Abstract
Cytomegalovirus (CMV) reactivation is one of the most common infections affecting allogeneic hematopoietic cell transplant recipients. Although available anti-CMV therapies have been evaluated for the prevention of CMV reactivation, their toxicity profile makes them unfavorable for use as primary prophylaxis; thus, they are routinely reserved for the treatment of CMV viremia or CMV end-organ disease. Pre-emptive CMV monitoring strategies have been widely accepted, and although they have been helpful in early detection, they have not affected the overall morbidity and mortality associated with CMV. Letermovir is a novel agent that was approved for primary prophylaxis in CMV-seropositive adult allogeneic hematopoietic cell transplant recipients. This review focuses on letermovir’s novel mechanism; clinical trials supporting its United States Food and Drug Administration (FDA) approval and subsequent follow-up analyses; clinical considerations, with an emphasis on pharmacology; and lessons learned from solid organ transplant recipients, as well as potential future directions.
Keywords: CMV infection, cytomegalovirus, hematopoietic cell transplant, letermovir, prevention, prophylaxis
Introduction
Cytomegalovirus (CMV) infection is a serious complication in allogeneic hematopoietic cell transplant (allo-HCT) recipients, and is associated with increased morbidity and mortality.1–3 CMV-seropositive patients undergoing allo-HCT are at increased risk of CMV infection, with up to 80% developing CMV reactivation in the absence of prophylaxis.2,4
Antiviral agents used in the treatment of CMV are associated with significant toxicities. Ganciclovir, a synthetic nucleoside that inhibits viral DNA synthesis, and its pro-drug, valganciclovir, are associated with myelosuppression, which may preclude their use in patients in the early post-transplant setting.1,5 Foscarnet, a pyrophosphate analogue that inhibits viral DNA polymerase, is nephrotoxic and can cause electrolyte imbalances that require close monitoring, and, in some cases, hospitalization for management.1,5 Because of the toxicity of these agents, they have fallen out of favor, at least for primary prophylaxis of CMV infections. Instead, a pre-emptive monitoring strategy has been adopted for allo-HCT recipients. This strategy involves close monitoring of CMV viral load via quantitative CMV DNA by polymerase chain reaction (PCR), pp65 antigenemia, or other molecular methods of detection. Once the virus reactivates, pre-emptive anti-CMV therapy is initiated to prevent progression to CMV end-organ disease.1,6 While this approach has decreased the incidence of CMV end-organ disease, the impact on overall morbidity and mortality that is associated with CMV viremia in allo-HCT recipients is still unclear.2,3,7
The development of a safe and effective agent for prophylaxis, one that would prevent CMV reactivation and disease while avoiding significant toxicities, has been an area of unmet need in CMV management. Letermovir was approved by the United States Food and Drug Administration (FDA) for the prophylaxis of CMV infection and disease in adult CMV-seropositive allo-HCT recipients in November 2017.8 Here, we summarize the available literature on letermovir, highlighting its use as primary prophylaxis for allo-HCT recipients, as well as discussing clinical considerations for the use of letermovir in practice and potential future directions.
Letermovir
Letermovir is a novel agent that represents a new class of non-nucleoside CMV inhibitors, the 3,4 dihydro-quinazoline-4-yl-acetic acid derivatives.9,10 Unlike other anti-CMV therapies that are currently available, letermovir has activity in the late stages of viral replication rather than against the viral DNA polymerase.10,11
Successful CMV viral replication requires cleavage of concatemeric DNA into functional monomers that are subsequently packaged into viral capsids. This process is performed by a group of proteins that are collectively known as a “terminase complex”; these include the protein subunits pUL56 and pUL89 and at least five additional proteins whose functions have yet to be fully elucidated.9,10,12,13 More recently, one of these proteins, pUL51, was further characterized; it likely represents a third component of the terminase complex that interacts with the previously described proteins, pUL56 and pUL89.13
Letermovir inhibits the viral terminase complex at pUL56 and pUL89, which leads to compromised viral replication by preventing genomes of proper unit length and the accumulation of immature viral DNA.9–11
In vitro studies have demonstrated that letermovir is one of the most potent anti-CMV agents identified to date, with reports illustrating up to 1000-fold potency compared with that of ganciclovir.9,10,14 Furthermore, given the absence of the mammalian counterpart of the viral terminase enzyme, mechanism-based adverse effects are unlikely, making letermovir an appealing alternative to current therapies.10,12,14 In addition, it has selective activity against only CMV14; as a result, antiviral prophylaxis to prevent herpes simplex virus and varicella zoster virus is recommended.
The approved dose of letermovir is 480 mg (240 mg if co-administered with cyclosporine) once daily. Presently, it is recommended to start letermovir at this dose in CMV seropositive adult recipients of an allo-HCT between day 0 and 28 and continue through day 100 post-transplant (Table 1).15,16
Table 1.
Letermovir overview.15
| Dose/frequency | Route | Dose adjustment | Pharmacokinetics | Drug interactions | Clinical pearls |
|---|---|---|---|---|---|
| 480 mg once daily 240 mg once daily with cyclosporine Start between days 0 and 28, continue through day 100 |
PO (tablet), with or without food IV containing hydroxylpropyl beta-cyclodextrin |
Renal impairment: no dose adjustment†
Hepatic impairment: no dose adjustment‡ |
F = 35–94%*
Vd = 45.5 l, 99% protein bound Hepatic metabolism through UGT1A1/1A3 (minor) t 1/2 = 12 h Excretion: feces, 93% (70% as unchanged drug) |
Substrate: CYP3A, CYP2D6, UGT1A1, UGT1A3; transporters P-gp, OATP1B1/3 Inhibitor: CYP3A (moderate) CYP2C8, P-gp, OAT3, OATP1B1/3 Inducer: CYP2C9, CYP2C19, CYP2B6, CYP3A |
28-day blister pack Letermovir decreases voriconazole concentration; recommend monitoring voriconazole levels Only active against CMV; recommend antiviral prophylaxis to prevent herpes simplex virus and varicella zoster virus Consider ordering CMV genotype to assess for resistance if patient develops clinically significant CMV infection on letermovir (>1000 IU/ml) |
CMV, cytomegalovirus; CrCL, creatinine clearance; F, bioavailability; IU, international units; t ½, half life; Vd, volume of distribution.
Bioavailability in healthy subjects = 94% without cyclosporine (240–480 mg once daily), hematopoietic cell transplant recipients without cyclosporine (480 mg once daily) = 35%, hematopoietic cell transplant recipients with cyclosporine (240 mg once daily) = 85%.
