Abstract
Background
US guidelines recommend genotype testing at human immunodeficiency virus (HIV) diagnosis (“baseline genotype”) to detect transmitted drug resistance (TDR) to nonnucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs), and protease inhibitors. With integrase strand inhibitor (INSTI)-based regimens now recommended as first-line antiretroviral therapy (ART), the of baseline genotypes is uncertain.
Methods
We used the Cost-effectiveness of Preventing AIDS Complications model to examine the clinical impact and cost-effectiveness of baseline genotype compared to no baseline genotype for people starting ART with dolutegravir (DTG) and an NRTI pair. For people with no TDR (83.8%), baseline genotype does not alter regimen selection. Among people with transmitted NRTI resistance (5.8%), baseline genotype guides NRTI selection and informs subsequent ART after adverse events (DTG AEs, 14%). Among people with transmitted NNRTI resistance (7.2%), baseline genotype influences care only for people with DTG AEs switching to NNRTI-based regimens. The 48-week virologic suppression varied (40%–92%) depending on TDR. Costs included $320/genotype and $2500–$3000/month for ART.
Results
Compared to no baseline genotype, baseline genotype resulted in <1 additional undiscounted quality-adjusted life-day (QALD), cost an additional $500/person, and was not cost-effective (incremental cost-effectiveness ratio: $420 000/quality-adjusted life-year). In univariate sensitivity analysis, clinical benefits of baseline genotype never exceeded 5 QALDs for all newly diagnosed people with HIV. Baseline genotype was cost-effective at current TDR prevalence only under unlikely conditions, eg, DTG-based regimens achieving ≤50% suppression of transmitted NRTI resistance.
Conclusions
With INSTI-based first-line regimens in the United States, baseline genotype offers minimal clinical benefit and is not cost-effective.
Keywords: HIV, cost-effectiveness, drug resistance, genotype
Genotype testing at human immunodeficiency virus diagnosis provides a small clinical benefit for a limited subset of people starting antiretroviral therapy and is not cost-effective given current treatment guidelines.
Guidelines from the US Department of Health and Human Services (DHHS) and the International AIDS Society USA (IAS–USA) recommend standard genotype resistance testing for people newly diagnosed with human immunodeficiency virus (HIV) [1, 2]. Standard genotype results are used to evaluate resistance to the nucleoside reverse transcriptase inhibitor (NRTI), nonnucleoside reverse transcriptase inhibitor (NNRTI), and protease inhibitor (PI) drug classes [3]. Resistance to integrase strand inhibitors (INSTIs) is evaluated with a separate INSTI-resistance test that is not routinely recommended prior to antiretroviral therapy (ART) initiation [1, 2] and is not cost-effective for routine screening [4].
Standard genotype at HIV diagnosis (“baseline genotype”) has 2 primary functions: to guide selection of initial ART, thereby optimizing viral suppression from the outset, and to establish a baseline resistance profile that can help in the selection of subsequent ART regimens, if changes are needed due to drug toxicity during viral suppression [1, 2]. When DHHS guidelines initially endorsed baseline genotype in 2006, NNRTI- and PI-based regimens were recommended as first-line ART [5]. In that context, a baseline genotype that identifies NRTI, NNRTI, and PI resistance mutations minimizes the use of inactive regimens [6, 7], although a Cochrane review found no eligible studies that evaluated the clinical benefit of baseline genotype [8]. Baseline genotype was shown to be cost-effective in that ART era if the prevalence of transmitted NNRTI resistance (NNRTI-R) was ≥1.5% [9].
Current United States treatment guidelines recommend an INSTI with an NRTI pair as first-line ART for most people with HIV (PWH) [1, 2]. Therefore, at ART initiation, baseline genotype now guides only the initial choice of NRTI pair given transmitted NRTI resistance (NRTI-R). This choice may not be critical, as limited data suggest that regimens that include an NRTI pair and a later-generation INSTI, such as dolutegravir (DTG) or bictegravir (BIC), remain effective in the setting of most transmitted NRTI-R mutations [10–12]. The activity of DTG or BIC plus NRTIs is uncertain in the setting of high-level NRTI-R (ie, both K65R and M184V/I), but these mutations are rarely transmitted in the United States [13–16].
PIs and NNRTIs are seldom prescribed as first-line ART in the United States, so baseline genotype results about transmitted PI resistance (PI-R) and NNRTI-R rarely affect initial regimen selection. However, baseline genotype may influence subsequent ART choice for people who switch from first-line INSTI-based ART to NNRTI- or PI-based ART due to adverse events (AEs). Under these circumstances, individuals are frequently virologically suppressed, so a genotype prior to regimen switch is infeasible. For these individuals, NNRTI- or PI-based regimens might not suppress transmitted NRTI-, NNRTI-, or PI-resistant virus [16, 17].
With the evolution of HIV treatment, uncertainty surrounding the role of baseline genotype has grown. We examined the clinical and economic impact of baseline genotype for people newly diagnosed with HIV in the United States.
METHODS
Analytic Overview
The Cost-effectiveness of Preventing AIDS Complications (CEPAC) model is a validated microsimulation model of HIV disease, clinical care, and costs [18, 19].The model simulates individuals throughout their lifetimes, tracking health outcomes and care costs [20].
We used CEPAC to compare 2 strategies at HIV diagnosis: no baseline genotype and baseline genotype. We modeled a cohort of adults newly diagnosed with HIV and starting ART in the United States, including 4 mutually exclusive subgroups: no transmitted drug resistance (no TDR, 83.8% of the cohort), transmitted NRTI-R (5.8%), transmitted NNRTI-R (7.2%), and transmitted PI-R (3.2%) [15]. We assumed no transmitted resistance to INSTIs [2, 21]. We selected input parameters that were most favorable to baseline genotype and varied them in sensitivity analysis.
We used a health sector perspective and discounted outcomes at 3%/year [22]. Model outcomes for all PWH, weighted by each of the subgroups, included life expectancy in quality-adjusted life-years (QALYs), lifetime HIV-related care costs (2018 USD), and incremental cost-effectiveness ratios (ICERs) expressed in dollars per QALY gained. We considered strategies with ICERs below $100 000/QALY to be cost-effective [22, 23]. To detail the clinical impact of baseline genotype for affected individuals, we reported clinical outcomes by subgroup; however, we did not assess cost-effectiveness by subgroup because these subgroups cannot be identified in the absence of a standard genotype.
