Abstract
Activation of glial cells has been shown to play an important role in chronic itch. However, whether glial cells play an important role in the development of psoriasis-induced chronic itch has not been fully elucidated. This study investigated the role of spinal glial cells in psoriasis-induced chronic itch. To develop a mouse model of psoriasis-induce chronic itch, we used 5% imiquimod cream to receive a daily topical application on the shaved back skin for seven consecutive days. The results showed that the expression of microglial marker ionized calcium binding adaptor molecule-1 was significantly increased after 5% imiquimod treatment in cervical spinal cord dorsal horn (C3–C4), and the intrathecal microglial inhibitor minocycline or PLX5622 diet suppressed both spontaneous itch and microglial activation. Furthermore, we found that the number of scratches and alloknesis score in female mice was significantly greater than in male mice after 5% imiquimod treatment. Our results indicate that microglia mediate chronic psoriatic itch induced by imiquimod.
Keywords: Itch, microglia, psoriatic, minocycline, PLX5622
Chronic itch is a common symptom of psoriasis associated with affected sleep quality and a significant reduction in patient’s quality of life. Activation of glial cells has been shown to play an important role in chronic itch. Increased expression of spinal ionized calcium binding adaptor molecule-1 (IBA-1), a microglial maker, were found in male mice with atopic dermatitis compared with control mice.1 Intrathecal injection of astrocyte inhibitor L-α-aminoadipate substantially reduced dry skin-induced chronic itch in male mice.2 However, the effect of spinal glial cells in the development of psoriasis-induced chronic itch has not been fully elucidated. We therefore investigated the role of spinal cord glial cells in psoriasis-induced chronic itch.
Six-week-old male and female C57BL/6J mice were maintained under clean conditions. All animal procedures were approved by the Animal Care and Use Committee of AnHui Medicine University. To develop a mouse model of psoriasis-induce chronic itch, we used 5% imiquimod (IMQ) cream (Med-Shine, Chengdu, China) to receive a daily topical application of 62.5 mg on the shaved back skin (2.5 cm × 2 cm) for seven consecutive days as described previously.3 The scratching behavior of IMQ-treated mice was video recorded for 1 h before IMQ treatments. After an hour of observation, the alloknesis score was assessed at five random sites at the edge of the application area using a von Frey filament (0.7 mN).4
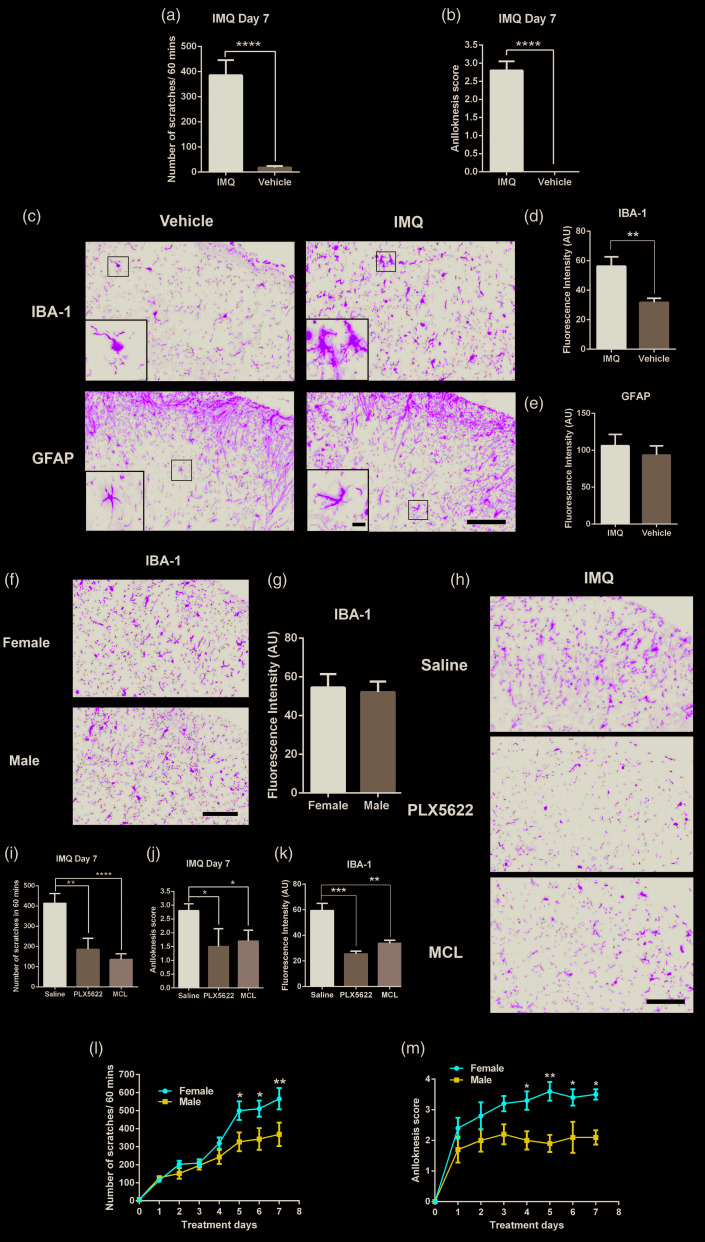
Seven days after 5% IMQ treatment, IMQ-treated mice showed a significant increase in the number of spontaneous scratching bouts and alloknesis score on day 7 compared to vehicle-(Vaseline Lanette cream) treated mice (Figure 1(a) and (b)). After behavioral observation on day 7, the expression of microglial marker IBA-1 and astrocyte marker glial fibrillary acidic protein (GFAP) in dorsal horn of cervical spinal cord (C3–C4) were elucidated by immunofluorescence (Figure 1(c)). Mice were deeply anesthetized with isoflurane and transcardially with phosphate-buffered saline followed by 4% paraformaldehyde. C3–C4 segments of the spinal cord were removed and then post-fixed overnight in the same fixative, the samples were dehydrated and cut into paraffin sections (4 µm). Briefly, the sections were washed with distilled water after dewaxing and then subjected to antigen