Patients with CrCL < 10 ml/min or patients on hemodialysis were excluded from the phase III trial. Caution should be used in patients with CrCL ⩽50 ml/min receiving IV letermovir.
Patients with moderate (Child Pugh Class B) or severe (Child Pugh Class C) liver dysfunction were excluded from the phase III trial.
Letermovir clinical studies for primary prophylaxis
Phase II study
A phase IIb, multi-center, double-blinded, dose-range study was conducted to evaluate the safety and efficacy of letermovir as prophylaxis (Table 2).17 CMV-seropositive allo-HCT recipients from a matched related or unrelated donor with evidence of engraftment within 40 days of transplant and undetectable CMV were eligible for enrollment. Patients were excluded if they had received an ex vivo T-cell-depleted graft, had undergone anti-CMV treatment after transplantation, or had past or present CMV end-organ disease, uncontrolled infection, or acute graft-versus-host disease (GVHD) of grade II or higher. Since letermovir is not active against herpes simplex virus or varicella zoster virus, the use of low-dose acyclovir, valacyclovir, or famciclovir was permitted.17
Table 2.
Letermovir primary prophylaxis: summary of clinical studies in hematopoietic cell transplant.
| Study design | No. of patients | Outcomes | |
|---|---|---|---|
| Clinical trials | |||
| Chemaly et al.17 | Phase IIb Prospective, randomized, placebo controlled, multi-center, dose ranging |
LTV (60 mg), n = 33 LTV (120 mg), n = 31 LTV (240 mg), n = 34 Placebo, n = 33 |
All-cause prophylaxis failure,-no. (%); p value LTV versus placebo LTV (60 mg) = 16 (48%), p = 0.32 LTV (120 mg) = 10 (32%), p = 0.01 LTV (240 mg) = 10 (29%), p = 0.007 Placebo = 21 (64%) Any drug-related adverse event, no. (%) LTV (60 mg) = 11 (33%) LTV (120 mg) = 4 (13%) LTV (240 mg) = 2 (6%) Placebo = 11 (33%) |
| Marty et al.16 | Phase III Prospective, randomized, placebo controlled, multi-center |
LTV, n = 325 Placebo, n = 170 |
CS-CMVi at week 14,-no. (%) LTV 62 (19.1%) versus 85 (50%), p < 0.001 CS-CMVi at week 24,-no. (%) LTV = 122 (37.5%) versus placebo = 103 (60.6%), p < 0.001 All-cause mortality at week 24 LTV = 10.2% versus placebo = 15.9%, p = 0.03 All-cause mortality at week 48 LTV = 20.9% versus placebo = 25.5%, p = 0.12 Any adverse event (LTV n = 373, placebo n = 192), no. (%) LTV = 365 (97.9% versus placebo = 192 (100%), p = 0.07 |
| Phase III follow-up analyses | |||
| Marty et al.18 | Analysis of patients with detectable CMV at randomization excluded from phase III trial | LTV, n = 48 Placebo, n = 22 |
CS-CMVi at week 14,-no. (%) LTV = 22 (45.8%) versus placebo = 20 (90.9%), p < 0.001 CS-CMVi at week 24,-no. (%) LTV = 31 (64.6%) versus placebo = 20 (90.9%), p < 0.01 All-cause mortality at week 24 LTV = 15% versus placebo = 18.2%, no reported p value All-cause mortality at week 48 LTV = 26.5% versus placebo = 40.9%, p = 0.268 |
| Ljungman et al.19 | Post hoc analysis of phase III data | Week 24 (59 deaths) LTV, n = 293 Placebo, n = 143 Week 48 (101 deaths) LTV, n = 264 Placebo, n = 130 |
HR all-cause mortality at week 24 (LTV versus placebo) 0.58 (95% CI, 0.35–0.98), p = 0.04 HR all-cause mortality at week 48 (LTV versus placebo) 0.74 (95% CI, 0.49–1.11), p = 0.14 HR of letermovir group with versus without CS-CMVi (week 48) 1.15 (95% CI, 0.56–2.37), p = 0.71 HR of placebo group with versus without CS-CMVi (week 48) 2.34 (95% CI, 1.17–4.67), p = 0.02 HR all-cause mortality CS-CMVi (LTV versus placebo) 0.45 (95% CI, 0.21–1.00), p = 0.05 |
CS-CMVi, clinically significant CMV infection; LTV, letermovir.
Patients were assigned in a 3:1 ratio to receive 60 mg, 120 mg, or 240 mg of letermovir or placebo for 12 weeks. Virologic failure was defined as either detectable CMV antigen or DNA at two consecutive time points, leading to pre-emptive treatment with anti-CMV therapy or evidence of CMV end-organ disease. The incidence and the time to onset of all-cause failure (discontinuing the study drug for virologic failure or any other reason) during the 12 weeks of study drug administration were the two primary efficacy endpoints.17
Between March 2010 and October 2011, 131 patients were assigned randomly to receive the study drug. The incidence of all-cause failure was lower in the groups that received 120 mg of letermovir [10 of 31 (32%)] and 240 mg of letermovir [10 of 34 (29%)] than in those that received placebo [21 of 33 (64%); p = 0.01 and p = 0.007, respectively]. There was no difference between 60 mg of letermovir and placebo [16 of 33 (48%), p = 0.32]. When evaluating virologic failure only, the incidence of detectable CMV was lower in patients who received letermovir than in those who received placebo in all arms. No cases of CMV end-organ disease were observed in this study.17
The most common reason for discontinuation of the study drug was CMV infection, which was more common in the placebo arm (letermovir = 26% versus placebo = 58%). In addition, the safety profiles of letermovir versus placebo were similar, with no hematologic toxicity associated with letermovir. The most frequent adverse event reported in both groups was gastrointestinal toxicity (letermovir = 66% versus placebo = 61%), such as nausea, vomiting, and diarrhea.17
Although a lower dose of letermovir was studied, a further analysis of this patient population revealed that patients who received the 240 mg dose without cyclosporine had drug exposure levels that were closer to those of the 60 mg and 120 mg daily doses, which were associated with more virologic failures.16,17 As a result, on the basis of all available safety data and exposure-response modeling and simulation, the dose of letermovir was increased to 480 mg once daily in patients who were not receiving cyclosporine, and continued at 240 mg once daily with cyclosporine.16 The promising results of this phase IIb trial afforded the opportunity to proceed with a phase III trial, not only with the adjusted letermovir dose but also starting the study drug prior to engraftment since no myelosuppression had been observed.
