Abstract
Cancer stem cells have been shown to be important in tumorigenesis processes, such as tumor growth, metastasis, and recurrence. As such, many three-dimensional models have been developed to establish an ex vivo microenvironment that cancer stem cells experience under in vivo conditions. Cancer stem cells propagating in three-dimensional culture systems show physiologically related signaling pathway profiles, gene expression, cell–matrix and cell–cell interactions, and drug resistance that reflect at least some of the tumor properties seen in vivo. Herein, we discussed the presently available Cancer stem cell three-dimensional culture models that use biomaterials and engineering tools and the biological implications of these models compared to the conventional ones.
Keywords: 3D culture; tumor model, cancer stem cells, biomaterials, tumor biology
Introduction
Recent statistics from the World Health Organization (WHO) report indicates that cancer is the leading cause of death globally, lowering the life expectancy of many populations across the world.1 Molecular mechanisms of oncogenesis have long been a topic of great interest in a wide range of fields. Cancer stem cells (CSCs) are a small tumor subpopulation of cells that have the potential of self-renewal and multi-differentiation. These aggressive cells are chemo- and radio-resistant, and contribute to the development and progression of malignancy (Figure 1(a)).2,3
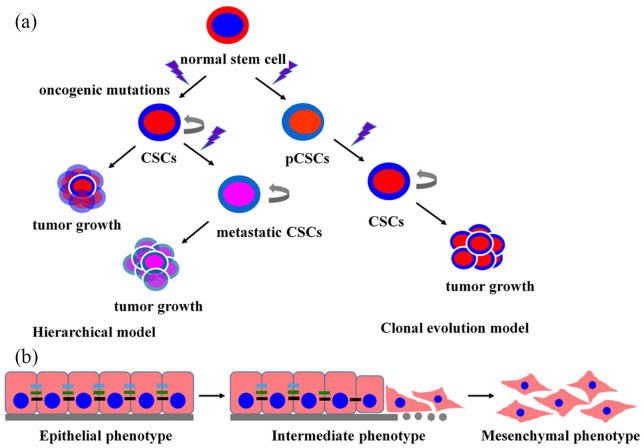
Figure 1.
Schematic illustration of cancer stem cell (CSC) models of tumorigenesis and the general features of EMT. (a) CSC models, including the hierarchical and clonal evolution prototypes. CSC subgroups showing self-renegeneration properties as well as capacity to differentiate at the apex of tumorigenesis hierarchy. (b) Schematic of EMT process: cancer cells lose their round, cobblestone-like morphology to become elongated with fibroblast-like morphology. CSCs, cancer stem cells; pCSC, precancerous stem cells; EMT, epithelial-mesenchymal transformation.
Compared with normal stem cells, CSCs show many unique features, including hyper-efficient mechanisms for DNA repair, expression of multidrug resistance-related ATP-binding cassette (ABC) membrane transporters, hypoxic niche resistance, and over-expression of anti-apoptotic proteins.4,5 In addition, the difference between CSCs and non-CSCs in the case of cancer may be attributed to epithelial-to-mesenchymal transition (EMT).6,7 EMT can be defined as a process whereby epithelial cells turn into mesenchymal cells, which are involved in the progression of malignant tumors. During EMT, cancer cells lose their round, cobblestone-like morphology to become elongated cells with fibroblast-like morphology, resulting in increased migration and invasion ability (Figure 1(b)).8,9 CSCs are considered a key treatment strategy for human cancers and represent new therapeutic targets.10 However, it has been shown that CSCs are difficult to culture in vitro, which is the main constraint in the study of CSC biology and drug discovery. Previous studies have shown that CSCs depend on a niche that regulates their proliferation and differentiation, analogous to normal stem cells.11
Considering the important part that CSCs plays during tumorigenesis, including tumor growth and radioresistance, it is essential to create tumor models similar to the in vivo condition. With the development of biotechnology methodologies, three-dimensional (3D) cell cultures are widely accepted as one of the most effective ways to elucidate the molecular mechanisms of CSCs. In the 3D culture systems, cells are grown to encourage cell–matrix and cell–cell interactions mimicking tumor microenvironment.12 Compared to two-dimensional (2D) cultures, the 3D culture systems allow cells to present more appropriate tissue physiology, anatomy, and structure.13–16 Signaling pathways in 3D culture also show different profiles in terms of cell migration, morphology, proliferation, and viability.15–18 Exemplar 3D culture models of CSCs are the serum-free culture suspension system and culture with a basement membrane scaffold.19,20
Many recent studies have reported various 3D culture models of CSCs using biomaterials and advanced technological tools. Herein, we summarize the developed 3D culture models of CSCs and discuss the biological implications of the models in terms of cell–cell and cell–matrix interactions, gene expression and signaling pathway profiles, and drug resistance. CSC studies in 3D models could contribute to further our understanding of tumorigenesis, tumor growth, metastasis, and recurrence behaviors occurring in vivo and assist in potential drug development for tumor therapy.21
3D culture models of CSCs
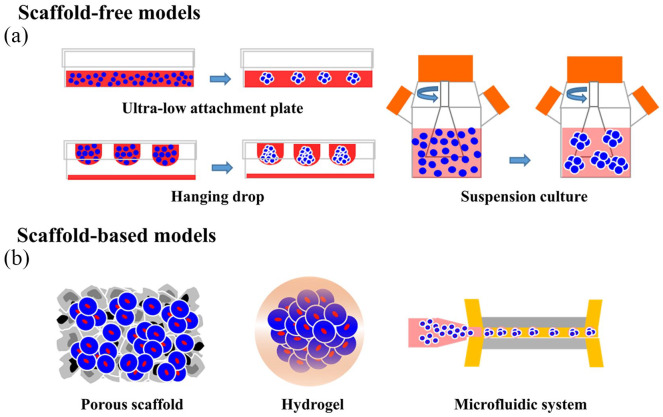
Numerous studies have demonstrated that certain cancer cells could re-acquire CSC traits via intrinsic stem-associated gene expression and extrinsic tumor microenvironment.22–26 Over the past few decades, many 3D culture methods of CSCs in the form of spheres have been developed. Typical methods for forming spheroids include scaffold-free methods such as the ultra-low attachment plate method, hanging drop method, suspension culture method, and scaffold-based techniques (Figure 2).27 Tumorspheres are primarily generated by a suspension of single cells in serum-free conditions.28,29 A subgroup of tumor cells that can survive in a serum-free culture is identified and isolated from a group of tumor cells, then used to form tumorsphere. These cells can proliferate and expand clonally devoid of serum supplements, suggesting that they may have stem cell-like features. However, recent evidence shows that tumorsphere culture-mediated enrichment of stem cell markers depends on the cell line and therefore, the resulting cells may not exhibit stemness.30 Tumorsphere formation has been achieved with many different tumor cells derived from prostate, skin, breast, and colon.
Figure 2.
Tumorspheres are 3D cancer stem cell (CSC) models generated by different methods. (a) Scaffold-free methods such as hanging drop, ultra-low attachment plate, and suspension culture. (b) Scaffold-based techniques, such as porous scaffolds, hydrogels, and microfluidic systems.
Scaffold-free 3D CSC culture models
Scaffold-free 3D CSC culture models mainly refers to the use of various physical methods to minimize cell attachment, which suspend the cancer cells onto the medium, and then promote cell aggregation into a spheroid. These methods contain ultra-low attachment plates, hanging drop and suspension culture.
Ultra-low attachment plates
The surface of the ultra-low attachment plate is coated with an inert substance, such as agarose or poly-hydroxyethyl methacrylate,31 which can minimize cell attachment.32 This method can isolate CSCs/tumor-initiating cells according to their distinctive anoikis-resistant capacity or anchorage-independent growth.33,34 For example, Gao and co-workers35 successfully isolated CSCs from multiple tumor cell lines by a non-adherent culture method, which has significant advantages over other methods. Im et al.36 developed CSC-like cells using A172 glioblastoma cells under conditions of non-adherent culture with serum deprivation. Krishnamurthy et al.37 applied ultra-low attachment plates to generate head and neck CSC for therapeutic strategies in head and neck cancer studies.
Advantages of this technique include the fact that it is a convenient procedure and multiple cell types (co-culture) can be incorporated.16,38 However, the major drawback of CSC spheroids formed with ultra-low attachment plates is that they vary in size. In addition, the mixture of attached cells and spheroids overwhelms assay chemistry.15,39
Hanging drop
The principle behind the hanging drop method is to use the surface tension of a droplet of cells and gravity to suspend the droplet of cells onto the underside of a lid, which could promote cell aggregation into a spheroid. Phosphate-buffered saline is routinely used to suspend the cells to prevent volatilization of the droplets.15,16,40,41 Raghavan et al.42 formed primary ovarian CSCs using a 3D hanging drop suspension platform to study CSC biology. Rodríguez et al.43 successfully constructed breast CSC using this approach to study the relevance of breast CSC number and human epidermal growth factor receptor 2 regulation. The hanging drop method has been shown to produce uniformly sized spheroids and is applicable to different cells.44–46
However, there are several major drawbacks concerning this method. First, this method is time-consuming to isolate and culture CSCs compared with the other methods. Second, the osmolarity of the droplet will be elevated owing to evaporation of media, which is not favorable for cell viability and long-term cultivation.15,16,47
Suspension culture
Suspension culture methods can achieve large-scale production of tumor spheroids using bioreactors, such as spinner flask and rotating flasks.31,48,49 These methods can decrease the effect of gravity by use of bioreactors and allow rapid production of large quantities of tumor spheroids.50 In the suspension culture method, the cell culture medium is stirred using a stirrer or rotating culture flask to prevent cell adhesion, thereby generating tumor spheroids. Appropriate control of stirring or rotating speed is critical for tumor spheroid generation; otherwise, these cells would be damaged by sheer force. Chang et al.51 successfully cultured hepatoma cell spheroids using rotating wall vessel bioreactors. In their study, spheroids of up to 1 mm in diameter could be obtained. Many tumorspheres have also been generated from the liver,51 neuroblastoma,52 breast,53 and melanoma54 using suspension culture methods.
