Abstract
Background:
The purpose of this study was to examine the prevalence and effects of current smoking on adverse outcomes among hospitalized COVID-19 patients.
Methods:
A systematic review of the literature (PubMed) identified 18 (from a total of 1398) relevant studies. Pooled current smoking prevalence was compared with the gender-adjusted and gender and age-adjusted, population-based expected prevalence by calculating prevalence odds ratio (POR). The association between current, compared with non-current and former, smoking and adverse outcome was examined. A secondary analysis was performed by including 12 pre-publications (30 studies in total). All analyses were performed using random-effects meta-analysis.
Results:
Among 6515 patients, the pooled prevalence of current smoking was 6.8% [95% confidence interval (CI): 4.8–9.1%]. The gender-adjusted POR was 0.20 (95% CI: 0.16–0.25, p < 0.001), and the gender and age-adjusted POR was 0.24 (95% CI: 0.19–0.30, p < 0.001). Current smokers were more likely to have an adverse outcome compared with non-current smokers [odds ratio (OR): 1.53, 95%CI: 1.06–2.20, p = 0.022] but less likely compared with former smokers (OR: 0.42, 95% CI: 0.27–0.74, p = 0.003). When pre-publications were added (n = 10,631), the gender-adjusted POR was 0.27 (95% CI: 0.19–0.38, p < 0.001) and the gender and age-adjusted POR was 0.34 (95% CI: 0.24–0.48, p < 0.001).
Conclusion:
This meta-analysis of retrospective observational case series found an unexpectedly low prevalence of current smoking among hospitalized patients with COVID-19. Hospitalized current smokers had higher odds compared with non-current smokers but lower odds compared with former smokers for an adverse outcome. Smoking cannot be considered a protective measure for COVID-19. However, the hypothesis that nicotine may have a protective effect in COVID-19 that is partially masked by smoking-related toxicity and by the abrupt cessation of nicotine intake when smokers are hospitalized should be explored in laboratory studies and clinical trials using pharmaceutical nicotine products.
Keywords: adverse outcome, COVID-19, hospitalization, inflammation, nicotine, SARS-CoV-2, smoking
Introduction
The coronavirus pandemic has generated an understandable concern about the effects of smoking on disease susceptibility and severity. Smoking is an established risk factor respiratory infections.1 Media reports and opinion pieces have made reasonable suggestions that smoking may be detrimental in case of infection with SARS-CoV-2, assuming that the gender differences in COVID-19 vulnerability and mortality may be due to the increased prevalence of smoking among Chinese men compared with women.2,3 However, until recently, clinical data were lacking. A study of 78 COVID-19 patients found that history of smoking increased the risk for disease progression by more than 14-fold.4 However, the association was weak and with a wide 95% confidence interval (CI) since only five smoking patients were included, of whom three progressed to severe disease. A meta-analysis of five case series from China found that current smokers had a statistically insignificant increase in the odds of progressing to severe COVID-19.5 However, another systematic review reported that smokers were more likely to be admitted to an intensive care unit (ICU), need mechanical ventilation, or die compared with non-smokers.6
Smoking is an established risk factor for a variety of diseases, including cardiovascular disease and chronic obstructive pulmonary disease (COPD), which appear to be risk factors for severe COVID-19 and adverse outcome.7 Thus, it would be expected for smokers to be over-represented among COVID-19 patients. Still, in a recent meta-analysis of 13 studies of hospitalized COVID-19 patients in China, an unexpectedly low prevalence of smoking, approximately one-fourth the population smoking prevalence, was found.8 To further explore this issue, we performed a systematic review of case series presenting data on the smoking status of hospitalized COVID-19 patients in order to:
Calculate the pooled prevalence of smoking among hospitalized COVID-19 patients and compare it with the expected prevalence based on population smoking rates.
Examine the association between current, compared with non-current and former, smoking and adverse outcome in COVID-19.
Methods
Studies included
A systematic search of the literature (PubMed) was performed for studies published until 25 April 2020 using the terms “(SARS-CoV-2 OR COVID-19 OR 2019-nCoV) AND (Clinical OR Mortality OR Outcome)” in the title or the abstract. Studies were included in the analysis if they satisfied all of the following criteria:
Present hospitalized patients with COVID-19
Classify patients according to disease severity, irrespective of the severity definition
Present data on the smoking status, separately for each severity classification.
Out of a total of 1398 studies, 19 studies fulfilling the previously mentioned criteria were found.4,9–26 One study was excluded because of unreliable data since the sum of current and former smokers did not correspond to the numbers presented in different severity subgroups.26 Thus, 18 studies were analyzed. All of the studies were retrospective observational case series. The PRISMA flow diagram is presented in the Supplemental Figure S1. Most of the studies were from China (n = 15), while two studies presented patients from the United States (US) and one from South Korea. Four of the studies recorded separately current and former smokers and were used to examine the association between current, rather than former, smoking and adverse outcome.9,13,20,21 The rest of the studies reported the smoking status as “smoking” or “history of smoking” and may have included former smokers. Three studies had missing smoking data on 14 of 1099, 44 of 645, and 22 of 476 patients.9,16,23 The number of patients with available smoking data (1085, 601 and 454, respectively) were used in the calculations. Another study reported unknown smoking history for 13 out of 487 patients (all with mild disease)17; they were also excluded from the analysis.
