELLA protein abundance by GAs or depletion of sucrose, supporting the bending growth.
MATERIALS AND METHODS
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia‐0 (Col‐0) was used as the wild type in this study. The phot1 (phot1‐5), phot2 (phot2‐1), phot1 phot2, rpt2‐2, phot1 rpt2‐2, and phot2 rpt2‐2 mutant seeds were kindly provided by Dr. Ken‐ichiro Shimazaki. The nph3‐6, phot1 nph3‐6, and phot2 nph3‐6 mutant seeds were kindly provided by Dr. Christian Fankhauser. The cry1 cry2 and phot1 phot2 cry1 cry2 mutant seeds were provided by Dr. Hong‐Quan Yang.
Seeds were surface sterilized and planted in Murashige and Skoog (MS) medium with 1.2% agar. Seeds were stratified at 4°C for 3 d and then exposed to white light for 24 h to induce uniform germination. After germination, the seedlings were incubated vertically for 3 d in darkness at 21–22°C. MS medium containing 1 or 10 µM GA3 were prepared for the treatments.
Measurement of hypocotyl curvature
Measurement of Arabidopsis hypocotyl phototropism was performed as described previously (Zhao et al. 2013) with slight modifications. To measure the phototropic curvature of hypocotyls, we irradiated 3‐d‐old etiolated seedlings with 1 or 100 μmol · m−2 s−1 unilateral blue light using blue light‐emitting diode lamps. The fluence rate was controlled by filters (film no. 72; Tokyo Butai Shoumei). After irradiation, photographs were taken and hypocotyl curvature was measured using e‐ruler software. Mean values and SD were calculated for each experiment.
Gene expression
Total RNA was extracted from Arabidopsis seedlings using TRIzol reagent (Invitrogen). Reverse transcription (RT) was performed using 5 μg of total RNA and Super Script II Reverse Transcriptase (Invitrogen). Gene expression was analyzed using RT‐PCR and quantitative (q) PCR according to previous methods (Zhao et al. 2013).
For qPCR analysis, the cDNA was diluted 10‐fold and used as a template. qPCR was performed with the Stratagene M33005 QPCR system using SYBR Green to monitor double‐stranded DNA products. ACTIN2 was used as an internal standard to normalize the data and amplified with the following primer pair: forward, 5’‐AACCACTATGTTCTCAGGCATCG‐3’; reverse 5’‐CCTGGACCTGCCTCATCATACT‐3’. The primers used for gene expression analysis by qPCR are listed in Table S1.
Immunodetection
Immunodetection was performed as previously described (Zhao et al. 2013). Briefly, proteins extracted from etiolated Arabidopsis seedling were subjected to SDS‐PAGE on an 8.0% gel and were electrophoretically transferred to polyvinylidene difluoride membranes (Roche). After blocking at room temperature for 2 h, the membranes were incubated with a monoclonal anti‐GFP antibody for 16 h at 4°C. The membranes were then washed three times for 10 min each in Tris‐buffered saline and Tween 20, reacted with a horseradish peroxidase‐conjugated anti‐goat IgG secondary antibody, and visualized using Lumi‐Light Western Blotting Substrate (Roche) in accordance with the manufacturer's instructions.
DR5::GUS staining
To determine the distribution of auxin, we used GUS staining driven by the DR5 promoter, an IAA‐responding promoter. The etiolated seedlings were incubated with GUS staining solution (100 mM Na‐phosphate buffer, pH 7.0, 2 mM K4Fe (CN)6, 2 mM K3Fe (CN)6, 0.1% Triton X‐100, and 1 mg/mL X‐Gluc). GUS staining was performed as previously reported using vacuum infiltration (Zhao et al. 2015). GUS‐stained hyalinized seedlings were photographed using a Leica MZ16 stereomicroscope with a PLAN‐APOX1 objective (Leica).