A major benefit of this method is that a large number of tumor spheroids are formed. Besides, oxygen and nutrients could be distributed evenly around the tumor spheroids. However, mechanical forces and shear stress generated by stirring may damage the cells. Meanwhile, it is difficult to obtain tumor spheroids that are uniform in size and shape with this method.
Biomaterial-based 3D CSC culture models
Biomaterials with different physical structures, such as porous foams and hydrogels, have been used to create 3D CSC culture systems.55–57 Furthermore, biomaterials combined with microfabrication technology have been demonstrated to generate more precisely controlled 3D CSC models. Biomaterial-based 3D CSC culture models can contribute not only to the understanding of biological behaviors of CSCs and the mechanisms underlying these events but also to the modeling of various tumors and screening of anti-cancer therapeutics.
Porous scaffolds
Numerous published articles have shown that owing to better mimicking the in vivo environment, porous scaffolds may provide a more favorable environment for tumor cells.58,59 Accumulating reports have demonstrated the critical role of porosity, pore shape, and size of the scaffold in cell functions, including growth, division, and migration.60 Various approaches have been used to prepare porous scaffolds including particle leaching, phase separation, and emulsification/freeze-drying, among others.60–65 Commonly used materials for constructing scaffolds are bioactive ceramics (hydroxyapatite, bioactive glasses), synthetic polymers (such as polyglycolic and polylactic acids), and natural polymers (including silk, collagen, chitosan-alginate (CA), and hyaluronic acid (HA)).66–69
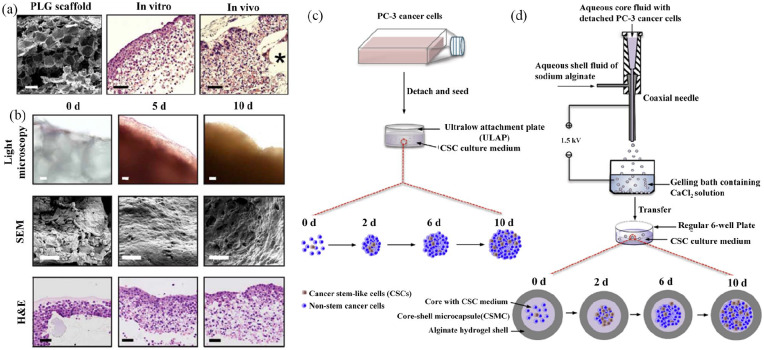
Polonio-Alcalá et al.70 found that polylactic acid (PLA) scaffolds can promote CSC proliferation and enrichment in breast cancer cells. Florczyk and coworkers71 showed that 3D CA scaffolds promoted enrichment of the CSC population, including the cells of prostate carcinoma, breast cancer, hepatocellular carcinoma (HCC), and glioblastoma cells. HA is a natural polymer that is widely chosen as a 3D tumor model material, as it is abundant in the extracellular matrix (ECM).72 Martínez-Ramos and Lebourg.73 reported that U87 astrocytoma cells cultured with HA showed significantly elevated expression of CSC-related proteins, suggesting that the 3D-HA scaffold is a valuable model for developing drugs targeted at CSC. Lee et al.74 reported a method using HA-based multilayer films to form, as well as culture pancreatic CSC colonies, which exhibited a dormant, slow-cycling phenotype, and increased expression of CSC-associated genes (OCT4, CXCR4, and CD44v6). One mechanism underlying CSC enrichment in porous scaffolds is that the scaffolds can isolate cancer cells by a unique pore structure that mimics the in vivo tumor niche (Figure 3(a) and (b)).55,55Collectively, porous scaffolds are simple and inexpensive to cultivate CSCs, thereby contributing to CSC research.
Figure 3.
3D cancer stem cell (CSC) models developed with porous scaffolds (a) and (b) and hydrogels (c) and (d). Characterization of tumor model (a) and (b): (a) Microstructure of cells loaded onto 3D PLG scaffolds visualized under scanning electron microscopy (SEM), and tumor-like tissue hematoxylin and eosin (H&E) stained specimens photomicrographs. Asterisk symbol (*) represent fragments of polymeric scaffolds. (b) Light microscopy, microstructure under SEM, and H&E-stained microphotographs of tumor-like tissues cultured in 3D PLG at different time points. Reproduced with permission from Fischbach et al.56 (2007, Nat Methods). Enrichment of PC-3 human prostate CSCs using conventional bulk suspension culture versus miniaturized 3D culture (c) and (d): (c) Schematic illustration of conventional bulk suspension culture in ultralow attachment plate. (d) Schematic illustration of miniaturized 3D culture in regular 6-well plates. The core of core-shell microcapsules (CSMCs) containing PC-3 prostate cells was prepared through coaxial electrospray by a coaxial needle. CSMCs were then cultured in regular 6-well plates for at least 10 days. Reproduced with permission from Rao et al.75 (2014, Biomaterials).
Porous scaffolds provide support for cell adhesion, growth, proliferation, metabolism and the formation of tumor spheroids. However, there are some limitations, such as low tensile strain strength and inadequate extensibility. In addition, the low usage efficiency of cancer cell due to their poor adhesion on synthetic polymers is a complication in 3D culture models of CSCs.
Hydrogels
Hydrogels are hydrophilic polymers and have advantages of biocompatibility and biodegradability.57,76 Because hydrogels are useful for cell growth and exchange of substances between cells, they have been widely applied in tumor models.77 The interconnected pores enable the transport of oxygen, nutrients, and metabolites.15 Hydrogels used for 3D culture are either natural or synthetic polymers.78 Rao et al.75 wrapped up human prostate cancer cells using alginate hydrogel to enrich CSCs for cancer research and therapy development (Figure 3(c) and (d)). Li et al.79 developed glioblastoma tumor-initiating cells (TICs) using a 3D thermo-reversible hydrogel, which showed sufficient, affordable glioblastoma TICs for drug discovery.
Pal et al.80 prepared scaffolds by impregnating hydrogels into electrospun scaffolds to study anti-cancer therapeutics against metastasis. The results showed that hydrogel-rich electrospun scaffolds could induce cancer cells to undergo EMT and drive non-CSC to CSC transformation, facilitating the enrichment of CSC phenotypes. Dai et al.81 prepared GAF hydrogel scaffolds using gelatin, fibrinogen, and alginate as raw materials, and the influence on survival rate as well as inherent characteristics of glioma stem cells with the scaffolds was investigated. In their study, glioma stem cells attained over 86% survival rate, and Nestin glioma stem cell markers showed high levels of expression with the GAF hydrogel scaffolds. Yang et al.82 encapsulated breast cancer cells within polyethylene glycol diacrylate (PEGDA) gel conjugated with CD44 binding peptide (CD44BP) to culture breast CSC. The flow cytometry results demonstrated that CD44BP conjugated to PEGDA gel could improve CD44 +/CD24− percentage, suggesting that this technique can maintain the stemness of breast CSC. Jabbari et al.83 also constructed a PEGDA hydrogel to generate breast CSCs, colorectal CSCs, and gastric CSCs.
Many authors have classified hydrogels into two categories based on their differences of raw materials: natural hydrogels (collagen hydrogels, fibrin hydrogels) and synthetic hydrogels (PEGDA hydrogels). Each type of these hydrogel systems have distinct advantages and disadvantages for specific 3D cell cultures, in terms of ranges of controllable properties and long- term cell viability. For example, the chemical structures of natural hydrogels similar to glycosaminoglycans and therefore provide natural hydrogels with controlled permeabilities. While natural polymers also have several disadvantageous features, such as poor mechanical properties. There are increasing efforts to modify gel-forming polymers or crosslinking molecules with multifunctional groups and also cross-link multipolymer systems, in order to further improve the controllability of the microstructures and properties of hydrogels.