Strategies and Model Structure
In both strategies, all simulated individuals start a first-line DTG-based regimen. In no baseline genotype, individuals initiate this regimen without knowledge of TDR; in baseline genotype, genotype results guide selection of the NRTI pair. In both strategies, upon virologic failure, standard and INSTI (if appropriate) genotype resistance tests are performed to guide selection of subsequent ART regimens. Individuals with resistant virus experience worsening immunosuppression, an increased risk of opportunistic infection and death, and associated costs; as per guidelines, people switch to a regimen with 3 active drugs within 3 months of observed treatment failure (“time to switch”) [1, 2]. Those who experience AEs on the DTG-based regimen (DTG AEs) require regimen switch without viremia, so a genotype cannot be obtained. With no baseline genotype, individuals with severe DTG AEs switch regimens empirically, which may result in treatment with an ineffective regimen. With baseline genotype, the baseline resistance profile informs selection of the next regimen.
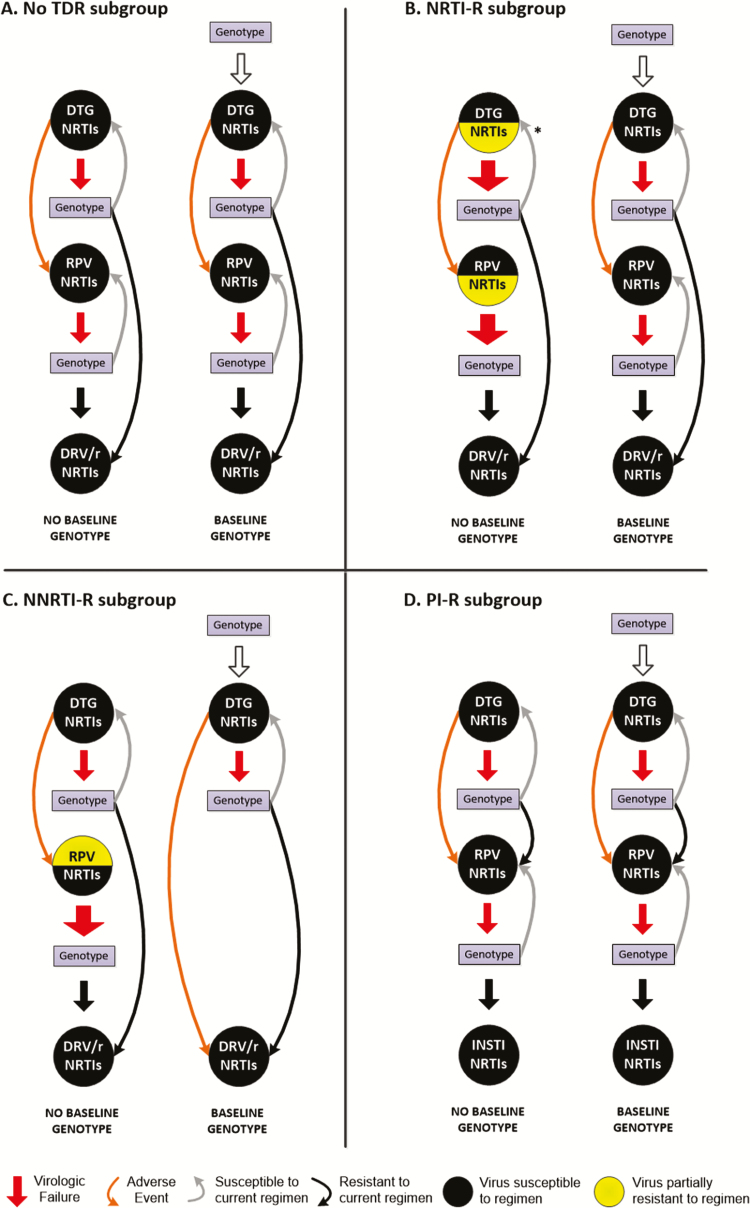
US guidelines do not outline a specific sequence of ART regimens after first-line INSTI-based ART [1, 2], so we modeled a regimen sequence to maximize the clinical impact of undetected TDR by including an NNRTI-based regimen as second-line therapy. For people with no TDR (Figure 1A), baseline genotype does not alter the choice or effectiveness of any ART regimen. For the NRTI-R subgroup (Figure 1B), the effectiveness of both the first-line DTG-based regimen and subsequent rilpivirine (RPV)-based regimen are reduced in no baseline genotype due to undiagnosed NRTI-R, leading to increased virologic failure. With baseline genotype, individuals with transmitted NRTI-R are always prescribed fully active regimens. For the NNRTI-R subgroup (Figure 1C), the effectiveness of the DTG-based regimen is unchanged in no baseline genotype, but individuals with DTG AEs switch to an RPV-based regimen with reduced effectiveness and increased virologic failure. With baseline genotype, individuals with transmitted NNRTI-R avoid treatment with RPV, switching instead to a darunavir/ritonavir (DRV/r)-based regimen. For the PI-R subgroup (Figure 1D), care is identical between strategies. The initial DTG-based regimen is fully active, and individuals with DTG AEs switch to a fully active RPV-based regimen. Upon observed treatment failure with RPV-based therapy, individuals switch to a second INSTI-based regimen rather than ineffective PI-based treatment because all individuals in both strategies have a genotype when prompted by treatment failure. Given that transmitted PI-R would influence the clinical value of baseline genotype only with PI-based treatment after DTG AEs, we investigated this scenario for the PI-R subgroup (Supplementary Figure 1).
Figure 1.