In addition, we sought to identify pre-publications (not peer-reviewed) on the pre-print server Medrxiv. The terms “smoking” and “COVID-19” were sought in the title or abstract. A total of 123 pre-publications were found, with 12 fulfilling the previously mentioned criteria.27–38 All of the studies were retrospective observational case series. Of these, nine were from China, two from the US, and one from Japan. Two of the studies recorded current and former smokers separately.27,31 The rest of the studies reported smoking status as “smoking” or “history of smoking” and may have included former smokers. One study from the US reported never smokers or unknown history of smoking as one group, without clarifying how many patients had missing data.27 Another study reported the smoking status and outcomes for 170 of 200 patients.29 In total, 30 studies (published and pre-publications) were used in the secondary analysis, whereas 6 studies were used to examine the association between current versus former smoking and adverse outcome.
Analysis
A cross-sectional analysis was performed. In two studies, both outpatients and hospitalized patients were presented.20,27 In one of them, 7162 patients were presented in total, of whom 5143 were ambulatory, 1037 were hospitalized in non-ICU units, 457 were hospitalized in ICU units, and for 525 patients the hospitalization status was unknown.20 Only hospitalized patients (n = 1494) were included to the analysis. In the other study, 2104 patients were ambulatory and 1999 were hospitalized.27 Of those hospitalized, 1582 were included in the analysis since 932 were classified as “discharged with no critical illness” and 650 as having “critical illness.” In studies where former smokers were not separately presented, all patients with a positive smoking history were classified as current smokers. When presented separately, former smokers were included in the non-current smoking group.
The pooled prevalence of current smoking was calculated using random-effects meta-analysis. Smoking prevalence in each study was compared with the expected prevalence based on gender-specific population smoking rates. The expected prevalence of smoking in each study was calculated using the gender distribution of patients in each study and the gender-specific population smoking prevalence. The following formula was used:
where SPE = expected smoking prevalence; PM = male prevalence among patients; SPP–M = population smoking prevalence in males; PF = female prevalence among patients; and SPP–F = population smoking prevalence in females.
The observed smoking prevalence was compared with the expected smoking prevalence by calculating prevalence odds ratio (POR).39 The population smoking rates used to calculate the gender-adjusted expected number of smokers in each study were (males and females, respectively): 50.5% and 2.1% for China,40 15.6% and 12.0% in the US,41 35.8% and 6.5% in South Korea,42 and 29.3% and 7.2% in Japan.43
The studies analyzed did not include data on the patients’ age distribution. Therefore, age-adjustment in the expected smoking prevalence calculation was performed by assuming that all patients were ⩾65 years old. This represents a worst-case scenario considering that people aged ⩾65 years have the lowest smoking rates compared with other adult age groups while the mean or median age of hospitalized COVID-19 patients was by far lower than 65 years in the studies examined (Table 1). Thus, this approach underestimates the expected smoking prevalence. The population smoking rates used to calculate the age and gender-adjusted expected number of smokers in each study were (males and females, respectively): 44.0% and 4.1% for China,40 10.1% and 7.7% in the US,44 17.9% and 1.9% in South Korea,45 and 21.2% and 5.4% in Japan.46,47 The formula mentioned previously was applied to calculate the gender and age-adjusted expected smoking prevalence. We report both gender-adjusted and gender and age-adjusted POR. The association between current (versus non-current) smoking and adverse outcome was performed by calculating the odds ratio (OR). In addition, the association between current smoking (versus former smoking) and adverse outcome was calculated using the studies that presented data for former smokers separately; never smokers were excluded from the latter analysis. All analyses were performed with random-effects meta-analyses using MetaXL v5.3, with heterogeneity evaluated through I2 and publication bias examined by visual assessment of funnel plots.
Table 1.
Characteristics of the studies included in the analysis.