Microfluidic devices
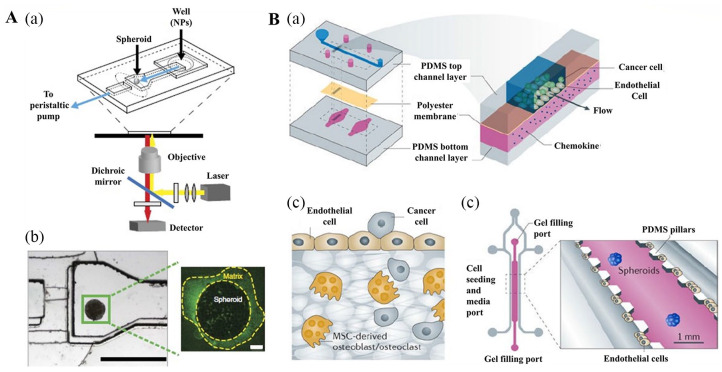
Microfluidics is a technique developed based on the advances in biology, physics, materials science, and engineering. In this technology, numerous experimental steps, such as sample preparation, reaction, separation, and assay, can be integrated into a microfluidic chip with diameters of 1 mm, which makes the detection process more miniaturized and intelligent. Owing to its high efficiency, excellent sensitivity, and exact controllability, this technique has been widely used in medical research.84 In recent years, microfluidics has been applied to the generation, isolation, and characterization of CSCs. Use of microfluidics can generate a large number of spheroids with uniform size and shape for high-throughput screening (HTS) (Figure 4).85,86 For example, microfluidic devices composed of microwells (250–450 μm) with vasculature-mimicking microfluidic channel connections could support growth of CSCs. Here, the glass plate was coated with a 3D growth matrix (hydrogel and a porous membrane) to promote cell aggregation and spheroid formation.15 Moreover, a microfluidic device was designed to control the flow rate to keep the cells in suspension, which may allow the formation of spheroids. Zhang et al.87 manufactured a mechanical separation chip via microfluidic technology to isolate and screen breast cancer cell lines. In their experiments, breast cancer cells with high flexibility and metastasis could easily pass through the mechanical separation chip, and these cells were identified to possess stem cell properties and the ability to form tumorspheres. Zhao et al.88 developed microfluidic devices that were prepared from polydimethylsiloxane by standard soft lithography and replica molding to investigate CSCs. The microfluidic devices consisted of 4 functional channels: main channels, endothelial cell channels, symmetric chambers, and fluidic channels. Many smaller horizontal bridge microposts connected all the chambers and parallel channels. Hexagonal columns formed numerous gaps between them to hold the matrigel. The polydimethylsiloxane was glued to a glass coverslip. The microfluidic device provided both 2D and 3D culture, as well as co-culture environments with no effect on cell viability.
Figure 4.
3D cancer stem cell (CSC) models developed using microfluidic devices. (A, a, b) Schematic of the tumor-on-a-chip. (A, a) Schematic of the polydimethylsiloxane microfluidic device at the microscope stage. (A, b) The microfluidic device (left) showing the channel width of 600 mm at the inlet, which extends to 1200 mm in the imaging chamber where the spheroid is immobilized. The height of the channel is 250 mm and decreases to 25 mm at the end of the imaging chamber, forming a dam. A spheroid (right) stained for 10 min with anti-Laminin-FITC, then flushed for 5 min with imaging media. (B, a-c) Schematic of the organ-on-a-chip model. Reproduced with permission from Albanese et al.85 (2013, Nat Commun). (B, a) An endothelium-on-a-chip microvascular established in a segmented microfluidic channel allowed endothelial cells cultured on a chemokine supplemented permeable membrane to undergo activation and basal stimulation during the investigation of attached circulating cancer cells in breast tumor metastasis. The impacts of chemokines, including tumor necrosis factor, were examined via incorporating the chemokines in the bottom channel. The endothelium pre-treated with tumor necrosis factor attracted more tumor cells compared to the untreated endothelium. (B, b) Migration of breast tumor cells to the bone was examined in microfluidic device with endothelial cell culture from human umbilical vein next to bone cells derived from mesenchymal stem cells of the human bone marrow held a 3D collagen gel. Movement of cancerous cell to the bone was detected. (B, c) To assess the epithelial-mesenchymal transition (EMT) during malignancy, spheroids of lung cancer were fixed in micropatterned 3D matrices connected closely to endothelial cells linning on the microchannel. EMT examination was performed with microfluorometry to identify cancer spheroids distribution. Reproduced with permission from Esch et al.86 (2015, Nat Rev Drug Discov).
High-throughput biosensor technologies as the basis of new-generation cell-based HTS techniques provide analytical information by the recognition of real time biological events employing a physical transducer. And this technique also has the advantage of generating a large number of spheroids with uniform size and shape for HTS.42 However, the main drawback of microfluidic devices is the complexity of its design and manufacture. Moreover, spheroids generated by microfluidic devices are difficult to collect for subsequent analysis.
Biological implications of 3D CSC models
The tumor microenvironment is different from that in a 2D culture. However, in a 3D culture, CSCs display different types of tumor biology, including sustained angiogenesis, tissue invasion, metastasis drug resistance, tumor–immune cell interactions, and EMT, which are much closer to the reality in humans.19,48,89,90
Hypoxia and metabolism in 3D CSC models
In general, CSCs requires a specific microenvironment to maintain self-renewal and asymmetric divisions where hypoxia is the predominant feature.91,92 When tumorspheres are enlarged beyond several hundred microns in diameter, the cells grown on the outermost layer of the tumorspheres could consume a lot of oxygen and nutrients, leading to preferential growth of cells located in the marginal zone. Meanwhile, the cells located in the innermost layers of tumorspheres undergo growth arrest or even necrosis due to an insufficient supply of oxygen and nutrients. Several studies have demonstrated that hypoxia could result in altered gene expression, promoting tumor angiogenesis, and metabolic shift in cancer cells.93,94 In addition to a condensed structure resulting from a tightly aligned cell layer, 3D cancer models reproduce unique features of tumor hypoxia and necrosis in the innermost layer,95 offering an efficient system to investigate hypoxic mimic biology of cancers and thus develop new anti-cancer therapies.96–98
Maintenance of stemness of CSCs is closely associated with the hypoxic environment.99,100 Hypoxia is obligatory for the formation of a CSC niche.22 This hypothesis was supported by studies that demonstrated that primitive hematopoietic stem cells inhabit areas with low oxygen pressure, given that they are likely to occupy regions that are far from the vessels.101,102 Emerging evidence also suggests that under a hypoxic environment, the stemness of breast cancer cells can be enhanced to increase malignancy and therapeutic resistance.100
The hypoxic tumor microenvironment could also activate CSC-related signaling pathways. The CSCs properties are regulated by a network of complex interacting signaling pathways. Some of them are critical for maintaining the stemness of CSCs, including the Wnt, Notch, and Hedgehog pathways. Studies in breast cancer cells have found that stem cell characteristics are largely mediated through the activation of Wnt/β-catenin and Notch signaling pathways under the hypoxic tumor microenvironment.103 This was proved by a study that hypoxia could enhance the stemness properties of glioblastoma and colorectal cancer by Notch, Hedgehog, and Wnt signaling pathways.104
Hypoxia is regarded as the main characteristic of the tumor microenvironment, which could contribute to the maintenance of stemness and promote tumor progression.105 There is mounting evidence that hypoxia induces stemness in differentiated progenitor and non-CSCs via stem gene activation and dedifferentiation.106–108 Hypoxia-inducible factors (HIFs) is important in maintaining stemness and EMT process under anoxia, as hypoxia induces stemness characteristics of CSCs via activating HIFs.92,109,110 The HIFs comprise a constitutively expressed subunit and an oxygen-associated α subunit (HIF-1α-3α).111 Many studies have reported that HIFs play a critical function in the regulation of the CSC phenotype.112–117
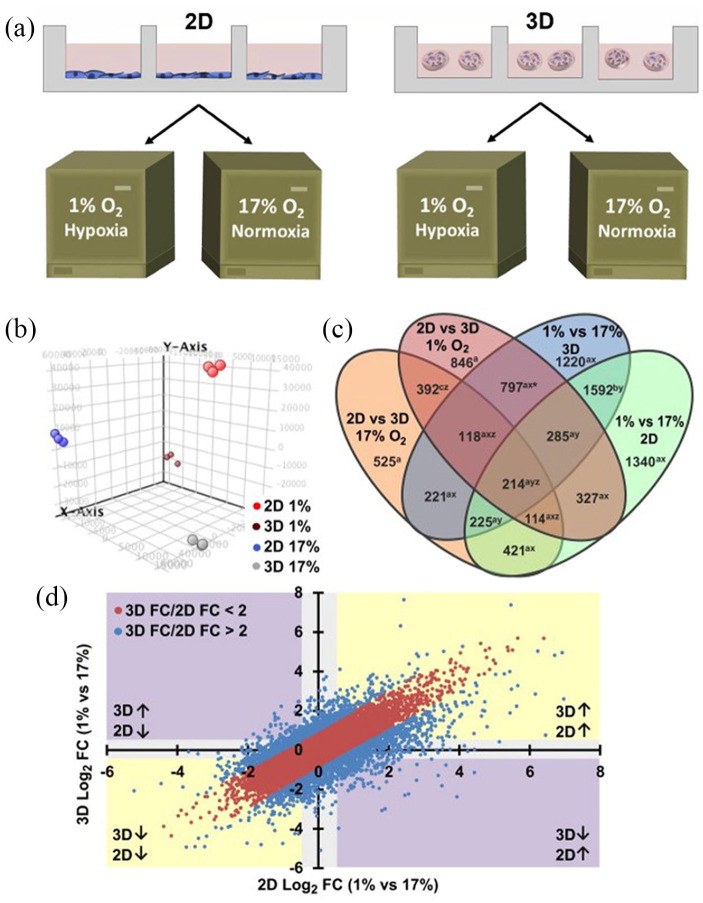
The cells are cultured in the 2D culture system uniform contact with oxygen, which cannot create a continuous oxygen concentration gradient. Thus, the 2D cell culture system does not mimic a hypoxic environment similar to in vivo conditions.118 Therefore, hypoxia level and hypoxia-controlled expression of genes are different between cells cultivated in 3D and 2D culture models. For example, Stankevicius et al.119 investigated the changes in gene expression associated with the maintenance of CSCs, such as genes involved in hypoxia, multipotency, CSC marker, and EMT in human colorectal carcinoma cell lines HT29 and DLD1 cultured in 2D vs 3D cell culture conditions. The authors selected hypoxia-related genes of GLUT1, CAIX, and VEGFA. The results showed that the HT29 and DLD1 cells cultured in a 3D lamin-rich-ECM environment showed higher levels of GLUT1, VEGFA, and CAIX gene expression relative to traditional 2D monolayer cell cultures. Klimkiewicz et al.120 built a 3D micro-melanoma tumor using a 3D system perfecta to study and compare melanoma cell monolayers and melanoma spheroids. The results of enzyme-linked immunosorbent assay showed that there was constant amounts of HIF-1α in melanoma spheroids cultured in the 3D system, while HIF-1α amounts rose and subsequently fell in 2D cell monolayers. In another study, clinical samples demonstrated a positive correlation between HIF-1α and estrogen receptor alpha (ERα) expression. DelNero et al.121 established a 3D culture alginate system with controlled oxygen, and examined expressed gene patterns of cancer cells grown in 2D and 3D in the same normoxic or hypoxic conditions using microarray assay. Microarray gene expression analysis of tumor cells grown in 2D vs 3D under hypoxic or ambient conditions showed a remarkable association of culture dimension and hypoxia reaction, indicating that response to hypoxia mainly depends on the condition where cell are grown either 2D or 3D environments (Figure 5). Whitman et al.122 observed a dramatic reduction in ERα in 2D cultures and the stabilization of ERα in 3D cultures.