A comparison of no baseline genotype and baseline genotype strategies for people newly diagnosed with human immunodeficiency virus (HIV). The figure indicates all treatment variations for adults in the United States with newly diagnosed HIV, including individuals with no TDR, NNRTI-R, NRTI-R, or PI-R. A, For people with no TDR, care is identical between strategies: individuals initiate a DTG-based regimen, switch to an RPV-based regimen in case of DTG adverse events (AEs), and switch to a DRV/r-based regimen if virologic resistance is diagnosed while on a DTG- or RPV-based regimen. B, For the NRTI-R subgroup, care differs between strategies. With no baseline genotype, first-line DTG-based antiretroviral therapy efficacy is reduced due to undetected NRTI-R. The larger red arrows reflect higher likelihood of virologic failure due to reduced efficacy given TDR. With DTG AEs, the efficacy of the subsequent RPV-based regimen is also reduced due to still undetected NRTI-R. With baseline genotype, TDR is diagnosed, so clinical outcomes are the same as those in no TDR. The asterisk in the no baseline genotype panel indicates that those who fail a DTG-based regimen due to undetected NRTI-R will be resuppressed on a DTG-based regimen with an NRTI pair to which they are susceptible. C, For the NNRTI-R subgroup, care differs only for individuals who experience DTG AEs. With no baseline genotype, individuals with DTG AEs switch empirically to an RPV-based regimen and are less likely to suppress. If not suppressed, individuals are evaluated with genotype and switched to a DRV/r-based regimen. In the baseline genotype strategy, transmitted NNRTI-R is identified at HIV diagnosis, and individuals switch directly to a DRV/r-based regimen after DTG AEs. D, For the PI-R subgroup, care is identical between strategies: individuals start on a DTG-based regimen and move to an RPV-based regimen in case of DTG AEs or in case of virologic failure with resistance on a DTG-based regimen. If individuals fail the RPV-based regimen due to resistance, they switch to a different INSTI-based regimen given diagnosis of transmitted PI-R on genotype at the time of failing the RPV-based regimen. Abbreviations: DRV/r, ritonavir-boosted darunavir; DTG, dolutegravir; INSTI, integrase strand inhibitor; NNRTI-R, nonnucleoside reverse transcriptase inhibitors resistance; NRTI-R, nucleoside reverse transcriptase inhibitors resistance; PI-R, protease inhibitors resistance; RPV, rilpivirine; TDR, transmitted drug resistance.
Input Parameters
Cohort Characteristics
Simulated individuals represent adults at HIV diagnosis in the United States; mean age 35 years; 81% men; mean initial CD4 count 346/μL (Table 1) [24, 25]. Prevalence of TDR is 5.8% for NRTI-R, 7.2% for NNRTI-R, and 3.2% for PI-R [15].
Table 1.
Base Case Input Parameters for an Analysis of Baseline Genotype at Human Immunodeficiency Virus Diagnosis Compared to No Baseline Genotype
| Parameter | Base Case Value | Range | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort characteristics | |||||||||
| Age, mean (SD), y | 35 (13) | … | [24] | ||||||
| Sex, male/female, % | 81/19 | … | [24] | ||||||
| Initial CD4 cell count, mean cells/µL (SD) | 346 (175) | … | [25] | ||||||
| HIV RNA distribution, % | [26] | ||||||||
| >100 000 copies/mL | 25 | … | |||||||
| 30 001–100 000 copies/mL | 42 | … | |||||||
| 10 001–30 000 copies/mL | 21 | … | |||||||
| 3001–10 000 copies/mL | 6 | … | |||||||
| ≤3000 copies/mL | 6 | … | |||||||
| Transmitted drug resistance prevalence | [15] | ||||||||
| NRTI-R, % | 5.8 | 3–40 | |||||||
| NNRTI-R, % | 7.2 | 5–40 | |||||||
| PI-R, % | 3.2 | 0–40 | |||||||
| ART efficacya | |||||||||
| Suppression (HIV RNA <50 copies/mL at 12 mo), mean, % | |||||||||
| Parameter | No TDR | NRTI-R | NNRTI-R | PI-R | Reference | ||||
| No BG | BG | No BG | BG | No BG | BG | No BG | BG | ||
| DTG-based regimenb | 92 | 92 | 82 c | 92 | 92 | 92 | 92 | 92 | [27, 28] |
| RPV-based regimenb | 83 | 83 | 57 d | 83 | 40 e | … | 83 | 83 | [29, 30] |
| DRV/r-based regimenb | 83 | 83 | 83 | 83 | 83 | 83 | … | … | [31] |
| Parameter | Base Case Value | Range | Reference | ||||||
| Regimen switch | |||||||||
| Time to regimen switch after observed failure, mo | 3 | 3–12 | [1] | ||||||
| Severe DTG AE requiring regimen change, % | 14 | 5–50 | [32] | ||||||
| Time from DTG start to AE, mo | 4 | … | [32, 33] | ||||||
| Costs (2018 USD) | |||||||||
| Laboratory tests | [34] | ||||||||
| Standard genotype resistance test | 320 | 0–500 | |||||||
| Integrase strand inhibitor genotype resistance test | 160 | … | |||||||
| Monthly routine care costs | 300–1200 | … | [35] | ||||||
| Monthly ART regimenf | [36] | ||||||||
| DTG-based regimen | 3000 | … | |||||||
| RPV-based regimen | 2500 | 1000–3500 | |||||||
| DRV/r-based regimen | 3000 | … |
Bold numbers indicate differences between strategies.
Abbreviations: AE, adverse event; ART, antiretroviral therapy; BG, baseline genotype; DRV/r, ritonavir-boosted darunavir; DTG, dolutegravir; HIV, human immunodeficiency virus; NNRTI-R, nonnucleoside reverse transcriptase inhibitors resistance; NRTI-R, nucleoside reverse transcriptase inhibitors resistance; PI-R, protease inhibitors resistance; RPV, rilpivirine; SD, standard deviation; TDR, transmitted drug resistance.
aExamined ranges for ART efficacy are reported in footnotes c, d, and e due to space restrictions.
bDTG- and RPV-based regimen suppression is based on treatment-naive patient populations given that these regimens are prescribed early in ART treatment. DRV/r-based regimen suppression is based on treatment-experienced patient populations given that this regimen is prescribed later in the care cascade.
cDTG-based regimen suppression with transmitted NRTI-R: base case, 82%; range, 40%–93%.
dRPV-based regimen suppression with transmitted NRTI-R: base case, 57%; range, 20%–80%.
eRPV-based regimen suppression with transmitted NNRTI-R: base case, 40%; range, 20%–80%.
fART regimen costs are based on average wholesale price and discounted by 23% for branded drugs. ART regimens costs do not differ by choice of NRTI.
HIV Care and ART Efficacy
Simulated individuals initiate ART and are monitored for virologic failure according to current DHHS/IAS–USA guidelines, with viral load testing at quarterly clinic visits [1, 2]. Without TDR, the probabilities of virologic suppression (HIV RNA <50 copies/mL) at 12 months are 92% (DTG-based regimen) and 83% (RPV or DRV/r-based regimens) (Table 1) [27, 30, 31]. For an individual with undiagnosed NRTI-R, we assumed a worst-case scenario (ie, all transmitted NRTI-resistant mutations are “high-level,” leading to complete inactivity of the NRTI pair). We estimated a reduced probability of virologic suppression at 12 months in the no baseline genotype strategy: 82% (DTG-based regimen) [28] and 57% (RPV-based regimen) [29]. For those with undiagnosed NNRTI-R, we estimated 40% suppression on an RPV-based regimen at 12 months in no baseline genotype.
