Abstract
Immune metabolism has been recognized as a new paradigm in the regulation of host immunity. In the environment, there are many micro-organisms including pathogenic and non-pathogenic and/or beneficial ones. Immune cells exhibit various responses against different types of microbes, which seem to be associated with changes in energy metabolism. In addition, dietary nutrition influences host metabolism and consequent responses by immune cells. In this review, we describe the complex network of immune metabolism from the perspectives of nutrition, micro-organisms and host immunity for the control of immunologic health and diseases.
Keywords: fatty acid, host defense, metabolite, symbiotic bacteria, vitamin B1
Introduction
Metabolism is the process whereby substances are synthesized or broken down in vivo by anabolic or catabolic reactions. Mammalian cells use six major metabolic pathways that have important roles in cell growth and survival: the aerobic glycolytic pathway, the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or Krebs cycle), the pentose phosphate pathway, fatty acid oxidation, fatty acid synthesis and the amino acid pathway.
The activity and importance of such metabolic pathways vary greatly depending on the type of cell and the active state of the cell. For example, cancer cells show an altered effect that is characterized by a shift to aerobic glycolysis, which is known as the Warburg effect and contributes to the rapid proliferation and survival of cancer cells (1). Immune cells are not exceptions and accumulating evidence has shown that cellular energy metabolism pathways such as aerobic glycolysis and the TCA cycle are altered during the differentiation and activation of immune cells. It is also known that metabolic products and intermediates that are generated during the pathways affect several cellular functions and signaling (2). Therefore, energy metabolism regulates the functions of immune cells.
There are many micro-organisms in the environment; some of them are pathogenic and others are non-pathogenic and/or beneficial. Various immune cells are deployed in the body where they work cooperatively with each other while exhibiting unique functions and they are responsible for defense against pathogens. Simultaneously, these immune cells show immunological tolerance to harmless and/or beneficial microbes and maintain immunological homeostasis. Thus, the immune response induced by various types of micro-organism is different, suggesting that the type of micro-organism also affects cellular energy metabolism.
In addition to micro-organisms, nutrients are essential for the development, maintenance and regulation of the host immune responses. Therefore, deficient or inappropriate intake of nutrients is frequently associated with increased risk of infectious, allergic and inflammatory diseases. Since nutrients are important factors not only for cellular components but also for energy metabolism, nutrition affects immune functions through the regulation of energy metabolisms in immune cells. In this review, we outline immune metabolism by focusing on the differences in metabolic changes caused by the type of micro-organisms and dietary nutrition.
Metabolic pathway in the acquired immune system
Naive T cells exhibit low metabolic activity and rely primarily upon fatty acid oxidation as a source of energy (2, 3). However, when naive T cells are activated, they acquire a large amount of glucose and increase glycolysis for rapid proliferation and differentiation into effector T cells. The changes are associated with the expression of glucose transporter 1 (glut1), a transporter that takes glucose into cells from extracellular environments, and activation of phosphatidylinositol 3′-kinase (PI3K), mammalian target of rapamycin (mTOR) and Akt (protein kinase B) (4). mTOR also promotes fatty acid synthesis and mitochondrial biogenesis (5).
The proliferating T cells require not only glucose but also glutamine, which is utilized primarily as a mitochondrial substrate, collectively suggesting that mitochondrial activity also plays an important role in T-cell activation. Indeed, mitochondrial metabolism plays a critical role in the expression of genes that drive T-cell activation through modulation of nuclear factor of activated T cells (NFAT), which is a key transcriptional factor for T-cell activation, by the generation of reactive oxygen species (ROS) via mitochondrial oxidative phosphorylation (OXPHOS) (6).
More interestingly, each differentiated effector T-cell subset exhibits unique metabolic requirements, which is thought to be closely related to their functions (7). Th1, Th2 and Th17 cells show a high level of glut1 expression on the cell surface and highly depend on glycolysis. In contrast, regulatory T cells (Treg cells) show a lower level of glut1 expression and a high level of fatty oxidation like naive T cells (7). Therefore, in glut1 transgenic mice, which highly express glut1 specifically in T cells, effector T cells, including Th1, Th2 and Th17 cells, were selectively increased although the T-cell-specific glut1 transgenic mice showed no overt signs of illness (7, 8). In contrast, AMP-activated protein kinase stimulation, which decreases glut1 expression and increases fatty oxidation, increases Treg generation (7).
Similarly, T-cell-specific deficiency of mTOR led to the enhanced generation of Treg cells with impaired differentiation of Th1, Th2 and Th17 cells (9). Of note, two different mTOR complexes, TORC1 and TORC2, exhibit disparate effects on the T-cell fate decision. Specific deletion of the Ras homologue enriched in brain (RHEB), which disrupts TORC1 activity, impairs Th1 and Th17 differentiation (10). In contrast, T cells deficient in the scaffolding protein raptor-independent companion of TOR (RICTOR), which is a critical adaptor protein for TORC2, fail to differentiate into Th2 cells (10).
