Abstract
Background:
Gaps in current product labels and a lack of detailed clinical guidelines leaves clinicians’ questions on the practical management of patients receiving cladribine tablets for the treatment of relapsing multiple sclerosis (MS) unanswered. We describe a consensus-based programme led by international MS experts with the aim of providing recommendations to support the use of cladribine tablets in clinical practice.
Methods:
A steering committee (SC) of nine international MS experts led the programme and developed 11 clinical questions concerning the practical use of cladribine tablets. Statements to address each question were drafted using available evidence, expert experiences and perspectives from the SC and an extended faculty of 33 MS experts, representing 19 countries. Consensus on recommendations was achieved when ⩾75% of respondents expressed an agreement score of 7–9, on a 9-point scale.
Results:
Consensus was achieved on 46 out of 47 recommendations. Expert-agreed practical recommendations are provided on topics including: the definition of highly active disease; patterns of treatment response and suboptimal response with cladribine tablets; management of pregnancy planning and malignancy risk, infection risk and immune function, and switching to and from cladribine tablets.
Conclusion:
These expert recommendations provide up-to-date relevant guidance on the use of cladribine tablets in clinical practice.
Keywords: cladribine tablets, consensus, disease modifying drugs, expert opinion, highly active disease, relapsing multiple sclerosis, switching, treatment response
Introduction
A considerable number of disease modifying drugs (DMDs) with different properties have been approved for the treatment of relapsing multiple sclerosis (RMS) over the two last decades (recently reviewed by Cree et al.).1 This evolving treatment landscape requires physicians to have a comprehensive understanding of the various treatment options, including their relevant mechanisms of action, clinical efficacy and safety, mode of administration and monitoring requirements, in order to be able to offer optimal care.2 The increasing number of treatment options also pose new challenges for neurologists, including when to start, switch and stop treatments, and other important issues around pregnancy.3
Clinical guidelines and treatment labels provide a framework, but rarely provide specific, detailed information on real-life usage, and are of limited help for individual decision making.3 This means that there are many unanswered questions about the practical use of these treatments.
Expert consensus recommendations can be developed to aid treatment decision-making when clinical recommendations are limited and there are gaps in guidance due to limited experience.4 Such consensus recommendations typically summarise opinions from an expert panel on particular, focussed topics and suggest treatment strategies based on collective knowledge and clinical experience.4
Cladribine tablets (MAVENCLAD®) are a short-course oral DMD for use in MS.5,6 At a cumulative dose of 3.5 mg/kg over 2 years, cladribine tablets were associated with significant improvement in clinical and imaging parameters in patients with highly active RMS in clinical trials (CLARITY and CLARITY Extension). Cladribine tablets were recently approved by the European Medicines Agency (EMA) and the United States (US) Food and Drug Administration (FDA), and various other regulatory authorities throughout the world.7,8 Cladribine is a deoxyadenosine analogue that selectively reduces B and T lymphocytes and is thought to interrupt the cascade of immune events central to the pathogenesis of MS.6
In order to address some of the unanswered questions relating to the use of cladribine tablets, we describe a consensus-based programme led by international MS experts with the aim of providing practical recommendations to support its use in real-life clinical practice. The questions focussed on six topics:
The definition of highly active disease;
The patterns of treatment response in patients treated with cladribine tablets;
Management of patients with evidence of disease activity while being treated with cladribine tablets;
Infection risk and immune function in patients being treated with cladribine tablets;
Management of pregnancy planning and malignancy risk in patients being treated with cladribine tablets;
Treatment switching to and from cladribine tablets and monitoring considerations.
The objective of the programme was to provide consensus-based practical recommendations on the use of Cladribine tablets in real-life practice addressing gaps not covered in current guidelines and labels.
Materials and methods
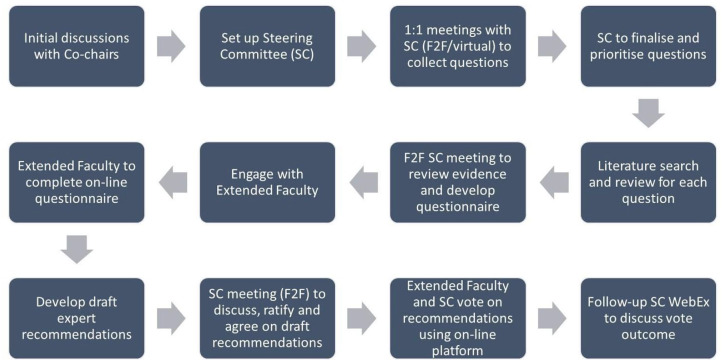
The consensus programme was based on a multi-step modified Delphi methodology, which took place between April 2018 and June 2019. The process is outlined in Figure 1. A steering committee (SC) of nine international MS experts with well-documented experience with cladribine tablets led the programme, co-chaired by P.S.S. and P.R. The SC in turn was supported by the identification of an extended faculty (EF) through the nomination of additional internationally recognised practicing neurologists with experience of caring for people with MS. A total of 76 international experts were invited to participate in the EF, with 33 taking part. A total of 19 countries were represented in the programme. The role of the EF was to review the available evidence, complete a questionnaire and finally vote on draft recommendations.
Figure 1.
Overview of the modified Delphi process for achieving consensus.
F2F, face to face; SC, steering committee.
