Abstract
The dendritic cell (DC) is recognized as a vital mediator of anti-tumor immunity. More recent studies have also demonstrated the important role of DCs in the generation of effective responses to checkpoint inhibitor immunotherapy. Metabolic programming of DCs dictates their functionality and can determine which DCs become immunostimulatory versus those that develop a tolerized phenotype capable of actively suppressing effector T-cell responses to cancers. As a result, there is great interest in understanding what mechanisms have evolved in cancers to alter these metabolic pathways, thereby allowing for their continued progression and metastasis. The therapeutic strategies developed to reverse these processes of DC tolerization in the tumor microenvironment represent promising candidates for future testing in combination immunotherapy clinical trials.
Keywords: β-catenin, fatty acid oxidation, indoleamine 2, 3-dioxygenase, lipid bodies, PPAR
Introduction
Dendritic cells (DCs) play a critical role in the generation of anti-tumor immunity (1). Indeed, recent data have shown the DC to be essential for the generation of effector T-cell responses to checkpoint inhibitor immunotherapy (2). Therefore, our understanding of the biochemical pathways that tumors leverage to interfere with DC function now has direct therapeutic relevance (3). Accumulating evidence has shown that multiple tumor types are associated with the accumulation of tolerized DC populations (4). Over the last several years, the concept of the tolerized DC has evolved to be defined as a DC sub-population that is more effective at driving regulatory T cell (Treg) differentiation than the activation of cluster of differentiation 8 (CD8)+ effector T cells (5, 6). This implies that tolerized DCs are reprogrammed to promote enhanced Treg development while also being impaired in their ability to conduct the process of antigen cross-presentation, a mechanism that is critical for tumor immunosurveillance and for generating responses to checkpoint inhibitor immunotherapy (2).
Despite this general understanding of the tolerized DC, the underlying signaling pathways responsible for driving this phenotypic switch within the tumor microenvironment (TME) have historically remained unclear. More recent reports have demonstrated that the metabolic state of the DC represents a fundamental regulator capable of dictating its function (7–11) (Fig. 1). This realization has raised several important questions about how cancers may manipulate these metabolic pathways in DCs to ultimately create an immunoprivileged microenvironment. This line of inquiry further carries immediate translational significance in immuno-oncology as these identified pathways represent promising pharmacologic targets for future development of novel immunotherapeutic combinations.
Fig. 1.
Several steps of cellular metabolism can have a direct impact on DC functionality. CPT1a, carnitine palmitoyltransferase 1a. PpIX, protoporphyrin IX.
While there are several tumor-derived factors that have been demonstrated to alter DC functionality within the TME in different settings, a relatively limited number of these mechanisms have been shown to target DC metabolic pathways. This mini-review will cover those tumor-dependent pathways where existing data support metabolic reprogramming of the DC as the primary mechanism of immune tolerization (Fig. 2).
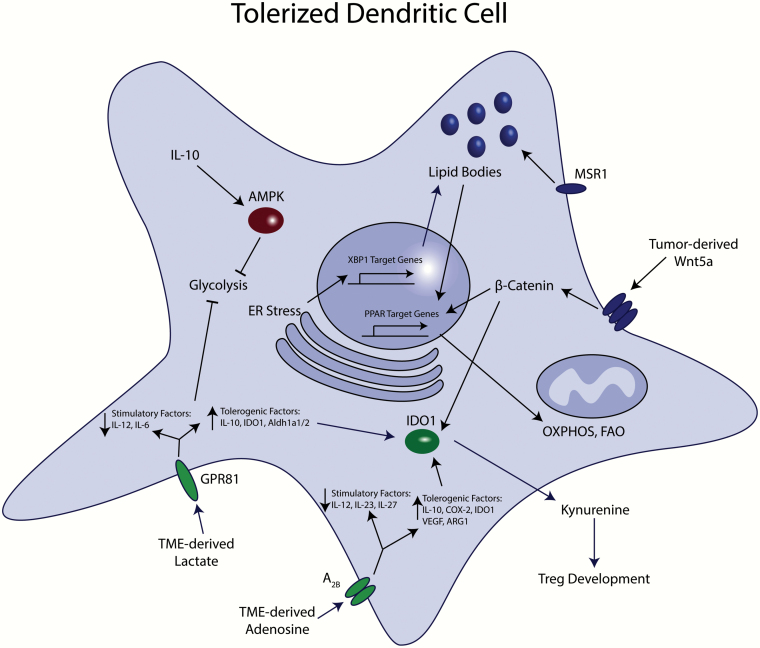
Fig. 2.