As further evidence for a T-cell fate decision, acetyl coenzyme A (acetyl-CoA) carboxylase 1 (ACC1)—an enzyme that catalyzes the conversion of acetyl-CoA to malonyl coenzyme A, a carbon donor for fatty acid synthesis—influences the development of Th17 cells (11). ACC1-mediated fatty acid synthesis is required for development of Th17 cells through production of phospholipids for cellular membranes (11).
As with T cells, B cells also show a metabolic shift to glycolysis during activation. For example, B-cell antigen receptor (BCR) stimulation induces B-cell proliferation and increased glucose uptake through activation of PI3K and Akt (12). Indeed, inhibition of glycolysis blocks BCR-mediated B-cell proliferation and antibody production, indicating that glycolysis is required for B-cell responses to antigen. B cells are also activated by other signals such as CD40L, B-cell-activating factor (BAFF) and interleukin (IL-4). Interestingly, IL-4 mediates B-cell survival by regulating glucose energy metabolism via a signal transducer and activator of transcription 6 (Stat6)-dependent pathway in a PI3K-independent manner (13).
For cell proliferation, it is necessary to synthesize cellular macromolecules in addition to the rapid production of adenosine triphosphate (ATP) by glycolysis. Indeed, fatty acid synthesis contributes to proliferation and differentiation of B cell through the induction of endogenous ATP-citrate lyase (ACLY) that produces acetyl-CoA from mitochondria-derived citrate (14). The mitochondrial activity is also required for B-cell functions. ROS production induced by mitochondrial OXPHOS appears to support BCR-mediated proliferation and regulate B-cell functions (15). Thus, during T-cell and B-cell activation, glycolysis is required for initial proliferation; on the other hand, mitochondrial function and fatty acid metabolism are closely linked to the later cellular events.
Immune metabolism in innate immune cells
Like acquired immunity, cells involved in innate immunity such as macrophages, dendritic cells (DCs) and neutrophils change their energy metabolism upon the recognition or capture of pathogens in order to eliminate the pathogens. The metabolic changes associated with infection have been well-characterized in macrophages (16, 17). In general, naive (M0) macrophages differentiate into M1 or M2 macrophages in response to stimulation with lipopolysaccharide (LPS) in combination with interferon (IFN)-γ or IL-4, respectively. M1 macrophages produce inflammatory cytokines and nitric oxide (NO) for the clearance of microbial infection. In contrast, M2 macrophages function in the resolution of inflammation and tissue repair.
M1 macrophages show high glycolysis, low OXPHOS and two breaks in the TCA cycle. These breaks occur at both isocitrate dehydrogenase and succinate dehydrogenase, resulting in increased levels of citrate and succinate. M1 macrophages also show increased activity of fatty acid synthesis, allowing the usage of citrate as a substrate for NO and prostaglandin production and of succinate to enhance IL-1β production through the stabilization of the transcription factor hypoxia-inducible factor-1α (HIF-1α) (18).
M2 macrophages show high levels of fatty acid oxidation, OXPHOS and an intact TCA cycle. The increased fatty acid oxidation and OXPHOS are induced by peroxisome proliferator-activated receptor-γ (PPARγ)-coactivator-1β (PGC-1β) in a STAT6-dependent manner in response to IL-4 (19). The increased OXPHOS is linked to M2 polarization and anti-inflammatory properties. Indeed, inhibition of OXPHOS by oligomycin or carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) decreases the expression level of arginase I, a key functional marker for M2 polarization. M2 macrophages also require glutamine and therefore glutamine deprivation decreases M2 polarization (16).
In addition, macrophages show dynamic changes of fatty acid synthesis during Toll-like receptor (TLR)-mediated activation. The intracellular content of anti-inflammatory mono- and poly- (ω-3, ω-7 and ω-9) unsaturated fatty acids was rapidly decreased at early time points of TLR4-mediated inflammation, whereas the late phase was characterized by increased intracellular unsaturated fatty acid levels, which contribute to the resolution of inflammation (20). The temporal pattern changes in specific lipid species were correlated with the changes in mRNAs encoding corresponding biosynthetic enzymes, which are regulated by sterol regulatory element-binding protein 1 (SREBP1) (20). In another report, the mTOR–Semaphorin 6D (Sema6D)–PPARγ axis plays critical roles in macrophage polarization through regulation of fatty acid uptake and metabolic reprogramming (21). Sema6D induces anti-inflammatory properties in macrophages by IL-4 stimulation, including Arg1 and IL-10 gene expression, and promotes fatty acid synthesis and uptake pathways in a PPARγ-dependent manner (21). Thus, fatty acid synthesis is likely to be associated with anti-inflammatory states of macrophages.