The SC outlined the areas of clinical focus and drafted 21 clinical questions to be addressed. These were ranked using a web-based platform, resulting in 11 prioritised questions, categorised into six topics. The questions included both contextual questions, relating to the definition of highly active RMS, and practical questions, relating to the use of cladribine tablets in different clinical scenarios. A comprehensive literature review was performed using the PICO (population, intervention, comparison, outcome) framework for each of the 11 questions. The level of evidence was assessed and agreed by the SC using the GRADE (grading of recommendations assessment, development, and evaluation) level of evidence ratings scale.9
A questionnaire was developed by the SC with draft answers based on the available evidence from the literature review, combined with their expert opinion, where evidence was lacking. The questionnaire was completed remotely via the on-line platform by the EF, following a review of the available evidence. The results from the questionnaire were used to develop draft clinical recommendations, which were then voted on by the SC and EF members. Consensus was achieved when ⩾75% of respondents agreed in the range 7–9 (on a 9-point scale). Each statement/recommendation was assigned a strength score (i.e. the median score) and a level of consensus, defined as the percentage of votes with a score of 7–9.10–14
Results
In total, 47 recommendations were drafted by the SC and voted on. Consensus was achieved on 46 of these recommendations. Not all EF members voted on all questions. The exact reasons for the few abstentions were unclear but could have been due to time constraints and the length of the on-line questionnaire and voting procedure. However, this did not affect the overall strength of the recommendations. A summary of the available evidence for each topic is provided in the following, and the recommendations for each question provided in Tables 1–6.
Table 1.
Defining highly active disease.
| Consensus recommendation | Strength of recommendation‡ | Level of consensus¶ |
|---|---|---|
| Q1a. What patient baseline characteristics and activity metrics indicate highly active disease: • If patients are treatment naïve? (Level of evidence: moderate) | ||
| Clinicians should consider the following activity metrics that may indicate highly active disease in a treatment naïve patient: • 1 prior clinical relapse in the last year AND evidence of subclinical MRI activity (Gd+ or new or enlarging T2 lesions) in a patient with poor prognostic factors (clinical, MRI or biomarker) OR • 2 or more clinical relapses in the last year, with or without MRI activity |
8 (7.8) | 88.2% (30/34) |
| Q1b. What patient activity metrics indicate highly active disease and suitability for high-efficacy treatment or escalation therapy: • If patients have had an appropriate course of a DMD? (Level of evidence: moderate) | ||
| Clinicians should consider the following activity metrics that may indicate highly active disease, and suitability for high-efficacy treatment or escalation therapy, in a patient who has had an appropriate course of another DMD:* • 1 prior clinical relapse in the last year with subclinical MRI activity (Gd+ or new or enlarging T2 lesions) OR • 2 prior clinical relapses in the last year without MRI activity OR • ⩾1 Gd+ lesions or ⩾ 2 new or enlarging T2 lesions in the last 12 months |
8 (7.8) | 88.2% (30/34) |
| *A new baseline MRI scan should be taken into consideration. The timing of the re-baseline scan may vary depending on the treatment.18,19 | ||
Median score on a 1–9 scale (mean score in brackets).
Percentage of votes with 7–9 on a 9-point scale.
DMD, disease modifying drug; Gd+, presence of gadolinium; MRI, magnetic resonance imaging.
Table 2.
Patterns of treatment response in patients treated with cladribine tablets.
| Consensus recommendations | Strength of recommendation‡ | Level of consensus¶ |
|---|---|---|
| Q2a. What are the patterns of treatment response with cladribine tablets? (Level of evidence: low) | ||
| A complete or durable treatment responder to cladribine tablets is a patient with no evidence of significant clinical or radiological activity after completion of the full recommended cumulative dose.* | 8 (8.0) | 93.9% (31/33) |
| In the absence of new disease activity in year 3, 4, or beyond, a patient is not a candidate for treatment switch to another DMD. | 9 (8.5) | 97.0% (32/33) |
| *A new baseline MRI scan should be taken into consideration. • Refer to Question 1b for the threshold of clinical or radiological activity in a patient following an appropriate course of a DMD that indicates a suboptimal responder | ||
| Q2b. What are the patterns of suboptimal response with cladribine tablets? (Level of evidence: low) | ||
| A patient with worsening or unchanged disease activity during the first 2 years of treatment with cladribine tablets, should be considered as a putative non- or suboptimal responder and is a candidate for treatment with a high-efficacy DMD. | 8 (7.6) | 84.8% (28/33) |
| • Refer to Question 1b for the threshold of clinical or radiological activity in a patient following an appropriate course of a DMD that indicates a suboptimal responder • Refer to Question 10 for ‘How to switch from cladribine tablets’ | ||
Median score on a 1–9 scale (mean score in brackets).
Percentage of votes with 7–9 on a 9-point scale.
DMD, disease modifying drug.
Table 3.
Managing patients with evidence of disease activity while being treated with cladribine tablets.