| Hospitalized cases n |
Age mean (SD) median (IQR) |
Males % |
Females % |
Hospitalized smokers n |
Hospitalized smokers Pooled prevalence % |
Expected smokers gender adjusted % |
Expected smokers gender and age-adjusted % |
Country | |
|---|---|---|---|---|---|---|---|---|---|
| Guan et al.9 | 1085 | 47 (35–58) | 58.1% | 41.9% | 137 | 12.6 (10.6–14.6) | 30.2% | 27.3% | China |
| Chen et al.10 | 274 | 62 (44–70) | 62.4% | 37.6% | 12 | 5.4 (2.4–8.3) | 32.3% | 29.0% | China |
| Zhou et al.11 | 191 | 56 (46–67) | 62.3% | 37.7% | 11 | 5.8 (2.5–9.1) | 32.3% | 29.0% | China |
| Mo et al.12 | 155 | 54 (42–66) | 55.5% | 44.5% | 6 | 3.9 (0.9–6.9) | 29.0% | 26.2% | China |
| Zhang et al.13 | 140 | 57 (25–87) | 50.7% | 49.3% | 2 | 1.4 (0.0–3.3) | 26.6% | 24.3% | China |
| Wan et al.14 | 135 | 47 (36–55) | 53.3% | 46.7% | 9 | 6.7 (2.5–10.9) | 27.9% | 25.4% | China |
| Liu et al.4 | 78 | 38 (33–57) | 50.0% | 50.0% | 5 | 6.4 (0.1–11.8) | 26.3% | 24.1% | China |
| Huang et al.15 | 41 | 49 (41–58) | 73.2% | 26.8% | 3 | 7.3 (0.0–15.3) | 37.5% | 33.3% | China |
| Zhang et al.16 | 601 | 35 (14.2) 47 (14) |
50.9% | 49.1% | 41 | 6.8 (4.9–9.1) | 26.7% | 24.4% | China |
| Shi et al.17 | 474 | 46 (19) | 53.2% | 46.8% | 40 | 8.4 (6.1–11.3) | 27.8% | 25.3% | China |
| Yang et al.18 | 52 | 52 (13) | 67.3% | 32.7% | 2 | 3.8 (0.5–13.2) | 34.7% | 31.0% | China |
| Kim et al.19 | 27 | 43 (13) | 53.6% | 46.4% | 5 | 18.5 (6.3–38.1) | 22.2% | 10.5% | South Korea |
| CDC20 | 1494 | 27 | 1.8 (1.2–2.6) | 13.7% | 8.8% | US | |||
| Li et al.21 | 544 | 60 (48–69) | 51.3% | 48.7% | 41 | 7.5 (5.4–10.1) | 26.9% | 24.6% | China |
| Wang et al.22 | 125 | 39 (14) | 56.8% | 43.2% | 16 | 12.8 (7.5–20.0) | 29.6% | 26.8% | China |
| Feng et al.23 | 454 | 53 (60–64) | 56.9% | 43.1% | 44 | 9.7 (7.1–12.8) | 29.7% | 26.8% | China |
| Ji et al.24 | 208 | 44 (16) | 56.3% | 43.8% | 19 | 9.2 (5.6–13.9) | 29.3% | 26.5% | China |
| Goyal et al.25 | 393 | 62 (49–74) | 60.6% | 39.4% | 20 | 5.1 (3.1–7.8) | 14.2% | 9.2% | US |
| Pre-publications | |||||||||
| Petrilli et al.27 | 1582 | 58 (46–71) 67 (56–77) |
63.3% | 36.7% | 90 | 5.7 (4.6–6.7) | 14.3% | 9.2% | US |
| Tabata et al.28 | 71 | 68 (47–75) | 54.9% | 45.1% | 13 | 18.3 (10.1–29.3) | 19.3% | 14.1% | japan |
| Fu et al.29 | 170 | 49.5% | 50.5% | 89 | 52.3 (44.6–60.1) | 18.1% | 13.2% | China | |
| Chen et al.30 | 97 | 48 (15–80) | 43.3% | 56.7% | 6 | 6.2 (2.3–13.0) | 23.1% | 21.4% | China |
| Rentsch et al.31 | 554 | 66 (60–71) | 95.4% | 4.6% | 159 | 28.7 (25.0–32.7) | 15.4% | 10.0% | US |
| Hu et al.32 | 323 | 61 (23–91) | 51.4% | 48.6% | 38 | 11.8 (8.5–15.8) | 27.0% | 24.6% | China |
| Luo et at.33 | 403 | 56 (39–68) | 47.9% | 52.1% | 29 | 7.2 (4.9–10.2) | 25.3% | 23.2% | China |
| Ma et al.34 | 84 | 48 (42–63) | 57.1% | 42.9% | 7 | 8.3 (3.4–16.4) | 29.8% | 26.9% | China |
| Luo et al.35 | 298 | 57 (40–69) | 50.3% | 49.7% | 21 | 7.0 (4.4–10.6) | 26.5% | 24.2% | China |
| Xu et al.36 | 69 | 57 (43–69) | 50.7% | 49.3% | 5 | 7.2 (2.4–16.1) | 26.7% | 24.3% | China |
| Cao et al.37 | 198 | 50 (16) | 51.0% | 49.0% | 11 | 5.6 (2.8–9.7) | 26.8% | 24.5% | China |
| Qi et al.38 | 267 | 48 (35–65) | 55.8% | 44.2% | 53 | 19.9 (15.2–25.2) | 29.1% | 26.4% | China |
| Total (published) | 6515 | 440 | 6.8 (4.8–9.1) | ||||||
| Total (all) | 11,104 | 961 | 9.0 (6.5–11.8) | ||||||
Blank cells represent non-available data or multiple age groups.
IQR, interquartile range; SD, standard deviation; US, United States.
Results
Study characteristics
The characteristics of the studies included in this analysis are presented in Table 1. Published studies included 6515 hospitalized patients, of whom 440 were current smokers. Together with the pre-publications, 10,631 patients, with 961 of them being smokers, were examined. Adverse outcomes ranged from non-specific “severe” to specific definitions based on clinical criteria or death. No publication bias was obvious from the funnel plot. Outcome definitions for each study are presented in Supplemental Table S1.