Like M1 macrophages, DCs show an increase of glucose uptake and glycolysis and a decline in OXHPOS when they are activated by LPS (22). The increased glycolysis is regulated in PI3K-dependent and Akt-dependent manner and these metabolic changes are essential for features of DC maturation such as IL-12 production and CD86 expression (22). In contrast to T cells, the metabolic switch is not linked to cellular proliferation but rather appears to support the synthesis, expression and secretion of a broad panel of immune mediators, including cytokines and co-stimulatory factors.
Immune metabolism in the defense against pathogenic bacteria
Immune responses vary depending on the type of microbe, which is presumably associated with the metabolic changes in the host cells. It is interesting to note that metabolic reprogramming is different in response to bacterial lysates from Escherichia coli, Staphylococcus aureus or Mycobacterium tuberculosis (23). All bacterial lysates equally increase glycolysis, whereas increased levels of mitochondrial activity vary among bacterial species (23). Consistently, diverse metabolic changes are induced by microbial stimulation of different TLR signaling pathways in CD14+ human monocytes (23). LPS–TLR4 stimulation induces increased glycolysis and lactate production together with low levels of mitochondrial respiration, whereas Pam3CysSK4 (P3C)–TLR2 increases both glycolysis and mitochondrial respiration (23). Thus, metabolic changes vary depending on the type of bacteria and stimulation; therefore, it becomes important to understand the different responses to individual bacteria.
Mycobacterium tuberculosis is a typical intracellular bacterium and can proliferate and survive inside cells after being engulfed by macrophages. Lung tissue-resident alveolar macrophages suppress inflammation and adaptive immunity under physiological conditions to maintain immunological homeostasis but can be activated by responding to microbial factors to play a critical role for host defense in the lung (24). Mycobacterium tuberculosis-infected lung tissue shows up-regulation of glycolytic enzymes and transporters for glucose uptake including those regulated by HIF-1α, and down-regulation of enzymes participating in the TCA cycle and OXPHOS, indicating that M. tuberculosis infection induces metabolism similar to the Warburg effect (25).
Consistent with this, similar metabolic changes, especially increased glycolysis, are also observed in human alveolar macrophages and play an important role in protection against M. tuberculosis infection (24). Inhibition of the metabolic shift decreases levels of IL-1β, a crucial cytokine in the innate immune response to M. tuberculosis and increases levels of anti-inflammatory IL-10, resulting in increased bacterial survival (24). These similar phenotypes are also observed in human monocyte-derived and murine bone marrow-derived macrophages (24), suggesting that the metabolic shift in M1 macrophages as described above occurs in human lung tissue.
As another mechanism, the expression of lactate dehydrogenase-A (LDH-A), which mediates glucose metabolism by converting lactate to pyruvate, was increased in M. tuberculosis-infected macrophages under the control of HIF-1α. In HIF-1α-deficient macrophages, the pyruvate concentration increases and is used as a feasible carbon source for intracellular growth by M tuberculosis. HIF-1α prevents the hijacking of pyruvate in macrophages, making this a fundamental host-protective mechanism against M. tuberculosis (26). In M. tuberculosis-infected macrophages, immune responsive gene 1 (Irg1) is highly expressed. Irg1 produces itaconic acid through the decarboxylation of cis-aconitate, a TCA cycle intermediate (27). Itaconic acid inhibits isocitrate lyase activity, an enzyme for bacterial growth, in M. tuberculosis. Therefore, depletion of Irg1 decreases intracellular itaconic acid levels as well as reducing anti-microbial activity during bacterial infections.
Listeria monocytogenes is a gram-positive bacterium that parasitizes intracellularly in phagocytic cells such as macrophages and causes listeriosis. Although there are few reports, a metabolic shift similar to that induced by M. tuberculosis infection seems to be induced by Listeria infection (28, 29). On the other hand, in regard to acquired immunity, Listeria infection induces robust CD8+ T-cell responses through innate inflammatory responses including IL-12, IL-18, IFN-γ and TNF-α production, which play a critical role in protective immune responses against Listeria infection.
After Listeria infection, activated CD8+ T cell showed increased expression of Acc1, suggesting that the fatty acid synthesis pathway plays an important role in CD8+ T-cell activation and functions (30). Indeed, T-cell-specific deletion of the Acc1 gene did not compromise effector CD8+ T-cell differentiation upon Listeria infection but did result in a severe defect in antigen-specific CD8+ T-cell accumulation because of the increased death of proliferating cells (30).