Figure 5.
Schematic diagram of cell culture model and overall changes in gene expression under different conditions. (a) OSCC-3 cells were cultured in traditional 2D monolayer cell cultures or microfabricated alginate disks and incubated in normoxia (17% O2) or hypoxia (1% O2) condition for a week, respectively. (b) GeneSpring GX 12.6.1 software was used to in principal component analysis (PCA) of microarray results. Each substrate assembling and oxygen concentration demonstrated the dependability of every treatment to produce autonomous and self-reliable gene expressed patterns. (c) Genes were related to dimensionality as well as oxygen level variations according to Venn diagram. (d) The 2D hypoxia vs normoxia (x-axis) and 3D hypoxia vs normoxia (y-axis) scatterplots indicate the degree (FC: fold change) and trend (↑ and ↓: up- and down-regulation, respectively) of gene expression variations. Reproduced with permission from DelNero et al.121 (2015, Biomaterials).
Angiogenesis in 3D CSC models
Angiogenesis in cancer provides oxygen and nutrients, which favors cancer cell growth and represents a prerequisite and biological underpinning of metastasis for tumor growth, invasion, progression, and metastasis.123,124 Recent findings suggest that CSCs are involved in promoting angiogenesis, thereby promoting cancer metastasis.125 Therefore, inhibiting cancer angiogenesis is considered an efficient therapeutic strategy. Growing CSCs in 3D can mimic tumor angiogenesis, which allows the evaluation of drug effects on angiogenesis.
Numerous studies have shown that CSCs can elevate the expression of angiogenic factors in hypoxic environments, suggesting that CSCs play pivotal roles in tumor progression and angiogenesis.120,125,126 CSCs may switch tumor neovascularization, resulting in promoting tumor development. Three pathways are known for tumor neovascularization by CSCs, namely production of proangiogenic factors, transdifferentiation, and formation of vasculogenic mimicry (VM).127 CSCs have been reported to contribute to the formation of VM, a unique pattern of blood supply, induced by endothelial cells and vascular smooth muscle-like cells, through non-endothelium lining channels.128–132
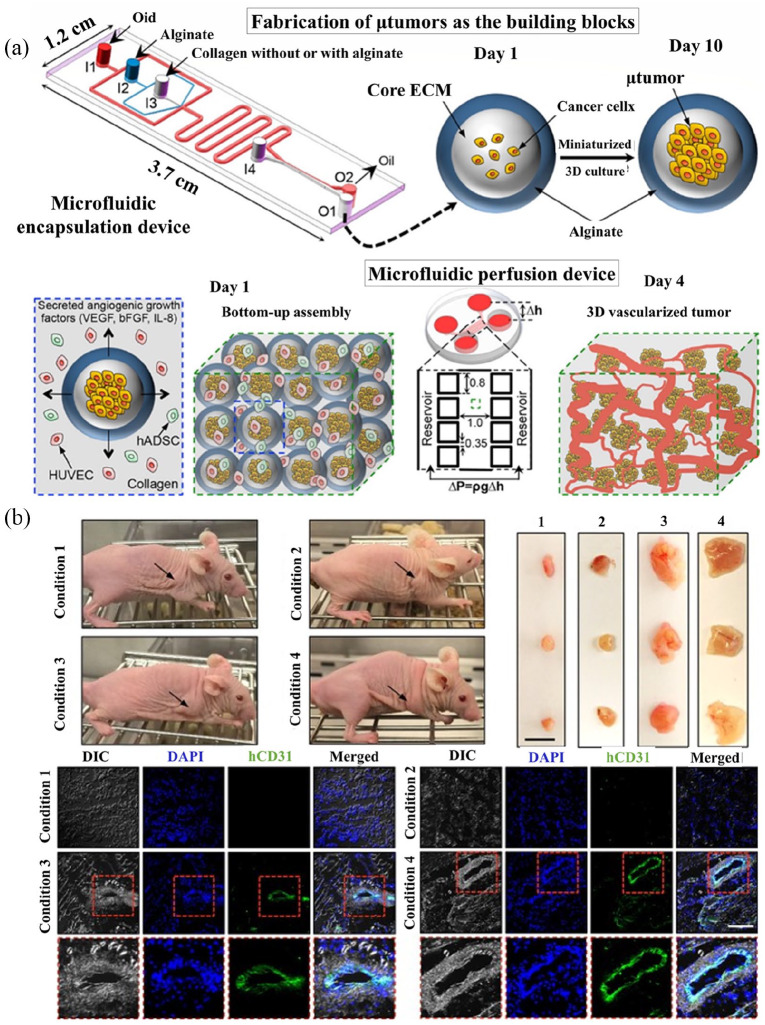
3D tumor models with CSCs have demonstrated some unique patterns of angiogenesis. For example, Bray et al.133 mimicked tumor angiogenesis by a hydrogel culture system built on glycosaminoglycan, which could recreate prostate and breast tumor vascularization. This microenvironment model often recreates tumor vascularization breast and prostate. The different types of cells grown within this model were more tolerant of chemotherapy than those in 2D cultures and exhibited a tumor inhibition profile as that seen in vivo. In another study, Chiew et al.134 developed a classical system to analyze cancer development and angiogenesis using ECs and HepG2 HCC cells. This model could resemble tumor angiogenesis under the HCC microenvironment, which enables investigation of the cellular signaling pathways involved in HCC progression. The results revealed that the 3D model exhibited similar levels of protein expression relative to HCC xenograft. Also, the 3D model showed increased expression of vital signaling proteins, including Akt/mTor and p70s6k, which was not observed in the 2D model. This could be attributed to strong association among liver cancer and ECs, thus facilitating the EC maturation, protein synthesis as well as the cancer cells development in 3D co-culture. Also, the levels of VEGF expression was higher in the 3D co-culture, indicating a higher secreted VEGF in 3D than 2D co-culture by HepG2-DsRed cells. Miller et al.135 reported the culture of primary human clear cell renal cell carcinoma (ccRCC) cells using 3D culture system (“ccRCC-on-a-chip”) to study tumor angiogenesis. Based on their findings, expression of key angiogenic factors, including ANGPTL4, PGF, and VEGFA, in primary human ccRCC cells is enhanced in 3D cultures compared to that in 2D monolayers. In study by Agarwal et al.,136 established “bottom-up” strategy for designing 3D vascularized human tumor which showed the ability to form complex 3D vascular networks that is controllable by incorporating cancerous cells into hydrogel-shelled microcapsules for reduced 3D culturing. In this study, the results showed that expression of vasculogenesis and angiogenesis-associated genes (e.g. VEGF) can indeed be discharged to the surrounding via the alginate shell and the secreted VEGF in the microcapsule confined 3D tumors was considerably higher than those released by 2D cultures. Also, the typical blood vessels dimension of the engineered derived tumors was higher compared to those formed from the 2D grown cells (Figure 6).
Figure 6.
(a) A scheme of the bottom-up approach of generating 3D vascularized human tumor. (b) In vivo tumorigenicity of the 3D-engineered system of encapsulated microtumors, HUVECs, and hADSCs in collagen. Reproduced with permission from Agarwal et al. (2017, ACS Nano).136
EMT in 3D CSC models
EMT has great significance for embryogenesis and maintains the integrity of the embryo.6 Recent studies have linked EMT with both metastatic progression of cancer and acquisition of stem cell characteristics, leading to treatment resistance, progression, and metastasis of malignant tumors.137–141 The activation of EMT processes in embryogenesis and tumor progression may induce changes in the physiological function of cells. Epithelial cells lose their round, cobblestone-like morphology and become elongated cells with fibroblast-like morphology, which results in a change in cell regulatory factors, enhancing cell motility.142
Considering the importance of EMT in drug resistance and tumor metastasis, it is necessary to establish a 3D culture design to mimic in vivo environments to be able to evaluate the reversibility of EMT and its role in tumorigenesis. Essentially, EMT is examined in 3D as well as 2D culture models. In 2D cultures, the cell form is confined to a “flat” plain, whereas in 3D models metastatic CSCs cells in vivo found at the edge of tumors forming aggregates, spherical forms, and colonies.143,144 Some studies showed that EMT activation is associated with the characteristics of stem cell traits for both neoplastic and normal cells.141,145 EMT is a crucial factor for CSC formation,141,146 and can result into transformation of epithelial to mesenchymal characteristics in cells, including high invasion and motility, bestowing them with stem cell-like features.147 EMT can lead to loss of polarity and phenotype in epithelial cells, and this includes their connection with the basement membrane. This can make them gain high invasion and migration, anti-apoptotic, and ECM degradation capacities, leading to drug resistance and metastasis of malignant tumors.