Phase III study
This phase III, multi-center, double-blinded, placebo-controlled study of letermovir enrolled eligible CMV-seropositive allo-HCT recipients (Table 2). They were randomly assigned, in a 2:1 ratio, to receive letermovir or placebo, starting between day 0 and 28 through week 14 (approximately day 100).16 Patients were excluded if they were less than 18 years old; had severe liver impairment, an estimated creatinine clearance of less than 10 ml/min, or detectable CMV DNA; or were currently undergoing or had recently undergone receipt of anti-CMV therapy.16 Patients were classified as high or low risk for CMV reactivation and CMV end-organ disease. Patients were considered high risk if they met one or more of the following criteria: haploidentical transplant; umbilical cord transplant; major human leukocyte antigen (HLA) mismatch at HLA-A, B, or DR donor (related or unrelated); ex vivo T-cell-depleted graft; and GVHD grade II or higher requiring ⩾1 mg/kg of prednisone or equivalent.16 Patients with clinically significant CMV infection (CS-CMVi) (i.e., CMV disease or CMV viremia leading to pre-emptive therapy) discontinued the trial regimen and began anti-CMV therapy.16
Between June 2014 and March 2016, 565 patients gave consent and were randomly assigned study drug. The baseline characteristics between the trial groups were well balanced, and, overall, 31% (175 of 565) were considered to be at high risk for CS-CMVi. The median time to begin letermovir or placebo was 9 days (range, 0–28 days) after transplantation, with a median duration of therapy of 82 days (range, 1–113 days) in the letermovir arm, and 56 days (range, 4–115 days) in the placebo arm.16
Of the patients who received the trial regimen, 495 (325 on letermovir and 170 on placebo) were included in the primary efficacy population for the primary endpoint of CS-CMVi through week 24; the other patients were excluded for detectable CMV DNA at the time of randomization.16 By week 24, fewer patients in the letermovir group had developed CS-CMVi than had those in the placebo group (letermovir = 37.5% versus placebo = 60.6%, p < 0.001). Of note, few patients in both arms had developed CMV end-organ disease, which involved the gastrointestinal tract in all cases (letermovir = 1.5% versus placebo = 1.8%).16
A more pronounced difference in the secondary end point of CS-CMVi was found between groups by week 14, again in favor of letermovir (letermovir = 19.1% versus placebo = 50%, p < 0.001). Interestingly, around week 18, the incidence of CS-CMVi increased in the letermovir group shortly after the discontinuation of therapy. This was reflective of an ongoing or new CMV risk, particularly in patients with GVHD who required corticosteroids.16
All-cause mortality at week 24 after transplantation was lower among patients who received letermovir (letermovir = 10.2% versus placebo = 15.9%, p = 0.03). However, all-cause mortality at week 48 was not statistically different between groups (letermovir = 20.9% versus placebo = 25.5%, p = 0.12). The prevention of CS-CMVi was consistent among all risk groups, and a lower mortality rate was more pronounced in the high-risk group, although the study was not powered to evaluate this endpoint.16
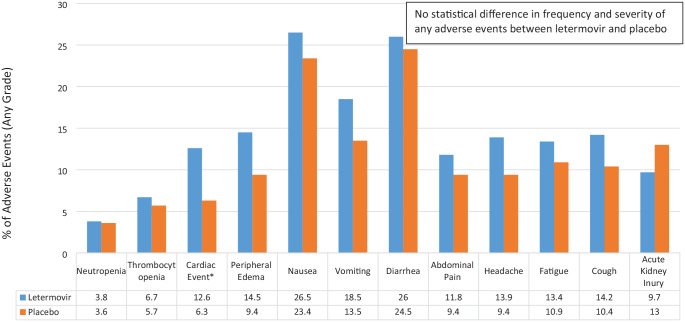
The most common reason for discontinuation of the trial regimen was CS-CMVi, which was more common in the placebo arm [82 of 194 (42.3%)]. No difference was noted between arms in regards to time to engraftment, relapse of hematologic disease, or incidence and grade of GVHD. In addition, the overall rates of adverse effects were similar between both groups, with no statistically significant difference noted (Figure 1).16
Figure 1.
Select LTV versus placebo adverse events (any grade) reported in phase III trial.16
*Cardiac event included (LTV versus placebo): atrial fibrillation (3.5% versus 1%), atrial flutter (1.1% versus 0%), cardiac failure (1.3% versus 0%), sinus tachycardia (1.1% versus 1.6%), and tachycardia (4% versus 2.1%). Further analysis did not show a relationship between letermovir and atrial arrhythmias.
LTV, letermovir.
Phase III study follow-up analyses
There have been two follow-up studies from the phase III trial with letermovir (Table 2) that have further evaluated outcomes in patients with detectable CMV DNA at the time of randomization and mortality analysis.18,19
A total of 70 patients from the phase III trial had detectable CMV DNA at randomization; however, since the viral load was verified in a central laboratory, the results were not known in real time. As a result, these patients continued on the study drug, but were excluded from the primary efficacy analysis.18 At the time of randomization, more patients with detectable CMV DNA by PCR were at high risk for CMV reactivation, received a myeloablative conditioning regimen, or received antithymocyte globulin, than did patients with undetectable CMV.18
More patients completed treatment with the study drug through week 14 in the letermovir group (25 of 48) than did those in the placebo group (2 of 22), with the main reason for discontinuation being CS-CMVi.18 A lower proportion of patients in the letermovir group had CS-CMVi at weeks 14 and 24 than did those in the placebo group (week 14: letermovir = 45.8% versus placebo = 90.9%, p < 0.001; week 24: letermovir = 64.6% versus placebo = 90.9%, p < 0.01). The proportion of CS-CMVi was lower in the letermovir arm at weeks 14 and 24 (week 14: letermovir = 33.1% versus placebo = 86.6%; week 24: letermovir = 51.8% versus placebo = 86.6%).18 Of note, all patients with detectable CMV at the time of randomization had higher rates of CS-CMVi than did all patients with undetectable CMV.18
The median time to CS-CMVi in the letermovir arm was longer than that in the placebo arm (letermovir = 156 days versus placebo = 34 days). Lastly, similar to patients with undetectable CMV, patients with detectable CMV at randomization had an all-cause mortality rate that favored letermovir at week 24 and week 48 (week 24: letermovir = 15% versus placebo = 18.2%; week 48: letermovir = 26.5% versus placebo = 40.9%).18 Although the results of this study were limited by the small sample size and low levels of CMV detection at randomization, they highlight the importance of early initiation of CMV prophylaxis with letermovir.