Host immune responses and metabolism for symbiotic commensal bacteria
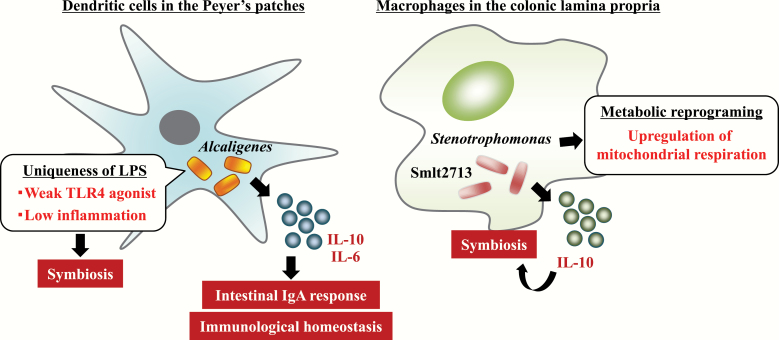
In addition to pathogenic micro-organisms, some micro-organisms are non-toxic or even beneficial to the host and thus host immunity establishes a symbiotic relationship with them. In this regard, we previously revealed unique bacterial communities in intestinal lymphoid tissues, including Peyer’s patches (PPs) (31). 16S rRNA sequencing and fluorescent in situ hybridization analysis allowed us to identify Alcaligenes spp., including Alcaligenes faecalis, which are a dominant genus within the PPs of mice; we termed them as lymphoid-tissue-resident commensal bacteria (LRCs) (31) (Fig. 1). A subsequent study showed that A. faecalis is captured by CD11c+ DCs in PPs after being taken up by M cells (32).
Fig. 1.
Modulation of immune metabolism and function by symbiotic bacteria. Alcaligenes and Stenotrophomonas are identified as predominant symbiotic bacteria in DCs in the PPs and colonic macrophages, respectively. They have unique systems for modulation of immune cell metabolism and function to establish their symbiotic environment.
An additional study showed that LRCs induced multiple members of the IL-10 cytokine family, including DC-derived IL-10 and type 3 innate lymphoid cell (ILC3)-derived IL-22, and provided protective effects in a murine colitis model (33, 34). Moreover, we previously demonstrated that A. faecalis activates DCs to produce moderate levels of IL-6 through the weak agonistic activity of its LPS against TLR4 without inducing excessive inflammatory activity, allowing Alcaligenes spp. to maintain their homeostatic relationship with host immunity (35). This evidence highlights that LRCs, including A. faecalis, interact directly with DCs in intestinal lymphoid tissue and regulate their immune functions to maintain immunologic homeostasis in the intestine (Fig. 1).
We further extended our knowledge by investigating bacteria that persistently colonize colonic macrophages and revealed the presence of Stenotrophomonas maltophilia in murine colonic macrophages (36) (Fig. 1). Interestingly, intracellular colonization by S. maltophilia in vitro led to increased mitochondrial respiration and robust IL-10 production, which are similar characteristics to those of M2 macrophages. Indeed, IL-10 production is critical for maintenance of the symbiotic condition, because intracellular colonization by S. maltophilia was impaired in IL-10-deficient macrophages (36). Moreover, we identified the smlt2713 gene as a critical bacterial factor for this symbiosis through the induction of IL-10 production (Fig. 1). The 25-kDa hypothetical protein is encoded by the smlt2713 gene and annotated as a bacterial protein exported by the type II secretion machinery. In line with this finding, an S. maltophilia smlt2713-deficient strain failed to establish persistent intracellular colonization in macrophages (36). Thus, unlike pathogenic bacteria, symbiotic bacteria induce unique host metabolic changes in the establishment of symbiotic relationships with immune cells.
Role of vitamin B1 in B-cell responses through regulation of immune metabolism
Another important factor involved in the control of immune metabolism is the nutritional condition. In mice in a fasting state, the number of B cells decreased in small intestinal PPs, reducing immune responses such as antigen-specific IgA antibody production against oral vaccines (37) (Fig. 2). As a mechanism, the expression of CXCL13 in bone marrow stromal cells is increased through metabolic reprogramming into Warburg-like aerobic glycolysis during fasting, so naive B cells migrate from the PPs to the bone marrow and the number of naive B cells in PPs apparently decreases (37). In addition, activated germinal center B-cell numbers in PPs are reduced by the induction of apoptosis by starvation (37) (Fig. 2). PPs are well-characterized small intestine-associated lymphoid structures, where antigens including oral vaccines are taken up for the subsequent induction of antigen-specific intestinal immune responses including IgA antibodies (38). Therefore, the nutrition-mediated maintenance of PPs is an important factor in the control of the efficacy of oral vaccines and prevention of infectious diseases in the intestine.
Fig. 2.
