Abstract
Lung hypoplasia is the main cause of congenital diaphragmatic hernia (CDH)‐associated death but pathogenesis remains unclear. MiR‐455‐5p is involved in lung hypoplasia. We hypothesized that nitrofen causes abnormal miR‐455‐5p expression during lung development and designed this study to determine the relationship between miR‐455‐5p, stimulated by retinoic acid 6 (STRA6), and retinol in a nitrofen‐induced CDH with lung hypoplasia rat model. Nitrofen or olive oil was administered to Sprague‐Dawley rats by gavage on day 9.5 of gestation, and the rats were divided into a nitrofen group and a control group (n = 6). The left lung of fetuses was dissected on day 15.5. The expression of miR‐455‐5p or STRA6 messenger RNA (mRNA) was determined by quantitative real‐time polymerase chain reaction. Average integrated optical density (IOD) of STRA6 protein was determined by immunofluorescence histochemistry. The average retinol level was detected by enzyme‐linked immunosorbent assay (n = 6 lungs, respectively). Compared with the control group, the nitrofen group exhibited significantly increased miR‐455‐5p expression levels (29.450 ± 9.253 vs 5.955 ± 2.330; P = .00045) and significantly decreased STRA6 mRNA levels (0.197 ± 0.097 vs 0.588 ± 0.184; P = .0047). In addition, the average IOD of the STRA6 protein was significantly lower in the nitrofen group (805.643 ± 291.182 vs 1616.391 ± 572.308, P = .015), and the average retinol level was significantly reduced (4.013 ± 0.195 vs 5.317 ± 0.337 µg/L, P = .000). In summary, the overexpression of miR‐455‐5p affected retinol absorption by downregulating STRA6 in the nitrofen‐induced CDH with lung hypoplasia rat model, and this downregulation may be one cause of CDH with lung hypoplasia.
Keywords: congenital diaphragmatic hernia, lung hypoplasia, miR‐455‐5p, STRA6
1. INTRODUCTION
Congenital diaphragmatic hernia (CDH) is a serious, deadly disease in neonates, and its incidence rate is approximately 1/2000 to 1/5000. 1 , 2 For severe CDH, the mortality rate is as high as 40% to 64% when associated with pulmonary arterial hypertension and respiratory failure caused by lung hypoplasia. 3 , 4 , 5 , 6 In addition to morphological changes, such as a reduction in bronchial branches, reduction in bronchial count, reduction in the number of alveoli, and reduction in alveoli, CDH with lung hypoplasia also exhibits abnormalities at the protein and molecular levels. 7 A nitrofen‐induced CDH with lung hypoplasia rat model is an ideal tool for studying CDH with lung hypoplasia. 8 , 9 Many studies have shown that the pathogenesis is similar between human CDH and the nitrofen‐induced CDH rat model with lung hypoplasia because both are caused by abnormal expression of genes and molecules in the retinoid signaling pathway. 7 , 10 , 11 Schmidt showed that retinol or retinoic acid (RA) could improve CDH with lung hypoplasia. 12 Stimulated by retinoic acid 6 (STRA6) is a specific retinol receptor on the cell membrane, which shows a robust retinol uptake activity. Its main biological function is to combine, with high affinity, with retinol‐binding protein (RBP) and to form the retinol‐RBP complex, which transports retinol from outside cells into cells and regulates the intracellular retinol content. 13 , 14 Mutations in the STRA6 gene can cause CDH, alveolar‐capillary hypoplasia, lung hypoplasia, and other congenital malformations. 15 Factors that cause changes in STRA6 gene expression may cause CDH with lung hypoplasia. This may be due to upstream microRNAs.
MicroRNAs, with a length of approximately 19 to 25 bp, are composed of noncoding RNA. MicroRNAs play important roles in the regulation of lung development. 16 We previously demonstrated that miR‐455‐5p is associated with lung development. Overexpression of miR‐455‐5p downregulates STRA6, leading to the reduction of the retinol level and the proliferation of rat lung alveolar type II cells. 17 We hypothesized that during lung development, nitrofen causes abnormal miR‐455‐5p expression. Therefore, this study was designed to determine the expression levels of miR‐455‐5p, STRA6, and retinol in a nitrofen‐induced CDH with lung hypoplasia rat model to verify the relationship between miR‐455‐5p and CDH with lung hypoplasia.