Primary analysis (published studies)
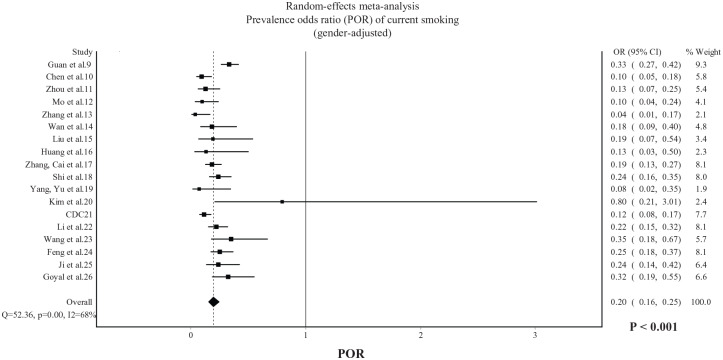
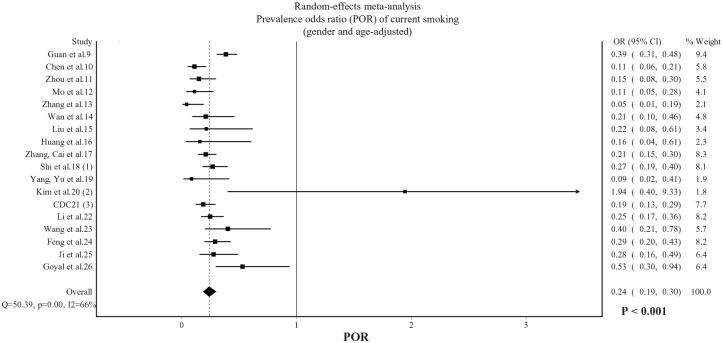
In published studies, the random-effects pooled prevalence of current smoking was 6.8% (95% CI 4.8–9.1%). The gender-adjusted POR, displayed in Figure 1, was 0.20 (95% CI: 0.16–0.25, p < 0.001) with substantial heterogeneity being observed (I2 = 68%). The gender and age-adjusted POR, displayed in Figure 2, was 0.24 (95% CI: 0.19–0.30, p < 0.001) with substantial heterogeneity being observed (I2 = 66%). In Chinese studies only, the gender-adjusted POR was 0.20 (95% CI: 0.16–0.26, p < 0.001) and the gender and age-adjusted POR was 0.22 (95% CI: 0.18–0.28, p < 0.001).
Figure 1.
POR of current smoking among hospitalized patients with COVID-19 (gender-adjusted). Data from 18 published studies.
POR, prevalence odds ratio.
Figure 2.
POR of current smoking among hospitalized patients with COVID-19 (gender and age-adjusted). Data from 18 published studies.
POR, prevalence odds ratio.
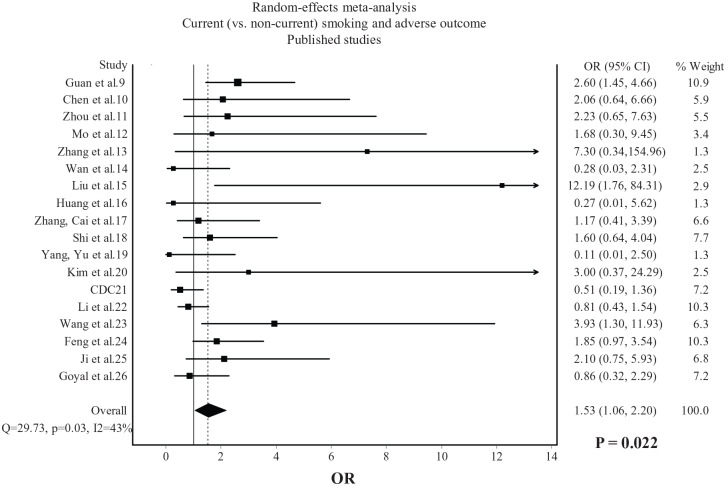
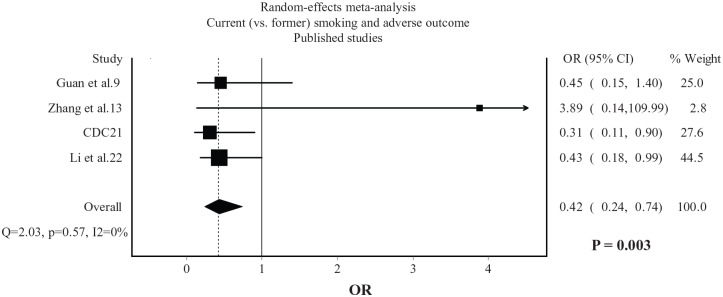
Current smokers were more likely than non-current smokers to experience an adverse outcome (OR: 1.53, 95% CI: 1.06–2.20, p = 0.022), with moderate heterogeneity being observed (I2 = 43%, Figure 3). Compared with former smokers, current smokers were less likely to experience an adverse outcome (OR: 0.42, 95% CI: 0.24–0.74, p = 0.003), with no heterogeneity being observed (I2 = 0%, Figure 4).
Figure 3.
Association between current (versus non-current) smoking and adverse outcome in COVID-19. Data from 18 published studies.
Figure 4.
Association between current (versus former) smoking and adverse outcome in COVID-19. Data from 4 published studies.
Secondary analysis (published studies and pre-publications)
From the secondary analysis, which included both published studies and pre-publications, the random-effects pooled prevalence of current smoking was 9.0% (95% CI: 6.5–11.8%). The gender-adjusted POR, displayed in Supplemental Figure S2, was 0.27 (95% CI: 0.19–0.38, p < 0.001), with considerable heterogeneity being observed (I2 = 93%). The gender and age-adjusted POR, displayed in Supplemental Figure S3, was 0.34 (95% CI: 0.24–0.48, p < 0.001), with considerable heterogeneity being observed (I2 = 93%). In Chinese studies only, the gender-adjusted POR was 0.24 (95% CI: 0.17–0.33, p < 0.001) and the gender and age-adjusted POR was 0.27 (95% CI: 0.20–0.38, p < 0.001).