A recent post hoc analysis of the phase III data further evaluated the effects of letermovir on all-cause mortality.19 As previously mentioned, an all-cause mortality benefit with letermovir was seen at week 24, and a numerical benefit with letermovir was seen at week 48.16 Of the 495 patients with undetectable CMV at the time of randomization, 437 had vital-status data available through week 48 [101 deaths (20.4%)]. A sensitivity analysis at week 24 and week 48 demonstrated results that were similar to those that had been previously reported for the incidence of all-cause mortality.16,19 In addition, lower Kaplan–Meier event rates for all-cause mortality were found for letermovir (week 24: letermovir = 12.1% versus 17.2%; p = 0.04; week 48: letermovir = 23.8% versus placebo = 27.6%; p = 0.21).19
Univariate and multivariable Cox models for time to all-cause mortality through week 24 and week 48 were calculated for possible risk factors associated with mortality. After adjusting for age, risk factors for CMV reactivation, and acute GVHD, the hazard ratio (HR) for all-cause mortality for the letermovir arm was 0.58 [95% confidence interval (CI), 0.35–0.98; p = 0.04] at week 24 and 0.74 (95% CI, 0.49–1.11; p = 0.14) at week 48.19
The incidence of all-cause mortality was also evaluated at week 48 in patients with or without CS-CMVi by week 24 after transplant. The incidence of all-cause mortality in the placebo group was substantially higher in patients with CS-CMVi, despite the use of pre-emptive therapy, than it was in patients without CS-CMVi [HR = 2.34 (95% CI = 1.17–4.67; p = 0.02)].19 This finding is consistent with those in the available literature that have demonstrated that CMV reactivation, regardless of the initiation of appropriate pre-emptive therapy, increases mortality.2,3 Contrariwise, the incidences of all-cause mortality in the letermovir group were similar, irrespective of CS-CMVi [HR = 1.15 (95% CI = 0.56–2.37; p = 0.71)].19 In a Cox regression model, and, after adjusting for other risk factors, the HR for all-cause mortality through week 48 in patients with CS-CMVi was 0.45 (95% CI = 0.21–1.00, p = 0.05) for letermovir versus placebo.19 This follow-up analysis supports the initial findings that suggest that letermovir reduces mortality by preventing, or maybe delaying, CS-CMVi.
Retrospective, single-center, real-world data
Three studies examined retrospective data on the use of letermovir as primary prophylaxis to provide further insight into its safety and efficacy.
Fooled et al. evaluated all adult patients who were CMV-seropositive and received an allo-HCT between March and October 2018.20 A total of 140 patients was included, with 111 receiving letermovir prophylaxis.20 The most common reason for not initiating letermovir prophylaxis was insurance approval and cost. Fewer patients on letermovir had CS-CMVi than did patients who did not receive prophylaxis [15 of 111 (14%) versus 13 of 29 (45%), p < 0.01]. Proven or probable CMV end-organ disease was similarly low in both groups [letermovir = 2 of 111 (2%) versus placebo = 2 of 29 (7%)]; further analysis is currently ongoing.20
Sharma et al. reported on the use of letermovir in cord blood transplant (CBT) recipients, as few CBT patients were enrolled in the phase III trial.16 This retrospective study evaluated three prophylactic strategies used in CBT recipients (double umbilical cord or haploidentical-cord transplant) at their institution from December 2009 through December 2018.21 Patients either received letermovir through day 100 (n = 32), 2 g of valacyclovir by mouth three times daily through day 100 (“valacyclovir day 100”) (n = 60), or 2 g of valacyclovir by mouth three times daily through hospital discharge, followed by 800 mg of acyclovir by mouth twice daily (“valacyclovir hospital discharge”) (n = 41). The CS-CMVi incidence was lower in the letermovir arm than in both arms with valacyclovir (0 of 32 letermovir, 6 of 60 valacyclovir day 100, and 9 of 41 valacyclovir hospital discharge; p = 0.06 and p = 0.005, respectively).21 There were also no delays in engraftment or graft failure in patients who received letermovir. Of note, among patients who were evaluated between days 100 and 180, late CMV reactivation was observed in patients who received letermovir (24%) or valacyclovir up to day 100 (18%),21 further supporting judicious monitoring of CMV events after day 100. Overall, letermovir prophylaxis was safe and effective in CBT recipients.
Lastly, Lin et al. performed a retrospective review of letermovir use in allo-HCT recipients between January and June 201822; the majority of patients were at high risk for CMV reactivation [27 of 39 (69%)], including ex vivo T-cell-depleted grafts.22 CS-CMVi was observed in 2 of the 39 patients (5%) receiving letermovir primary prophylaxis. In addition, the median duration of letermovir use was 116 days (range, 12–221 days), with 29 patients continuing beyond day 100. Only one patient (3.4%) in this group had CS-CMVi.22 This study underlines the safety and efficacy of extended primary prophylaxis beyond day 100 with letermovir.