2. MATERIALS AND METHODS
2.1. Establishment of the nitrofen‐induced CDH with lung hypoplasia rat model
A total of 12 adult female pathogen‐free Sprague‐Dawley (SD) rats (Guangdong Medical Animal Experimental Center, Guangdong, China) and 4 SPF grade male SD rats were assigned to the control group (n = 6) and the nitrofen group (n = 6) according to random numbering. Female and male SD rats were housed overnight in the same cage at a ratio of 3:1. The next day, vaginal smears showing sperm under microscopy were regarded as the 0.5th day of gestation. On day 9.5 of gestation, rats in the nitrofen group were injected with 100 mg of nitrofen dissolved in olive oil (100 mg/mL) (Sigma‐Aldrich) via a gavage needle after light anesthesia (inhalation of 2% volatile isoflurane (RWD Life Science Ltd, Shenzhen, China). Rats in the control group were only administered 1 mL of olive oil. In the nitrofen and control groups, a total of 45 and 62 fetuses, respectively, were harvested by cesarean section from pregnant rats under anesthesia on day 15.5 of gestation. The fetal rats were euthanized by carbon dioxide asphyxiation. The fetal rats underwent thoracotomy and were dissected under sterile conditions using a Nikon SMZ100 stereomicroscope. The left lungs of the fetal rats were collected and immediately placed into cryopreservation tubes, which were frozen on dry ice and stored in liquid nitrogen for total RNA extraction and an enzyme‐linked immunosorbent assay (ELISA). Specimens used for morphological analysis or immunofluorescence histochemistry experiments were placed in 10% paraformaldehyde (Beijing Leagene Biotech Co, Ltd, China) for preservation. Paraffin sections were then prepared by pathologists. To reduce experimental error, only one fetus was selected from each pregnant rat for each experimental method (six lungs for each group from six different litters).
All animal procedures were performed according to the current guidelines for the management and welfare of laboratory animals. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Sun Yat‐Sen University Laboratory Animal Center, Guangdong, China (No. IACUC‐DB‐16‐0620) where all of the animal experiments were performed.
2.2. Total RNA extraction
Lung specimens from fetal rats in the two groups were removed from liquid nitrogen (n = 6 per group). Single specimens were placed in 1.5‐mL EP tubes, and 0.5 mL TRIzol (Invitrogen, Carlsbad, CA) solution was added to fully homogenize the specimens. After standing, 0.1 mL of chloroform was added (Guangzhou Chemical Reagent Factory, Guangzhou, China). The mixture was shaken on a vortex for approximately 15 seconds and then allowed to stand for 5 minutes at room temperature, followed by centrifugation at 4°C for 15 minutes (12 000 g). The upper aqueous phase was carefully transferred into a new 1.5‐mL EP tube, and 0.25 mL of isopropanol (Guangzhou Chemical Reagent Factory) was added. After mixing thoroughly, the mixture was placed at room temperature for 10 minutes and centrifuged at 4°C for 10 minutes (12000g). The supernatant was discarded, and the precipitate was retained. Then, 0.5 mL of precooled 75% ethanol (Guangzhou Chemical Reagent Factory) was added, RNA precipitates were washed by shaking, followed by centrifugation at 4°C for 5 minutes (7500g). After the supernatant was discarded carefully, the EP tube was placed in a vacuum dryer for 3 minutes, and then 10 μL of RNase‐free dH2O was added to dissolve the RNA. Samples were placed on ice before determining the concentration. One microliter of total RNA was used for determining the RNA concentration using a nucleic acid protein quantitation analyzer (Eppendorf, Hamburg, Germany). Agarose gel electrophoresis (1%, BioWest, Spain) was used to verify the integrity of the extracted total RNA and to exclude DNA contamination.