Phytohormone measurements
Phytohormone measurements were performed as previously described (Chen et al. 2012). Fresh etiolated Arabidopsis seedlings were harvested, weighed, immediately frozen in liquid nitrogen, and stored at −80°C until needed. To extract phytohormones, Arabidopsis seedlings (120 mg fresh weight) were frozen in liquid nitrogen and finely ground followed by extraction with modified Bieleski solvent (methanol/H2O, 80/20, v/v) at 4°C for 12 h. [2H5]IAA (15.0 ng/g), [2H2] GA4 (1.00 ng/g), [2H2] GA8 (1.00 ng/g), [2H2] GA12 (1.00 ng/g), [2H2] GA19 (1.00 ng/g), [2H2] GA24 (2.00 ng/g), and [2H2] GA34 (2.00 ng/g) were added to samples as internal standards (I.S.) prior to grinding. The purification of acidic phytohormones from plant matrix were performed by tandem solid‐phase extraction (SPE) followed by liquid–liquid extraction (LLE). Then, 3‐bromoactonyltrimethylammonium bromide (BTA) was applied to derive acidic phytohormones by incorporating a positively charged quaternary ammonium group. Afterwards, nano‐LC−ESI‐Q‐TOF‐MS platform was used for quantitative determination of BTA‐derived acidic phytohormones.
ACKNOWLEDGEMENTS
We thank Dr. Ken‐ichiro Shimazaki (Kyushu University, Japan) for providing the phot1 (phot1‐5), phot2 (phot2‐1), phot1 phot2, rpt2‐2, phot1 rpt2‐2 and phot2 rpt2‐2 mutants, Dr. Christian Fankhauser (University of Lausanne, Switzerland) for providing the nph3‐6, phot1 nph3‐6, phot2 nph3‐6, and pks1 pks2 pks4 mutant seeds, Jian Xu (Huazhong Agricultural University, China) for providing the DR5‐GFP and DR5‐GUS seeds. This work was supported by the National Key Research and Development Program of China (2016YFD0101900), Science and Technology Innovation Talents in Universities of Henan Province (17HASTIT035) and by National Natural Science Foundation of China (31670289 and 31570294).
AUTHOR CONTRIBUTIONS
Xo.Z. and Xn.Z. designed the research; Q.P.Z., J.D.Z., N.N.L. and X.N.W. performed the experiments; Xo.Z. and Xn.Z. analyzed the data; Xn.Z. contributed reagents/materials; Xo.Z., Xn.Z. and Q.P.Z. wrote the article. All authors have read and approved this manuscript.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.12813/suppinfo
Table S1. List of all the primers has been used in text
Online on Apr. 2, 2019
Contributor Information
Xiang Zhao, Email: xzhao@henu.edu.cn.
Xiao Zhang, Email: xzhang@henu.edu.cn.
REFERENCES
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR (1998) Cryptochrome blue‐light photoreceptors of Arabidopsis implicated in phototropism. Nature 392: 720–723 [DOI] [PubMed] [Google Scholar]
- Briggs WR, Tseng TS, Cho HY, Swartz TE, Sullivan S, Bogomolni RA, Kaiserli E, Christie JM (2007) Phototropins and their LOV domains: Versatile plant blue‐light receptors. J Integr Plant Biol 49: 4–10 [Google Scholar]
- Chen ML, Fu XM, Liu JQ, Ye TT, Hou SY, Huang YQ, Yuan BF, Wu Y, Feng YQ (2012) Highly sensitive and quantitative profiling of acidic phytohormones using derivatization approach coupled with nano‐LC‐ESI‐Q‐TOF‐MS analysis. J Chromatogr B 905: 67 –74 [DOI] [PubMed] [Google Scholar]
- Christie JM, Murphy AS (2013) Shoot phototropism in higher plants: New light through old concepts. Am J Bot 100: 35–46 [DOI] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NP H1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (1985) Kinetic separation of phototropism from blue‐light inhibition of stem elongation. Photochem Photobiol 42: 745–751 [DOI] [PubMed] [Google Scholar]
- de Carbonnel M, Davis P, Roelfsema MR, Inoue S, Schepens I, Lariguet P, Geisler M, Shimazaki K, Hangarter R, Fankhauser C (2010) The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarsy E, Schepens I, Okajima K, Hersch M, Bergmann S, Christie J, Shimazaki K, Tokutomi S, Fankhauser C (2012) Phytochrome Kinase Substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J 31: 3457–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Galvan‐Ampudia CS, Demarsy E, Langowski L, Kleine‐Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, Friml J (2011) Light‐mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis . Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Esmon CA, Tinsley AG, Ljung K, Sandberg G, Hearne LB, Liscum E (2006) A gradient of auxin and auxin‐dependent transcription precedes tropic growth responses. Proc Natl Acad Sci USA 103: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Christie JM (2015) Plant phototropic growth. Curr Biol 25: 384–389 [DOI] [PubMed] [Google Scholar]