Adverse Events
Of those on DTG-based regimens, 14% experience AEs, including sleep disturbance, gastrointestinal discomfort, weight gain, and psychiatric symptoms sufficiently severe to prompt regimen switch [32, 37], on average 4 months after DTG initiation (Table 1) [32, 33].
Costs
Each standard genotype cost $320 and HIV RNA tests cost $110 (Table 1) [34]. Routine HIV care costs range from $300 to $1200/month, depending on CD4 count [38]. DTG- and DRV/r-based regimens cost $3000/month, while RPV-based regimens cost $2500/month [36].
Sensitivity Analyses
To assess the influence of changes in key model inputs, we performed univariate sensitivity analyses on clinical and cost parameters, including the prevalence of different TDR and rates of virologic failure. We examined the impact of lower barriers to resistance in earlier-generation INSTIs (eg, raltegravir) by reducing the effectiveness of INSTI-based regimens. In the base case, we assumed that no clinically relevant NRTI-R emerged due to the use of inactive RPV-based regimens. To investigate the potential for selecting clinically significant “emergent NRTI-R,” we conducted univariate sensitivity analysis on the effectiveness of a DRV/r-based regimen for individuals in the NNRTI-R subgroup who are treated with an inactive RPV-based regimen after DTG AEs in no baseline genotype. We simultaneously varied the most influential parameters on cost-effectiveness outcomes in multivariate sensitivity analysis.
RESULTS
Base Case
For all PWH, the no baseline genotype strategy resulted in 27.959 undiscounted QALYs, which increased to 27.962 QALYs with baseline genotype, a gain of <1 undiscounted quality-adjusted life-day (QALD; Table 2). Discounted per-person lifetime costs were $620 200 and $620 700 for no baseline genotype and baseline genotype. Baseline genotype was not cost-effective compared to no baseline genotype (ICER, $420 000/QALY).
Table 2.
Base Case Results for an Analysis of Baseline Genotype Compared to No Baseline Genotype at Human Immunodeficiency Virus Diagnosis
| Cohort | Strategy | Undiscounted | Discounted | |||||
|---|---|---|---|---|---|---|---|---|
| QALYs | ∆ QALDsa | QALYs | ∆ QALYs | Cost ($) | ∆ ($) | Incremental Cost-effectiveness Ratio ($/QALY) | ||
| All people with human immunodeficiency virus | No baseline genotype | 27.959 | <1 | 16.152 | 620 200 | |||
| Baseline genotype | 27.962 | 16.153 | 0.001 | 620 700 | 500 | 420 000 | ||
| Subgroup | ||||||||
| No transmitted drug resistance (83.8%) | No baseline genotype | 27.962 | … | … | … | … | … | |
| Baseline genotype | 27.962 | 0 | … | … | … | … | … | |
| Nucleoside reverse transcriptase inhibitors resistance (5.8%) | No baseline genotype | 27.926 | … | … | … | … | … | |
| Baseline genotype | 27.962 | 13 | … | … | ||||
| Nonnucleoside reverse transcriptase inhibitors resistance (7.2%) | No baseline genotype | 27.960 | … | … | … | … | … | |
| Baseline genotype | 27.962 | <1 | … | … | … | … | … | |
| Protease inhibitors resistance (3.2%) | No baseline genotype | 27.962 | … | … | … | … | … | |
| Baseline genotype | 27.962 | 0 | … | … | … | … | … |
Abbreviations: QALY, quality-adjusted life-years; QALDs, quality-adjusted life-days.
aDifferences in life expectancy between the 2 strategies are small, so we report these differences in QALDs.
We projected undiscounted clinical outcomes for the 4 TDR subgroups. With no TDR, undiscounted life expectancy was identical between strategies (27.962 QALYs). With NRTI-R, clinical outcomes were worse with the no baseline genotype strategy (27.926 QALYs); baseline genotype resulted in a gain of 13 QALDs. No baseline genotype resulted in 27.960 QALYs in people with NNRTI-R, and <1 QALD was gained with baseline genotype. The undiscounted life expectancy was identical between strategies (27.962 QALYs) with PI-R.
Sensitivity Analyses
Univariate Sensitivity Analysis
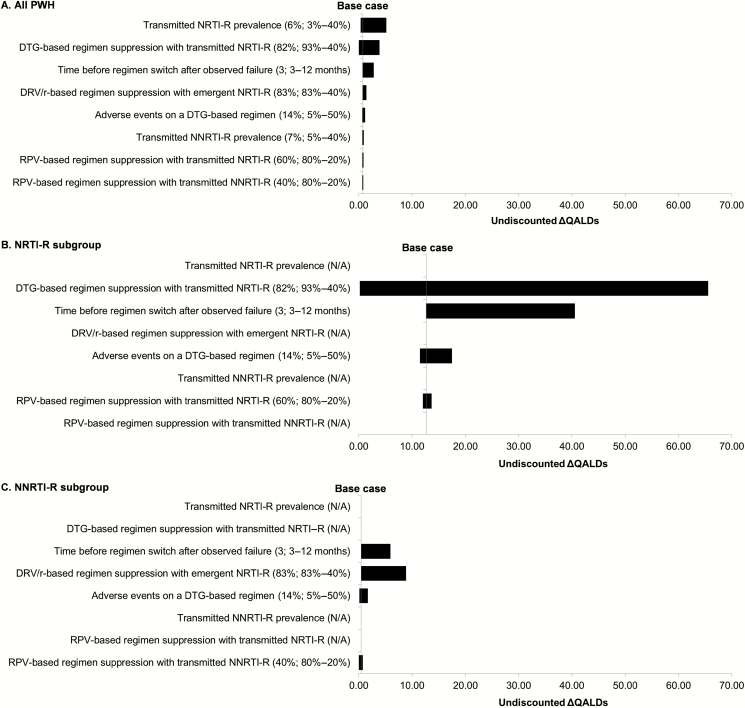
The maximum clinical impact was 5 QALDs among all PWH, even at extreme values of parameter estimates. Differences in clinical outcomes were greatest with increasing prevalence of transmitted NRTI-R, reduced suppression of transmitted NRTI-R with a DTG-based regimen, or longer time to switch (Figure 2A).