Signaling pathways involved in tumor-mediated metabolic reprogramming of DCs. Several factors generated within the TME contribute to suppressed DC-dependent effector T-cell activation and enhanced generation of local Tregs. The Wnt–β-catenin pathway can also dominantly inhibit DC glycolysis (not shown). Common themes include inhibition of glycolysis, promotion of OXPHOS and the stimulation of IDO1 activity.
DC lipid stores, fatty acid oxidation and the peroxisome proliferator-activated receptors
During activation, DCs functionally depend on the process of glycolysis because of their increased bioenergetic demands as opposed to oxidative phosphorylation (OXPHOS), which predominates in immature or tolerogenic DCs (9). This enhanced glycolytic state of the DC was described as a critical driver of enhanced fatty acid synthesis, a process necessary for the expansion of the endoplasmic reticulum (ER) and the increased production capacity of secreted protein factors (10). An early report indicating that interleukin-10 (IL-10) antagonizes lipopolysaccharide (LPS)-induced DC glycolysis and maturation by promoting the activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) raised the possibility that extrinsic paracrine signaling pathways may be capable of promoting the generation of an immunotolerant microenvironment by modulating DC metabolism (9).
It was another observation reported by Herber et al. in 2010 describing increased lipid deposition in tumor-associated DC populations that implicated the alteration of DC metabolism as an important driver of tumor-mediated immune evasion. These effects were further shown to be promoted by class A macrophage scavenger receptor type 1 (Msr1, CD204)-mediated lipid uptake and that this process impaired DC-dependent stimulation of T-cell responses (12). Notably, small-molecule inhibition of Msr1 also normalized the stimulatory capacity of these DCs. How these alterations in lipid levels led to impaired DC functionality was uncertain until a more recent report described the molecular mechanism linking oxidized lipid bodies with the binding of the heat shock protein-70 (Hsp70) chaperone and the defective transport of complexes of major histocompatibility complex (MHC) class I with peptide (MHC:peptide), which ultimately suppressed cross-presentation of antigens by DCs (13). Tumor-derived oxysterols have also been shown to suppress C–C chemokine receptor type 7 (CCR7) expression on DCs, thereby inhibiting DC migration to secondary lymphoid tissues and suppressing the generation of an anti-tumor immune response (14).
An additional study has further suggested that lipid peroxidation in DCs promotes the activation of X-box binding protein 1 (XBP1), a mediator of the unfolded protein response (UPR) to ER-associated stress induced by tumors (15). XBP1, in turn, activates a lipid biosynthetic program and the subsequent accumulation of increased lipid stores. XBP1-deficient DCs were shown to better support enhanced CD8+ T-cell activation while also being less efficient at promoting Treg differentiation (15). The increased level of reactive oxygen species (ROS) within tumor-associated DCs was implicated as a critical contributor to the generation of reactive by-products capable of forming stable adducts with ER-associated chaperones, thus promoting protein-folding stress. While this phenomenon was demonstrated to occur in a model of ovarian cancer, whether this process is an important contributor to lipid accumulation in DCs in other cancer types and in patients remains unclear.
In addition to increased lipid stores, various authors have also noted that the process of fatty acid oxidation (FAO), a mitochondrial aerobic process that serves to convert fatty acids to cellular energy, plays an active role in driving DCs toward a tolerized phenotype (7, 16). Presumably, the increased lipid stores in tumor-associated DCs can serve to fuel the process of FAO through the stimulation of the peroxisome proliferator-activated receptors (PPARs) (17). The PPARs represent a family of transcription factors that respond to various lipid ligands including fatty acids that, in turn, activate pathways important in FAO.