2.3. Complementary DNA reverse transcription reaction
An iScript reverse transcription supermix kit (Bio‐Rad) was used for reverse transcription. The reaction system was prepared according to the instruction manual. The reaction conditions were as follows: 25°C for 5 minutes (initiation), 46°C for 20 minutes (reverse transcription), and 95°C for 1 minute (termination). The samples were stored at 4°C. For reverse transcription of microRNA (miRNA) into complementary DNA (cDNA), miRNA‐specific primers (synthesized by Guangzhou Ribo Biotechnology Co, Ltd) were also used. The obtained cDNA was stored at −20°C until the subsequent quantitative real‐time polymerase chain reaction (QPCR) assay.
2.4. Quantitative real‐time polymerase chain reaction
The relative expression levels of miR‐455‐5p or STRA6 mRNA were quantified via dye‐based QPCR using an iTaq Universal STBR Green Supermix kit (Bio‐Rad). The reaction system was prepared according to the instruction manual. The primers for miR‐455‐5p were designed and synthesized by Guangzhou Ruibo Biotechnology Co, Ltd. U6 was selected as an internal reference gene. The primer sequences for STRA6 are provided in Table 1. Glyceraldehyde‐3‐phosphate dehydrogenase was selected as an internal reference gene. The conditions for both reactions were as follows: predenaturation at 95°C for 30 seconds and 40 cycles of denaturation at 95°C for 5 seconds and annealing at 60°C for 30 seconds. Samples were stored at 4°C after the reaction was completed. A fluorescence quantitative QPCR system (CFX96; Bio‐Rad) was used. All experiments were run in duplicate for each sample and primer pair.
Table 1.
The sequences of primers
| Name of primer | Primer sequence | Product size, bp |
|---|---|---|
| STRA6‐forward | 5′‐TCCAAGCGTAGCCTTCTGTC‐3′ | 131 |
| STRA6‐reverse | 5′‐CCACCTGGTAAGTGGCTGTT‐3′ | |
| GAPDH‐forward | 5′‐TGCCACTCAGAAGACTGTGG‐3′ | 129 |
| GAPDH‐reverse | 5′‐TTCAGCTCTGGGATGACCTT‐3′ |
Abbreviations: GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; STRA6, stimulated by retinoic acid 6.
2.5. The average integrated optical density of STRA6 protein in fetal lungs via immunofluorescence histochemistry
Paraffin sections of lungs from the two groups were deparaffinized with xylene and rehydrated with ethanol and distilled water (n = 6 per group). Antigen retrieval was performed using an antigen retrieval kit and ethylenediaminetetraacetic acid antigen retrieval buffer, pH 9.0 (Servicebio, Wuhan, China). The sections were washed with phosphate‐buffered saline and then incubated with primary goat anti‐rat STRA6 antibody (Everest Biotech, Bicester, UK) at 4°C overnight. CY3‐labeled donkey anti‐goat fluorescent secondary antibody (Servicebio) was added, and the sections were incubated at room temperature for 50 minutes. Nuclei were counterstained with 4′,6‐diamidino‐2‐phenylindole staining solution and mounted with antiquenching mounting medium. Each section was placed under a Nikon inverted fluorescence microscope for observation and to collect three images selected randomly at ×200 magnification (UV excitation wavelength, 420 nm; CY3 red excitation wavelength, 590 nm). Image pro‐plus 6.0 software was used to analyze the integrated optical density (IOD) of each fluorescent image, and finally, the average IOD was calculated.
2.6. Measurement of the average retinol level in fetal lungs by ELISA
A rat vitamin A (retinol) ELISA kit (Cusabio Technology LLC, China) was used. Standard references were prepared, and then a standard curve was plotted. The fetal lung samples from the two groups were removed from liquid nitrogen (n = 6 per group), and the tissues were ground and homogenized. Sterile water was added, and the cells were lysed by repeated freezing and thawing (three times); the lysed cells were then centrifuged for 15 minutes (12 000 g), and the supernatant was transferred to a new EP tube for analysis. After conducting the assay, a microplate reader (K3; Thermo Fisher Scientific) was used to perform measurements (wavelength 450 nm).
2.7. Statistical analysis
All numerical data are presented as the mean ± SD. Differences between two groups were tested using an unpaired t test. Statistical significance was defined as a P value of less than .05.