Current smokers were more likely than non-current smokers to experience an adverse outcome (OR: 1.59, 95% CI: 1.15–2.21, p = 0.006) with moderate heterogeneity being observed (I2 = 67%, Supplemental Figure S4). Compared with former smokers, current smokers were less likely to experience an adverse outcome (OR: 0.61, 95% CI: 0.36–1.02, p = 0.056) with moderate heterogeneity being observed (I2 = 54%, Supplemental Figure S5).
Discussion
The main finding of this systematic review was the unusually low prevalence of current smoking among hospitalized COVID-19 patients. Smoking prevalence was less than one-fourth the expected prevalence based on gender-adjusted population smoking rates. Even when age-adjustment was performed by calculating expected prevalence based on the age group with the lowest population smoking rates (age ⩾ 65 years), smoking prevalence in hospitalized COVID-19 patients was still approximately one-third the expected prevalence (66% lower). Current smokers had higher odds for adverse outcome compared with non-current smokers. In contrast, current smokers were less likely to have an adverse outcome compared with former smokers.
An important limitation is that the analysis was unadjusted for confounding factors such as comorbidities that appear to be associated with higher risk for an adverse outcome in COVID-19.48 Sociodemographic factors may also be associated with reduced access of smokers to hospital care. Older age is usually associated with lower current smoking and higher former smoking prevalence, which could explain the lower odds for adverse outcome in current compared with former smokers. However, China has a high smoking prevalence even in older age groups. Liu et al. reported a prevalence of >50% in smoking males aged 60–69 years and >40% in those >70 years.40,49 Moreover, we performed age-adjustment by calculating the expected prevalence based on the adult age group with the lowest population smoking prevalence. Still, the difference between observed and expected smoking prevalence was substantial. There were no available data to further adjust expected prevalence based on geographically-specific population smoking rates. The possibility for inaccurate recording, false-reporting, or inability to report the smoking status due to critical condition of patients admitted to the hospitals, as well as the lack of an objective assessment of the smoking status, should also be considered. Still, the findings that smokers are under-represented by approximately 3- to >4-fold compared with population smoking rates could be explained only by unusually extensive under-reporting of the current smoking status. Finally, many studies reported smoking history instead of current smoking, which might include former smokers and thus overestimate current smoking prevalence among hospitalized COVID-19 patients.
Low prevalence of current smokers among hospitalized COVID-19 patients has been observed in case series outside China too.20,25,27,50 While it is highly unlikely for the majority of cigarette smoke compounds to have any potential benefit considering their toxic characteristics and oxidative stress and inflammation-promoting properties, some researchers have hypothesized that these findings might imply a protective effect of nicotine.50–53 It was recently reported that many of the clinical manifestations of COVID-19 could be explained by a dysfunction of the nicotinic cholinergic system, and it was hypothesized that nicotine could modulate the immune response by restoring the function of the cholinergic anti-inflammatory pathway.52 Severe COVID-19 appears to represent a hyper-inflammatory response that could result from dysregulation of the immune system and a failure to return to homeostasis after being activated to combat viral invasion. SARS-CoV-2 activates the innate immune system, which, if left unregulated, can increase vascular permeability, cause migration of fluid and blood cells into the alveoli, and result in respiratory failure.54 This is commonly called “cytokine storm”, and is characterized by the release of several pro-inflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6. Elevated levels of these cytokines have been associated with poor prognosis in COVID-19 patients.11,55 The importance of this process in severe COVID-19 has been recognized by the scientific community, and clinical trials of immunosuppressants and cytokine inhibitors are already underway based on biological plausibility and despite warnings and precautions against the use of such medication in the presence of active infection.56–60 The cholinergic anti-inflammatory pathway, mediated mainly through the vagus nerve and alpha 7 nicotinic acetylcholine receptors, has well-established immunomodulatory effects that are present in a variety of cells such as macrophages and bronchial epithelial and endothelial cells.61–64 It represents a reflex mechanism based on a bi-directional communication between the immune and nervous systems. Activation of the cholinergic anti-inflammatory pathway through electrical stimulation of the vagus nerve has been shown to reduce levels of pro-inflammatory cytokines and control the acute hyper-inflammatory response in animal models.65,66 Therefore, dysregulation of the cholinergic nervous system could explain the clinical manifestations of severe COVID-19 and the inability to suppress and control the inflammatory response. Notably, risk factors for severe COVID-19 such as diabetes, heart disease, ageing and obesity are characterized by autonomic nervous system imbalance.67–70 These patients are expected to be more sensitive to further compromise of the cholinergic nervous system due to SARS-CoV-2 infection.