In addition to their role in lipid metabolism, all three PPAR isoforms, PPAR-α, PPAR-β/δ and PPAR-γ, have also been implicated in the regulation of various autoimmune and inflammatory diseases while impacting the biology of several different immune cell populations, including DCs (18). PPAR-γ, in particular, has been demonstrated to play a role in the suppression of DC migration and DC-mediated T-cell activation and it was found that agonists of this pathway inhibit the expression of DC-derived cytokines such as IL-6, tumor necrosis factor-α (TNF-α), IL-15 and IL-12 while promoting DC IL-10 expression (19–23).
Consistent with these findings, various short-chain fatty acids generated by gut microbiota also suppress the expression of several pro-inflammatory chemokines by DCs (24). Indeed, small-molecule ligands for both PPAR-α and PPAR-β/δ suppress DC IL-12 expression along with allogeneic stimulation of T-cell proliferation, indicating that these PPAR isoforms may behave in a similar manner or at least have functional overlap with PPAR-γ (20, 25). This is further supported by a study demonstrating that PPAR-β/δ signaling drives the accumulation of IL-10-expressing myeloid cells and promotes the progression of a pre-clinical model of non-small cell lung cancer (26).
It has been postulated that the ability of the PPARs to modulate cytokine expression is secondary to the process of transrepression, where PPARs effectively inhibit the activation of other transcription factors such as nuclear factor-κB (NF-κB) (27). Whether the relative activity and downstream gene targets of the different PPARs may vary depending on the specific DC sub-population remains less clear. In addition, given that there are differences in the ligand repertoire for each PPAR isoform, it is possible that the individual PPARs may have different roles depending on the physiologic setting of the DC.
Despite the ability of the PPARs to suppress pro-inflammatory cytokines and chemokines in DCs, it has remained uncertain how DC FAO is mechanistically linked to the promotion of Treg differentiation. An earlier report noted that PPAR-γ induces retinoic acid (RA) synthesis by promoting retinol production, as well as the expression of the retinal metabolizing enzyme, aldehyde dehydrogenase 1 family member A2 (Aldh1a2) (28). This is significant since subsequent reports have shown CD103+ DCs and lamina propria DCs in the gut to drive Treg differentiation partially via the production of RA via Aldh1a1 (29, 30).
The role of DC-expressed indoleamine 2,3-dioxygenase (IDO1) has also been demonstrated in several studies to play an important role in promoting DC-dependent Treg differentiation and activation (5, 31, 32). However, how IDO1 is modulated by DC metabolism and whether IDO1 contributes to the tolerized state created by DC FAO have remained important questions in the field. The protoporphyrin IX (PpIX) prosthetic group plays a critical rate-limiting role in regulating the enzymatic activity of IDO1 (33–35). On this basis, we demonstrated that increased FAO in DCs drives heme biosynthetic flux via succinyl CoA of the tricarboxylic acid (TCA) cycle in order to generate increased levels of PpIX (36). This work further showed DC FAO to enhance IDO1 enzymatic activity and ultimately generate increased numbers of Tregs via this metabolic pathway both in vitro and in vivo.
Tumor-mediated regulation of DC metabolic reprogramming
While these studies have shed light on how DC lipid metabolism drives the generation of an immunotolerant state, our understanding of the underlying biochemical pathways utilized by cancers to manipulate DC metabolism and to generate an environment permissive for cancer growth remains limited.
One tumor-derived factor that contributes to DC metabolic re-reprogramming is the metabolic by-product, lactic acid. It has been well documented that tumor-infiltrating immune cell populations encounter an acidic microenvironment generated through the Warburg effect (aerobic glycolysis) and that high levels of lactic acid derived from tumor cells and/or tumor-associated stromal cells are an important contributor to this process. Importantly, studies have shown tumor-derived lactic acid to suppress anti-tumor immunity and promote resistance to checkpoint inhibitor immunotherapy (37). Consistent with these findings, lactic acid suppresses DC IL-12 expression and inhibits DC-dependent antigen presentation in vitro (38).