3. RESULTS
3.1. Relative expression levels of miR‐455‐5p in fetal lungs
Compared with those in the control group, the expression levels of miR‐455‐5p in the nitrofen group were significantly higher (29.450 ± 9.253 vs 5.955 ± 2.330, P = .00045 [<.05]) (Figure 1).
Figure 1.
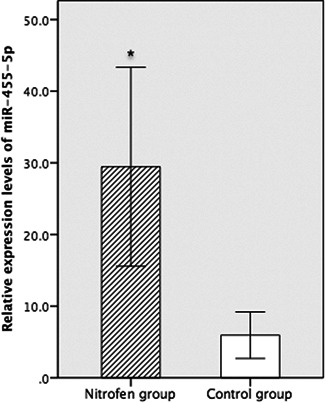
Compared with that in the control group, the relative expression of miR‐455‐5p was significantly lower in the nitrofen group (n = 6 per group). *P < .05
3.2. Relative expression levels of STRA6 mRNA in fetal lungs
The expression levels of STRA6 mRNA in the nitrofen group were significantly lower than those in the control group (0.197 ± 0.097 vs 0.588 ± 0.184, P = 0.0047 [<.05]) (Figure 2).
Figure 2.
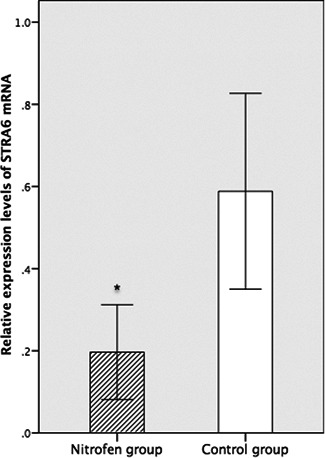
Relative expression levels of STRA6 mRNA in the nitrofen group were significantly lower than those in the control group (n = 6 per group). mRNA, messenger RNA; STRA6, stimulated by retinoic acid 6. *P < .05
3.3. The average IOD of STRA6 protein in fetal lungs
The average IOD of the STRA6 protein in the nitrofen group was significantly lower than that in the control group (805.643 ± 291.182 vs 1616.391 ± 572.308, P = .015 [<.05]) (Figure 3), indicating that the relative expression level of STRA6 protein was significantly lower in the nitrofen group than in the control group.
Figure 3.
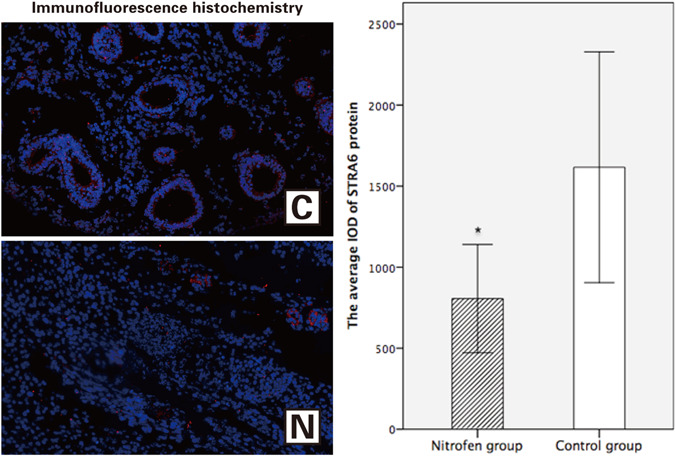
Immunofluorescence histochemistry at ×200 magnification: the control group (C) and the nitrofen group (N) (red indicates positive STRA6 expression). The average integrated optical density of STRA6 in the control group was significantly higher than that in the nitrofen group. (n = 6 per group). *P < .05 [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Average retinol level in fetal lungs
The average retinol level in the nitrofen group was significantly lower than that in the control group (4.013 ± 0.195 vs 5.317 ± 0.337 µg/L, P = .000 [<.05]) (Figure 4).
Figure 4.