- Fu X, Richards DE, Fleck B, Xie D, Burton N, Harberd NP (2004) The Arabidopsis mutant sleepy1gar2‐1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16: 1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C, Rabenold JJ, Liscum E (2007) Functional ecology of a blue light photoreceptor: Effects of phototropin‐1 on root growth enhance drought tolerance in Arabidopsis thaliana . New Phytol 173: 91–99 [DOI] [PubMed] [Google Scholar]
- Gallego‐Bartolome J, Alabadi D, Blazquez MA (2011) DELLA‐induced early transcriptional changes during etiolated development in Arabidopsis thaliana . PLoS ONE 6: e23918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MD, Fuster‐Almunia C, Ocana‐Cuesta J, Alonso JM, Perez‐Amador MA (2019) RGL2 controls flower development, ovule number and fertility in Arabidopsis . Plant Sci 281: 82–92 [DOI] [PubMed] [Google Scholar]
- Goyal A, Karayekov E, Galvao VC, Ren H, Casal JJ, Fankhauser C (2016) Shade promotes phototropism through phytochrome B‐controlled auxin production. Curr Biol 26: 3280–3287 [DOI] [PubMed] [Google Scholar]
- Goyal A, Szarzynska B, Fankhauser C (2013) Phototropism: At the crossroads of light‐signaling pathways. Trends Plant Sci 18: 393–401 [DOI] [PubMed] [Google Scholar]
- Guo H, Duong H, Ma N, Lin C (1999) The Arabidopsis blue light receptor cryptochrome 2 is a nuclear protein regulated by a blue light‐dependent post‐transcriptional mechanism. Plant J 19: 279–287 [DOI] [PubMed] [Google Scholar]
- Haga K, Tsuchida‐Mayama T, Yamada M, Sakai T (2015) Arabidopsis ROOT PHOTOTROPISM2 contributes to the adaptation to high‐intensity light in phototropic responses. Plant Cell 27: 1098–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauvermale AL, Ariizumi T, Steber CM (2012) Gibberellin signaling: A theme and variations on DELLA repression. Plant Physiol 160: 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Sponsel V (2015) A century of gibberellin research. J Plant Growth Regul 34: 740–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Liu J, Dong H, Sun J (2019) The blue light receptor CRY1 interacts with BZR1 and BIN2 to modulate the phosphorylation and nuclear function of BZR1 in repressing BR signaling in Arabidopsis . Mol Plant 12: 689 –703 [DOI] [PubMed] [Google Scholar]
- Hirose F, Inagaki N, Hanada A, Yamaguchi S, Kamiya Y, Miyao A, Hirochika H, Takano M (2012) Cryptochrome and phytochrome cooperatively but independently reduce active gibberellin content in rice seedlings under light irradiation. Plant Cell Physiol 53: 1570–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohm T, Preuten T, Fankhauser C (2013) Phototropism: Translating light into directional growth. Am J Bot 100: 47–59 [DOI] [PubMed] [Google Scholar]
- Inada S, Ohgishi M, Mayama T, Okada K, Sakai T (2004) RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana . Plant Cell 16: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi AK, Konjevic R, Whitelam G, Gordon W, Poffand KL (1997) Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thaliana seedlings. Physiol Plantarum 101: 278–282 [Google Scholar]
- Jia KP, Luo Q, He SB, Lu XD, Yang HQ (2014) Strigolactone‐regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis . Mol Plant 7: 528–540 24126495 [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high‐light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kami C, Hersch M, Trevisan M, Genoud T, Hiltbrunner A, Bergmann S, Fankhauser C (2012) Nuclear phytochrome A signaling promotes phototropism in Arabidopsis . Plant Cell 24: 566–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B, Grancher N, Koyffmann V, Lardemer D, Burney S, Ahmad M (2008) Multiple interactions between cryptochrome and phototropin blue‐light signalling pathways in Arabidopsis thaliana . Planta 227: 1091–1099 [DOI] [PubMed] [Google Scholar]
- Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C, de Carbonnel M, Alonso JM, Ecker JR, Liscum E, Fankhauser C (2006) PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascève G, Leymarie J, Olney MA, Liscum E, Christie JM, Vavasseur A, Briggs WR (1999) Arabidopsis contains at least four independent blue‐light‐activated signal transduction pathways. Plant Physiol 120: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Feke A, Li MW, Adamchek C, Webb K (2018) Decoys untangle complicated redundancy and reveal targets of circadian clock F‐box proteins. Plant Physiol 177: 1170–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, An F, Li W, Ma M, Feng Y, Zhang X, Guo H (2016b) DELLA proteins interact with FLC to repress flowering transition. J Integr Plant Biol 58: 642–655 [DOI] [PubMed] [Google Scholar]
- Li K, Yu R, Fan LM, Wei N, Chen H (2016a) DELLA‐mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis . Nat Commun 7: 11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Van den Ende W, Rolland F (2014) Sucrose induction of anthocyanin biosynthesis is mediated by DELLA. Mol Plant 7: 570–572 [DOI] [PubMed] [Google Scholar]
- Lian N, Liu X, Wang X, Zhou Y, Li H, Li J, Mao T (2017) COP1 mediates dark‐specific degradation of microtubule‐associated protein WDL3 in regulating Arabidopsis hypocotyl elongation. Proc Natl Acad Sci USA 114: 12321–12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Todo T (2005) The cryptochromes. Genome Biol 6: 220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Askinosie SK, Leuchtman DL, Morrow J, Willenburg KT, Coats DR (2014) Phototropism: Growing towards an understanding of plant movement. Plant Cell 26: 38–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liu B, Zhao C, Pepper M, Lin C (2011) The action mechanisms of plant cryptochromes. Trends Plant Sci 16: 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Li X, Guo Y, Chu J, Fang S, Yan C, Noel J, Liu H (2016) Cryptochrome 1 interacts with PIF4 to regulate high temperature‐mediated hypocotyl elongation in response to blue light. Proc Natl Acad Sci USA 113: 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur MJ, Kwaaitaal M, Mateos MA, Maio F, Kini RK (2019) The SUMO conjugation complex self‐assembles into nuclear bodies independent of SIZ1 and COP1. Plant Physiol 179: 168–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E (1999) Arabidopsis NPH3: A NPH1 photoreceptor‐interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Nagashima A, Uehara Y, Sakai T (2008) The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N‐1‐naphthyphthalamic acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant Cell Physiol 49: 1250–1255 [DOI] [PubMed] [Google Scholar]
- Ohgishi M, Saji K, Okada K, Sakai T (2004) Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis . Proc Natl Acad Sci USA 101: 2223–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale UV, Liscum E (2007) Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1‐interacting protein NPH3. J Biol Chem 282: 19992–20001 [DOI] [PubMed] [Google Scholar]
- Petersen J, Inoue SI, Kelly SM, Sullivan S, Kinoshita T, Christie JM (2017) Functional characterization of a constitutively active kinase variant of Arabidopsis phototropin 1. J Biol Chem 292: 13843–13852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusová H, Fendrych M, Friml J (2015) Intracellular trafficking and PIN‐mediated cell polarity during tropic responses in plants. Curr Opin Plant Biol 23: 116–123 [DOI] [PubMed] [Google Scholar]
- Sakaguchi J, Matsushita T (2019) DWARF4 accumulation in root tips is enhanced via blue light perception by cryptochromes. Plant Cell Environ 42: 1615–1629 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T (2019) Determination of auxin flow during phototropic responses using fluorescent auxin analogs. Methods Mol Biol 1924: 157–163 [DOI] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2: A signal transducer of the phototropic response in Arabidopsis . Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Montesino R, Bouza‐Morcillo L, Marquez J, Ghita M, Duran‐Nebreda S, Gomez L, Holdsworth MJ, Bassel G, Onate‐Sanchez L (2019) A regulatory module controlling GA‐mediated endosperm cell expansion is critical for seed germination in Arabidopsis . Mol Plant 12: 71–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi‐Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, Matsuoka M (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F‐box mutant. Science 299: 1896–1898 [DOI] [PubMed] [Google Scholar]
- Schepens I, Boccalandro HE, Kami C, Casal JJ, Fankhauser C (2008) PHYTOCHROME KINASE SUBSTRATE4 modulates phytochrome‐mediated control of hypocotyl growth orientation. Plant Physiol 147: 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager‐Lavelle A, Gath NN (2019) The role of a class III gibberellin 2‐oxidase in tomato internode elongation. Plant J 97: 603–615 [DOI] [PubMed] [Google Scholar]