Figure 2.
Tornado diagrams of univariate sensitivity analyses for the clinical outcomes of baseline genotype compared to no baseline genotype among people newly diagnosed with human immunodeficiency virus. These 3 tornado diagrams show the difference in undiscounted QALDs between baseline genotype and no baseline genotype. A, All newly diagnosed PWH in the United States. B, The NRTI-R subgroup. C, The NNRTI-R subgroup. Input parameters are displayed on the y-axis; base case values are listed in parentheses. Following the semicolon, the input value that results in the smallest undiscounted ∆ QALDs is listed before the hyphen, and the input that results in the largest undiscounted ∆ QALDs is listed after the hyphen. Base case results are demonstrated by the vertical line. Abbreviations: DRV/r, ritonavir-boosted darunavir; DTG, dolutegravir; N/A, not applicable; NNRTI-R, nonnucleoside reverse transcriptase inhibitors resistance; NRTI-R, nucleoside reverse transcriptase inhibitors resistance; PWH, people with human immunodeficiency virus; QALDs, quality-adjusted life-days; RPV, rilpivirine; TDR, transmitted drug resistance.
The impact of key parameters on clinical outcomes was greater for the TDR subgroups, but the difference between strategies remained limited. Within the NRTI-R subgroup (Figure 2B), the most influential parameter was suppression of transmitted NRTI-R with a DTG-based regimen; when only 40% of individuals with transmitted NRTI-R achieved virologic suppression with a DTG-based regimen, baseline genotype resulted in a gain of 66 QALDs compared to no baseline genotype. Within the NNRTI-R subgroup (Figure 2C), the maximum gain with baseline genotype (9 QALDs) occurred when those in the no baseline genotype strategy experienced poor likelihood of suppression (40%) on third-line DRV/r-based regimens due to emergent NRTI-R.
Cost-effectiveness Thresholds
For all PWH, baseline genotype was not cost-effective compared to no baseline genotype except in extreme scenarios—≤50% of individuals with transmitted NRTI-R suppressed on a DTG-based regimen, prevalence of transmitted NRTI-R ≥14%, ≥18-month time to switch, or ≤22% of individuals with emergent NRTI-R suppressed on DRV/r-based regimens. Baseline genotype was not cost-effective even at $0 per baseline genotype because the cost savings did not outweigh lower costs of no baseline genotype due to increased use of relatively less costly RPV-based regimens. Increasing costs of the RPV-based regimen resulted in lower ICERs for baseline genotype; however, even when the RPV-based regimen cost the same as DTG and DRV/r regimens ($3000/month), baseline genotype was not cost effective (ICER, $260 000/QALY) (Supplementary Figure 2).
Multivariate Sensitivity Analysis
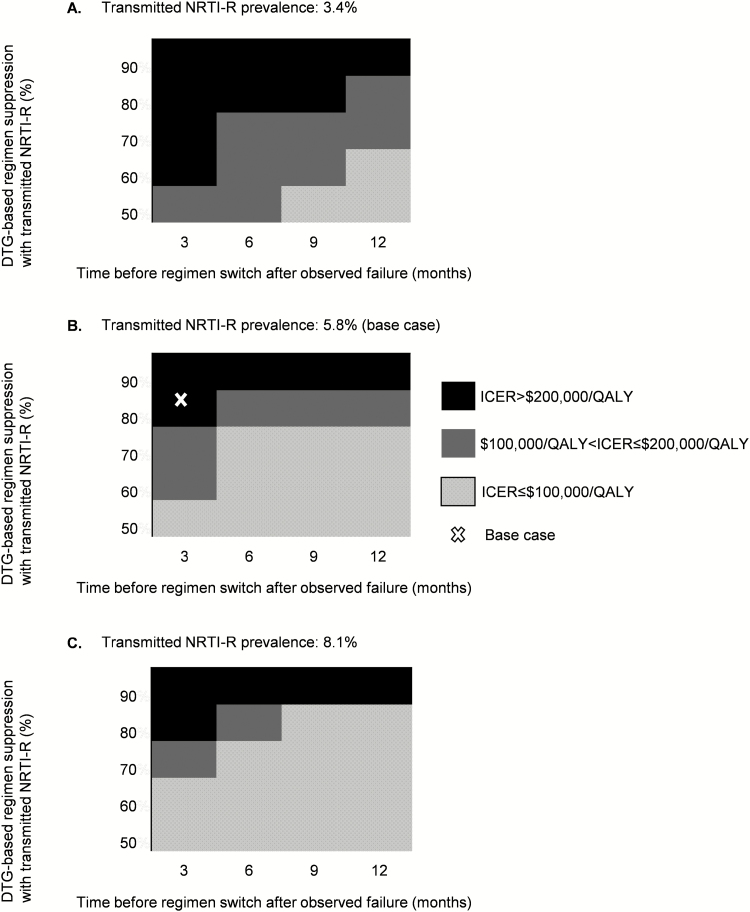
We varied suppression of transmitted NRTI-R with a DTG-based regimen (50%–90%) [28, 39] and prevalence of transmitted NRTI-R (3.4%–8.1%) [13, 15] (Supplementary Figure 4), as well as time to switch (3–12 months; Figure 3). At base case NRTI-R prevalence, baseline genotype was cost-effective if ≤50% of individuals with NRTI-R suppressed on a DTG-based regimen (Figure 3B). At the highest reported prevalence of NRTI-R in the United States (8.1%), baseline genotype became cost-effective only if ≤60% of individuals with NRTI-R suppressed on DTG-based regimens or if time to switch was ≥6 months (Figure 3C).
Figure 3.
Baseline genotype is cost-effective compared to no baseline genotype only at high prevalence of transmitted drug resistance, low likelihood of suppressing NRTI-R virus with DTG-based regimen, and prolonged time to switch. On the horizontal axis, we varied the number of months individuals were observed on a failing antiretroviral therapy regimen before switching to a new regimen (3–12 months). On the vertical axis, we varied the likelihood of suppressing transmitted NRTI-R with a DTG-based regimen (50%–90%). Each panel represents a different prevalence of transmitted NRTI-R virus, as follows: 3.4% (A), base case 5.8% (B), and 8.1% (C). The white X indicates the base case in (B). Baseline genotype was cost-effective compared to no baseline genotype at the base case transmitted NRTI-R only when DTG-based regimen suppression was ≤50% with NRTI-R or when DTG-based regimen suppression was ≤70% and individuals spent at least 6 months observed on the failing regimen before switching to a new regimen. Abbreviations: DTG, dolutegravir; ICER, incremental cost-effectiveness ratio; NRTI-R, nucleoside reverse transcriptase inhibitors resistance; QALY, quality-adjusted life-years.