| Consensus recommendations | Strength of recommendation‡ | Level of consensus¶ |
|---|---|---|
| Q3a. How would you manage a patient who has taken the first course of cladribine tablets but has evidence of new disease activity in year 1? (Level of evidence: moderate) | ||
| After the first treatment course of cladribine tablets in year 1, a patient with disease activity less than pre-treatment levels, might not necessarily be an indication for treatment discontinuation.*19,29 | 8 (8.3) | 97.0% (32/33) |
| Corticosteroids should be used to treat the relapse according to local guidelines. Clinicians may wait and monitor the patient and provide cladribine tablets at the beginning of year 2 in order to allow the patient to receive the recommended cumulative dose. | 9 (8.4) | 97.0% (32/33) |
| *Disease activity in the first 3–6 months of treatment with cladribine tablets may be a carry-over from a patient’s prior treatment, especially for those switching from lymphocyte trafficking agents (fingolimod or natalizumab). | ||
| Q3b. How would you manage a patient who has worsening disease activity during the first two years of treatment with cladribine tablets? (Level of evidence: very low) | ||
| During the first two years of treatment with cladribine tablets, a patient with increasing disease activity above pre-treatment levels, may be a candidate for a treatment switch to another high-efficacy DMD.* | 8 (8.0) | 87.9% (29/33) |
| Corticosteroids should be used to treat relapses according to local guidelines. | 9 (8.7) | 97.0% (32/33) |
| *Disease activity in the first 3–6 months of treatment with cladribine tablets may be a carry-over from a patient’s prior treatment, especially for those switching from lymphocyte trafficking agents (fingolimod or natalizumab). • Refer to Question 1b for the threshold of clinical or radiological activity in a patient following an appropriate course of a DMD that indicates a suboptimal responder • Refer to Question 10 for ‘How to switch from cladribine tablets’. | ||
| Q4a. How would you manage a patient who has taken the indicated two courses of cladribine tablets but has evidence of new/reappearing disease activity only in year 3–4? (Level of evidence: low) | ||
| Clinicians should consider a switch to another high-efficacy DMD in a patient with a complete but non-durable response to cladribine tablets with evidence of new/reappearing disease activity in year 3–4 | 7 (6.7) | 60.6% NOT ACHIEVED |
| Clinicians should consider treatment options and associated risks and discuss with the patient. | 9 (8.6) | 100% (33/33) |
| • Refer to Question 1b for the threshold of clinical or radiological activity in a patient following an appropriate course of a DMD that indicates a suboptimal responder • Refer to Question 10 for ‘How to switch from cladribine tablets’ | ||
| Q4b. How would you manage a patient who has taken the indicated two courses of cladribine tablets but has evidence of new/reappearing disease activity only beyond year 4? (Level of evidence: low) | ||
| Treatment options for a patient with a complete but non-durable response to cladribine tablets with evidence of new/reappearing disease activity beyond year 4 could include: • Consideration of a switch to another high-efficacy DMD after thorough risk/benefit analysis. • Consideration of re-initiation with cladribine tablets, after thorough risk/benefit analysis. • Benefit of additional treatment with cladribine tablets in response to disease activity beyond year 2 has not been investigated. The incidence of lymphopenia and other adverse events is increased with additional treatment in years 3 or 4. Re-initiation of therapy after year 4 has also not been investigated. • Clinicians should consider treatment options and associated risks and discuss with the patient. |
8 (8.3) | 97.0% (32/33) |
Median score on a 1–9 scale (mean score in brackets).
Percentage of votes with 7–9 on a 9-point scale.
DMD, disease modifying drug.
Table 4.
Infection risk and immune function in patients being treated with cladribine tablets.
| Consensus recommendations | Strength of recommendation‡ | Level of consensus¶ |
|---|---|---|
| Q5a. How are patients with severe lymphopenia on cladribine tablets managed? (Level of evidence: moderate/low) | ||
| A patient with grade 3 or 4 lymphopenia on cladribine tablets may be at an increased risk of infection and should be actively monitored for signs and symptoms of infections. Clinicians should consider appropriate prophylactic treatment based on the individual patient’s risk. | 8 (8.2) | 93.8% (30/32) |
| A patient with grade 3 or 4 lymphopenia should be actively monitored for signs and symptoms particularly suggestive of herpes zoster. A patient should also be informed about the signs and symptoms of herpes zoster. If such signs and symptoms occur, anti-viral treatment should be initiated immediately. | 9 (8.6) | 96.9% (31/32) |
| Q5b. Do patients with severe lymphopenia on cladribine tablets need anti-viral prophylaxis against herpes zoster? Which anti-herpes therapy should be used prophylactically? (Level of evidence: moderate/low) | ||
| Initiation of anti-viral prophylaxis with a licenced anti-viral drug should be recommended in a patient with grade 4 lymphopenia.* | 8 (7.7) | 84.4% (27/32) |
| Initiation of anti-viral prophylaxis with a licenced anti-viral drug may be considered in a patient with grade 3 lymphopenia. Special consideration should be given to any patient at risk of herpes zoster infection such as elderly patients.*,** | 8 (7.4) | 75% (24/32) |
| Anti-viral prophylaxis should be maintained until severity of lymphopenia is reduced. | 8 (7.7) | 83.9% (26/31) |
| Vaccination with Shingrix may be considered for any patient at increased risk of herpes zoster infection (for example those with age ⩾50, previous herpetic exacerbations) | 8 (7.4) | 81.3% (26/32) |
| *Anti-viral prophylaxis could include: 200 mg acyclovir/day, 400 mg acyclovir/day, or 500 mg valaciclovir/day **Grade 3 lymphopenia was more common in year 2 of the CLARITY study, and duration of lymphopenia was longer. Zoster infection is more common in older patients. The history of the patient should be taken into consideration including the patient age, prior duration of lymphopenia and previous infection with varicella zoster virus. • Refer to Questions 6 and 7 for recommendations on vaccinations | ||
| Q6. What vaccinations are recommended as part of the de-risking strategy before patients are initiated with cladribine tablets? (Level of evidence: very low) | ||
| Clinicians should review a patient’s vaccination status before initiation with cladribine tablets and consult their local vaccination guidelines.* | 9 (8.4) | 93.8% (30/32) |