- Stone BB, Esmon CA, Liscum E (2005) Phototropins, other photoreceptors, and associated signaling: The lead and supporting cast in the control of plant movement responses. Curr Top Dev Biol 66: 215–238 [DOI] [PubMed] [Google Scholar]
- Stowe‐Evans EL, Luesse DR, Liscum E (2001) The enhancement of phototropin‐induced phototropic curvature in Arabidopsis occurs via a photoreversible phytochrome A‐dependent modulation of auxin responsiveness. Plant Physiol 126: 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, Cheng Y, Lim J, Zhao Y, Ballare CL, Sandberg G, Noel JP, Chory J (2008) Rapid synthesis of auxin via a new tryptophan‐dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida‐Mayama T, Nakano M, Uehara Y, Sano M, Fujisawa N, Okada K, Sakai T (2008) Mapping of the phosphorylation sites on the phototropic signal transducer, NPH3. Plant Sci 174: 626–633 [Google Scholar]
- Tsuchida‐Mayama T, Sakai T, Hanada A, Uehara Y, Asami T, Yamaguchi S (2010) Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J 62: 653–662 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YL, Eisinger W, Ehrhardt D, Kubitscheck U, Baluska F, Briggs W (2008) The subcellular localization and blue‐light‐induced movement of phototropin 1‐GFP in etiolated seedlings of Arabidopsis thaliana . Mol Plant 1: 103–117 [DOI] [PubMed] [Google Scholar]
- Wang S, Li L, Xu P, Lian H, Wang W, Xu F, Mao Z, Zhang T, Yang H (2018) CRY1 interacts directly with HBI1 to regulate its transcriptional activity and photomorphogenesis in Arabidopsis . J Exp Bot 69: 3867–3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WX, Lian HL, Zhang LD, Mao ZL, Li XM, Xu F, Li L, Yang HQ (2016) Transcriptome analyses reveal the involvement of both C and N termini of Cryptochrome 1 in its regulation of phytohormone‐responsive gene expression in Arabidopsis . Front Plant Sci 7: 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP (2003) Second positive phototropism results from coordinated co‐action of the phototropins and cryptochromes. Plant Physiol 132: 1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP (2006) Phototropism: Bending towards enlightenment. Plant Cell 18: 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59: 225–251 [DOI] [PubMed] [Google Scholar]
- Zhao QP, Wang XN, Li NN, Zhu ZY, Mu SC, Zhao X, Zhang X (2018a) Functional analysis of MAX2 in phototropins‐mediated cotyledon flattening in Arabidopsis . Front Plant Sci 9:1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang J, Yuan J, Wang XL, Zhao QP, Kong PT, Zhang X (2015) NITRIC OXIDE‐ASSOCIATED PROTEIN1 (AtNOA1) is essential for salicylic acid‐induced root waving in Arabidopsis thaliana . New Phytol 207: 211–224 [DOI] [PubMed] [Google Scholar]
- Zhao X, Wang YL, Qiao XR, Wang J, Wang LD, Xu CS, Zhang X (2013) Phototropins function in high‐intensity blue light‐induced hypocotyl phototropism in Arabidopsis by altering cytosolic calcium. Plant Physiol 162: 1539–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yu X, Foo E, Symons GM, Lopez J, Bendehakkalu KT, Xiang J, Weller JL, Liu X, Reid JB, Lin C (2007a) A study of gibberellin homeostasis and cryptochrome‐mediated blue light inhibition of hypocotyl elongation. Plant Physiol 145: 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhao QP, Xu CY, Wang J, Zhu JD, Shang BS, Zhang X (2018b) Phot2‐regulated relocation of NPH3 mediates phototropic response to high‐intensity blue light in Arabidopsis thaliana . J Integr Plant Biol 60: 562–577 [DOI] [PubMed] [Google Scholar]
- Zhao XY, Yu XH, Liu XM, Lin CT (2007b) Light regulation of gibberellins metabolism in seedling development. J Integr Plant Biol 49: 21–27 [Google Scholar]
- Zheng H, Zhang F, Wang S, Su Y, Ji X, Jiang P, Chen R, Hou S, Ding Y (2018) MLK1 and MLK2 Coordinate RGA and CCA1 activity to regulate hypocotyl elongation in Arabidopsis thaliana . Plant Cell 30: 67–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltowski BD, Imaizumi T (2014) Structure and function of the ZTL/FKF1/LKP2 group proteins in Arabidopsis . The Enzymes 35: 213–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.12813/suppinfo
Table S1. List of all the primers has been used in text