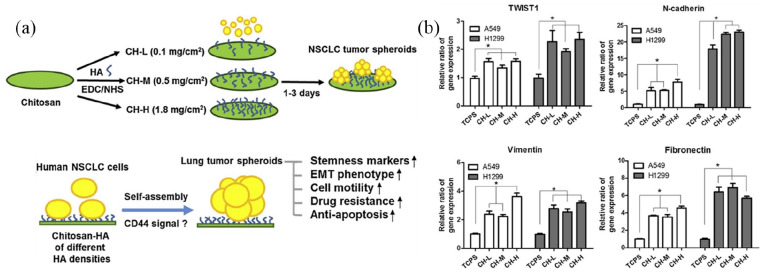
The 3D model has been widely used to investigate molecular events in EMT.123 To reflect CSC and EMT properties in a 3D context, Liu et al.148 constructed a collagen scaffold to research adenoid cystic carcinoma (ACC) cell biological function in 3D culture system. Here, ACC-83 cells seeded in collagen scaffold were compared to ACC-83 cells in 2D culture. The EMT and angiogenesis associated genes expressions were considerably increased in 3D culture. Moreover, the collagen scaffold could improve ACC-83 cell migration, invasion and resistance to chemotherapeutic agents. From this, they concluded that the collagen scaffolds provide a new platform for CSC research in diseases. Also, to explain the effect of composition and biophysical features of ECM on pancreatic cancer EMT, Puls et al.149 also cultured pancreatic ductal adenocarcinoma in a type I collagen oligomer 3D matrices, suggesting that classic EMT changes could also be observed in 3D models of the mammary gland acinus. On the contrary, the frequently used 2D in vitro cell culture system does not model the conditions of in vivo cancer EMT. Huang and Hsu150 used chitosan-coated and HA as the material to produce chitosan-hyaluronic acid (CH) membranes. Human non-small cell lung cancer (NSCLC) cells were seeded onto CH membranes to promote generation of tumor spheroid. In this study, they examined the biological function of tumor spheroid by comparing NSCLC cells on CH membranes (3D culture) or tissue culture polystyrene (conventional 2D culture). The result showed that compared with the conventional 2D culture, the expression levels of EMT- and stemness-associated genes were considerably increased in 3D culture. Besides, the NSCLC seeded on the CH membranes displayed more aggressive characteristics and resistant to antineoplastic drugs. These results illustrated that CH was valuable for CSC research and antineoplastic drugs development. (Figure 7).
Figure 7.
(a) Synthesis of chitosan-hyaluronic acid (CH) matrices under diverse hyaluronic acid (HA) concentrations and formation of tumor spheroids from non-small cell lung cancer (NSCLC) cells via CD44 signaling modulation. (b) Features of EMT from qRT-PCR, denoted by expressed TWIST1, N-cadherin, vimentin, and fibronectin mRNA in a week. Reproduced with permission from Huang et al.150 (2014, Biomaterials).
Drug resistance in 3D CSC models
Multidrug resistance (MDR) refers to the fact that when cancer cells are exposed to a type of chemotherapeutics for long-term, they could acquire resistance to that type of chemotherapeutics, and also develop cross-resistance to other chemotherapeutics.151,152 MDR remains a tough clinical problem for researchers and a difficult issue in the treatment of cancer, leading to tumor recurrence and progression.153
Studies have indicated that CSCs play crucial role in the emergence of MDR, which is the main cause of tumor treatment failure.152,154 Resistance to these treatments can be subcategorized into intrinsic and acquired. MDR of tumors can be categorized into intrinsic resistance and acquired drug resistance. Intrinsic resistance is a preexisting factor before the start of chemotherapy, thus inducing certain treatments useless. Acquired drug resistance occurs gradually during the course of chemotherapy, and seems to be the main reason for tumor treatment failure.154
3D CSC culture models could simulate the in vivo situation to study therapy resistance because they can reflect real drug responses. Studies have indicated that tumorspheres cultured in 3D models may show increased treatment resistance compared with cancer cells cultured in traditional 2D culture, reflecting the real resistance level against an anti-cancer drug.39,123,155,156 Many mechanisms of CSC resistance have been explored. First is the overexpression of ABC transporters. These are complex molecular pumps which mainly act as catalyst in active transport, by hydrolysing ATP. In a clinical setting, these may involve pumping out of the drug by ABC transporters, directing drugs and removal via exocytosis in vesicles, as well as low drug uptake.157 Second is the overactivation of the DNA damage response (DDR). Radiotherapy is a local tumor treatment used to kill tumor cells, while CSCs overactivate DDR, which produces drug-tolerant states.158,159 Third is cell-cycle promotion and/or cell metabolic alterations. Most CSCs are in the state of cell-cycle quiescence, except the state of self-renewal and cell division, which reduces damage from anticancer drugs. This is because some anticancer drugs are cell-cycle-specific agents, which only act on cancer cells in the proliferating phase.152,160 Finally, apoptosis evasion and activation of pro-survival pathways is another resistance mechanism, as one of the key mechanisms of chemotherapy treatment is inducing cancer cell apoptosis.151 When cancer cell apoptosis is disrupted, cancer cells could show resistance to chemotherapy treatment. In addition, the tumor microenvironment, activation of aldehyde dehydrogenase, and developmental pathways also play an important role in CSC resistance.161–163 In conclusion, CSCs play a key role in tumorigenesis and promote MDR phenotype via multiple mechanisms.
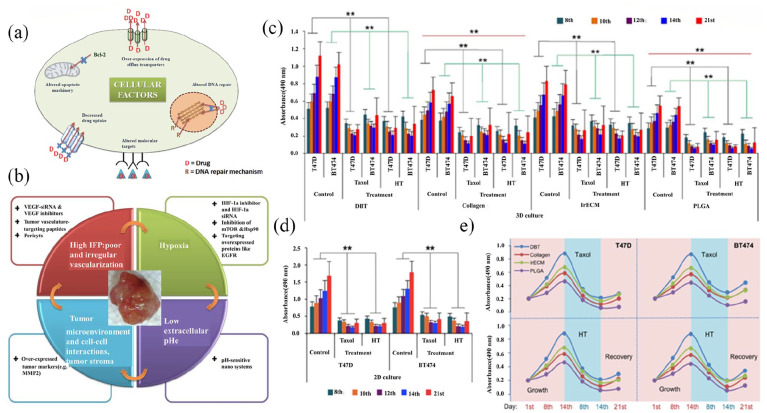
Rija and Li164 introduced a fabricated reconstructable tissue matrix scaffold system native tissue from ECM tissue-like structure and pliability to test effectiveness of two anti-cancer medications, taxol and 4-hydroxytamoxifen (HT), in 2D and 3D cultures. The BT474 and T47D cells were seeded onto 2D and 3D surface scaffolds and evaluated with Live/Dead Cell assay and CCK-8 reagent post the anti-cancer drugs administering. According to the results, BT474 and T47D cells exhibited a faster proliferation with distinct growth tendency in the 3D scaffolds among the scaffold sets, and higher robust growth was observed in 2D models. The administered medication suppressed the proliferation of cells in the 3D and 2D groups in a time-dependent manner, but the impact of drug suppression in 3D cultures was lower than in 2D. Thus, indicating that 3D cell cultures have better biological and clinical feasibility than 2D cultures (Figure 8).
Figure 8.
Drug resistance in cancer cells (a) Mechanisms of intrinsic multidrug resistance in cancer cells. (b) Intrinsic relationship between multidrug resistance and physiological factors. Reproduced with permission from Patel et al.165 (2013, Adv Drug Deliv Rev). The susceptibilities of T47D and BT474 cells to 4-hydroxytamoxifen and paclitaxel from different cultivation methods. (c) and (d). (c) The histogram shows absorbance values of T47D and BT474 cells treated with 4-hydroxytamoxifen and paclitaxel from four different scaffolds. (d) Proliferation of T47D and BT474 cells treated with 4-hydroxytamoxifen and paclitaxel cultured in 2D culture were assessed by measuring the absorbance. (e) Proliferation/inhibition curve plots. Reproduced with permission from Rija et al.164 (2017, Sci Adv).
Concluding remarks
In this communication, we discussed about different 3D models of cancer for CSCs enrichment, putting an emphasis on the biomaterials- and engineering-based approaches and designs. Development of 3D culture models similar to the conditions of in vivo tumorigenesis enables better understanding the key events including tumorigenesis, tumor growth, metastasis, and recurrence in the in vitro conditions that resemble tumor microenvironment in humans. Even with the active studies in this area, current 3D models of cancer for CSC enrichment are limited in terms of variability in cell size and homogeneity, protocol standardization and mass production. Tackling these issues with advanced 3D CSC models may facilitate tumor modeling and drug development in the near future.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by National Natural Science Foundation of China (81760531), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07048459, 2019R1A6A1A11034536, and Global Research Development Center Program 2018K1A4A3A01064257).