| Vaccination for varicella zoster virus is recommended in any antibody-negative patient prior to initiation of cladribine therapy. | 9 (8.7) | 96.9% (31/32) |
| *A review of a patient’s vaccination status includes the patient history and may also include a check of antibody titres. | ||
| Q7. How do you manage vaccinations after treatment with cladribine tablets; inactivated component vaccines versus live attenuated vaccines? (Level of evidence: low) | ||
| Cladribine tablets should not be initiated within 4–6 weeks after vaccination with live or attenuated live vaccines. | 9 (8.6) | 100% (31/31) |
| Any use of live attenuated vaccines should be avoided during treatment with cladribine tablets. Users should wait for the leukocytes/lymphocytes to return to normal wherever possible. | 9 (8.4) | 96.8% (30/31) |
| If an inactivated component vaccination is essential for a patient, clinicians should wait for the lymphocyte levels to return to within the normal range. | 8 (7.3) | 77.4% (24/31) |
| For certain multi-dose vaccinations,* clinicians may consider giving the first dose of the vaccine 4–6 weeks before treatment initiation with cladribine tablets. Subsequent vaccine dose(s) should be given at a later date, after initiation with cladribine tablets, once lymphocyte counts have recovered | 8 (8.1) | 93.5% (29/31) |
| *Relevant multi-dose vaccinations include those for HBV, HPV, VZV, measles, pneumococcus | ||
| Q8. How should latent or active infections be managed before initiation of cladribine tablets? [e.g. positive PPD/Quantiferon test for TB, HPV (cervical screening), HBV/HCV test, PML] (Level of evidence: low) | ||
| Cladribine tablets are contraindicated in a patient with HIV or an active chronic infection (e.g. HBV, HCV, VZV, Syphilis, TB, PML etc.), and a delay in initiation of cladribine tablets should be considered in a patient with an acute infection until the infection is fully controlled.* • In any case of infection (latent or active), a relevant specialist should be contacted (e.g. infectious disease, pulmonologist, hepatologist etc.). • The infection should be diagnosed, managed, and treated according to local guidelines. |
9 (8.5) | 96.8% (30/31) |
| Screening for PML is recommended in any patient previously treated with natalizumab, particularly those who are JCV antibody positive, and a baseline MRI (within 3 months) should be performed before initiation of cladribine tablets. Additional CSF analysis should be considered | 9 (7.7) | 83.3% (26/31) |
| *Clinicians should consider a patient’s prior treatment since those switching from a DMD associated with lymphopenia, may be at an increased risk from latent infections. | ||
Median score on a 1–9 scale (mean score in brackets).
Percentage of votes with 7–9 on a 9-point scale.
CSF, cerebrospinal fluid; DMD, disease modifying drug; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; JCV, John Cunningham virus; MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy; PPD, purified protein derivative; TB, tuberculosis; VZV, varicella-zoster virus.
Table 5.
Pregnancy planning management and malignancy risk in patients being treated with cladribine tablets.
| Consensus recommendations | Strength of recommendation‡ | Level of consensus¶ |
|---|---|---|
| Q9a. How should pregnancy planning be managed in patients on cladribine tablets? (Level of evidence: very low) | ||
| Based on human experience with other substances inhibiting DNA synthesis, cladribine tablets could cause congenital malformations when administered during pregnancy. Studies in animals have shown reproductive toxicity. There is very limited pregnancy data from the clinical trial programme. | 8.5 (8.3) | 96.9% (31/32) |
| Cladribine tablets are contraindicated and should not be administered during pregnancy. Subsequent courses of cladribine tablets may be delayed during this time. | 9 (8.8) | 100% (32/32) |
| Breast-feeding is contraindicated during dosing with cladribine tablets and for 10 days after the last dose. | 9 (8.5) | 93.8% (30/32) |
| Before initiation of treatment both in year 1 and year 2, women of childbearing potential and males who could potentially father a child should be counselled regarding the potential for risk to the foetus and the need for effective contraception for at least 6 months after the last dose of cladribine tablets.* | 8 (8.4) | 100% (32/32) |
| Any unforeseen pregnancy within 6 months after the last dose of cladribine tablets is not necessarily an indication for a termination of the pregnancy. Any further administrations of cladribine tablets should, however, be discontinued immediately or delayed in this event. Patients should be counselled about potential risks to the foetus and referred to a high-risk pregnancy clinic. | 9 (8.5) | 96.9% (31/32) |
| *It is currently unknown whether cladribine may reduce the effectiveness of systemically acting hormonal contraceptives. Therefore, women using systemically acting hormonal contraceptives should add a barrier method during cladribine treatment and for at least 4 weeks after the last dose in each treatment year.24 | ||
| Q9b. Do cladribine tablets result in an increased risk of malignancy? (Level of evidence: moderate) | ||
| Cladribine tablets may increase the risk of malignancies, as seen with other high-efficacy DMDs. • There was a higher incidence of malignancies in clinical studies and long-term follow up of patients treated with a cumulative dose of 3.5 mg/kg cladribine tablets compared with placebo*; however, when compared with a matched reference population, there was no evidence for an increased risk.** |
8 (7.7) | 86.7% (26/30) |
| Clinicians should instruct patients to observe the standard guidelines for cancer screening.*** | 9 (8.5) | 100% (30/30) |
| Cladribine tablets are contraindicated in patients with an active malignancy. | 9 (8.2) | 90.0% (27/30) |
| *Included all studies that used cladribine tablets monotherapy, matching the recommended dose: CLARITY, CLARITY EXT and ORACLE-MS + follow-up in PREMIERE. **The rate of malignancies observed with cladribine tablets during the clinical development programme in MS was similar to the expected rate in the GLOBOCAN reference population [8.00 observed events in the monotherapy oral cohort versus 8.27 expected events, respectively; SIR: 0.97 (95% CI 0.44, 1.85)]. Non-melanoma skin cancer was excluded due to inconsistent reporting in GLOBOCAN. Data is adjusted for country of origin, age and gender.30 ***Physicians should direct patients to country-specific screening regimens, which may be found on cancer society or local health authority web sites. | ||
median score on a 1–9 scale (mean score in brackets).
percentage of votes with 7–9 on a 9-point scale.