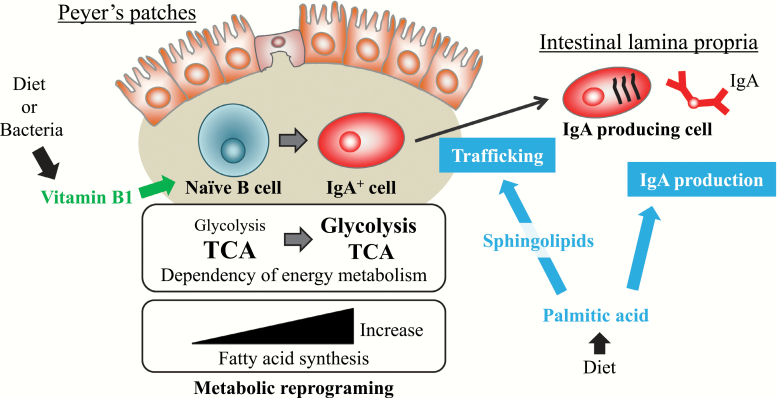
Role of dietary nutrition and its metabolism in intestinal IgA responses. The nutritional conditions are an important factor in the control of immune metabolism and function. Naive B cells have a high dependency on the TCA cycle for energy generation, and therefore depletion of dietary vitamin B1, an essential cofactor in the TCA cycle, results in decreased B-cell numbers in the PPs and a reduction of intestinal IgA responses against oral vaccine antigens. Dietary palmitic acid enhances intestinal IgA production through a direct effect on IgA-producing cells and an indirect effect via endogenous sphingolipid metabolism.
From the perspective of a specific nutrient and immune function, we focus on vitamin B1, which is also known as thiamine (39–41). The TCA cycle is an important biochemical cycle of aerobic metabolism via citrate, α-ketoglutarate, succinyl-CoA, fumarate and oxaloacetate, which occurs in the matrix of the mitochondrion and generates ATP through OXPHOS (39, 41). As major routes in which the TCA cycle takes up the substrate, glucose-derived pyruvate and fatty acids are converted to acetyl-CoA, which enters the TCA cycle. Glutamate is also converted to α-ketoglutarate and enters the TCA cycle. Vitamin B1 acts as an essential cofactor for enzymes such as pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, which are both involved in the TCA cycle (39, 40).
Previously, we found a different dependency on vitamin B1 during B-cell differentiation in the small intestine (41) (Fig. 2). In the small intestine, naive B cells differentiate into IgA+ B cells in the PPs by class-switching of the immunoglobulin and then differentiate into IgA-producing plasma cells (IgA+ PCs) in the small intestine lamina propria (iLP). Both naive B cells in PPs and IgA+ PCs in iLP use the TCA cycle but only IgA+ PCs undergo glycolysis (41). These metabolic differences reflected their dependencies on vitamin B1, an essential cofactor for the TCA cycle. Indeed, depletion of dietary vitamin B1 decreases only naive B cells in PPs but does not affect IgA+ PCs in iLP in mice (41). Since PPs are the primary sites of induction of antigen-specific IgA responses, PP regression induced by vitamin B1 deficiency leads to decreased IgA antibody responses to oral vaccines in mice (41) (Fig. 2).
In addition to diets, commensal bacteria, including gut microbiota, contribute to vitamin metabolism (39). Most intestinal commensal bacteria are obligate anaerobic bacteria belonging to the phyla Firmicutes, Bacteroidetes and Actinobacteria, which are dominant phyla among human gut microbiota. Bacteria belonging to Bacteroidetes such as Bacteroides and Prevotella and Actinobacteria such as Bifidobacterium are most likely to be able to synthesize the vitamin B family and vitamin K but many of the bacteria belonging to the Firmicutes such as Faecalibacterium lack the genes involved in the synthesis pathways of these vitamins, suggesting that it is impossible to synthesize vitamins (39, 42).
Thus, there are bacteria that can synthesize vitamins and there are bacteria that are not able to synthesize vitamins. On the other hand, it is believed that many bacteria in the intestine possess vitamin transporters, even in bacteria that do not have a vitamin synthesis pathway. Therefore, many bacteria in the intestine are believed to take up vitamins and use them. Taken together, intestinal commensal bacteria are one of the key factors that influence host immunity through vitamin-mediated energy metabolism.
Dietary vitamin B1 is absorbed by the small intestinal epithelium via thiamine transporters and is transported to the blood for distribution throughout the body (43). Therefore, it is thought that dietary vitamin B1 does not reach the colon; however, commensal bacteria are mainly present in the colon, indicating that bacterial vitamin B1 is absorbed directly by the colon (39). Thus, dietary vitamin B1 and bacterial vitamin B1 appear to have immunological roles at different places in the intestine.