Clinical considerations
Since letermovir has activity in the late stages of viral replication, traditional CMV monitoring strategies with pp65 antigen or DNA detection may not be accurate biomarkers of response if the drug is used for the treatment of CMV infections beyond its indication; instead, they will detect prophylactic failures in the setting of persistently rising CMV titers. Furthermore, these biomarkers may be detectable for prolonged periods of time or have transient increases after the initiation of letermovir as therapy for CS-CMVi, contributing to the difficulty in interpreting laboratory findings.18,23 Monitoring with pp67 RNA may help in assessing response to letermovir; however, pp67 RNA is not commercially available and would need to be validated in this setting prior to implementation.24
Dosing and administration
As previously mentioned, the dose of letermovir is 480 mg (240 mg if given concomitantly with cyclosporine), administered by mouth (with or without food) or intravenously (over 1 h) once daily, starting between day 0 and 28 and continuing through day 100.15
Letermovir is minimally excreted in urine (<2%). A phase I trial determined that patients with renal impairment (moderate, 30–59 ml/min/1.73 m2, and severe, <30 ml/min/1.73 m2, using the modification of diet renal disease equation) experienced higher exposure to letermovir than did healthy subjects.25 Nevertheless, there was a weak correlation between clearance and eGFR; in addition, letermovir was well tolerated among all groups, with no adverse effects that were attributable to the drug.25 On the basis of this information, no letermovir adjustment is recommended in patients with renal impairment; however, there is insufficient data to make recommendations for patients with CrCL ⩽10 ml/min or patients on hemodialysis.15 A case report describes the use of oral letermovir, prescribed under eIND, in a patient who was on hemodialysis for the treatment of refractory CMV disease.26 The patient was initiated on 120 mg once daily, with the dose eventually increasing to 240 mg once daily. Pharmacokinetic sampling revealed no alterations in levels with hemodialysis.26 It is also recommended to use caution with patients receiving the intravenous (IV) formulation with CrCL ⩽50 ml/min because of the potential accumulation of hydroxylpropyl beta-cyclodextrin.15
Letermovir undergoes hepatic metabolism and is excreted primarily via the liver by bile (this is further described, along with its other pharmacokinetic properties, below); therefore, impaired hepatic function may affect letermovir concentration and exposure. In a phase I trial by Kropeit and colleagues,27 patients with moderate (Child Pugh Class B) and severe (Child Pugh Class C) hepatic impairment were found to have a 1.6- and 3.8-fold increase in the area under the receiver operating characteristic curve (AUC), respectively, compared with healthy subjects.27 Although generally well tolerated, lower dosages of letermovir were used compared with the determined therapeutic dose. Of note, patients with moderate or severe liver dysfunction were excluded from the phase III trial.16 Currently, there is no letermovir dose adjustment recommendation for patients with mild-to-moderate hepatic impairment, and the drug is not recommended for patients with severe hepatic impairment.15
Letermovir has a large tablet size; an oral solution, although initially evaluated in phase I studies, is not commercially available.28 Presently, it is not recommended to divide, crush, or chew the tablets because of the lack of data available on tablet manipulation15; however, letermovir tablets are formulated as immediate release, and the film coating is essentially non-functional except for appearance, which makes crushing a viable option.29 An IV solution is available that contains hydroxypropyl beta-cyclodextrin, which is an excipient that is used to increase the solubility of the drug and reduce irritation at the injection site.15,30 There was a recent shortage of the IV formulation, and, with its return, there has been an updated recommendation to administer IV letermovir with a sterile 0.2-micron polyethersulfone in-line filter and the use of IV bags and infusion set materials that are free of the plasticizer bis(2-ethylhexyl) phthalate (DEHP).31
Pharmacokinetics
Letermovir is absorbed quickly, with a median time to peak concentration of 1.5 h.28 Its bioavailability was 94% in healthy subjects, 35% in HCT patients without cyclosporine, and up to 85% in HCT patients receiving cyclosporine.15 Although food intake may decrease the rate and extent of absorption, the AUC was not affected; therefore, letermovir may be taken with or without food.15,28 Letermovir has a mean steady-state volume of distribution of 45.5 l following IV administration in HCT recipients and is 99% protein bound. It undergoes hepatic metabolism through UGT1A1/1A3 (minor), and its route of elimination is hepatic uptake through OATP1B1/3. The drug is excreted mainly in the feces (93%) as unchanged (70%), with minimal excretion in the urine.15 Lastly, letermovir has a mean terminal half-life of 12 h.15,28
Drug–drug interactions
In vitro results showed that letermovir is a substrate of CYP3A, CYP2D6, UGT1A1, UGT1A3, and the transporters P-gp and OATP1B1/3. Letermovir is considered a moderate CYP3A inhibitor and inhibits CYP2C8, P-gp, OAT3, and OATP1B1/3. It is also an inducer of CYP2C9, CYP2C19, CYP2B6, and CYP3A.15 As letermovir has the potential for a number of drug–drug interactions, given its pharmacokinetic properties, this review will focus on its interactions with immunosuppressants and antifungals that are commonly used in the HCT patient population.
Kropeit et al. reported that letermovir (80 mg twice daily) increased the exposure of cyclosporine and tacrolimus in healthy volunteers. In addition, cyclosporine was found to alter letermovir pharmacokinetics.32 A 5-phase I trial design validated and expanded on these findings; it also evaluated sirolimus and mycophenolate mofetil and the use of higher doses of letermovir for co-administration (240 mg or 480 mg once daily).33 Letermovir increased the AUC of cyclosporine, tacrolimus, and sirolimus 1.7-, 2.4-, and 3.4-fold, and the maximum plasma concentration by 1.1-, 1.6-, and 2.8-fold, respectively.33 The increases in the exposure of these immunosuppressants support the known CYP3A inhibition of letermovir. Although dose adjustments were not required for concomitant immunosuppressants in the letermovir clinical studies, judicious drug monitoring should be employed. Two recently published retrospective studies evaluating the effect of letermovir on tacrolimus and/or cyclosporine concentrations determined that empiric dose adjustments for the addition of letermovir is not warranted; however, azoles were also administered concomitantly and potentially mitigating the impact of the interactions.34,35 Cyclosporine increased the letermovir AUC and maximum plasma concentration by 2.1- and 1.5-fold, respectively; it also decreased both apparent total body clearance and volume of distribution.33 This observed effect is likely due to cyclosporine inhibiting the liver uptake transporters OATP1B1/3, which are responsible for letermovir elimination, and supports the dose adjustment to 240 mg of letermovir when given concomitantly.33 Lastly, the co-administration of letermovir with mycophenolate mofetil had no meaningful effect on the pharmacokinetics of mycophenolate mofetil, and vice versa.33
Posaconazole and voriconazole are commonly used in HCT patients to prevent opportunistic fungal infections. Two pharmacokinetic trials conducted in healthy female subjects evaluated the interactions between these azole antifungals and 480 mg of letermovir. Posaconazole’s AUC ratio and mean Cmax ratio were unchanged when it was co-administered with letermovir (0.98 and 1.11, respectively), suggesting that letermovir has no clinically meaningful effect on posaconazole concentrations.36 Conversely, when given concomitantly with letermovir, voriconazole’s mean AUC ratio decreased to 0.56 and its mean Cmax ratio decreased to 0.61. This effect is attributed to the induction of CYP2C9 and CYP2C19 by letermovir.36 In addition, two case reports have demonstrated the significance of this interaction, with a decrease in voriconazole serum concentrations when the drugs were administered concomitantly, and an increase in serum concentrations after letermovir therapy had been completed.37 As a result, voriconazole levels should be monitored with increased frequency when it is co-administered with letermovir. To our knowledge, the pharmacokinetic effect of letermovir on other antifungals, such as fluconazole, isavuconazonium, and caspofungin, have not been evaluated.
Resistance in letermovir prophylaxis
Letermovir resistance has been described in both in vitro and in vivo studies.38–43 The viral mutations that confer letermovir resistance are linked primarily to the UL56 component of the terminal complex, with mutations at UL89 and UL51 also reported.38
Both phase IIb and phase III trials characterized resistance mutations in patients with CMV breakthrough infections. Lischka et al. performed UL56 genotyping in 12 of the 15 patients considered to have virologic failure in the phase IIb study. Six amino acid substitutions were detected in five patients, including the known letermovir resistance mutation V236M in one patient in the 60-mg dose group, whereas the other variants were shown to be inert with regards to letermovir susceptibility.41 This finding supports the emergence of resistance, likely because of suboptimal dosing.