Alternative Sequence of ART Regimens
To assess the impact of treatment variation for people with transmitted PI-R, we examined an alternative pathway with a DRV/r-based regimen as second-line therapy. At 60% suppression of PI-R with a DRV/r-based regimen, baseline genotype added <1 QALD for the PI-R subgroup and was not cost-effective compared to no baseline genotype (Supplementary Table 1). Even at 20% suppression of PI-R, baseline genotype still provided the PI-R subgroup <1 additional QALD (Supplementary Figure 3).
DISCUSSION
In this modeling analysis of the current INSTI treatment era, we found that obtaining a standard genotype at the time of HIV diagnosis had minimal clinical impact and was not cost-effective. A baseline genotype offered no clinical benefit to most people newly diagnosed with HIV. Those with transmitted drug resistance accrued little lifetime benefit and comprised only 6.8% of all those newly diagnosed—5.8% with NRTI-R plus 1% with transmitted NNRTI-R who also experience DTG AEs. We projected an average population benefit of <1 QALD. This benefit is far less than that of other HIV interventions in the United States, such as expanded HIV testing, improved engagement in care, and preexposure prophylaxis [18, 40].
These findings contrast with those from a 2005 CEPAC model-based analysis in which baseline genotype led to an undiscounted clinical benefit of 1 quality-adjusted life-month and was cost-effective in the United States (ICER, $23 900/QALY) [9]. Substantial advances in HIV therapy have changed the value of baseline resistance testing. New generations of ART are more potent, better tolerated, and more active against resistant virus [1, 2]. When efavirenz was widely used in first-line regimens, a baseline genotype that demonstrated NNRTI-R helped clinicians avoid selecting treatment with a high likelihood of failure, thereby avoiding more complex, more costly, and less effective regimens. By contrast, with INSTI-based first-line regimens that include DTG and BIC, high-level transmitted NRTI-R that reduces treatment efficacy is exceedingly rare [10]; our model-based results suggest that baseline genotype is favored only when the prevalence of high-level NRTI-R is ≥14%. In addition, because guidelines recommend routine viral load testing, virologic failure is generally detected quickly for people who remain in care, and clinicians can then perform genotype testing to identify drug resistance [1, 2]. Thus, even when people with undiagnosed transmitted resistance start a partially active regimen, the duration of virologic failure should be limited. Last, more treatment options are now available, so durable virologic suppression can typically be achieved even after virologic failure to a given regimen.
Although we selected input parameters to favor the baseline genotype strategy, we still found that it had minimal clinical impact and was not cost-effective. We assumed that all transmitted NRTI mutations were clinically significant, although data suggest that most NRTI mutations do not affect virologic suppression in INSTI-based regimens [10–13]. Similarly, we estimated that individuals with transmitted NRTI-R would be far less likely to achieve virologic suppression with DTG- or RPV-based regimens than has been commonly reported [10–13, 41, 42]. Although one study of 11 adults with transmitted NRTI-R reported 50% efficacy of an INSTI-based regimen [39], even DTG monotherapy studies have shown >85% suppression at 12 months [28]. For individuals with NNRTI-R, we assumed much lower virologic suppression with RPV-based regimens (40%) than has been reported for other NNRTIs (66%) [43]. Notably, the newly approved NNRTI doravirine, with its distinctive resistance profile, is likely to be more effective than RPV in suppressing NNRTI-R; its use would further reduce the clinical impact of baseline genotype [44, 45]. Finally, the most serious concern for people with NNRTI-R is that spending time on a partially active RPV-based regimen might select for emergent NRTI-R that decreases the efficacy of subsequent therapy. In an extreme scenario where adults with transmitted NNRTI-R treated with an RPV-based regimen had only a 40% chance of suppressing on subsequent regimens (compared to 74% suppression reported for DRV/r monotherapy) [46, 47], we projected a gain of just 9 QALDs.
While obtaining a baseline genotype offers little clinical benefit for individual patients, collecting resistance data at the population level could offer public health benefits. The US Centers for Disease Control and Prevention and local jurisdictions have used baseline genotype results for molecular surveillance to detect and respond to transmission clusters and track trends in transmitted drug resistance [48, 49]. Given limited individual benefit, genotype testing should perhaps be financed expressly for this purpose, rather than by people with HIV and payers.
This analysis has several limitations. We modeled the value of obtaining a resistance genotype at diagnosis given current US treatment guidelines, which recommend 2 NRTIs and 1 INSTI as first-line therapy. Treatment options are continuously evolving, and we did not model the possible value of this strategy in the setting of long-acting injectable ART, 2-drug regimens, or other nascent HIV therapies [50–52]. We also did not model the use of more costly archive genotype testing for suppressed patients; the rare occurrence of transmitted resistance to the second-generation INSTIs, DTG and BIC [53]; transmitted multidrug resistance; or the potential for increased HIV transmissions due to undiagnosed resistance. This analysis is limited in scope to the United States; the conclusions may differ in settings with different resistance considerations, HIV treatment policies, and costs, such as low- and middle-income countries that are rolling out DTG-based regimens but may have relatively higher prevalence of transmitted high-level NRTI-R [54].
In conclusion, for people starting DTG-based regimens in the United States, obtaining a baseline genotype offers minimal clinical benefit; a similar conclusion is likely for BIC-based regimens [55]. Baseline genotypes provide no benefit to most adults with a new HIV diagnosis and only a very small increase in projected survival to those who do benefit. This analysis projects a mean gain of <1 QALD among all people starting ART. At $320/test, this results in an ICER of $420 000/QALY, which offers poor value relative to other HIV interventions [18, 19, 40]. Given currently recommended HIV treatment regimens in the United States, a resistance genotype at HIV diagnosis is not cost-effective; inclusion of this test in baseline evaluation of adults newly diagnosed with HIV should be reconsidered.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge Taige Hou for his programming expertise and Dr Rajesh Gandhi for his critical review of the manuscript.
Disclaimer. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH) or the Massachusetts General Hospital Executive Committee on Research.