Nicotine is an agonist of the nicotinic cholinergic system and has been shown to prevent acute respiratory distress syndrome, the hallmark of severe COVID-19, in animal models.71 Therefore, we present the hypothesis that nicotine intake could be the reason for the low prevalence of smoking among hospitalized COVID-19 patients. This would seemingly be in disagreement with the higher odds of adverse outcomes among hospitalized COVID-19 smokers. However, it should be emphasized that smokers experience abrupt cessation of nicotine intake after hospital admission and are unlikely to receive pharmaceutical nicotine replacement therapies while hospitalized. As a result, the effects of nicotine will rapidly wean off within hours after admission considering that the plasma half-life of nicotine is 1–2 h. Therefore, the higher odds for adverse outcome in current, compared with non-current, smokers are not contradictory to the hypothesis that nicotine may be beneficial for COVID-19. In addition, any hypothesized benefits of nicotine are expected to be masked by the well-established adverse effects of smoking and smoking-related comorbidities, which could also explain the higher odds for adverse outcome in hospitalized smokers. Thus, it is possible that COVID-19 severity and outcome may differ between smokers without and with smoking-related disease. Therefore, smoking cannot be recommended or used as a protective or therapeutic measure. However, pharmaceutical nicotine products have been available for years and have been used therapeutically even in non-smokers for longer periods of time compared with the few days that would be needed for a clinical trial of nicotine in COVID-19 patients.72,73 The safety profile and lack of dependence potential that has been observed in these studies, with pharmaceutical nicotine being administered to elderly non-smokers at high doses and for several weeks, offers a rationale for future clinical trials of nicotine in COVID-19 patients. In addition, such a clinical trial would not need to administer nicotine as a substitute for other standardized therapeutic measures.
Another concern in relation to nicotine and COVID-19 refers to its effects on angiotensin converting enzyme 2 (ACE2) expression, since this enzyme is used as a receptor by SARS-CoV-2 for cell entry.74 Recent data support that nicotine upregulates ACE2, which is suggested as a potential mechanism to increase susceptibility and severity of COVID-19.75,76 However, this is in contrast with previous data that nicotine and smoking down-regulate ACE2.77 Moreover, up-regulation of ACE2 does not necessarily imply more fulminant disease. Concerns that up-regulation of ACE2 by ACE-inhibitors and angiotensin receptor blockers could be linked to adverse prognosis in hypertensive COVID-19 patients were raised early in the COVID-19 pandemic.78 However, recent studies found either no adverse effect or a protective effect associated with the use of these medications.79,80 In fact, down-regulation of ACE2 immediately after viral cell entry is proposed as an important mechanism favoring the progression and severity of disease.81 ACE2 deficiency has been observed with ageing, in diabetes mellitus, and in heart disease, all of which appear to be risk factors for severe COVID-19.82–84 In addition, children and young women, who usually experience mild COVID-19, were found to have higher levels of ACE2 than older people.85 Thus, it is possible that ACE2 upregulation is protective against severe COVID-19,78,86 and the contradictory data about the effects of nicotine on ACE2 expression need to be clarified in future experimental studies.
In conclusion, this systematic review and meta-analysis of retrospective, observational case series identified a low prevalence of current smoking among hospitalized COVID-19 patients but higher odds of adverse outcome for current compared with non-current smokers. Smoking cannot be considered a protective measure against COVID-19 (or any other condition) due to associated risk for cardiovascular, respiratory and cancer morbidity and mortality. Thus, smokers should still be encouraged to quit. While limitations are applicable to this analysis and other unknown reasons or confounding factors could, at least partially, explain these findings, the hypothesis that nicotine may be protective against severe COVID-19 is biologically plausible and should be explored through laboratory and, eventually, clinical trials using pharmaceutical nicotine products.
Supplemental Material
Supplemental material, PRISMA_checklist-filled for Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis by Konstantinos Farsalinos, Anastasia Barbouni, Konstantinos Poulas, Riccardo Polosa, Pasquale Caponnetto and Raymond Niaura in Therapeutic Advances in Chronic Disease
Supplemental material, Supplementary_file for Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis by Konstantinos Farsalinos, Anastasia Barbouni, Konstantinos Poulas, Riccardo Polosa, Pasquale Caponnetto and Raymond Niaura in Therapeutic Advances in Chronic Disease
Supplemental material, Supplementary_file_TAJ for Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis by Konstantinos Farsalinos, Anastasia Barbouni, Konstantinos Poulas, Riccardo Polosa, Pasquale Caponnetto and Raymond Niaura in Therapeutic Advances in Chronic Disease
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Konstantinos Farsalinos  https://orcid.org/0000-0001-6839-4710
https://orcid.org/0000-0001-6839-4710
Riccardo Polosa  https://orcid.org/0000-0002-8450-5721
https://orcid.org/0000-0002-8450-5721
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Konstantinos Farsalinos, Department of Pharmacy, Laboratory of Molecular Biology and Immunology, University of Patras, Panepistimiopolis, 26500, Greece; Department of Public and Community Health, School of Public Health, University of West Attica, Egaleo, Attica, Greece.
Anastasia Barbouni, Department of Public and Community Health, School of Public Health, University of West Attica, Egaleo, Attica, Greece.
Konstantinos Poulas, Department of Pharmacy, Laboratory of Mol. Biology and Immunology, University of Patras, Panepistimiopolis, Greece.
Riccardo Polosa, Center of Excellence for the Acceleration of Harm Reduction, University of Catania, Catania, Italy; Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy.
Pasquale Caponnetto, Center of Excellence for the Acceleration of Harm Reduction, University of Catania, Catania, Italy.
Raymond Niaura, Departments of Social and Behavioral Science and Epidemiology, College of Global Public Health, New York University, New York, USA.