Douglas et al. performed genomic analyses of some patients enrolled in the phase III trial.43 CMV resistance among patients receiving letermovir prophylaxis was low (three patients) and included known resistance-associated variants of UL56 V236M and C325W in one patient each, in addition to the novel variants E237G and R369T.43
Letermovir cross-resistance with ganciclovir and foscarnet is unlikely, highlighting the differences in the mechanism of action.44 Pilorge et al. noted polymorphisms in UL56 and UL89 at similar rates in both resistant and non-resistant isolates from patients with resistance to ganciclovir, cidofovir, or foscarnet.44
It is important to note that, although CMV resistance in the setting of letermovir primary prophylaxis was uncommon in both clinical trials, this may not be true in the setting of treatment. Letermovir use in patients with a higher CMV viral load or CMV end-organ disease may be limited because of the emergence of resistance. An ongoing phase II trial [ClinicalTrials.gov identifier: NCT03728426] that is evaluating the use of letermovir for refractory or resistant CMV infection will provide additional information in relation to resistance to letermovir treatment.
Lessons from solid organ transplant recipients
HCT patients are a subset of the population that is at increased risk for CMV infection. The potential role of letermovir in CMV prevention in solid organ transplant recipients is an area of increasing interest. Currently, letermovir is not approved for use in the solid organ transplant population; however, a phase III, randomized, double-blind study [ClinicalTrials.gov identifier: NCT03443869] is currently recruiting adult kidney transplant recipients to evaluate letermovir versus valganciclovir for the prevention of CMV disease through 52 weeks after transplantation.
Letermovir prophylaxis in solid organ transplant recipients
Data on the use of letermovir in solid organ transplant recipients remain limited for CMV prophylaxis. The available data show mixed responses, indicating that caution is required when using letermovir off label. Chong et al. reported the successful use of letermovir for secondary prophylaxis in a heart transplant recipient with ganciclovir-resistant (UL97) CMV after an undetectable CMV viral load was achieved with foscarnet.45 On the other hand, another report noted rapid letermovir resistance after the initiation of letermovir for secondary prophylaxis in a lung transplant recipient with ganciclovir-resistant CMV infection.46
A single-center review of letermovir use in nine thoracic organ transplant recipients reported mixed results for both the prophylaxis and treatment of CMV disease (eight patients received letermovir for prophylaxis and two for treatment).47 Of the eight patients who received letermovir prophylaxis (two primary and six secondary), three developed CMV DNAemia and were considered treatment failures. Of note, most of these patients were considered to be at high risk for CMV infection because of CMV mismatches (donor+/recipient–) or a history of CMV infection in those receiving letermovir as secondary prophylaxis.47
Letermovir treatment in solid organ transplant recipients
At present, letermovir is not recommended for the treatment of CMV infection in HCT or solid organ transplant recipients; limited data exist for this treatment off label. In one of the first reported uses of letermovir for the treatment of CMV infections, compassionate use was initiated for multi-drug-resistant CMV disease in a lung transplant recipient. The initiation of letermovir at 120 mg daily, later increased to 240 mg daily for a total of 49 days, and a reduction in immunosuppression were associated with a rapid resolution of CMV disease (lungs, gastrointestinal tract, and retinas), with no observed relapse or recurrent CMV infection for >3 months after the discontinuation of treatment.26
In addition, letermovir was evaluated as pre-emptive treatment in kidney transplant recipients in a phase IIa, randomized, open-label study. A total of 27 patients with active CMV infection were randomly assigned to receive 40 mg of letermovir twice daily, 80 mg of letermovir once daily, or the local standard of care (ganciclovir or valganciclovir) for 14 days.23 All patients experienced a statistically significant decrease in CMV DNA load, but patients who received letermovir had a slower decline in viral load, which was attributed to letermovir’s mechanism of action.23 It is also unclear whether these results would have been different if letermovir had been given at higher doses, such as the currently FDA-approved dose of 480 mg for prophylaxis. Nevertheless, this exploratory proof-of-concept trial is the only study thus far that has evaluated letermovir as an alternative to standard pre-emptive therapies for CMV infections.
In the study by Aryal et al., two patients (one lung and one heart transplant recipient) were treated with letermovir for CMV DNAemia. One patient failed to respond, whereas the other patient, after a slight rise in his CMV DNA in blood, had a sustained response and remained on therapy for 10 months.47 Lastly, Turner et al. described the use of high dose letermovir (720–960 mg) as salvage therapy for ganciclovir-resistant CMV retinitis in four solid organ transplant recipients.48 Whereas all patients experienced clinical improvement, with resolution of retinitis, three had recurrence of CMV DNAemia and two developed resistance to letermovir (UL56).48
These overall mixed results highlight the need for careful consideration when using letermovir off label, especially in the setting of treatment, given concerns about the development of resistance. More clinical studies are needed to further elucidate the role of letermovir in both HCT and solid organ transplant recipients for the treatment of CMV DNAemia or CMV end-organ disease.
Future directions
Unanswered questions still exist regarding the use of letermovir as prophylaxis, including extended primary prophylaxis beyond day 100 and secondary prophylaxis.
An ongoing multi-center, phase III, double-blind, placebo-controlled clinical trial [ClinicalTrials.gov identifier: NCT03930615] is evaluating the safety and efficacy of letermovir prophylaxis extended beyond 100 days. This study focuses on delayed CMV reactivation and hypothesizes that continued letermovir prophylaxis until day 200 is superior to placebo in preventing CS-CMVi in HCT recipients who are at high risk for CMV infections beyond day 100.
Secondary prophylaxis is the initiation of letermovir after the receipt of pre-emptive therapy for CS-CMVi. Two retrospective studies have reported on the use of letermovir for secondary prophylaxis. Lin et al. described letermovir secondary prophylaxis in 14 patients, in whom letermovir was continued for a median of 125 days (range, 18–270 days), with no recurrent CMV reactivation.22 A second, larger retrospective study from the French Compassionate Program reported on letermovir secondary prophylaxis in 80 patients who had at least one CS-CMVi (infection or disease).49 Letermovir was given for a median of 118 days (range, 26–396 days). Of the 80 patients, 50 had current or previous GVHD, and 14 had CMV end-organ disease after transplant.49 Four patients developed CMV breakthrough infections, including one with CMV end-organ disease. CMV resistance testing was completed in these four patients; three had UL56 mutation C325Y or W,49 which is consistent with letermovir resistance. Both of these studies demonstrated the potential for letermovir use as secondary prophylaxis, but a prospective trial will need to be completed to further expand on the above findings.