Role of dietary and bacterial fatty acid metabolites in host immune metabolism
Fatty acids are key components of many cellular structures and associate with cellular signaling through several receptors. Therefore, fatty acids play an important role in the control of cell metabolism. As an example of the importance of fatty acid metabolism in immune function, we reported that dietary palm oil promotes intestinal IgA antibody production in mice (44, 45) (Fig. 2). Palm oil contains a large amount of palmitic acid, which occurs in membrane phospholipids and has multiple fundamental biological functions (46, 47). We found two pathways by which palmitic acid enhances IgA antibody production: a direct pathway in which palmitic acid directly affects IgA-producing cells and an indirect pathway where palmitic acid acts through its metabolites such as sphingolipids (Fig. 2).
When we co-cultured IgA-producing cells and palmitic acid in vitro, IgA antibody production was enhanced by the direct action of palmitic acid in a dose-dependent manner (45). Sphingolipids, which are endogenously produced from palmitic acid by serine-palmitoyl transferase, are involved in several cellular events including differentiation, proliferation, migration and apoptosis (46, 47). In this regard, we found that sphingosine 1 phosphate is associated with differentiation of naive B cells to IgA+ B cells in PPs and migration of IgA+ B cells into iLP (48–50). Thus, fatty acid metabolism plays an important role in immunological function, especially small intestinal IgA immune responses. Taken together, it has been clarified that dietary nutrients regulate immune function through the metabolism of immune cells.
In addition, intestinal bacteria contribute to fatty acid metabolism for the modulation of the immune system (51–53). Conjugated linoleic acid is a well-known bacterial fatty acid metabolite, which is used widely as a functional food (52, 54, 55). Conjugated linoleic acid such as (9Z,11E)-CLA isomer is a potent agonist of PPAR-α and increases catabolism of lipids in the liver (56). In addition, conjugated linoleic acid modulates macrophage function and induces anti-inflammatory M2 macrophages in a PPAR-γ-dependent manner (53). Another bacterial metabolite, the linolenic acid metabolite 10-hydroxy-cis-12-octadecenoic acid (HYA), acts as a G-protein-coupled receptor 40 (GPR40) agonist and enhances the barrier function of the intestinal epithelium by increasing the expression of tight-junction-related molecules, resulting in suppression of colitis in mice (51, 57). The α-linolenic acid metabolites 13-hydroxy-9(Z),15(Z)-octadecadienoic acid and 13-oxo-9(Z),15(Z)-octadecadienoic acid induce the differentiation of anti-inflammatory M2 macrophages through GPR40 (58). Thus, bacterial fatty acid metabolites have several biological functions, including modulation of the differentiation and function of immune cells.
When considering the signaling pathways and roles of receptors for bacterial fatty acid metabolites, PPARs play an important role in energy metabolism, including fatty acid metabolism, glucose metabolism and mitochondrial function in several tissues and cells (59). GPR120 and GPR40 also regulate energy metabolism through the regulation of glucose metabolism (60–62). Taken together, bacterial fatty acid metabolites could modulate cell metabolism associated with immunological function.
Conclusion
Accumulating evidence reveals mechanisms of metabolic regulation and roles in immune functions; however, there is still a lack of information about the metabolic changes and their roles in the defense against individual pathogenic micro-organisms. Metabolic changes to commensals seem to be different from those to pathogens. Moreover, both the diet and microbial effects on nutrition are also involved in the regulation of host metabolism. When considering immune metabolism, we need an understanding of complex interactions between host immunity, nutrition and micro-organisms. Further research will lead to the development of new therapies and prevention of infectious and immune diseases.
Funding
The work related to this review article was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Japan Society for the Promotion of Science under grant numbers JP18H02150 (J.K.), JP18H02674 (J.K.), JP17K09604 (J.K.) and JP18K17997 (K.H.); the Japan Agency for Medical Research and Development (AMED) under grant numbers JP17fk0108223h0002 (J.K.), JP17ek0410032s0102 (J.K.), JP17fk0108207h0002 (J.K.), JP17ek0210078h0002 (J.K.), JP17ak0101068h0001 (J.K.), JP17gm1010006s0101 (J.K.), JP18ck0106243h0003 (J.K.) and JP19ek0410062h0001 (J.K.); the Ministry of Health, Labour, and Welfare of Japan under grant number JP19KA3001 (K.H.); the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (J.K.); the Terumo Foundation for Life Sciences and Arts (J.K.); the ONO Medical Research Foundation (J.K.); the Canon Foundation (J.K.); and Cross-ministerial Strategic Innovation Promotion Program (J.K.); the Joint Research Project of the Institute of Medical Science, the University of Tokyo (J.K.) and Public/Private R&D Investment Strategic Expansion PrograM (PRISM).