ORCID iDs: Hae-Won Kim  https://orcid.org/0000-0001-6400-6100
https://orcid.org/0000-0001-6400-6100
Yanhua Xuan  https://orcid.org/0000-0001-6817-219X
https://orcid.org/0000-0001-6817-219X
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Pützer BM, Solanki M, Herchenröder O. Advances in cancer stem cell targeting: how to strike the evil at its root. Adv Drug Deliv Rev 2017; 120: 89–107. [DOI] [PubMed] [Google Scholar]
- 3. Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010; 29: 4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monzani E, Facchetti F, Galmozzi E, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer 2007; 43(5): 935–946. [DOI] [PubMed] [Google Scholar]
- 5. Trumpp A, Wiestler OD. Mechanisms of disease: cancer stem cells—targeting the evil twin. Nat Clin Pract Oncol 2008; 5(6): 337–347. [DOI] [PubMed] [Google Scholar]
- 6. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9(4): 265–273. [DOI] [PubMed] [Google Scholar]
- 7. Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol 2013; 15: 338–344. [DOI] [PubMed] [Google Scholar]
- 8. Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 2017; 14(10): 611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev 2009; 28(1–2): 15–33. [DOI] [PubMed] [Google Scholar]
- 10. Kievit FM, Florczyk SJ, Leung MC, et al. Proliferation and enrichment of CD133+ glioblastoma cancer stem cells on 3D chitosan-alginate scaffolds. Biomaterials 2014; 35(33): 9137–9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiao S, Zhao Y, Li C, et al. An alginate-based platform for cancer stem cell research. Acta Biomater 2016; 37: 83–92. [DOI] [PubMed] [Google Scholar]
- 12. Thoma CR, Zimmermann M, Agarkova I, et al. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 2014; 69–70: 29–41. [DOI] [PubMed] [Google Scholar]
- 13. Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 2012; 125: 3015–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sant S, Johnston PA. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov Today Technol 2017; 23: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nath S, Devi GR. Three-dimensional culture systems in cancer research: focus on tumor spheroid model. Pharmacol Ther 2016; 163: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang BW, Gao JQ. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J Control Release 2018; 270: 246–259. [DOI] [PubMed] [Google Scholar]
- 17. Kumar HR, Zhong X, Hoelz DJ, et al. Three-dimensional neuroblastoma cell culture: proteomic analysis between monolayer and multicellular tumor spheroids. Pediatr Surg Int 2008; 24(11): 1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oktem G, Sercan O, Guven U, et al. Cancer stem cell differentiation: TGFβ1 and versican may trigger molecules for the organization of tumor spheroids. Oncol Rep 2014; 32(2): 641–649. [DOI] [PubMed] [Google Scholar]
- 19. Bielecka ZF, Maliszewska-Olejniczak K, Safir IJ, et al. Three-dimensional cell culture model utilization in cancer stem cell research. Biol Rev 2017; 92: 1505–1520. [DOI] [PubMed] [Google Scholar]
- 20. Mehta S, Shelling A, Muthukaruppan A, et al. Predictive and prognostic molecular markers for cancer medicine. Ther Adv Med Oncol 2010; 2(2): 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaughan CA, Singh S, Grossman SR, et al. Gain-of-function p53 activates multiple signaling pathways to induce oncogenicity in lung cancer cells. Mol Oncol 2017; 11(6): 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015; 16: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Fiore R, Marcatti M, Drago-Ferrante R, et al. Mutant p53 gain of function can be at the root of dedifferentiation of human osteosarcoma MG63 cells into 3AB-OS cancer stem cells. Bone 2014; 60: 198–212. [DOI] [PubMed] [Google Scholar]
- 24. Kumar SM, Liu S, Lu H, et al. Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation. Oncogene 2012; 31: 4898–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suvà ML, Rheinbay E, Gillespie SM, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell 2014; 157: 580–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fessler E, Borovski T, Medema JP. Endothelial cells induce cancer stem cell features in differentiated glioblastoma cells via bFGF. Mol Cancer 2015; 14: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zanoni M, Piccinini F, Arienti C, et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep 2016; 6: 19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445: 111–115. [DOI] [PubMed] [Google Scholar]
- 29. Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005; 65: 5506–5511. [DOI] [PubMed] [Google Scholar]
- 30. Calvet CY, André FM, Mir LM. The culture of cancer cell lines as tumorspheres does not systematically result in cancer stem cell enrichment. PLoS ONE 2014; 9: e89644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stadler M, Walter S, Walzl A, et al. Increased complexity in carcinomas: analyzing and modeling the interaction of human cancer cells with their microenvironment. Semin Cancer Biol 2015; 35: 107–124. [DOI] [PubMed] [Google Scholar]
- 32. Vinci M, Gowan S, Boxall F, et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 2012; 10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duan J, Qiu W, Xu S, et al. Strategies for isolating and enriching cancer stem cells: well begun is half done. Stem Cells Dev 2013; 22: 2221–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tirino V, Desiderio V, Paino F, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J 2013; 27(1): 13–24. [DOI] [PubMed] [Google Scholar]
- 35. Gao W, Wu D, Wang Y, et al. Development of a novel and economical agar-based non-adherent three-dimensional culture method for enrichment of cancer stem-like cells. Stem Cell Res Ther 2018; 9: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Im CN, Yun HH, Yoo HJ, et al. Enhancement of SOX-2 expression and ROS accumulation by culture of A172 glioblastoma cells under non-adherent culture conditions. Oncol Rep 2015; 34(2): 920–928. [DOI] [PubMed] [Google Scholar]
- 37. Krishnamurthy S, Dong Z, Vodopyanov D, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res 2010; 70: 9969–9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ho WY, Yeap SK, Ho CL, et al. Development of multicellular tumor spheroid (MCTS) culture from breast cancer cell and a high throughput screening method using the MTT assay. PLoS ONE 2012; 7(9): e44640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mehta G, Hsiao AY, Ingram M, et al. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 2012; 164: 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jørgensen A, Young J, Nielsen JE, et al. Hanging drop cultures of human testis and testis cancer samples: a model used to investigate activin treatment effects in a preserved niche. Br J Cancer 2014; 110: 2604–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu LY, Di Carlo D, Lee LP. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdevices 2008; 10(2): 197–202. [DOI] [PubMed] [Google Scholar]
- 42. Raghavan S, Ward MR, Rowley KR, et al. Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays. Gynecol Oncol 2015; 138(1): 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodríguez CE, Berardi DE, Abrigo M, et al. Breast cancer stem cells are involved in Trastuzumab resistance through the HER2 modulation in 3D culture. J Cell Biochem 2018; 119(2): 1381–1391. [DOI] [PubMed] [Google Scholar]
- 44. Ham SL, Joshi R, Thakuri PS, et al. Liquid-based three-dimensional tumor models for cancer research and drug discovery. Exp Biol Med (Maywood) 2016; 241(9): 939–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lamichhane SP, Arya N, Kohler E, et al. Recapitulating epithelial tumor microenvironment in vitro using three dimensional tri-culture of human epithelial, endothelial, and mesenchymal cells. BMC Cancer 2016; 16: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kelm JM, Timmins NE, Brown CJ, et al. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 2003; 83: 173–180. [DOI] [PubMed] [Google Scholar]
- 47. Lee WG, Ortmann D, Hancock MJ, et al. A hollow sphere soft lithography approach for long-term hanging drop methods. Tissue Eng Part C Methods 2010; 16(2): 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He J, Xiong L, Li Q, et al. 3D modeling of cancer stem cell niche. Oncotarget 2018; 9: 1326–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 2013; 18(5–6): 240–249. [DOI] [PubMed] [Google Scholar]
- 50. Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv 2014; 32: 1256–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chang TT, Hughes-Fulford M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng Part A 2009; 15(3): 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Redden RA, Doolin EJ. Microgravity assay of neuroblastoma: in vitro aggregation kinetics and organoid morphology correlate with MYCN expression. In Vitro Cell Dev Biol Anim 2011; 47(4): 312–317. [DOI] [PubMed] [Google Scholar]
- 53. Kaur P, Ward B, Saha B, et al. Human breast cancer histoid: an in vitro 3-dimensional co-culture model that mimics breast cancer tissue. J Histochem Cytochem 2011; 59(12): 1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marrero B, Messina JL, Heller R. Generation of a tumor spheroid in a microgravity environment as a 3D model of melanoma. In Vitro Cell Dev Biol Anim 2009; 45(9): 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kievit FM, Florczyk SJ, Leung MC, et al. Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials 2010; 31(22): 5903–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds. Nat Methods 2007; 4(10): 855–860. [DOI] [PubMed] [Google Scholar]
- 57. Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 2009; 103: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Z, Tang M, Zhao J, et al. Looking into the future: toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Adv Mater 2018; 30(17): e1705388. [DOI] [PubMed] [Google Scholar]