Lastly, letermovir’s approval came 12 years after pivotal guidelines on CMV prevention and management were published; therefore, letermovir was not included.50 A more recent publication from the 2017 European Conference on Infections in Leukemia provided guidelines for CMV management in patients with hematologic malignancies and those who had undergone allo-HCT. Letermovir was recommended for primary prophylaxis (evidence from at least one properly designed randomized, controlled trial strongly supports the recommendation for use) and did not distinguish between low- and high-risk patients.51 Given limited data, no recommendation was provided for letermovir secondary prophylaxis or treatment.51 Updates to the 2009 guidelines by the American Society of Transplant and Cellular Therapy are in development, and those recommendations will help to further solidify letermovir’s current place in the management of CMV infections after HCT.
Conclusion
Letermovir’s unique mechanism of action, limited toxicity profile, and proven efficacy in CMV prevention, including its all-cause mortality benefit through week 24, has launched it to the forefront of CMV management. As we garner more experience with letermovir use, we will continue to further our understanding of its use not only as primary prophylaxis but in other areas of interest, including the prevention of late CMV reactivation with extended primary prophylaxis, secondary prophylaxis, treatment, and the prevention of resistance.
Acknowledgments
The authors would like to acknowledge and thank Scientific Publications, Research Medical Library for their thorough review of this manuscript.
Footnotes
Conflict of interest statement: R. Chemaly has served as a consultant to Oxford Immunotec, Merck, Chimerix, Shire/Takeda, Astellas, and Clinigen, and has received research funding paid to his institution from Oxford Immunotec, AiCuris, Viracor, Merck, Shire/Takeda, Chimerix, and Novartis. TL Shigle and VW Handy have no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly supported by National Institutes of Health National Cancer Institute (grant number P30CA016672).
ORCID iD: Roy F. Chemaly  https://orcid.org/0000-0002-1322-9555
https://orcid.org/0000-0002-1322-9555
Contributor Information
Terri Lynn Shigle, Division of Pharmacy, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Victoria Wehr Handy, Division of Pharmacy, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Roy F. Chemaly, Department of Infectious Diseases, Infection Control, and Employee Health, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4000, USA.
References
- 1. Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009; 113: 5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 2016; 127: 2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green ML, Leisenring W, Xie H, et al. CMV viral load and mortality after hematopoietic cell transplantation: a cohort study in the era of preemptive therapy. Lancet Haematol 2016; 3: e119–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am 2011; 25: 151–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest 2011; 121: 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Heiden P, Marijt E, Falkenburg F, et al. Control of cytomegalovirus viremia after allogeneic stem cell transplantation: a review on CMV-specific T cell reconstitution. Biol Blood Marrow Transplant 2018; 24: 1776–1782. [DOI] [PubMed] [Google Scholar]
- 7. Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis 2014; 59: 473–481. [DOI] [PubMed] [Google Scholar]
- 8. Department of Health and Human Services. PREVYMIS™(letermovir) tablet and injection: NDA approval letter, 2017. [Google Scholar]
- 9. Lischka P, Hewlett G, Wunberg T, et al. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother 2010; 54: 1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldner T, Hewlett G, Ettischer N, et al. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol 2011; 85: 10884–10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melendez DP, Razonable RR. Letermovir and inhibitors of the terminase complex: a promising new class of investigational antiviral drugs against human cytomegalovirus. Infect Drug Resist 2015; 8: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bogner E. Human cytomegalovirus terminase as a target for antiviral chemotherapy. Rev Med Virol 2002; 12: 115–127. [DOI] [PubMed] [Google Scholar]
- 13. Borst EM, Kleine-Albers J, Gabaev I, et al. The human cytomegalovirus UL51 protein is essential for viral genome cleavage-packaging and interacts with the terminase subunits pUL56 and pUL89. J Virol 2013; 87: 1720–1732.23175377 [Google Scholar]
- 14. Marschall M, Stamminger T, Urban A, et al. In vitro evaluation of the activities of the novel anticytomegalovirus compound AIC246 (letermovir) against herpesviruses and other human pathogenic viruses. Antimicrob Agents Chemother 2012; 56: 1135–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prevymis (letermovir). Prescribing information: Merck Co., Inc., Whitehouse Station, NJ: https://www.merck.com/product/usa/pi_circulars/p/prevymis/prevymis_pi.pdf (2020, accessed March 16, 2020). [Google Scholar]
- 16. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017; 377: 2433–2444. [DOI] [PubMed] [Google Scholar]
- 17. Chemaly RF, Ullmann AJ, Stoelben S, et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 2014; 370: 1781–1789. [DOI] [PubMed] [Google Scholar]
- 18. Marty FM, Ljungman PT, Chemaly RF, et al. Outcomes of patients with detectable CMV DNA at randomization in the phase III trial of letermovir for the prevention of CMV infection in allogeneic hematopoietic cell transplantation. Am J Transplant. Epub ahead of print 18 January 2020. DOI: 10.1111/ajt.15764. [DOI] [PubMed] [Google Scholar]
- 19. Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic-cell transplantation. Clin Infect Dis. Epub ahead of print 10 April 2020. DOI: 10.1093/cid/ciz490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foolad F, Shigle TL, Handy VW, et al. A single center experience of letermovir for the prevention of CMV infection in CMV-seropositive allogeneic cell transplant recipients. Biol Blood Marrow Transplant 2019; 25: S275. Abstract 396. [Google Scholar]
- 21. Sharma P, Gakhar N, MacDonald J, et al. Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation. Bone Marrow Transplant. Epub ahead of print 29 October 2019. DOI: 10.1038/s41409-019-0730-y. [DOI] [PubMed] [Google Scholar]
- 22. Lin A, Maloy M, Su Y, et al. Letermovir for primary and secondary cytomegalovirus prevention in allogeneic hematopoietic cell transplant recipients: real-world experience. Transpl Infect Dis 2019; 21: e13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stoelben S, Arns W, Renders L, et al. Preemptive treatment of Cytomegalovirus infection in kidney transplant recipients with letermovir: results of a phase 2a study. Transpl Int 2014; 27: 77–86. [DOI] [PubMed] [Google Scholar]