CI, confidence interval; DMD, disease modifying drug; MS, multiple sclerosis; SIR, standardised incidence ratio.
Table 6.
Treatment switching to and from cladribine tablets and monitoring considerations.
| Consensus recommendations | Strength of recommendation‡ | Level of consensus¶ |
|---|---|---|
| Q10. When switching to cladribine tablets, what are the washout periods/baseline requirements for different DMDs? Are there any specific treatment classes that preclude cladribine tablets as a next switch?* (Level of evidence: very low) | ||
| Switch decisions should be made after a thorough risk/benefit analysis.19,29 | 9 (8.8) | 100% (31/31) |
| Due to a lack of clinical evidence for treatment switches in MS, caution should be taken in a patient who is switching from a prior treatment due to adverse events that may also occur with cladribine tablets. | 9 (8.1) | 90% (28/31) |
| *Due to a lack of clinical evidence for treatment switches in MS, recommendations are based on individual treatment risks or carry-over risks. | ||
| Glatiramer acetate/Interferon-beta • Possible treatment effects on blood (e.g. lymphopenia, leukopenia, thrombopenia), and/or liver and kidney parameters should have subsided • Recommended safety interval: none generally required |
9 (8.7) | 100% (31/31) |
| Dimethyl fumarate • Possible treatment effects on the differential blood count, should have subsided following the last dose of dimethyl fumarate • Possible additional treatment effects on blood (e.g. severe lymphopenia), liver/kidney parameters should have subsided • Recommended safety interval: none generally required |
9 (8.4) | 93.5% (29/31) |
| Teriflunomide • Possible treatment effects on the immune system and liver values should have subsided • Washout necessary – it must be documented that teriflunomide is no longer detectable in the blood • Recommended safety interval: normally around 4 weeks |
8 (7.9) | 90.3% (28/31) |
| Fingolimod • Possible treatment effects on the differential blood count, should have subsided following the last dose of fingolimod. There should be no cytopenia. • Possible treatment effects on other blood parameters and liver values, as well as vital signs, should have subsided • Recommended safety interval: normally around 4 weeks |
8 (7.7) | 80% (25/31) |
| Natalizumab • Possible effects on the immune system (e.g. lymphocytosis, cytopenia) should have subsided • PML should be excluded (e.g. MRI including FLAIR sequence immediately before start of treatment). A CSF examination including a JCV-PCR should be considered beforehand in patients with positive JCV antibody status and a treatment duration of >12 months) • Recommended safety interval: normally around 4–8 weeks |
8 (7.8) | 90.3% (28/31) |
| Alemtuzumab • Possible treatment effects on the immune system (e.g. cytopenia) should have subsided (lymphocyte typing is optional e.g. T and B cells) • Clinical and laboratory monitoring (including platelets, creatinine, TSH and urine sediment) must be continued for 4 years following the last alemtuzumab infusion • Recommended safety interval: normally around 6–12 months |
8 (8.3) | 96.8% (30/31) |
| Ocrelizumab • Differential blood count must be ascertained before treatment initiation (lymphocyte typing is optional e.g. • CD19+ B cells) • Any treatment effects on the immune system (e.g. cytopenia) should have subsided • Recommended safety interval: normally around 6–12 months |
8 (8.0) | 93.1% (27/29) |
| Q11. How do you switch from cladribine tablets? What DMDs can patients use after cladribine tablets? If the patient’s lymphocyte counts have not recovered to LLN but a treatment switch is required, what is the recommended course of action? (Level of evidence: very low) | ||
| Potential additive effects on the immune system should be considered when choosing subsequent DMDs following treatment with cladribine tablets. | 9 (8.5) | 93.5% (29/31) |
| Treatment-specific effects on lymphocyte counts should have ideally subsided before switching from cladribine tablets. | 8 (8.4) | 100% (31/31) |
| The waiting time is defined by the clinical need to switch. Cases of treatment non-response should be decided on an individual risk/benefit analysis. | 9 (8.6) | 100% (31/31) |
| Caution is recommended in switching from cladribine tablets to natalizumab in any patient who is JCV antibody positive. | 9 (8.4) | 93.5% (29/31) |
Median score on a 1–9 scale (mean score in brackets).
percentage of votes with 7–9 on a 9-point scale.
CSF, cerebrospinal fluid; DMD, disease modifying drug; FLAIR, fluid-attenuated inversion recovery; JCV, John Cunningham virus; LLN, lower limit of normal; MRI, magnetic resonance imagining; MS, multiple sclerosis; PCR, polymerase chain reaction; PML, progressive multifocal leukoencephalopathy; TSH, thyroid stimulating hormone.
Definition of highly active disease
Currently, there is no universally accepted definition of highly active MS, yet many MS treatments include this in their labelled indication. Clinical trials lack consistent definitions for ‘highly active’ MS in subgroup analyses (reviewed by Fernandez).15 In general, patients with highly active MS will have frequent relapses and/or an increasing burden of brain magnetic resonance imaging (MRI) lesions.16 Other features that may be taken into consideration include severity and localization of relapses, clinical or MRI disease activity on treatment, burden of T2 lesions and presence of gadolinium (Gd+) enhancing lesions.