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Liberti M. V. and Locasale J. W. 2016. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pearce E. L. and Pearce E. J. 2013. Metabolic pathways in immune cell activation and quiescence. Immunity 38:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang R., Dillon C. P., Shi L. Z. et al. . 2011. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frauwirth K. A., Riley J. L., Harris M. H. et al. . 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity 16:769. [DOI] [PubMed] [Google Scholar]
- 5. Waickman A. T. and Powell J. D. 2012. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol. Rev. 249:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sena L. A., Li S., Jairaman A. et al. . 2013. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michalek R. D., Gerriets V. A., Jacobs S. R. et al. . 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186:3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobs S. R., Herman C. E., Maciver N. J. et al. . 2008. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 180:4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delgoffe G. M., Kole T. P., Zheng Y. et al. . 2009. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delgoffe G. M., Pollizzi K. N., Waickman A. T. et al. . 2011. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berod L., Friedrich C., Nandan A. et al. . 2014. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20:1327. [DOI] [PubMed] [Google Scholar]
- 12. Doughty C. A., Bleiman B. F., Wagner D. J. et al. . 2006. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 107:4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dufort F. J., Bleiman B. F., Gumina M. R. et al. . 2007. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J. Immunol. 179:4953. [DOI] [PubMed] [Google Scholar]
- 14. Dufort F. J., Gumina M. R., Ta N. L. et al. . 2014. Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for atp-citrate lyase in lipopolysaccharide-induced differentiation. J. Biol. Chem. 289:7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wheeler M. L. and Defranco A. L. 2012. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J. Immunol. 189:4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jha A. K., Huang S. C., Sergushichev A. et al. . 2015. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42:419. [DOI] [PubMed] [Google Scholar]
- 17. Mills E. L. and O’Neill L. A. 2016. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 46:13. [DOI] [PubMed] [Google Scholar]
- 18. Tannahill G. M., Curtis A. M., Adamik J. et al. . 2013. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vats D., Mukundan L., Odegaard J. I. et al. . 2006. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oishi Y., Spann N. J., Link V. M. et al. . 2017. SREBP1 contributes to resolution of pro-inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 25:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang S., Nakanishi Y., Kioi Y. et al. . 2018. Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Nat. Immunol. 19:561. [DOI] [PubMed] [Google Scholar]
- 22. Krawczyk C. M., Holowka T., Sun J. et al. . 2010. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115:4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lachmandas E., Boutens L., Ratter J. M. et al. . 2016. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat. Microbiol. 2:16246. [DOI] [PubMed] [Google Scholar]
- 24. Gleeson L. E., Sheedy F. J., Palsson-McDermott E. M. et al. . 2016. Cutting edge: Mycobacterium tuberculosis induces aerobic glycolysis in human alveolar macrophages that is required for control of intracellular bacillary replication. J. Immunol. 196:2444. [DOI] [PubMed] [Google Scholar]
- 25. Shi L., Salamon H., Eugenin E. A., Pine R., Cooper A. and Gennaro M. L. 2015. Infection with Mycobacterium tuberculosis induces the Warburg effect in mouse lungs. Sci. Rep. 5:18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caputa G., Castoldi A. and Pearce E. J. 2019. Metabolic adaptations of tissue-resident immune cells. Nat. Immunol. 20:793. [DOI] [PubMed] [Google Scholar]
- 27. Michelucci A., Cordes T., Ghelfi J. et al. . 2013. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl Acad. Sci. USA 110:7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan Z., Xie N., Banerjee S. et al. . 2015. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J. Biol. Chem. 290:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gillmaier N., Götz A., Schulz A., Eisenreich W. and Goebel W. 2012. Metabolic responses of primary and transformed cells to intracellular Listeria monocytogenes. PLoS One 7:e52378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee J., Walsh M. C., Hoehn K. L., James D. E., Wherry E. J. and Choi Y. 2014. Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity. J. Immunol. 192:3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obata T., Goto Y., Kunisawa J. et al. . 2010. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc. Natl Acad. Sci. USA 107:7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sato S., Kaneto S., Shibata N. et al. . 2013. Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer’s patch M cells. Mucosal Immunol. 6:838. [DOI] [PubMed] [Google Scholar]