- 59. Rahmati M, Pennisi CP, Mobasheri A, et al. Bioengineered scaffolds for stem cell applications in tissue engineering and regenerative medicine. Adv Exp Med Biol 2018; 1107: 73–89. [DOI] [PubMed] [Google Scholar]
- 60. Knight E, Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat 2015; 227(6): 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Locs J, Zalite V, Berzina-Cimdina L, et al. Ammonium hydrogen carbonate provided viscous slurry foaming—a novel technology for the preparation of porous ceramics. J Eur Ceram Soc 2013; 33: 3437–3443. [Google Scholar]
- 62. Sauceau M, Fages J, Common A, et al. New challenges in polymer foaming: a review of extrusion processes assisted by supercritical carbon dioxide. Prog Polym Sci 2011; 36: 749–766. [Google Scholar]
- 63. Khorasani MM, Ghaffarian SR, Goldansaz SH, et al. Solid-state microcellular foaming of PE/PE composite systems, investigation on cellular structure and crystalline morphology. Compos Sci Technol 2010; 70: 1942–1949. [Google Scholar]
- 64. Zhao J, Han W, Tu M, et al. Preparation and properties of biomimetic porous nanofibrous poly(l-lactide) scaffold with chitosan nanofiber network by a dual thermally induced phase separation technique. Mater Sci Eng C 2012; 32: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 65. Xiong Z. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr Mater 2002; 46: 771–776. [Google Scholar]
- 66. Li Z, Ramay HR, Hauch KD, et al. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005; 26(18): 3919–3928. [DOI] [PubMed] [Google Scholar]
- 67. Muzzarelli RAA. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr Polym 2011; 83: 1433–1445. [Google Scholar]
- 68. Lasprilla AJR, Martinez GAR, Lunelli BH, et al. Poly-lactic acid synthesis for application in biomedical devices — a review. Biotechnol Adv 2012; 30(1): 321–328. [DOI] [PubMed] [Google Scholar]
- 69. Saini P, Arora M, Kumar MNVR. Poly(lactic acid) blends in biomedical applications. Adv Drug Deliv Rev 2016; 107: 47–59. [DOI] [PubMed] [Google Scholar]
- 70. Polonio-Alcalá E, Rabionet M, Guerra A, et al. Screening of additive manufactured scaffolds designs for triple negative breast cancer 3D cell culture and stem-like expansion. Int J Mol Sci 2018; 19: 3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Florczyk SJ, Kievit FM, Wang K, et al. 3D porous chitosan-alginate scaffolds promote proliferation and enrichment of cancer stem-like cells. J Mater Chem B 2016; 4: 6326–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dicker KT, Gurski LA, Pradhan-Bhatt S, et al. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 2014; 10(4): 1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Martínez-Ramos C, Lebourg M. Three-dimensional constructs using hyaluronan cell carrier as a tool for the study of cancer stem cells. J Biomed Mater Res B Appl Biomater 2015; 103(6): 1249–1257. [DOI] [PubMed] [Google Scholar]
- 74. Lee IC, Wu YC, Hung WS. Hyaluronic acid-based multilayer films regulate hypoxic multicellular aggregation of pancreatic cancer cells with distinct cancer stem-cell-like properties. ACS Appl Mater Interfaces 2018; 10: 38769–38779. [DOI] [PubMed] [Google Scholar]
- 75. Rao W, Zhao S, Yu J, et al. Enhanced enrichment of prostate cancer stem-like cells with miniaturized 3D culture in liquid core-hydrogel shell microcapsules. Biomaterials 2014; 35(27): 7762–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee J-H, Kim H-W. Emerging properties of hydrogels in tissue engineering. J Tissue Eng 2018; 9: 29623184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ruedinger F, Lavrentieva A, Blume C, et al. Hydrogels for 3D mammalian cell culture: a starting guide for laboratory practice. Appl Microbiol Biotechnol 2015; 99(2): 623–636. [DOI] [PubMed] [Google Scholar]
- 78. Huang G, Wang L, Wang S, et al. Engineering three-dimensional cell mechanical microenvironment with hydrogels. Biofabrication 2012; 4(4): 042001. [DOI] [PubMed] [Google Scholar]
- 79. Li Q, Lin H, Wang O, et al. Scalable production of glioblastoma tumor-initiating cells in 3 dimension thermoreversible hydrogels. Sci Rep 2016; 6: 31915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pal M, Chen H, Lee BH, et al. Epithelial-mesenchymal transition of cancer cells using bioengineered hybrid scaffold composed of hydrogel/3D-fibrous framework. Sci Rep 2019; 9: 8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dai X, Ma C, Lan Q, et al. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 2016; 8: 045005. [DOI] [PubMed] [Google Scholar]
- 82. Yang X, Sarvestani SK, Moeinzadeh S, et al. Effect of CD44 binding peptide conjugated to an engineered inert matrix on maintenance of breast cancer stem cells and tumorsphere formation. PLoS ONE 2013; 8(3): e59147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jabbari E, Sarvestani SK, Daneshian L, et al. Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells. PLoS ONE 2015; 10: e0132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sreepadmanabh M, Toley BJ. Investigations into the cancer stem cell niche using in-vitro 3-D tumor models and microfluidics. Biotechnol Adv 2018; 36(4): 1094–1110. [DOI] [PubMed] [Google Scholar]
- 85. Albanese A, Lam AK, Sykes EA, et al. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat Commun 2013; 4: 2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 2015; 14(4): 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang W, Kai K, Choi DS, et al. Microfluidics separation reveals the stem-cell-like deformability of tumor-initiating cells. Proc Natl Acad Sci 2012; 109: 18707–18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhao Y, Yan X, Li B, et al. Three-dimensional co-culture microfluidic model and its application for research on cancer stem-like cells inducing migration of endothelial cells. Biotechnol Lett 2017; 39(9): 1425–1432. [DOI] [PubMed] [Google Scholar]
- 89. Zhao Y, Dong Q, Li J, et al. Targeting cancer stem cells and their niche: perspectives for future therapeutic targets and strategies. Semin Cancer Biol 2018; 53: 139–155. [DOI] [PubMed] [Google Scholar]
- 90. Koren E, Fuchs Y. The bad seed: cancer stem cells in tumor development and resistance. Drug Resist Updat 2016; 28: 1–12. [DOI] [PubMed] [Google Scholar]
- 91. Picco N, Gatenby RA, Anderson ARA. Stem cell plasticity and niche dynamics in cancer progression. IEEE Trans Biomed Eng 2017; 64(3): 528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schöning JP, Monteiro M, Gu W. Drug resistance and cancer stem cells: the shared but distinct roles of hypoxia-inducible factors HIF1α and HIF2α. Clin Exp Pharmacol Physiol 2017; 44(2): 153–161. [DOI] [PubMed] [Google Scholar]
- 93. Loizzi V, Del Vecchio V, Gargano G, et al. Biological pathways involved in tumor angiogenesis and bevacizumab based anti-angiogenic therapy with special references to ovarian cancer. Int J Mol Sci 2017; 18: 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Semenza GL. Dynamic regulation of stem cell specification and maintenance by hypoxia-inducible factors. Mol Aspects Med 2016; 47–48: 15–23. [DOI] [PubMed] [Google Scholar]
- 95. Ravi M, Ramesh A, Pattabhi A. Contributions of 3D cell cultures for cancer research. J Cell Physiol 2017; 232(10): 2679–2697. [DOI] [PubMed] [Google Scholar]
- 96. Casson J, Davies OG, Smith CA, et al. Mesenchymal stem cell-derived extracellular vesicles may promote breast cancer cell dormancy. J Tissue Eng 2018; 9: 30627418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lim W, Kim HS. Exosomes as therapeutic vehicles for cancer. Tissue Eng Regen Med 2019; 16: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Han SW, Kim YY, Kang WJ, et al. The use of normal stem cells and cancer stem cells for potential anti-cancer therapeutic strategy. Tissue Eng Regen Med 2018; 15: 365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wan ACA. Primitive cancer cell states: a target for drug screening. Trends Pharmacol Sci 2019; 40(3): 161–171. [DOI] [PubMed] [Google Scholar]
- 100. Kim H, Lin Q, Glazer PM, et al. The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells. Breast Cancer Res 2018; 20: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chow DC, Wenning LA, Miller WM, et al. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. modified Kroghian models. Biophys J 2001; 81: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roy IM, Biswas A, Verfaillie C, et al. Energy producing metabolic pathways in functional regulation of the hematopoietic stem cells. IUBMB Life 2018; 70(7): 612–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yan Y, Liu F, Han L, et al. HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J Exp Clin Cancer Res 2018; 37: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kahlert UD, Mooney SM, Natsumeda M, et al. Targeting cancer stem-like cells in glioblastoma and colorectal cancer through metabolic pathways. Int J Cancer 2017; 140: 10–22. [DOI] [PubMed] [Google Scholar]
- 105. Carnero A, Lleonart M. The hypoxic microenvironment: a determinant of cancer stem cell evolution. Bioessays 2016; 38(Suppl. 1): S65–74. [DOI] [PubMed] [Google Scholar]
- 106. Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med 2013; 17(1): 30–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bennewith KL, Durand RE. Quantifying transient hypoxia in human tumor xenografts by flow cytometry. Cancer Res 2004; 64: 6183–6189. [DOI] [PubMed] [Google Scholar]
- 108. Brurberg KG, Thuen M, Ruud Rofstad EK. Fluctuations in pO2 in irradiated human melanoma xenografts. Radiat Res 2006; 165(1): 16–25. [DOI] [PubMed] [Google Scholar]
- 109. Semenza GL. Regulation of the breast cancer stem cell phenotype by hypoxia-inducible factors. Clin Sci 2015; 129: 1037–1045. [DOI] [PubMed] [Google Scholar]