retrieval. After nonspecific blocking in goat serum, the sections were incubated with primary antibodies against IBA-1 (1:100, Abcam, ab178847) and GFAP (1:100, Boster, BM4287) at 4°C overnight. Next day, the sections were incubated with fluorescein isothiocyanate-conjugated secondary antibodies. The sections were scanned with a Pannoramic MIDI II (3DHISTECH, Hungary) scanner and randomly photographed on spinal cord dorsal horn. The results were analyzed by Image J. The intensity of IBA-1 in IMQ-treated mice was significantly greater compared to vehicle-treated mice (Figure 1(d)). There is no significant difference in the intensity of IBA-1 in the spinal dorsal horn between male and female mice after 5% IMQ treatment (Figure 1(f) and (g)). Moreover, GFAP intensity was not significantly different to vehicle-treated mice (Figure 1(e)). Thereafter, we examined the effects of microglial inhibitor minocycline (MCL, Sigma, M9511) and PLX5622 (MedChemExpress, HY-114153), a colony stimulating factor 1 receptor (CSF1R) inhibitor, which can result in depletion of microglia in previously report5 on IMQ-treated mice. MCL was dissolved in saline, intrathecal saline, or MCL and administered at a dose of 50 µg in 5 µL three times a week for two weeks. Seven days after 5% IMQ treatment, PLX5622 formulated in rodent chow by Research Diets at a concentration of 1200 ppm. Mice were provided PLX5622 diet for seven days. Intrathecal MCL and PLX5622 diet significantly decreased the number of scratching bouts (Figure 1(i)) and alloknesis score (Figure 1(j)) compared with control mice. We employed immunofluorescence to examine the expression of IBA-1 in cervical spinal cord (C3–C4) and found that after intrathecal MCL treatment or PLX5622 diet, expression had significantly decreased (Figure 1(h) and (k)).
Figure 1.
Scratching bouts (a) and alloknesis score (b) in IMQ treated-mice on day 7, ****P < 0.0001, n = 10 (male = 5, female = 5) mice per group. (c) Immunofluorescence showing the expression of IBA-1 and GFAP in spinal cord dorsal horn (C3–C4) in IMQ- and vehicle-treated mice after seven days treatment. Scale bars, 100 µm, 10 µm in insert. Quantitative analysis of IBA-1 (d) and GFAP (e) immuofluorescence intensity of IMQ- and vehicle-treated mice after seven days treatment. **P < 0.01, n = 5 (male = 3, female = 2) mice per group. (f) Immunofluorescence showing the expression of IBA-1 in spinal cord dorsal horn (C3-C4) in IMQ- treated male and female mice after seven days treatment, n = 4 mice per group. Scale bars, 100 µm. (g) Quantitative analysis of IBA-1 immuofluorescence intensity in male and female mice after seven days treatment, n = 4 mice per group. n = 4 mice per group. (h) Immunofluorescence showing the expression of IBA-1 in saline-, minocycline-, and PLX5622-treated male mice. Scale bars, 100 µm. Scratching bouts (i) and alloknesis score (j) in saline-, minocycline-, and PLX5622-treated mice after seven days IMQ treatment,*P < 0.05; **P < 0.01; ****P < 0.0001, n = 10 (male = 5, female = 5) mice in saline- and minocycline-treated per group, n= 4 (male =2, female = 2) mice in PLX5622- treated group. (k) Quantitative analysis of IBA-1 immuofluorescence intensity in saline-, minocycline-, and PLX5622-treated mice. **P < 0.01, n = 4 (male =2, female = 2) mice per group. Scratching bouts (l) and alloknesis score (m) in male and female mice after IMQ treatment. *P < 0.05; **P < 0.01, n = 7 mice per group. Data are presented as means ± SEM, Student’s t test or two-way ANOVA.