- 33. Fung T. C., Bessman N. J., Hepworth M. R. et al. . 2016. Lymphoid-tissue-resident commensal bacteria promote members of the IL-10 cytokine family to establish mutualism. Immunity 44:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sonnenberg G. F., Monticelli L. A., Alenghat T. et al. . 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shibata N., Kunisawa J., Hosomi K. et al. . 2018. Lymphoid tissue-resident Alcaligenes LPS induces IgA production without excessive inflammatory responses via weak TLR4 agonist activity. Mucosal Immunol. 11:693. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi I., Hosomi K., Nagatake T. et al. . 2020. Persistent colonization of non-lymphoid tissue-resident macrophages by Stenotrophomonas maltophilia. Int. Immunol. 32:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagai M., Noguchi R., Takahashi D. et al. . 2019. Fasting-refeeding impacts immune cell dynamics and mucosal immune responses. Cell 178:1072. [DOI] [PubMed] [Google Scholar]
- 38. Kunisawa J., Kurashima Y. and Kiyono H. 2012. Gut-associated lymphoid tissues for the development of oral vaccines. Adv. Drug Deliv. Rev. 64:523. [DOI] [PubMed] [Google Scholar]
- 39. Yoshii K., Hosomi K., Sawane K. and Kunisawa J. 2019. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hosomi K. and Kunisawa J. 2017. The specific roles of vitamins in the regulation of immunosurveillance and maintenance of immunologic homeostasis in the Gut. Immune Netw. 17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kunisawa J., Sugiura Y., Wake T. et al. . 2015. Mode of bioenergetic metabolism during B cell differentiation in the intestine determines the distinct requirement for vitamin B1. Cell Rep. 13:122. [DOI] [PubMed] [Google Scholar]
- 42. Magnúsdóttir S., Ravcheev D., de Crécy-Lagard V. and Thiele I. 2015. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Said H. M. 2011. Intestinal absorption of water-soluble vitamins in health and disease. Biochem. J. 437:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kunisawa J. and Kiyono H. 2012. Immunological function of sphingosine 1-phosphate in the intestine. Nutrients 4:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kunisawa J., Hashimoto E., Inoue A. et al. . 2014. Regulation of intestinal IgA responses by dietary palmitic acid and its metabolism. J. Immunol. 193:1666. [DOI] [PubMed] [Google Scholar]
- 46. Hannun Y. A. and Obeid L. M. 2018. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 19:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carta G., Murru E., Banni S. and Manca C. 2017. Palmitic acid: physiological role, metabolism and nutritional implications. Front. Physiol. 8:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kunisawa J., Gohda M., Kurashima Y., Ishikawa I., Higuchi M. and Kiyono H. 2008. Sphingosine 1-phosphate-dependent trafficking of peritoneal B cells requires functional NFkappaB-inducing kinase in stromal cells. Blood 111:4646. [DOI] [PubMed] [Google Scholar]
- 49. Kunisawa J., Kurashima Y., Gohda M. et al. . 2007. Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood 109:3749. [DOI] [PubMed] [Google Scholar]
- 50. Gohda M., Kunisawa J., Miura F. et al. . 2008. Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer’s patches for intestinal IgA responses. J. Immunol. 180:5335. [DOI] [PubMed] [Google Scholar]
- 51. Kishino S., Takeuchi M., Park S. B. et al. . 2013. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl Acad. Sci. USA 110:17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ogawa J., Kishino S., Ando A., Sugimoto S., Mihara K. and Shimizu S. 2005. Production of conjugated fatty acids by lactic acid bacteria. J. Biosci. Bioeng. 100:355. [DOI] [PubMed] [Google Scholar]
- 53. Hosomi K., Kiyono H. and Kunisawa J. 2019. Fatty acid metabolism in the host and commensal bacteria for the control of intestinal immune responses and diseases. Gut Microbes 23:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. den Hartigh L. J. 2019. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives. Nutrients 11:E370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Evans N. P., Misyak S. A., Schmelz E. M., Guri A. J., Hontecillas R. and Bassaganya-Riera J. 2010. Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARgamma. J. Nutr. 140:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moya-Camarena S. Y., Vanden Heuvel J. P., Blanchard S. G., Leesnitzer L. A. and Belury M. A. 1999. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J. Lipid Res. 40:1426. [PubMed] [Google Scholar]
- 57. Miyamoto J., Mizukure T., Park S. B. et al. . 2015. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J. Biol. Chem. 290:2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ohue-Kitano R., Yasuoka Y., Goto T. et al. . 2018. α-Linolenic acid-derived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. FASEB J. 32:304. [DOI] [PubMed] [Google Scholar]
- 59. Nakamura M. T., Yudell B. E. and Loor J. J. 2014. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 53:124. [DOI] [PubMed] [Google Scholar]
- 60. Song T., Yang Y., Zhou Y., Wei H. and Peng J. 2017. GPR120: a critical role in adipogenesis, inflammation, and energy metabolism in adipose tissue. Cell. Mol. Life Sci. 74:2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huang J., Xue M., Zhang J. et al. . 2019. Protective role of GPR120 in the maintenance of pregnancy by promoting decidualization via regulation of glucose metabolism. EBioMedicine 39:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hara T., Kashihara D., Ichimura A., Kimura I., Tsujimoto G. and Hirasawa A. 2014. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim. Biophys. Acta 1841:1292. [DOI] [PubMed] [Google Scholar]