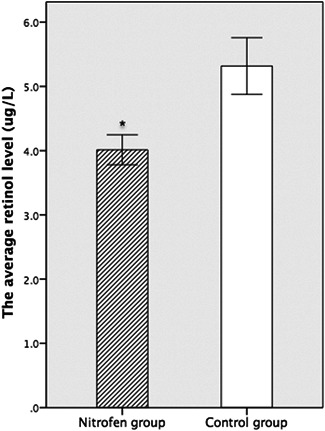
The average retinol level (μg/L) in the nitrofen group was significantly lower than that in the control group (n = 6 per group). *P < .05
4. DISCUSSION
The main cause of death in CDH is lung hypoplasia, which can cause lung hypertension and respiratory failure but the pathogenic mechanism of CDH with lung hypoplasia is still unclear. MicroRNAs are small noncoding RNAs comprising 22 nucleotides that regulate gene expression by targeting mRNAs for cleavage or translational repression at the posttranscriptional level. An increasing number of microRNAs have been found to regulate downstream signaling pathways and to be associated with lung development. 18 Huo et al 19 found that increased miR‐455 expression in late gestation after intrauterine hypoxia exposure may be a cause of lung injury in a hypoxic rat model. Martinez‐Anton et al 20 discovered that miR‐455 decreased and was involved in changes in epithelial cell marker mRNA levels. Transfection with miR‐455‐3p resulted in changes in target MUC1 protein expression. They believed that changes in specific miRNAs during human bronchial epithelial cell differentiation control gene and protein expression important for differentiation. In addition, using a miR‐455‐5p adenoviral expression vector that was constructed and used to transfect rat lung alveolar type II cells, we previously demonstrated that miR‐455‐5p overexpression reduces rat lung alveolar type II cell proliferation by downregulating STRA6. 17 Therefore, we used the nitrofen‐induced CDH rat model to obtain further verification. In this study, as determined by QPCR using samples of hypoplastic lungs from the nitrofen‐induced CDH rat model, relative miR‐455‐5p expression was significantly increased. Thus, we believe that the overexpression of miR‐455‐5p is involved in lung hypoplasia. To understand the mechanism connecting mir‐455‐5p and lung hypoplasia, we continued to examine the expression of its downstream target gene STRA6.
STRA6 (stimulated by the retinoic acid gene 6 homolog) is located on chromosome 8qq24, has a gene size of 2722 bp and is a specific membrane receptor for RBP in the retinoid signaling pathway. STRA6 combines with RBP to form the retinol‐RBP complex, which transports extracellular retinol into cells and regulates intracellular retinol levels. 14 Multiple clinical syndromes characterized by pulmonary hypoplasia have been identified and are associated with genetic variations in STRA6. Pasutto et al 15 discovered that mutations in STRA6 can result in a broad range of malformations, including CDH, alveolar‐capillary hypoplasia, and lung hypoplasia. Golzio et al 21 found that a homozygous insertion/deletion in exon 2 or a homozygous insertion in exon 7 of the STRA6 gene can lead to Matthew‐Wood syndrome, which is primarily characterized by CDH, lung hypoplasia, and microphthalmia. Segel et al 22 found that some patients were compound heterozygotes for two novel STRA6 missense mutations associated with pulmonary hypoplasia‐diaphragmatic hernia‐anophthalmia‐cardiac defect syndrome, which is characterized by pulmonary hypoplasia, diaphragmatic hernia, cardiac malformations, and anophthalmia. We examined STRA6 mRNA expression in hypoplastic lungs of the nitrofen‐induced CDH rat model using QPCR and the IOD of STRA6 protein via immunofluorescence histochemistry. The results showed that relative STRA6 mRNA expression and the average IOD of the STRA6 protein were significantly decreased. Therefore, we speculate that decreased STRA6 expression can directly decrease the intracellular retinol level and lead to abnormalities in the retinoid signaling pathway.