A recent murine study demonstrated that lactic acid signals via the Gi-protein-coupled receptor, GPR81, in colonic DCs to suppress IL-6 and IL-12 expression while promoting the expression of IL-10, IDO1 and the retinal-metabolizing enzymes, Aldh1a1 and Aldh1a2 (39). Consistent with these findings, GPR81−/− mice were further found to exhibit diminished levels of Tregs in the colonic mucosa and to have an enhanced susceptibility to dextran sulfate sodium (DSS)-induced colitis. This was shortly followed by an additional report showing that paracrine lactate–GPR81 signaling contributes to diminished DC-dependent T-cell stimulation in vitro and to enhanced progression of a murine breast cancer model in vivo (40). In plasmacytoid DCs (pDCs), lactic acid inhibits type I interferon (IFN) expression and promotes IDO1-dependent Treg generation in a breast cancer model (41). This effect, mediated by both GPR81 and the lactate monocarboxylate transporters, MCT-1 and MCT-2, is also accompanied by a significant inhibition in pDC glycolysis and is consistent with the previously reported role of glycolysis in pDC-induced type I IFN production (42).
In addition to an acidic microenvironment, developing cancers also generate regions of hypoxia that promote local expression of hypoxia inducible factor-1α (HIF-1α). Previous studies have shown a role for HIF-1α in driving the development of an immunotolerant M2 macrophage subset and promoting tumor progression in vivo. Consistent with this, HIF-1α has been more recently implicated as an important contributor to DC-mediated Treg differentiation in the intestinal mucosa and a repressor of DC-dependent T helper type 1 (TH1) polarization in atherosclerosis (43, 44). Additional work has shown the pharmacologic inhibition of HIF-1α to enhance the anti-tumor efficacy of a DC-based cancer vaccine in a 4T1 breast cancer model (45). On the basis of these cumulative studies, we can deduce that lactic acid drives DC tolerization by promoting OXPHOS in the TME. Further studies are needed to confirm this hypothesis and to investigate whether this process is also FAO-dependent.
In addition to lactic acid, tumor-associated hypoxia also promotes the accumulation of adenosine in the TME. Adenosine is an enzymatic degradation product of adenosine triphosphate (ATP). Like lactate, adenosine causes a tolerogenic phenotype in tumor-infiltrating DCs and ultimately leads to tumor progression. Adenosine has multiple mechanisms that converge to inhibit the immunostimulatory properties of DCs via activation of the adenosine receptor 2B (A2B adenosine receptor) (46). For example, treatment of human DCs with adenosine receptor agonists diminishes the secretion of IL-12 family cytokines (IL-12, IL-23, IL-27) during DC maturation (47, 48).
Additionally, adenosine receptor agonism leads to increased production of numerous immunosuppressive and tolerogenic factors by DCs including IL-10, cyclooxygenase-2 (COX-2), IDO1, arginase I (ARG1) and ARG2, thrombospondin and vascular endothelial growth factor (VEGF) (49, 50). While all of these alterations are expected to hamper TH1 polarization and impair the activation of cytolytic CD8+ T cells, additional studies have also shown that MHC:peptide engagement of the T-cell antigen receptor (TCR) in the presence of adenosine leads to T-cell anergy and the generation of lymphocyte-activation gene-3 (Lag-3)+ Tregs (51, 52).
Although the impact of adenosine on the regulation of DC metabolism has not been investigated thoroughly, our understanding of the downstream signaling pathway of the A2B adenosine receptor does allow us to postulate that the elevation in cytoplasmic cyclic AMP (cAMP) that occurs in response to the activation of this pathway is likely to increase the activity of AMPK, a well-described inducer of catabolic metabolism (53, 54). Previous work has shown that elevated AMPK activity suppresses DC glycolysis and inhibits DC activation (as mentioned in the previous section), thus suggesting that adenosine-induced suppression of DC functionality is likely to depend on a metabolic shift from glycolysis toward OXPHOS. Further studies are necessary to formally link these two signaling pathways.
Because of the impact of adenosine on DC function, researchers have initiated studies to target the adenosine signaling axis with the goal of reversing DC tolerization and promoting DC-mediated CD8+ T-cell activation. In mouse models of melanoma and sarcoma, Perrot et al. demonstrated that preventing the production of adenosine from ATP in the TME by blocking the ecto-nucleotidases, CD39 and CD73, can promote tumor immunity by enhancing DC-dependent effector T-cell activation (55). These findings have supported the development of ongoing clinical studies designed to test pharmacologic inhibitors of CD39 and CD73 activity in various immunotherapy trials in cancer patients (56).