References
- 1. Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect 2013; 67: 169–184. [DOI] [PubMed] [Google Scholar]
- 2. Berlin I, Thomas D, Le Faou AL, et al. COVID-19 and smoking. Nicotine Tob Res. Epub ahead of print 3 April 2020. DOI: 10.1093/ntr/ntaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wenham C, Smith J, Morgan R; Gender and COVID-19 Working Group. COVID-19: the gendered impacts of the outbreak. Lancet 2020; 395: 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020; 133: 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med 2020; 75: 107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vardavas CI, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis 2020; 18: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emami A, Javanmardi F, Pirbonyeh N, et al. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med 2020; 8: e35. [PMC free article] [PubMed] [Google Scholar]
- 8. Farsalinos K, Barbouni A, Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med. Epub ahead of print 9 May 2020. DOI: 10.1007/s11739-020-02355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. Epub ahead of print 16 March 2020. DOI: 10.1093/cid/ciaa270. [DOI] [Google Scholar]
- 13. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. Epub ahead of print 19 February 2020. DOI: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 14. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. Epub ahead of print 21 March 2020. DOI: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Cai H, Hu J, et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis 2020; 94: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi Y, Yu X, Zhao H, et al. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care 2020; 24: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim ES, Chin BS, Kang CK, et al. ; Korea National Committee for Clinical Management of COVID-19. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci 2020; 35: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. Epub ahead of print 12 April 2020. DOI: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis 2020; 95: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multi-center study of clinical features. Am J Respir Crit Care Med 2020; 201: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji D, Zhang D, Xu J, et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. Epub ahead of print 9 April 2020. DOI: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. Epub ahead of print 17 April 2020. DOI: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. Epub ahead of print 17 April 2020. DOI: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv 2020.04.08.20057794; DOI: 10.1101/2020.04.08.20057794. [DOI] [Google Scholar]
- 28. Tabata S, Imai K, Kawano S, et al. The clinical characteristics of COVID-19: a retrospective analysis of 104 patients from the outbreak on board the Diamond Princess cruise ship in Japan. medRxiv 2020.03.18.20038125; DOI: 10.1101/2020.03.18.20038125. [DOI] [Google Scholar]
- 29. Fu L, Fei J, Xiang HX, et al. Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. medRxiv 2020.03.13.20035329; DOI: 10.1101/2020.03.13.20035329. [DOI] [Google Scholar]
- 30. Chen M, Tu C, Tan C, et al. Key to successful treatment of COVID-19: accurate identification of severe risks and early intervention of disease progression. medRxiv 2020.04.06.20054890; DOI: 10.1101/2020.04.06.20054890. [DOI] [Google Scholar]
- 31. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. medRxiv 2020.04.09.20059964; DOI: 10.1101/2020.04.09.20059964. [DOI] [Google Scholar]
- 32. Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. medRxiv 2020.03.25.20037721; DOI: 10.1101/2020.03.25.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo X, Xia H, Yang W, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv 2020.03.19.20033175; DOI: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 34. Ma KL, Liu ZH, Cao CF, et al. COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv 2020.03.19.20034124. DOI: 10.1101/2020.03.19.20034124. [DOI] [Google Scholar]
- 35. Luo X, Zhou W, Yan X, et al. Prognostic value of C-reactive protein in patients with COVID-19. medRxiv 2020.03.21.20040360. DOI: 10.1101/2020.03.21.20040360. [DOI] [Google Scholar]
- 36. Xu Y, Li YR, Zeng Q, et al. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. medRxiv 2020.03.08.20031658. DOI: 10.1101/2020.03.08.20031658. [DOI] [Google Scholar]
- 37. Cao M, Zhang D, Wang Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv 2020.03.04.20030395. DOI: 10.1101/2020.03.04.20030395. [DOI] [Google Scholar]
- 38. Qi D, Yan X, Tang X, et al. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study. medRxiv 2020.03.01.20029397. DOI: 10.1101/2020.03.01.20029397. [DOI] [Google Scholar]
- 39. Tamhane AR, Westfall AO, Burkholder GA, et al. Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med 2017; 36: 3760. [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization. Global Adult Tobacco Survey (GATS). Fact sheet China 2018, https://www.who.int/docs/default-source/wpro—documents/countries/china/2018-gats-china-factsheet-cn-en.pdf?sfvrsn=3f4e2da9_2 (accessed 1 May 2019).
- 41. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. 2018, https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm (accessed 1 May 2020).
- 42. Statista. Share of cigarette smokers in South Korea from 2008 to 2018, by gender. 2020, https://www.statista.com/statistics/645282/south-korea-smoking-rate-by-gender/ (accessed 1 May 2019).
- 43. World Health Organization. WHO report on the global tobacco epidemic, 2019. Country profile, Japan. 2019, https://www.who.int/tobacco/surveillance/policy/country_profile/jpn.pdf (accessed 1 May 2020).
- 44. Statista. Percentage of adults in the U.S. who were current cigarette smokers as of 2016, by age and gender, https://www.statista.com/statistics/673619/smoking-prevalence-among-men-us-by-age/ (accessed 6 May 2020).
- 45. Chang Y, Kang HY, Lim D, et al. Long-term trends in smoking prevalence and its socioeconomic inequalities in Korea, 1992-2016. Int J Equity Health 2019; 18: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Statista. Smoking rate of men in Japan as of May 2017, by age, https://www.statista.com/statistics/741056/japan-male-smoking-rate-by-age/ (accessed 6 May 2020).