The retinoid signaling pathway is one of the most relevant signaling pathways in CDH with lung hypoplasia. Maintenance of a proper level of retinol and its active metabolites (RA) is required for lung development. Retinol metabolic abnormalities may lead to a variety of congenital malformations, including in the respiratory system. 23 James et al 24 established hyperoxia‐induced lung injury in a C57BL/6 mouse model and treated the mice with a VitA‐RA mixture. They discovered that the VitA‐RA mixture increased lung retinol stores and lung compliance while reducing hyperoxia‐induced alveolar simplification. Thus, they concluded that the VitA‐RA mixture is a method of treating VitA deficiency and hyperoxia‐induced lung injury during lung development. Both human CDH and nitrofen‐induced CDH are caused by abnormalities in the retinoid signaling pathway and share similar mechanisms. 7 Yu et al 25 found that the nitrofen‐induced rat model had a CDH incidence rate of 41%, while the nitrofen + vitamin A group had a CDH incidence rate of 27%. Thebaud et al 26 , 27 showed that vitamin A administration not only reduced nitrofen‐induced CDH incidence in pregnant rats but also promoted lung development and maturation and reduced CDH severity. Nakazawa et al 28 showed that vitamin A levels were lower in nitrofen‐induced hypoplastic lungs from fetal rats than in lungs from the normal group. We also found that the average retinol level, determined by ELISA, significantly decreased in this model.
In conclusion, based on the above‐mentioned results and the mechanism of action of microRNAs, we believe that the overexpression of miR‐455‐5p results in partial degradation of STRA6 mRNA, leading to decreased STRA6 protein expression and reduced translocation of retinol into cells, which may be a cause of CDH with lung hypoplasia in the nitrofen‐induced CDH with lung hypoplasia rat model. However, the study results are still limited because the etiology of CDH with lung hypoplasia is complex. A next step will be to test the outcomes of the present study by injecting pregnant rats with a miR‐455‐5p adenoviral expression vector to interfere with the absorption of vitamin A and determine whether this interference causes CDH or lung hypoplasia in the fetus.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
This study was supported by the Project funded by the China Postdoctoral Science Foundation (Postdoc No. 241006), the Science and Technology Planning Project 2019 of the Bureau of Science and Technology of Foshan City (No. 1920001000338), and the Science and Technology Planning Project of Guangdong Province (No. 2014A020212025).
Zheng J, He Q, Tang H, et al. Overexpression of miR‐455‐5p affects retinol (vitamin A) absorption by downregulating STRA6 in a nitrofen‐induced CDH with lung hypoplasia rat model. Pediatric Pulmonology. 2020;55:1433–1439. 10.1002/ppul.24739
Contributor Information
Xiangming Mao, Email: mxm631221@126.com.
Guoqing Liu, Email: liuguoqing-fs@hotmail.com.
REFERENCES
- 1. Lally KP. Congenital diaphragmatic hernia—the past 25 (or so) years. J Pediatr Surg. 2016;51(5):695‐698. [DOI] [PubMed] [Google Scholar]
- 2. Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million California births, 1989‐1997. Birth Defects Res A Clin Mol Teratol. 2006;76(3):170‐174. [DOI] [PubMed] [Google Scholar]
- 3. Haroon J, Chamberlain RS. An evidence‐based review of the current treatment of congenital diaphragmatic hernia. Clin Pediatr. 2013;52(2):115‐124. [DOI] [PubMed] [Google Scholar]
- 4. Congenital Diaphragmatic Hernia Study Group. Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg. 2001;36(1):141‐145. [DOI] [PubMed] [Google Scholar]
- 5. Mohseni‐Bod H, Bohn D. Pulmonary hypertension in congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16(2):126‐133. [DOI] [PubMed] [Google Scholar]
- 6. Kim DH, Park JD, Kim HS, et al. Survival rate changes in neonates with congenital diaphragmatic hernia and its contributing factors. J Korean Med Sci. 2007;22(4):687‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tovar JA. Congenital diaphragmatic hernia. Orphanet J Rare Dis. 2012;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xia H, Migliazza L, Diez‐Pardo JA, Tovar JA. The tracheobronchial tree is abnormal in experimental congenital diaphragmatic hernia. Pediatr Surg Int. 1999;15(3‐4):184‐187. [DOI] [PubMed] [Google Scholar]