In an effort to identify additional pathways in the TME that may promote DC tolerization, our group initially focused on the regulation of the immunoregulatory enzyme, IDO1, previously identified to play a critical role in driving DC-dependent Treg development (see previous section). Analysis of the Ido1/IDO1 promoter led us to the identification of the Wnt5a–β-catenin signaling pathway as an important candidate for promoting tumor-mediated DC tolerization (57). Using a transgenic model of melanoma, this study demonstrated tumor-expressed Wnt5a to drive β-catenin-dependent expression of IDO1 by nearby DCs and for this process to potently stimulate the differentiation of Tregs in vitro as well as in vivo. To support the relevance of this pathway in the regulation of anti-tumor immunity, this study further showed the inhibition of Wnt5a release by melanomas to enhance the efficacy of checkpoint inhibitor immunotherapy.
While performing these studies, we further observed that Wnt5a inhibited DC glycolysis, prompting additional investigation into the metabolic impact of the Wnt5a–β-catenin signaling pathway. We therefore launched a series of experiments demonstrating paracrine Wnt5a signaling to drive DC FAO and subsequent DC-mediated Treg development along with concurrent suppression of effector CD8+ T-cell activation (36). This pathway was found to trigger the formation of β-catenin:PPAR-γ complexes resulting in downstream induction of the mitochondrial fatty acid transporter, carnitine palmtoyltransferase-1a (CPT1a). This study further showed DC-targeted genetic and pharmacologic inhibition of the Wnt5a–β-catenin signaling pathway to suppress melanoma progression and to augment the efficacy of antibodies to programmed cell death protein-1 (anti-PD-1).
Remaining questions and future directions
While each of these tumor-dependent mechanisms induces the tolerization of DCs in situ, these pathways are restricted to local DC populations within the tumor bed. Whether and how cancers manipulate DC FAO within more distant lymph node tissues remains unanswered. Given that this long-range mechanism would be expected to lead to a more dramatic impact on the priming of naive T-cell populations, this has become a scientific priority for our research group. It seems likely that these mechanisms may ultimately vary between different solid tumor types; however, this requires further study.
In addition, it remains unclear whether there are separate tumor-derived signals that can modulate various steps of DC metabolic reprogramming such as pathways that promote DC lipid uptake along with distinct signals capable of directing the transcriptional programs necessary for DC FAO. There is emerging evidence of a close bidirectional relationship between the programming of DC metabolism and epigenetic regulation (58, 59). An improved understanding of this interplay, how it integrates with the PPAR-dependent pathways discussed above and how it is influenced by the TME will be important areas of future research. Insights into the intricacies of the mechanisms utilized by cancers to modulate DC metabolism promise to lead to the identification of novel pharmacologic, as well as genetic, targets capable of reversing DC tolerization and potently enhancing anti-tumor immunity.
Functionally similar to what we observe in DCs, FAO has also been demonstrated to promote myeloid-derived suppressor cell activity as well as the M2 polarization of macrophages (60, 61). These findings suggest that the use of systemic inhibitors of these pathways could also promote anti-tumor immunity through alternative mechanisms. Indeed, the anti-diabetic agent metformin has been shown to inhibit FAO and promote glycolysis in tumor-associated myeloid cell populations while suppressing the development of a murine osteosarcoma model (62). Further studies have shown metformin therapy to augment anti-PD-1 antibody immunotherapy by suppressing tumor hypoxia in pre-clinical models (63).
We have further demonstrated systemic inhibition of CPT1a with the pharmacological inhibitor, etomoxir, to suppress the progression of a transgenic melanoma model in a CD8+ T-cell-dependent manner while also enhancing the activity of anti-PD-1 blockade (36). How much this effect is CPT1a-specific in situ remains unclear (64). Reports indicating a role for FAO in the development of memory CD8+ T cells suggest that more targeted, cell-specific strategies may be necessary to target DC FAO in future clinical protocols (65–67). More elegant approaches for selectively manipulating DCs in situ are therefore an area of unmet need and are predicted to make a significant impact on the field of immuno-oncology.