The consensus recommendation for the definition of highly active MS is provided in Table 1 for both treatment naïve patients (Question 1a), and those who have received an appropriate course of a DMD (Question 1b). Additional factors associated with poor prognosis may also be taken into consideration, alongside activity metrics, when deciding whether to initiate a high-efficacy therapy in treatment naïve patients with MS including those with ⩾1 spinal cord lesions, incomplete recovery from relapses, accrual of physical or cognitive impairment and a short inter-attack interval.16,17
Patterns of treatment response in patients treated with cladribine tablets
Clinical judgement about response to treatment and acceptance of any on-treatment disease activity is often subjective and can vary between individual physicians. There is no consensus on the definition of treatment failure, or suboptimal treatment response in MS, nor indeed specifically for patients receiving cladribine tablets.
There are published decision models that may support the identification of patients failing on current treatment including the Canadian MS Working Group model20 and the multifactorial MS decision model.21 Both of these use a traffic light-based system to flag whether a change of therapy should be considered for a given patient. However, there has been no wide implementation of these models across the MS community.
The European Academy of Neurology/European Committee of Treatment and Research in Multiple Sclerosis (EAN/ECTRIMS) recommendation suggests ‘combining MRI with clinical measures to evaluate disease evolution’.18 The American Academy of Neurology (AAN) recommends including ⩾1 relapses, ⩾2 MRI lesions or increased disability when assessing suboptimal treatment response.19 There is a strong debate in the MS community whether physicians should treat to ‘no evidence of disease activity’ (NEDA), defined as an absence of relapses, disability worsening and MRI activity.17,22,23 However, NEDA can be difficult to sustain in the long term, and can depend on the mechanism of action of the DMD and timing of the re-baseline scan.22,23
Consensus recommendations for the definitions of optimal (Question 2a) and suboptimal (Question 2b) treatment responses with cladribine tablets are provided in Table 2.
Management of patients with evidence of disease activity while being treated with cladribine tablets
Disease activity in the first 2 years
The full recommended dose of cladribine tablets is 3.5 mg/kg given as two courses 12 months apart.24 Following completion of the two treatment courses in years 1 and 2, The patient is observed in years 3 and 4 without any additional planned treatment with cladribine tablets. Any patient with residual activity in the first 12 months should receive the full dose of cladribine tablets, since a lower cumulative dose appears to result in significantly lowered efficacy.5 In CLARITY, 56.1% of patients receiving cladribine tablets were disease activity-free during year 1 (defined as having no relapses, no 6-month sustained change in EDSS score, no new T1 gadolinium-enhancing lesions and no active T2 lesions).25
When considering disease activity in the first year, the timing of the maximal effect of cladribine tablets on B and T cells may need to be considered.24 This is recognised in the AAN guidelines, which state that relapses or new MRI lesions may develop after initiation of a disease-modifying therapy but before it becomes effective.19 Across clinical studies, the largest proportion of patients with grade 3 or 4 lymphopenia was seen 2 months after the first cladribine dose in each year. The greatest reduction in lymphocyte count is reached at 4 months after the first dose.7,24 It is a possibility that residual disease activity at less than 6 months may indicate that the maximum effect on lymphocytes has not been reached.
The CLARITY study protocol allowed relapses in years 1–2 to be treated as per clinical practice with short-term systemic corticosteroid therapy. This occurred in 23% of patients receiving cladribine tablets versus 46% of patients receiving placebo [odds ratio (OR) 95% confidence interval (CI): 0.34 (0.26, 0.46) p < 0.001].7,26,27
Disease activity in years 3, 4 or beyond
Cladribine tablets are efficacious in patients with MS for up to 4 years after the initial dose in clinical trials; re-initiation of therapy after year 4 has not been investigated.24 The CLARITY extension study (CLARITY EXT) was a safety study in which 867 patients from the original CLARITY trial were enrolled. Of these, 98 patients who received cladribine tablets during CLARITY, received placebo in CLARITY EXT (CP group), and no further doses of cladribine tablets, and 186 patients received an additional two treatment courses of cladribine tablets, 12 months apart in years 3 and 4 (CC group; 7 mg/kg cumulative dose). All efficacy analyses were exploratory. The incidence of lymphopenia events during CLARITY EXT increased in the CC group compared with the CP group (36.6% versus 9.2%, respectively).8 Furthermore, there was an increase in lymphopenia leading to discontinuation (11.8% versus 0%, respectively). However, these findings occurred in the absence of the requirement to achieve a lymphocyte count of at least 800 cells/mm3 before initiation of cladribine tablets in year 2, as per current practice.24 In patients with ⩾800 cells/mm3 prior to administration of subsequent courses in years 2, 3 and 4, the incidence of lymphopenia dropped to 11% and 12% in years 3 and 4, respectively.28 During the extension period, 75.6% of patients in the CP group stayed relapse free, compared with 81.2% of patients in the CC group.8 Additional exploratory MRI analyses demonstrated that cladribine tablets had a durable effect on MRI outcomes in the majority of patients, an effect that was sustained up to the end of the extension period.
Consensus recommendations for the management of patients with evidence of disease activity during: Year 1 (Question 3a); the first 2 years (Question 3b); in years 3 or 4 (Question 4a); or beyond year 4 (Question 4b) while being treated with cladribine tablets are provided in Table 3. Consensus was not achieved for one recommendation in answer to Question 4a. The reasons provided by the EF for this was that they would consider re-treatment with cladribine tablets in this instance. Re-treatment in year 3 or 4 has not been formally investigated in a clinical trial setting, in addition to an increased incidence of lymphopenia and other adverse events following additional cladribine tablets treatment in year 3 or 4 in the CLARITY EXT study was observed.