- 24. Hebart H, Lengerke C, Ljungman P, et al. Prospective comparison of PCR-based vs late mRNA-based preemptive antiviral therapy for HCMV infection in patients after allo-SCT. Bone Marrow Transplant 2011; 46: 408–415. [DOI] [PubMed] [Google Scholar]
- 25. Kropeit D, Scheuenpflug J, Erb-Zohar K, et al. Pharmacokinetics and safety of letermovir, a novel anti-human cytomegalovirus drug, in patients with renal impairment. Br J Clin Pharmacol 2017; 83: 1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaul DR, Stoelben S, Cober E, et al. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant 2011; 11: 1079–1084. [DOI] [PubMed] [Google Scholar]
- 27. Kropeit D, McCormick D, Erb-Zohar K, et al. Pharmacokinetics and safety of the anti-human cytomegalovirus drug letermovir in subjects with hepatic impairment. Br J Clin Pharmacol 2017; 83: 2678–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kropeit D, McCormick D, Von Richter O, et al. Phase I safety and PK data of the novel anti-HCMV terminase inhibitor AIC246. In: Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 2010. [Google Scholar]
- 29. Medication information request: crushing letermovir. Merck & Co., Inc., Global Medical Information, 2019. [Google Scholar]
- 30. Erb-Zohar K, Kropeit D, Scheuenpflug J, et al. Intravenous hydroxypropyl beta-cyclodextrin formulation of letermovir: a phase I, randomized, single-ascending, and multiple-dose trial. Clin Transl Sci 2017; 10: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Food & Drug Administration. Important Prescribing Information for PREVYMIS™ (letermovir) Injection. Merck & Co., Inc., Kenilworth, NJ, 2019. [Google Scholar]
- 32. Kropeit D, von Richter O, Stobernack HP, et al. Pharmacokinetics and safety of letermovir coadministered with cyclosporine A or tacrolimus in healthy subjects. Clin Pharmacol Drug Dev 2018; 7: 9–21. [DOI] [PubMed] [Google Scholar]
- 33. McCrea JB, Macha S, Adedoyin A, et al. Pharmacokinetic drug-drug interactions between letermovir and the immunosuppressants cyclosporine, tacrolimus, sirolimus, and mycophenolate mofetil. J Clin Pharmacol 2019; 59: 1331–1339. [DOI] [PubMed] [Google Scholar]
- 34. Maples KT, Maloy M, Devlin S, et al. Lack of a significant pharmacokinetic interaction between letermovir and calcineurin inhibitors in allogeneic HCT recipients. Bone Marrow Transplant. Epub ahead of print 14 January 2020. DOI: 10.1038/s41409-020-0785-9. [DOI] [PubMed] [Google Scholar]
- 35. Hikasa S, Shimabukuro S, Osugi Y, et al. Tacrolimus concentration after letermovir initiation in hematopoietic stem cell transplantation recipients receiving voriconazole: a retrospective, observational study. Int J Med Sci 2020; 17: 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marshall WL, McCrea JB, Macha S, et al. Pharmacokinetics and tolerability of letermovir coadministered with azole antifungals (posaconazole or voriconazole) in healthy subjects. J Clin Pharmacol 2018; 58: 897–904. [DOI] [PubMed] [Google Scholar]
- 37. Duong A, Sweet A, Jain R, et al. Clinically significant drug interaction: letermovir and voriconazole. J Antimicrob Chemother 2020; 75: 775–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chou S. A third component of the human cytomegalovirus terminase complex is involved in letermovir resistance. Antiviral Res 2017; 148: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldner T, Hempel C, Ruebsamen-Schaeff H, et al. Geno- and phenotypic characterization of human cytomegalovirus mutants selected in vitro after letermovir (AIC246) exposure. Antimicrob Agents Chemother 2014; 58: 610–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goldner T, Zimmermann H, Lischka P. Phenotypic characterization of two naturally occurring human Cytomegalovirus sequence polymorphisms located in a distinct region of ORF UL56 known to be involved in in vitro resistance to letermovir. Antiviral Res 2015; 116: 48–50. [DOI] [PubMed] [Google Scholar]
- 41. Lischka P, Michel D, Zimmermann H. Characterization of cytomegalovirus breakthrough events in a phase 2 prophylaxis trial of letermovir (AIC246, MK 8228). J Infect Dis 2016; 213: 23–30. [DOI] [PubMed] [Google Scholar]
- 42. Komatsu TE, Hodowanec AC, Colberg-Poley AM, et al. In-depth genomic analyses identified novel letermovir resistance-associated substitutions in the cytomegalovirus UL56 and UL89 gene products. Antiviral Res 2019; 169: 104549. [DOI] [PubMed] [Google Scholar]
- 43. Douglas CM, Barnard R, Holder D, et al. Letermovir resistance analysis in a clinical trial of cytomegalovirus prophylaxis for hematopoietic stem cell transplant recipients. J Infect Dis. Epub ahead of print 16 March 2020. DOI: 10.1093/infdis/jiz577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pilorge L, Burrel S, Ait-Arkoub Z, et al. Human cytomegalovirus (CMV) susceptibility to currently approved antiviral drugs does not impact on CMV terminase complex polymorphism. Antiviral Res 2014; 111: 8–12. [DOI] [PubMed] [Google Scholar]
- 45. Chong PP, Teiber D, Prokesch BC, et al. Letermovir successfully used for secondary prophylaxis in a heart transplant recipient with ganciclovir-resistant cytomegalovirus syndrome (UL97 mutation). Transpl Infect Dis 2018; 20: e12965. [DOI] [PubMed] [Google Scholar]
- 46. Cherrier L, Nasar A, Goodlet KJ, et al. Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am J Transplant 2018; 18: 3060–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aryal S, Katugaha SB, Cochrane A, et al. Single-center experience with use of letermovir for CMV prophylaxis or treatment in thoracic organ transplant recipients. Transpl Infect Dis 2019; 21: e13166. [DOI] [PubMed] [Google Scholar]
- 48. Turner N, Strand A, Grewal DS, et al. Use of letermovir as salvage therapy for drug-resistant cytomegalovirus retinitis. Antimicrob Agents Chemother 2019; 63: e00755-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robin C, Thiebaut A, Alain S, et al. Letermovir for secondary prophylaxis of cytomegalovirus infection and disease after allogeneic hematopoietic cell transplantation: results from the french compassionate program. Biol Blood Marrow Transplant. Epub ahead of print 5 February 2020. DOI: 10.1016/j.bbmt.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 50. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15: 1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European conference on infections in leukaemia (ECIL 7). Lancet Infect Dis 2019; 19: e260–e272. [DOI] [PubMed] [Google Scholar]