Financial support. This publication was made possible by funding from the NIH (R01AI042006, R37AI093269, K01HL123349 to E. P. H., and T32AI007433 to C. M. D.) and the Steve and Deborah Gorlin MGH Research Scholars Award (to R. P. W.).
Potential conflicts of interest. C. M. D. reports grants from the National Institute of Mental Health, IMPAACT Network, and Harvard University Center for AIDS Research outside the submitted work. P. E. S. is a scientific advisory board member for Gilead, GlaxoSmithKline/ViiV Healthcare, Merck, and Janssen; he has received grant support to his institution from Gilead, Merck, and GlaxoSmithKline/ViiV Healthcare. All other authors reported no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents 2018. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 25 April 2019.
- 2. Günthard HF, Calvez V, Paredes R, et al. . Human immunodeficiency virus drug resistance: 2018 recommendations of the International Antiviral Society–USA Panel. Clin Infect Dis 2019; 68:177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. HIV drug resistance database. Stanford University; Available at: https://hivdb.stanford.edu/. Accessed 25 April 2019. [Google Scholar]
- 4. Koullias Y, Sax PE, Fields NF, Walensky RP, Hyle EP. Should we be testing for baseline integrase resistance in patients newly diagnosed with human immunodeficiency virus? Clin Infect Dis 2017; 65:1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents 2006. Available at: https://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL05042006050.pdf. Accessed 25 April 2019.
- 6. Little SJ, Holte S, Routy JP, et al. . Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002; 347:385–94. [DOI] [PubMed] [Google Scholar]
- 7. Kantor R, Smeaton L, Vardhanabhuti S, et al. ; AIDS Clinical Trials Group A5175 Study Team Pretreatment HIV drug resistance and HIV-1 subtype C are independently associated with virologic failure: results from the multinational PEARLS (ACTG A5175) clinical trial. Clin Infect Dis 2015; 60:1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aves T, Tambe J, Siemieniuk RA, Mbuagbaw L. Antiretroviral resistance testing in HIV-positive people. Cochrane Database Syst Rev 2018; 11:CD006495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sax PE, Islam R, Walensky RP, et al. . Should resistance testing be performed for treatment-naive HIV-infected patients? A cost-effectiveness analysis. Clin Infect Dis 2005; 41:1316–23. [DOI] [PubMed] [Google Scholar]
- 10. White KL, Kulkarni R, Wilkom M, et al. . Abstract 532: pooled week 48 efficacy and baseline resistance: B/F/TAF in treatment-naive patients. In: 25th Conference on Retroviruses and Opportunistic Infections Boston, MA; 4–7 March 2018 Available at: http://www.croiconference.org/sessions/pooled-week-48-efficacy-and-baseline-resistance-bftaf-treatment-naive-patients. [Google Scholar]
- 11. Sörstedt E, Carlander C, Flamholc L, et al. . Effect of dolutegravir in combination with nucleoside reverse transcriptase inhibitors (NRTIs) on people living with HIV who have pre-existing NRTI mutations. Int J Antimicrob Agents 2018; 51:733–8. [DOI] [PubMed] [Google Scholar]
- 12. Aboud M, Kaplan R, Lombaard J, et al. . Abstract 5633: superior efficacy of dolutegravir (DTG) plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) compared with lopinavir/ritonavir (LPV/r) plus 2 NRTIs in second-line treatment - 48-week data from the DAWNING Study. In: 22nd International AIDS Conference Amsterdam, Netherlands, 23–27 July 2018 Available at: https://programme.aids2018.org/Abstract/Abstract/5633. [Google Scholar]
- 13. Margot NA, Wong P, Kulkarni R, et al. . Commonly transmitted HIV-1 drug resistance mutations in reverse-transcriptase and protease in antiretroviral treatment-naive patients and response to regimens containing tenofovir disoproxil fumarate or tenofovir alafenamide. J Infect Dis 2017; 215:920–7. [DOI] [PubMed] [Google Scholar]
- 14. Ross LL, Shortino D, Shaefer MS. Changes from 2000 to 2009 in the prevalence of HIV-1 containing drug resistance-associated mutations from antiretroviral therapy-naive, HIV-1-infected patients in the United States. AIDS Res Hum Retroviruses 2018; 34:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rhee SY, Clutter D, Fessel WJ, et al. . Trends in the molecular epidemiology and genetic mechanisms of transmitted human immunodeficiency virus type 1 drug resistance in a large US clinic population. Clin Infect Dis 2019; 68:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levintow SN, Okeke NL, Hué S, et al. . Prevalence and transmission dynamics of HIV-1 transmitted drug resistance in a southeastern cohort. Open Forum Infect Dis 2018; 5:ofy178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodman DD, Zhou Y, Margot NA, et al. . Low level of the K103N HIV-1 above a threshold is associated with virological failure in treatment-naive individuals undergoing efavirenz-containing therapy. AIDS 2011; 25:325–33. [DOI] [PubMed] [Google Scholar]
- 18. Borre ED, Hyle EP, Paltiel AD, et al. . The clinical and economic impact of attaining national HIV/AIDS strategy treatment targets in the United States. J Infect Dis 2017; 216:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ross EL, Weinstein MC, Schackman BR, et al. . The clinical role and cost-effectiveness of long-acting antiretroviral therapy. Clin Infect Dis 2015; 60:1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CEPAC Model. Medical Practice Evaluation Center, Massachusetts General Hospital. Available at: https://www.massgeneral.org/mpec/cepac/. Accessed 25 April 2019. [Google Scholar]
- 21. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness— the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014; 371:796–7. [DOI] [PubMed] [Google Scholar]
- 22. Sanders GD, Neumann PJ, Basu A, et al. . Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016; 316:1093–103. [DOI] [PubMed] [Google Scholar]
- 23. Anderson JL, Heidenreich PA, Barnett PG, et al. ; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation 2014; 129:2329–45. [DOI] [PubMed] [Google Scholar]
- 24. Division of HIV/AIDS Prevention. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2016. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Centers for Disease Control and Prevention, 2016; 28:1–125. [Google Scholar]
- 25. Division of HIV/AIDS Prevention. HIV surveillance report: monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2016. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB prevention. Centers for Disease Control and Prevention, 2016; 23:1–51. [Google Scholar]
- 26. Daar ES, Tierney C, Fischl MA, et al. ; AIDS Clinical Trials Group Study A5202 Team Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sax PE, Pozniak A, Montes ML, et al. . Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390:2073–82. [DOI] [PubMed] [Google Scholar]
- 28. Blanco JL, Marcelin AG, Katlama C, Martinez E. Dolutegravir resistance mutations: lessons from monotherapy studies. Curr Opin Infect Dis 2018; 31:237–45. [DOI] [PubMed] [Google Scholar]
- 29. Armenia D, Di Carlo D, Calcagno A, et al. . Pre-existent NRTI and NNRTI resistance impacts on maintenance of virological suppression in HIV-1-infected patients who switch to a tenofovir/emtricitabine/rilpivirine single-tablet regimen. J Antimicrob Chemother 2017; 72:855–65. [DOI] [PubMed] [Google Scholar]
- 30. Cohen CJ, Molina JM, Cahn P, et al. ; ECHO Study Group; THRIVE Study Group Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naive HIV-1-infected patients: pooled results from the phase 3 double-blind randomized ECHO and THRIVE trials. J Acquir Immune Defic Syndr 2012; 60:33–42. [DOI] [PubMed] [Google Scholar]
- 31. Madruga JV, Berger D, McMurchie M, et al. ; TITAN Study Group Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet 2007; 370:49–58. [DOI] [PubMed] [Google Scholar]
- 32. de Boer MG, van den Berk GE, van Holten N, et al. . Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS 2016; 30:2831–4. [DOI] [PubMed] [Google Scholar]
- 33. Cid-Silva P, Llibre JM, Fernández-Bargiela N, et al. . Clinical experience with the integrase inhibitors dolutegravir and elvitegravir in HIV-infected patients: efficacy, safety and tolerance. Basic Clin Pharmacol Toxicol 2017; 121:442–6. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Medicare & Medicaid Services. Clinical laboratory fee schedule 2018 Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/. Accessed 25 April 2019.