Results showed that spinal microglia play an important role in IMQ-induced chronic psoriatic itch in both male and female mice. Itch and pain are different somatic sensations with many similarities. Although previous reports indicate that microglia are required for chronic pain hypersensitivity in male mice rather than in female mice,6 we did not find the same sex difference in microglia-mediated, IMQ-induced chronic itch. However, we found that the number of scratches and alloknesis score in female mice was significantly greater than in male mice after 5% IMQ treatment (Figure 1(l) and (m)). This suggests that female mice may be more sensitive to 5% IMQ-induced chronic psoriatic itch and alloknesis than male mice. IMQ is a toll-like receptor 7 (TLR7) agonist and TLR7 expression in primary sensory neurons can be activated by ligands to elicit an itch response.7 However, there is no research focusing on the sex different on expression of TLR7 in sensory neurons. It remains to be tested whether the expression of TLR7 difference in sensory neurons induces those sex differences. Moreover, although activation of TLR7 in immune cells initiates signaling cascades leading to a variety of transcriptional changes to promote inflammation,8 5% IMQ induced events in skin which are independent of TLR7 and even independent of the major active compound IMQ.9 There are at least two immune pathways, IMQ and component of the vehicle, which are required for IMQ induced chronic psoriatic skin lesions which may existed unknown sex difference. The phenomenon of increased sensitivity of female mice to 5% IMQ-induced psoriatic itch and alloknesis suggests that sex differences in the above process may exist. Although the cause of those differences is unclear, it eludes to the possible effect of sex differences in itch-related experiments in future studies.
Acknowledgment
The authors would like to thank Dake Huang for their contributions to the study.
Author Note
Jingcheng Zhang is also affiliated with Department of Biliary and Pancreatic Surgery, Anhui Provincial Hospital Affiliated with Anhui Medical University.
Author Contributions
ZHX, ZQQ, and JCZ performed all experiments and then analyzed the data. ZHX and YW designed the experiments as well as discussed and completed the final version of the paper. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partly supported by Key Fund Project of Anhui Provincial Department of Education (KJ 2019A0946).
ORCID iD
Zhehao Xu https://orcid.org/0000-0002-2252-9058
References
- 1.Torigoe K, Tominaga M, Ko KC, Takahashi N, Matsuda H, Hayashi R, Ogawa H, Takamori K. Intrathecal minocycline suppresses itch-related behavior and improves dermatitis in a mouse model of atopic dermatitis. J Invest Dermatol 2016; 136: 879–881. [DOI] [PubMed] [Google Scholar]
- 2.Liu T, Han Q, Chen G, Huang Y, Zhao L-X, Berta T, Gao Y-J, Ji R-R. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016; 157: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakai K, Sanders KM, Youssef MR, Yanushefski KM, Jensen L, Yosipovitch G, Akiyama T. Mouse model of imiquimod-induced psoriatic itch. Pain 2016; 157: 2536–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai K, Akiyama T. Disinhibition of touch-evoked itch in a mouse model of psoriasis. J Invest Dermatol 2019; 139: 1407–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, Szeto GL, Wu J, Stoica BA, Faden AI, Loane DJ. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci 2020; 40: 2960–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-S, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji R-R, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015; 18: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci 2010; 13: 1460–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taves S, Ji RR. Itch control by Toll-like receptors. Handb Exp Pharmacol 2015; 226: 135–150. [DOI] [PubMed] [Google Scholar]
- 9.Walter A, Schäfer M, Cecconi V, Matter C, Urosevic-Maiwald M, Belloni B, Schönewolf N, Dummer R, Bloch W, Werner S, Beer H-D, Knuth A, van den Broek M. Aldara activates TLR7-independent immune defence. Nat Commun 2013; 4: 1560. [DOI] [PubMed] [Google Scholar]