- 9. Migliazza L, Otten C, Xia H, Rodriguez JI, Diez‐Pardo JA, Tovar JA. Cardiovascular malformations in congenital diaphragmatic hernia: human and experimental studies. J Pediatr Surg. 1999;34(9):1352‐1358. [DOI] [PubMed] [Google Scholar]
- 10. Coste K, Beurskens LW, Blanc P, et al. Metabolic disturbances of the vitamin A pathway in human diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. 2015;308(2):L147‐L157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sluiter I, Veenma D, van Loenhout R, et al. Etiological and pathogenic factors in congenital diaphragmatic hernia. Eur J Pediatr Surg. 2012;22(5):345‐354. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt AF, Goncalves FL, Figueira RL, Scorletti F, Peiro JL, Sbragia L. Combined antenatal therapy with retinoic acid and tracheal occlusion in a rat model of congenital diaphragmatic hernia. Pediatr Surg Int. 2016;32(6):591‐598. [DOI] [PubMed] [Google Scholar]
- 13. Kawaguchi R, Yu J, Honda J, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315(5813):820‐825. [DOI] [PubMed] [Google Scholar]
- 14. Kawaguchi R, Zhong M, Kassai M, Ter‐Stepanian M, Sun H. Vitamin A transport mechanism of the multitransmembrane cell‐surface receptor STRA6. Membranes. 2015;5(3):425‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasutto F, Sticht H, Hammersen G, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80(3):550‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350‐353. [DOI] [PubMed] [Google Scholar]
- 17. Zheng J, He Q, Tang H, Xia H. miR‐455‐5p overexpression reduces rat lung alveolar type II cell proliferation by downregulating STRA6. Anat Rec. 2019;302(11):2062‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281‐297. [DOI] [PubMed] [Google Scholar]
- 19. Huo H, Luo Z, Wang M, et al. MicroRNA expression profile in intrauterine hypoxia‐induced pulmonary hypoplasia in rats. Exp Ther Med. 2014;8(3):747‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez‐Anton A, Sokolowska M, Kern S, et al. Changes in microRNA and mRNA expression with differentiation of human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2013;49(3):384‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golzio C, Martinovic‐Bouriel J, Thomas S, et al. Matthew‐Wood syndrome is caused by truncating mutations in the retinol‐binding protein receptor gene STRA6. Am J Hum Genet. 2007;80(6):1179‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Segel R, Levy‐Lahad E, Pasutto F. Pulmonary hypoplasia‐diaphragmatic hernia‐anophthalmia‐cardiac defect (PDAC) syndrome due to STRA6 mutations‐‐what are the minimal criteria? Am J Med Genet A. 2009;149A(11). 10.1002/ajmg.a.v149a:11 [DOI] [PubMed] [Google Scholar]
- 23. Clagett‐Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347‐381. [DOI] [PubMed] [Google Scholar]
- 24. James ML, Ross AC, Nicola T, Steele C, Ambalavanan N. VARA attenuates hyperoxia‐induced impaired alveolar development and lung function in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2013;304(11). 10.1152/ajplung.00257.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu J, Gonzalez S, Diez‐Pardo JA, Tovar JA. Effects of vitamin A on malformations of neural‐crest‐controlled organs induced by nitrofen in rats. Pediatr Surg Int. 2002;18(7):600‐605. [DOI] [PubMed] [Google Scholar]
- 26. Thébaud B, Tibboel D, Rambaud C, et al. Vitamin A decreases the incidence and severity of nitrofen‐induced congenital diaphragmatic hernia in rats. Am J Physiol. 1999;277(2 pt 1):L423‐L429. [DOI] [PubMed] [Google Scholar]
- 27. Thébaud B, Barlier‐Mur AM, Chailley‐Heu B, et al. Restoring effects of vitamin A on surfactant synthesis in nitrofen‐induced congenital diaphragmatic hernia in rats. Am J Respir Crit Care Med. 2001;164(6):1083‐1089. [DOI] [PubMed] [Google Scholar]
- 28. Nakazawa N, Montedonico S, Takayasu H, Paradisi F, Puri P. Disturbance of retinol transportation causes nitrogen‐induced hypoplastic lung. J Pediatr Surg. 2007;42(2). 10.1016/j.jpedsurg.2006.10.028 [DOI] [PubMed] [Google Scholar]