Conclusions
It has become clear that many of the molecular underpinnings of DC functionality reside within cellular metabolic pathways (Fig. 1). Given the fundamental importance of metabolic reprogramming in DC activity and its downstream impact on the generation of anti-tumor immunity, it is logical to conclude that these metabolic pathways are critical targets for manipulation by evolving cancers to evade immunosurveillance (Fig. 2). While different cancer types have likely developed diverse strategies for altering DC metabolism in the TME, additional investigation will be important to identify potential downstream pathways that are shared among the various tumor-derived factors generated to impair DC activity. This will allow for the future development of therapeutic strategies capable of potently reversing these pathways of DC tolerization in a larger number of patients with a broader range of tumor types.
In light of the importance of DCs in the generation of anti-tumor immunity and the development of responses to checkpoint inhibitor therapy, those agents capable of modulating tumor-dependent alterations in DC metabolism will be promising candidates for future combination immunotherapy studies.
Funding
This work was supported in part by a Damon Runyon Foundation Physician Scientist Award (to N.C.D.), a National Institutes of Health (NIH) grant K08 CA191063-04 (to B.A.H.), an Alliance for Cancer Gene Therapy Young Investigator Award (to B.A.H.), a Duke University Health Scholar Award (to B.A.H.) and a Duke Strong Start Physician Scientist Award (to B.A.H.).
Conflicts of interest statement: B.A.H. receives research funding from Merck & Co., Tempest Therapeutics, Leap Therapeutics and A*STAR (Agency for Science, Technology and Research) Singapore.
References
- 1. Banchereau, J. and Steinman, R. M. 1998. Dendritic cells and the control of immunity. Nature 392:245. [DOI] [PubMed] [Google Scholar]
- 2. Salmon, H., Idoyaga, J., Rahman, A.et al. 2016. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spranger, S., Dai, D., Horton, B. and Gajewski, T. F. 2017. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gabrilovich, D. 2004. Mechanisms and functional significance of tumor-induced dendritic cell defects. Nat. Rev. Immunol. 4:941. [DOI] [PubMed] [Google Scholar]
- 5. Munn, D. H., Sharma, M. D., Lee, J. R.et al. 2002. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297:1867. [DOI] [PubMed] [Google Scholar]
- 6. Mellor, A. L. and Munn, D. H. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4:762. [DOI] [PubMed] [Google Scholar]
- 7. Everts, B. and Pearce, E. J. 2014. Metabolic control of dendritic cell activation and function: recent advances and clinical implications. Front. Immunol. 5:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Neill, L. A. and Pearce, E. J. 2016. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krawczyk, C. M., Holowka, T., Sun, J.et al. 2010. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115:4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Everts, B., Amiel, E., Huang, S. C.et al. 2014. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 15:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pearce, E. J. and Everts, B. 2015. Dendritic cell metabolism. Nat. Rev. Immunol. 15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herber, D. L., Cao, W., Nefedova, Y.et al. 2010. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 16:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramakrishnan, R., Tyurin, V. A., Tuyrin, V. A.et al. 2014. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J. Immunol. 192:2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villablanca, E. J., Raccosta, L., Zhou, D.et al. 2010. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med. 16:98. [DOI] [PubMed] [Google Scholar]
- 15. Cubillos-Ruiz, J. R., Silberman, P. C., Rutkowski, M. R.et al. 2015. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161:1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malinarich, F., Duan, K., Hamid, R. A.et al. 2015. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. J. Immunol. 194:5174. [DOI] [PubMed] [Google Scholar]
- 17. Zechner, R., Zimmermann, R., Eichmann, T. O.et al. 2012. FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kidani, Y. and Bensinger, S. J. 2012. Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol. Rev. 249:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nencioni, A., Grünebach, F., Zobywlaski, A., Denzlinger, C., Brugger, W. and Brossart, P. 2002. Dendritic cell immunogenicity is regulated by peroxisome proliferator-activated receptor gamma. J. Immunol. 169:1228. [DOI] [PubMed] [Google Scholar]