- 110. Zhang C, Samanta D, Lu H, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A 2016; 113: E2047–E2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Triner D, Shah YM. Hypoxia-inducible factors: a central link between inflammation and cancer. J Clin Invest 2016; 126: 3689–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang H, Lu H, Xiang L, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A 2015; 112: E6215–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cheloni G, Tanturli M, Tusa I, et al. Targeting chronic myeloid leukemia stem cells with the hypoxia-inducible factor inhibitor acriflavine. Blood 2017; 130: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chanmee T, Ontong P, Izumikawa T, et al. Hyaluronan production regulates metabolic and cancer stem-like properties of breast cancer cells via hexosamine biosynthetic pathway-coupled HIF-1 signaling. J Biol Chem 2016; 291: 24105–24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cao L, Fan X, Jing W, et al. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-κB-HIF-1α pathway. Oncotarget 2015; 6: 6627–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mahkamova K, Latar N, Aspinall S, et al. Hypoxia increases thyroid cancer stem cell-enriched side population. World J Surg 2018; 42(2): 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qin J, Liu Y, Lu Y, et al. Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression. Sci Rep 2017; 7: 10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Eguchi T, Sogawa C, Okusha Y, et al. Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS ONE 2018; 13: e0191109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stankevicius V, Kunigenas L, Stankunas E, et al. The expression of cancer stem cell markers in human colorectal carcinoma cells in a microenvironment dependent manner. Biochem Biophys Res Commun 2017; 484: 726–733. [DOI] [PubMed] [Google Scholar]
- 120. Klimkiewicz K, Weglarczyk K, Collet G, et al. A 3D model of tumour angiogenic microenvironment to monitor hypoxia effects on cell interactions and cancer stem cell selection. Cancer Lett 2017; 396: 10–20. [DOI] [PubMed] [Google Scholar]
- 121. DelNero P, Lane M, Verbridge SS, et al. 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials 2015; 55: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Whitman NA, Lin ZW, Kenney RM, et al. Hypoxia differentially regulates estrogen receptor alpha in 2D and 3D culture formats. Arch Biochem Biophys 2019; 671: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kimlin LC, Casagrande G, Virador VM. In vitro three-dimensional (3D) models in cancer research: an update. Mol Carcinog 2013; 52(3): 167–182. [DOI] [PubMed] [Google Scholar]
- 124. Folkman J, Merler E, Abernathy C, et al. Isolation of a tumor factor responsible for angiogenesis. J Exp Med 1971; 133: 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Markowska A, Sajdak S, Markowska J, et al. Angiogenesis and cancer stem cells: new perspectives on therapy of ovarian cancer. Eur J Med Chem 2017; 142: 87–94. [DOI] [PubMed] [Google Scholar]
- 126. Yadav AK, Desai NS. Cancer stem cells: acquisition, characteristics, therapeutic implications, targeting strategies and future prospects. Stem Cell Rev Rep 2019; 15(3): 331–355. [DOI] [PubMed] [Google Scholar]
- 127. Ping YF, Bian XW. Concise review: contribution of cancer stem cells to neovascularization. Stem Cells 2011; 29: 888–894. [DOI] [PubMed] [Google Scholar]
- 128. Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010; 468: 829–833. [DOI] [PubMed] [Google Scholar]
- 129. Bussolati B, Grange C, Sapino A, et al. Endothelial cell differentiation of human breast tumour stem/progenitor cells. J Cell Mol Med 2009; 13(2): 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shen R, Ye Y, Chen L, et al. Precancerous stem cells can serve as tumor vasculogenic progenitors. PLoS ONE 2008; 3: e1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Alvero AB, Fu HH, Holmberg J, et al. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells 2009; 27(10): 2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010; 468: 824–828. [DOI] [PubMed] [Google Scholar]
- 133. Bray LJ, Binner M, Holzheu A, et al. Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 2015; 53: 609–620. [DOI] [PubMed] [Google Scholar]
- 134. Chiew GGY, Wei N, Sultania S, et al. Bioengineered three-dimensional co-culture of cancer cells and endothelial cells: a model system for dual analysis of tumor growth and angiogenesis. Biotechnol Bioeng 2017; 114(8): 1865–1877. [DOI] [PubMed] [Google Scholar]
- 135. Miller CP, Tsuchida C, Zheng Y, et al. A 3D human renal cell carcinoma-on-a-chip for the study of tumor angiogenesis. Neoplasia 2018; 20(6): 610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Agarwal P, Wang H, Sun M, et al. Microfluidics enabled bottom-up engineering of 3D vascularized tumor for drug discovery. ACS Nano 2017; 11: 6691–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Taube JH, Herschkowitz JI, Komurov K, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A 2010; 107: 15449–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–939. [DOI] [PubMed] [Google Scholar]
- 139. Frixen UH, Behrens J, Sachs M, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991; 113(1): 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sabbah M, Emami S, Redeuilh G, et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat 2008; 11(4–5): 123–151. [DOI] [PubMed] [Google Scholar]
- 141. Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15: 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Marotta LLC, Polyak K. Cancer stem cells: a model in the making. Curr Opin Genet Dev 2009; 19(1): 44–50. [DOI] [PubMed] [Google Scholar]
- 144. Oskarsson T, Batlle E, Massagué J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 2014; 14: 306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhou P, Li B, Liu F, et al. The epithelial to mesenchymal transition (EMT) and cancer stem cells: implication for treatment resistance in pancreatic cancer. Mol Cancer 2017; 16: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cai Z, Cao Y, Luo Y, et al. Signalling mechanism(s) of epithelial–mesenchymal transition and cancer stem cells in tumour therapeutic resistance. Clin Chim Acta 2018; 483: 156–163. [DOI] [PubMed] [Google Scholar]
- 147. Wang F, Ma L, Zhang Z, et al. Hedgehog signaling regulates epithelial-mesenchymal transition in pancreatic cancer stem-like cells. J Cancer 2016; 7: 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu LJ, Zhang J, Xiao ZF, et al. Three-dimensional collagen scaffold enhances the human adenoid cystic carcinoma cancer stem cell and epithelial-mesenchymal transition properties. J Biomed Mater Res B Appl Biomater 2014; 102(4): 772–780. [DOI] [PubMed] [Google Scholar]
- 149. Puls TJ, Tan X, Whittington CF, et al. 3D collagen fibrillar microstructure guides pancreatic cancer cell phenotype and serves as a critical design parameter for phenotypic models of EMT. PLoS ONE 2017; 12: e0188870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Huang YJ, Hsu SH. Acquisition of epithelial-mesenchymal transition and cancer stem-like phenotypes within chitosan-hyaluronan membrane-derived 3D tumor spheroids. Biomaterials 2014; 35(38): 10070–10079. [DOI] [PubMed] [Google Scholar]
- 151. Cao Y, Li Z, Mao L, et al. The use of proteomic technologies to study molecular mechanisms of multidrug resistance in cancer. Eur J Med Chem 2019; 162: 423–434. [DOI] [PubMed] [Google Scholar]
- 152. Moitra K. Overcoming multidrug resistance in cancer stem cells. Biomed Res Int 2015; 2015: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mohammad IS, He W, Yin L. Understanding of human ATP binding cassette superfamily and novel multidrug resistance modulators to overcome MDR. Biomed Pharmacother 2018; 100: 335–348. [DOI] [PubMed] [Google Scholar]
- 154. Garcia-Mayea Y, Mir C, Masson F, et al. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin Cancer Biol 2020; 60: 166–180. [DOI] [PubMed] [Google Scholar]
- 155. Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 2008; 3(9–10): 1172–1184. [DOI] [PubMed] [Google Scholar]
- 156. Fennema E, Rivron N, Rouwkema J, et al. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 2013; 31(2): 108–115. [DOI] [PubMed] [Google Scholar]
- 157. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer 2002; 2(1): 48–58. [DOI] [PubMed] [Google Scholar]
- 158. Chu G. Cellular responses to cisplatin: the roles of DNA-binding proteins and DNA repair. J Biol Chem 1994; 269: 787–790. [PubMed] [Google Scholar]
- 159. Ding J, Miao ZH, Meng LH, et al. Emerging cancer therapeutic opportunities target DNA-repair systems. Trends Pharmacol Sci 2006; 27(6): 338–344. [DOI] [PubMed] [Google Scholar]
- 160. Graham SM, Jørgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002; 99: 319–325. [DOI] [PubMed] [Google Scholar]
- 161. Kirtane AR, Kalscheuer SM, Panyam J. Exploiting nanotechnology to overcome tumor drug resistance: challenges and opportunities. Adv Drug Deliv Rev 2013; 65(13–14): 1731–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Markman JL, Rekechenetskiy A, Holler E, et al. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev 2013; 65(13–14): 1866–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Núñez C, Capelo JL, Igrejas G, et al. An overview of the effective combination therapies for the treatment of breast cancer. Biomaterials 2016; 97: 34–50. [DOI] [PubMed] [Google Scholar]
- 164. Rijal G, Li W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci Adv 2017; 3: e1700764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Patel NR, Pattni BS, Abouzeid AH, et al. Nanopreparations to overcome multidrug resistance in cancer. Adv Drug Deliv Rev 2013; 65(13–14): 1748–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]