- 47. Statista. Smoking rate of women in Japan as of May 2018, by age group, https://www.statista.com/statistics/741170/japan-female-smoking-rate-by-age/ (accessed 6 May 2020).
- 48. Guan WJ, Liang WH, Zhao Y, et al. ; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J 2020; 55: 2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu S, Zhang M, Yang L, et al. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health 2017; 71: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rossato M, Russo L, Mazzocut S, et al. Current smoking is not associated with COVID-19. Eur Respir J 2020; 55: 2001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garufi G, Carbognin L, Orlandi A, et al. Smoking habit and hospitalization for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related pneumonia: the unsolved paradox behind the evidence. Eur J Intern Med. Epub ahead of print 23 April 2020. DOI: 10.1016/j.ejim.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Farsalinos K, Niaura R, Le Houezec J, et al. Editorial: nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol Rep 2020; 7: 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Volti G, Caruso M, Polosa R. Smoking and SARS-CoV-2 disease (COVID-19): dangerous liaisons or confusing relationships? J Clin Med 2020; 9: E1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020; 34: 1. [DOI] [PubMed] [Google Scholar]
- 55. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. Epub ahead of print 3 March 2020. DOI: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 2020; 55: 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Georgiev T. Coronavirus disease 2019 (COVID-19) and anti-rheumatic drugs. Rheumatol Int. Epub ahead of print 30 March 2020. DOI: 10.1007/s00296-020-04570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Slatter H. FDA approves phase III clinical trial of tocilizumab for COVID-19 pneumonia. CancerNetwork. March 26, 2020, https://www.cancernetwork.com/news/fda-approves-phase-iii-clinical-trial-tocilizumab-covid-19-pneumonia (accessed 4 April 2020).
- 59. Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020; 395: 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. ACTERMA®. Highlights of prescribing information: ACTERMA (tocilizumab), https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125276s107_125472s018lbl.pdf (accessed 14 May 2020).
- 61. Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421: 384–388. [DOI] [PubMed] [Google Scholar]
- 62. Tracey KJ. The inflammatory reflex. Nature 2002; 420: 853–859. [DOI] [PubMed] [Google Scholar]
- 63. Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 2014; 1843: 2563–2582. [DOI] [PubMed] [Google Scholar]
- 64. Wang Y, Pereira EF, Maus AD, et al. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol Pharmacol 2001; 60: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 65. Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462. [DOI] [PubMed] [Google Scholar]
- 66. Blalock JE. Harnessing a neural-immune circuit to control inflammation and shock. J Exp Med 2002; 195: F25–F28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Costa J, Moreira A, Moreira P, et al. Effects of weight changes in the autonomic nervous system: a systematic review and meta-analysis. Clin Nutr 2019; 38: 110–126. [DOI] [PubMed] [Google Scholar]
- 68. Carnethon MR, Golden SH, Folsom AR, et al. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the atherosclerosis risk in communities study, 1987-1998. Circulation 2003. May 6; 107: 2190–2195. [DOI] [PubMed] [Google Scholar]
- 69. Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int 2010; 10(Suppl. 1): S127–S136. [DOI] [PubMed] [Google Scholar]
- 70. Armour JA. Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res 1999; 41: 41–54. [DOI] [PubMed] [Google Scholar]
- 71. Mabley J, Gordon S, Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation 2011; 34: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Villafane G, Thiriez C, Audureau E, et al. High-dose transdermal nicotine in Parkinson’s disease patients: a randomized, open-label, blinded-endpoint evaluation phase 2 study. Eur J Neurol 2018; 25: 120–127. [DOI] [PubMed] [Google Scholar]
- 73. Newhouse P, Kellar K, Aisen P, et al. Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology 2012; 78: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang H, Penninger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020; 46: 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brake SJ, Barnsley K, Lu W, et al. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J Clin Med 2020; 9: 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 2020; 55: 2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oakes JM, Fuchs RM, Gardner JD, et al. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 2018; 315: R895–R906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; 382: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mehra MR, Desai SS, Kuy S, et al. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. Epub ahead of print 1 May 2020. DOI: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. Epub ahead of print 1 May 2020. DOI: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Verdecchia P, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. Epub ahead of print 20 April 2020. DOI: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xie X, Chen J, Wang X, et al. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci 2006; 78: 2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract 2020; 162: 108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kassiri Z, Zhong J, Guo D, et al. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail 2009; 2: 446–455. [DOI] [PubMed] [Google Scholar]
- 85. Ciaglia E, Vecchione C, Puca AA. COVID-19 Infection and circulating ACE2 levels: protective role in women and children. Front Pediatr 2020; 8: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. Epub ahead of print 4 March 2020. DOI: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PRISMA_checklist-filled for Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis by Konstantinos Farsalinos, Anastasia Barbouni, Konstantinos Poulas, Riccardo Polosa, Pasquale Caponnetto and Raymond Niaura in Therapeutic Advances in Chronic Disease
Supplemental material, Supplementary_file for Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis by Konstantinos Farsalinos, Anastasia Barbouni, Konstantinos Poulas, Riccardo Polosa, Pasquale Caponnetto and Raymond Niaura in Therapeutic Advances in Chronic Disease
Supplemental material, Supplementary_file_TAJ for Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis by Konstantinos Farsalinos, Anastasia Barbouni, Konstantinos Poulas, Riccardo Polosa, Pasquale Caponnetto and Raymond Niaura in Therapeutic Advances in Chronic Disease