- 20. Jakobsen, M. A., Petersen, R. K., Kristiansen, K., Lange, M. and Lillevang, S. T. 2006. Peroxisome proliferator-activated receptor alpha, delta, gamma1 and gamma2 expressions are present in human monocyte-derived dendritic cells and modulate dendritic cell maturation by addition of subtype-specific ligands. Scand. J. Immunol. 63:330. [DOI] [PubMed] [Google Scholar]
- 21. Angeli, V., Hammad, H., Staels, B., Capron, M., Lambrecht, B. N. and Trottein, F. 2003. Peroxisome proliferator-activated receptor gamma inhibits the migration of dendritic cells: consequences for the immune response. J. Immunol. 170:5295. [DOI] [PubMed] [Google Scholar]
- 22. Gosset, P., Charbonnier, A. S., Delerive, P.et al. 2001. Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. Eur. J. Immunol. 31:2857. [DOI] [PubMed] [Google Scholar]
- 23. Thompson, P. W., Bayliffe, A. I., Warren, A. P. and Lamb, J. R. 2007. Interleukin-10 is upregulated by nanomolar rosiglitazone treatment of mature dendritic cells and human CD4+ T cells. Cytokine 39:184. [DOI] [PubMed] [Google Scholar]
- 24. Nastasi, C., Candela, M., Bonefeld, C. M.et al. 2015. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 5:16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubrac, S., Stoitzner, P., Pirkebner, D.et al. 2007. Peroxisome proliferator-activated receptor-alpha activation inhibits Langerhans cell function. J. Immunol. 178:4362. [DOI] [PubMed] [Google Scholar]
- 26. Park, J., Lee, S. E., Hur, J.et al. 2015. M-CSF from cancer cells induces fatty acid synthase and PPARβ/δ activation in tumor myeloid cells, leading to tumor progression. Cell Rep. 10:1614. [DOI] [PubMed] [Google Scholar]
- 27. Daynes, R. A. and Jones, D. C. 2002. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2:748. [DOI] [PubMed] [Google Scholar]
- 28. Szatmari, I., Pap, A., Rühl, R.et al. 2006. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 203:2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coombes, J. L., Siddiqui, K. R., Arancibia-Cárcamo, C. V.et al. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204:1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun, C. M., Hall, J. A., Blank, R. B.et al. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J. Exp. Med. 204:1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Munn, D. H. and Mellor, A. L. 2007. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 117:1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma, M. D., Baban, B., Chandler, P.et al. 2007. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Invest. 117:2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Metz, R., Duhadaway, J. B., Rust, S.et al. 2010. Zinc protoporphyrin IX stimulates tumor immunity by disrupting the immunosuppressive enzyme indoleamine 2,3-dioxygenase. Mol. Cancer Ther. 9:1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Littlejohn, T. K., Takikawa, O., Skylas, D., Jamie, J. F., Walker, M. J. and Truscott, R. J. 2000. Expression and purification of recombinant human indoleamine 2, 3-dioxygenase. Protein Expr. Purif. 19:22. [DOI] [PubMed] [Google Scholar]
- 35. Thomas, S. R., Salahifar, H., Mashima, R., Hunt, N. H., Richardson, D. R. and Stocker, R. 2001. Antioxidants inhibit indoleamine 2,3-dioxygenase in IFN-gamma-activated human macrophages: posttranslational regulation by pyrrolidine dithiocarbamate. J. Immunol. 166:6332. [DOI] [PubMed] [Google Scholar]
- 36. Zhao, F., Xiao, C., Evans, K. S.et al. 2018. Paracrine Wnt5a-β-catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity 48:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feichtinger, R. G. and Lang, R. 2019. Targeting L-lactate metabolism to overcome resistance to immune therapy of melanoma and other tumor entities. J. Oncol. 2019:2084195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gottfried, E., Kunz-Schughart, L. A., Ebner, S.et al. 2006. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 107:2013. [DOI] [PubMed] [Google Scholar]
- 39. Ranganathan, P., Shanmugam, A., Swafford, D.et al. 2018. GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J. Immunol. 200:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown, T. P., Bhattacharjee, P., Ramachandran, S.et al. 2020. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 39:3292. [DOI] [PubMed] [Google Scholar]