Infection risk and immune function in patients being treated with cladribine tablets
Lymphopenia and infection risk (including herpes zoster)
A pooled safety analysis showed the incidence of infection was similar between cladribine tablet-exposed cohort and placebo groups, except for herpes zoster.30 Infections or infestations, including herpes zoster were more common in patients receiving cladribine tablets during periods with Grade 3 or 4 lymphopenia.30
Guidance on the management of lymphopenia and herpes zoster is provided in the Mavenclad Summery of Product Characteristics (SmPC) and in the German Competence Network guidance.24,31 Consensus recommendations on the management of lymphopenia and infections in patients on cladribine tables are provided in Table 4 (Question 5). Whereas consensus was reached on recommendations regarding use of antiviral prophylaxis in patients with lymphopenia (Question 5b), five EF members (16%) did not agree that antiviral treatment should be considered in patients with grade 4 lymphopenia. Reasons provided for this included ‘a lack of indication for prophylaxis with anti-viral treatments in patients with lymphopenia’, ‘no scientific evidence’, ‘more evidenced on risk/benefit ratio is needed for “continuous” antiviral therapy’, ‘lack of evidence based documentation for efficacy and duration of anti-viral prophylaxis’ and ‘lack of sufficient evidence that prophylactic antivirals significantly reduces the risk of shingles to justify routine use’.
Vaccinations
There are no comprehensive studies available on vaccines and cladribine tablets, and the effects of cladribine tablets on the immunological memory acquired by previous vaccinations have not been studied. Certain vaccines may be recommended by physicians to their patients before initiation of cladribine tablets as part of a de-risking strategy; however, this may vary by physician and by country.
Guidance on vaccinations is provided in the Mavenclad SmPC and in the German Competence Network guidance.24,31 Consensus recommendations on vaccinations for patients receiving cladribine tables are provided in Table 4 (Questions 6 and 7).
Management of latent or active infections
Active infection with HIV, tuberculosis or hepatitis must be excluded before initiation of cladribine tablets.24 Latent infections may be activated upon treatment with cladribine tablets, therefore screening must be performed.24
Guidance on the management of latent and active infections is provided in the Mavenclad SmPC and in the German Competence Network guidelines.24,31 Guidance on John Cunningham virus activation and screening for progressive multifocal leukoencephalopathy is also provided in EAN/ECTRIMS recommendations.18 Consensus recommendations for the management of latent or active infections are provided in Table 4 (Question 8).
Management of pregnancy planning and malignancy risk in patients being treated with cladribine tablets
Management of pregnancy planning
There are no clinical studies that have investigated the effect of cladribine tablets on pregnancy outcomes. There are limited data from the cladribine clinical programme on outcomes from women exposed to cladribine (n = 44) and from women whose partners had been exposed to cladribine (n = 10); however, the numbers are too small to draw conclusions.32
Guidance on the management of pregnancy planning is provided in the Mavenclad SmPC and in the German Competence Network guidelines.24,31 Consensus recommendations on the management of pregnancy planning are provided in Table 5 (Question 9a). Two EF members did not strongly agree with the recommendation on breast feeding, both giving a score of 6. The reason provided for not scoring in the range 7–9 was the duration of abstaining from breast feeding; 7 days was seen to be sufficient.
Risk of malignancy with cladribine tablets
In a pooled analysis of clinical studies and long-term follow up of patients treated with cladribine tablets 3.5 mg/kg, events of malignancies were observed more frequently in cladribine tablets-treated patients compared with patients who received placebo (0.29 versus 0.15 events per 100PY, respectively) (included all studies that used cladribine tablets monotherapy, matching the recommended dose: CLARITY, CLARITY EXT and ORACLE-MS, plus follow-up in PREMIERE).30 However, in an independent analysis of key MS clinical trials, there was no observed increased incidence of malignancy associated with cladribine tablets, and the incidence rate was similar to that reported for other DMDs.30,33 Furthermore, the incidence of malignancies observed with cladribine tablets 3.5 mg/kg was almost identical to the expected rate of malignancies from the GLOBOCAN matched reference population (0.97, 95% CI 0.44–1.85).30 There was no increase in malignancies over time in patients treated with cladribine tablets, and no increase in the types of malignancies known to be associated with severe immunosuppression (e.g. non melanoma skin cancer, virally associated tumours and haematological malignancies).30
Consensus recommendations regarding the risk of malignancy with cladribine tablets are provided in Table 5 (Question 9b). Consensus was achieved on all three recommendations around the risk of malignancy. Three EF members (10%) did not vote in the range 7–9 regarding contraindicated use of cladribine tablets in active malignancy. Two of these EF members provided a score of 5 (neither agree nor disagree) and one gave a score of 3. Reasons for this included ‘there is no evidence that cladribine tablets are contraindicated in active malignancy – it should be at clinician’s discretion depending upon the malignancy’ and ‘I would think it would depend on the type of malignancy’. One voting expert gave no reason for their allocated score.
Treatment switching to and from cladribine tablets and monitoring considerations
Switching to cladribine tablets
There are no randomised clinical studies investigating a switch in DMD to cladribine tablets. However, there are currently a number of treatments for RMS with different mechanisms of action that are candidates for treatment sequencing with cladribine tablets; therefore, advice on switching is necessary and important.