- 35. Centers for Medicare & Medicaid Services. Medicare physician fee schedule 2018 Available at: http://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed 25 April 2019.
- 36. RED BOOK Online(R) search—MICROMEDEX® [cited 25 April 2019]. Available at: http://www.micromedexsolutions.com.ezp-prod1.hul.harvard.edu/micromedex2/librarian/PFDefaultActionId/redbook.ModifyRedBookSearch.
- 37. Norwood J, Turner M, Bofill C, et al. . Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schackman BR, Fleishman JA, Su AE, et al. . The lifetime medical cost savings from preventing HIV in the United States. Med Care 2015; 53:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spertilli Raffaelli C, Rossetti B, Paglicci L, et al. . Impact of transmitted HIV-1 drug resistance on the efficacy of first-line antiretroviral therapy with two nucleos(t)ide reverse transcriptase inhibitors plus an integrase inhibitor or a protease inhibitor. J Antimicrob Chemother 2018; 73:2480–4. [DOI] [PubMed] [Google Scholar]
- 40. Fu R, Owens DK, Brandeau ML. Cost-effectiveness of alternative strategies for provision of HIV preexposure prophylaxis for people who inject drugs. AIDS 2018; 32:663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hill AM, Venter F. The unexpected success of NRTIs in second-line treatment. Lancet Infect Dis 2018; 18:3–5. [DOI] [PubMed] [Google Scholar]
- 42. Cahn P, Pozniak AL, Mingrone H, et al. ; Extended SAILING Study Team Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 43. Carey D, Puls R, Amin J, et al. ; ENCORE1 Study Group Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect Dis 2015; 15:793–802. [DOI] [PubMed] [Google Scholar]
- 44. Colombier MA, Molina JM. Doravirine: a review. Curr Opin HIV AIDS 2018; 13:308–14. [DOI] [PubMed] [Google Scholar]
- 45. Smith SJ, Pauly GT, Akram A, et al. . Rilpivirine and doravirine have complementary efficacies against NNRTI-resistant HIV-1 mutants. J Acquir Immune Defic Syndr 2016; 72:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arribas JR, Clumeck N, Nelson M, Hill A, van Delft Y, Moecklinghoff C. The MONET trial: week 144 analysis of the efficacy of darunavir/ritonavir (DRV/r) monotherapy versus DRV/r plus two nucleoside reverse transcriptase inhibitors, for patients with viral load <50 HIV-1 RNA copies/mL at baseline. HIV Med 2012; 13:398–405. [DOI] [PubMed] [Google Scholar]
- 47. Girard PM, Antinori A, Arribas JR, et al. . Week 96 efficacy and safety of darunavir/ritonavir monotherapy vs. darunavir/ritonavir with two nucleoside reverse transcriptase inhibitors in the PROTEA trial. HIV Med 2017; 18:5–12. [DOI] [PubMed] [Google Scholar]
- 48. Division of HIV/AIDs Prevention. Detecting, investigating, and responding to HIV transmission clusters. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB prevention. Centers for Disease Control and Prevention, 2017; 2:1–132. [Google Scholar]
- 49. Oster AM, Wertheim JO, Hernandez AL, Ocfemia MC, Saduvala N, Hall HI. Using molecular HIV surveillance data to understand transmission between subpopulations in the United States. J Acquir Immune Defic Syndr 2015; 70:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. . Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390:1499–510. [DOI] [PubMed] [Google Scholar]
- 51. Casado JL, Monsalvo M, Rojo AM, Fontecha M, Rodriguez-Sagrado MA. Dolutegravir and rilpivirine for the maintenance treatment of virologically suppressed HIV-1 infection. Expert Rev Clin Pharmacol 2018; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 52. Cahn P, Madero JS, Arribas JR, et al. . Abstract 13210: non-inferior efficacy of dolutegravir (DTG) plus lamivudine (3TC) versus DTG plus tenofovir/emtricitabine (TDF/FTC) fixed-dose combination in antiretroviral treatment-naïve adults with HIV-1 infection— 48-week results from the GEMINI studies. In: 22nd International AIDS Conference Amsterdam, The Netherlands; 23–27 July 2018 Available at: https://programme.aids2018.org/Abstract/Abstract/13210. [Google Scholar]
- 53. McGee KS, Okeke NL, Hurt CB, McKellar MS. Canary in the coal mine? Transmitted mutations conferring resistance to all integrase strand transfer inhibitors in a treatment-naive patient. Open Forum Infect Dis 2018; 5:ofy294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016; 16:565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deeks ED. Bictegravir/emtricitabine/tenofovir alafenamide: a review in HIV-1 infection. Drugs 2018; 78:1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.