- 41. Raychaudhuri, D., Bhattacharya, R., Sinha, B. P.et al. 2019. Lactate induces pro-tumor reprogramming in intratumoral plasmacytoid dendritic cells. Front. Immunol. 10:1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bajwa, G., DeBerardinis, R. J., Shao, B., Hall, B., Farrar, J. D. and Gill, M. A. 2016. Cutting edge: critical role of glycolysis in human plasmacytoid dendritic cell antiviral responses. J. Immunol. 196:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flück, K., Breves, G., Fandrey, J. and Winning, S. 2016. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 9:379. [DOI] [PubMed] [Google Scholar]
- 44. Chaudhari, S. M., Sluimer, J. C., Koch, M.et al. 2015. Deficiency of HIF1α in antigen-presenting cells aggravates atherosclerosis and type 1 T-helper cell responses in mice. Arterioscler. Thromb. Vasc. Biol. 35:2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kheshtchin, N., Arab, S., Ajami, M.et al. 2016. Inhibition of HIF-1α enhances anti-tumor effects of dendritic cell-based vaccination in a mouse model of breast cancer. Cancer Immunol. Immunother. 65:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilson, J. M., Ross, W. G., Agbai, O. N.et al. 2009. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J. Immunol. 182:4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Challier, J., Bruniquel, D., Sewell, A. K. and Laugel, B. 2013. Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8(+) T-cell priming capacity. Immunology 138:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Panther, E., Idzko, M., Herouy, Y.et al. 2001. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 15:1963. [DOI] [PubMed] [Google Scholar]
- 49. Novitskiy, S. V., Ryzhov, S., Zaynagetdinov, R.et al. 2008. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 112:1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ben Addi, A., Lefort, A., Hua, X.et al. 2008. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A(2B) receptor. Eur. J. Immunol. 38:1610. [DOI] [PubMed] [Google Scholar]
- 51. Zarek, P. E., Huang, C. T., Lutz, E. R.et al. 2008. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silva-Vilches, C., Ring, S. and Mahnke, K. 2018. ATP and its metabolite adenosine as regulators of dendritic cell activity. Front. Immunol. 9:2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haskó, G., Csóka, B., Németh, Z. H., Vizi, E. S. and Pacher, P. 2009. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 30:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Omar, B., Zmuda-Trzebiatowska, E., Manganiello, V., Göransson, O. and Degerman, E. 2009. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell. Signal. 21:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perrot, I., Michaud, H. A., Giraudon-Paoli, M.et al. 2019. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 27:2411. [DOI] [PubMed] [Google Scholar]
- 56. Hammami, A., Allard, D., Allard, B. and Stagg, J. 2019. Targeting the adenosine pathway for cancer immunotherapy. Semin. Immunol. 42:101304. [DOI] [PubMed] [Google Scholar]
- 57. Holtzhausen, A., Zhao, F., Evans, K. S.et al. 2015. Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunol. Res. 3:1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boukhaled, G. M., Corrado, M., Guak, H. and Krawczyk, C. M. 2019. Chromatin architecture as an essential determinant of dendritic cell function. Front. Immunol. 10:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu, P. S., Wang, H., Li, X.et al. 2017. α-Ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18:985. [DOI] [PubMed] [Google Scholar]
- 60. Nomura, M., Liu, J., Rovira, I. I.et al. 2016. Fatty acid oxidation in macrophage polarization. Nat. Immunol. 17:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hossain, F., Al-Khami, A. A., Wyczechowska, D.et al. 2015. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol. Res. 3:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Uehara, T., Eikawa, S., Nishida, M.et al. 2019. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int. Immunol. 31:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scharping, N. E., Menk, A. V., Whetstone, R. D., Zeng, X. and Delgoffe, G. M. 2017. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol. Res. 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Raud, B., Roy, D. G., Divakaruni, A. S.et al. 2018. Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. Cell Metab. 28:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pearce, E. L., Walsh, M. C., Cejas, P. J.et al. 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pan, Y., Tian, T., Park, C. O.et al. 2017. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chowdhury, P. S., Chamoto, K., Kumar, A. and Honjo, T. 2018. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8+ T cells and facilitates anti-PD-1 therapy. Cancer Immunol. Res. 6:1375. [DOI] [PubMed] [Google Scholar]