Specific guidance on switching to cladribine tablets is limited. The German Competence Network provide the most comprehensive guidance to date, detailing individual treatments and suggested washout/safety interval times.31 This has been used as a basis for the development of the consensus recommendations on switching to cladribine tablets as provided in Table 6 (Question 10), which may act as a guide to support switching decisions; however, individual patients may vary and decisions should be made on the specific case.
Switching from cladribine tablets
There are no Class 1 clinical trials specifically investigating a treatment switch from cladribine tablets to other DMDs. In patients treated with cladribine 3.5 mg/kg in CLARITY, approximately 75% remained relapse-free when given placebo during CLARITY Extension.8 Indeed, in one study, 4 years after the last dose of cladribine tablets 3.5 mg/kg, approximately 25% of patients had switched to another DMD.34 Across the clinical development programme, 21.5% (124/576) of patients had a record of using another DMD post-treatment with cladribine tablets; the majority of these (56%) received treatment with interferon β-1a.34
There are very few data on the outcomes of patients who have switched from cladribine tablets to other DMDs, coming only from real-world observations, therefore guidance on the management of this switch is lacking. The PREMIERE long-term follow-up registry includes some patients that have been treated with other DMDs following treatment with cladribine tablets (n = 941).5 In a snapshot analysis before the study end, the highest proportion of patients switched to IFN β: 23.0%, followed by glatiramer acetate: 9.7%.5 No specific pattern in the reported serious adverse events and no unexpected safety findings were observed.
Consensus recommendations for switching from cladribine tablets to another DMD are provided in Table 6 (Question 11).
Conclusion
The recommendations described here are the collective opinions of an international group of MS experts. The SC identified and prioritised a number of essential questions concerning the practical use of cladribine tablets in real-life clinical situations. Recommendations were drafted based on a review of the literature and expert opinion and were voted on through a rigorous and transparent process.
The strengths of the recommendations provided here are the result of the large number of experts involved (both SC and EF) and the breadth of geographical representation. Limitations include the lack of available class 1 evidence to support the development of many recommendations.
The recommendations reflect status of knowledge in 2019 and will be updated in a timely fashion when new evidence and/or novel data emerges. The consensus recommendations should provide practical, specific advice to all health-care providers (involved in the treatment and management of patients with MS, address gaps in existing guidance and ultimately improve care.
Acknowledgments
Emma East of Vivid Medical Communications provided editorial assistance, funded by Merck KGaA, Darmstadt, Germany. The SC would like to thank all the experts who contributed their knowledge to this programme by completing the questionnaire and voting on the draft recommendations. Listed below are 29 EF members who are happy to be cited in this document:
Mohammed Al Jumah1, Thomas Berger2, Alexey Boyko3, Helmut Butzkuven4, Marinella Clerico5, Joao Correia de Sa6, Sara Eichau7, Juha-Pekka Erälinna8, Oscar Fernández9, Mark Freedman10, Claudio Gasperini11, Joachim Havla12, Eva Havrdová13, Dana Horáková14, Gunnar Juliusson15, Juerg Kesselring16, Natalia Khachanova17, Joanna Kitley18, Myhr Kjell-Morten19, Marcello Moccia20, Ester Moral21, Thomas Mueller22, Jiwon Oh23, David Paling24, Tobias Ruck25, Johann Sellner26, Aksel Siva27, Natalia Totolyan28, Tjalf Ziemssen29
1National Neuroscience Institute, King Fahad Medical City (KFMC), Riyadh, Kingdom of Saudi Arabia
2Department of Neurology, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria
3Department of Neurology, Neurosurgery & Medical Genetics of the Pirogov Russian National Research Medical University (RNRMU), Department of Neuroimmunology of the Federal Center of Cerebrovascular Pathology and Stroke, Moscow, Russia
4MS and Neuroimmunology Unit, Department of Neuroscience, Alfred Centre, Monash University, Melbourne, Australia
5Department of Clinical and Biological Sciences, University of Torino, Torino, Italy
6CHLN Hospital de Santa Maria, Faculdade Medicina, Lisboa, Portugal
7Multiple Sclerosis Unit (UEMAC), Virgen Macarena Hospital, Seville, Spain
8University of Helsinki, Finland and University of Turku, Finland
9Instituto de Investigación Biomédica de Málaga (IBIMA), Hospital Regional Universitario de Málaga, Málaga 29010, Spain
10Multiple Sclerosis Research Unit, Professor of Neurology, Division of Neurology, Department of Medicine, The Ottawa Hospital, Canada
11Department of Neuroscience, San Camillo Forlanini Hospital, 00152 Rome, Italy
12Institute of Clinical Neuroimmunology, Ludwig-Maximilians University, Marchioninistr. 15, 81377, Munich, Germany
13Department of Neurology and Center of Clinical Neuroscience, First Faculty of Medicine Charles University and General University Hospital, Katerinska 30, Prague 2, 12000, Czech Republic
14Department of Neurology and Center of Clinical Neuroscience, First Faculty of Medicine Charles University and General University Hospital, Katerinska 30, Prague 2, 12000, Czech Republic
15Department of Hematolog, Skåne University Hospital, Lund University, Lund, Sweden
16Department of Neurology & Neurorehabilitation, Kliniken Valens, Taminaplatz 1, CH-7317 Valens, Switzerland
17Russian National Research Medical University, Department of Neurology, Neurosurgery and Medical Genetics, Moscow, Russia
18Wessex Neurological Centre, Southampton General Hospital, Southampton, SO16 6YD, UK
19Department of Neurology, Haukeland University Hospital, and the Department of Clinical Medicine, University of Bergen, Bergen, Norway
20Department of Neurosciences, Federico II University of Naples, Italy
21Department of Neurology, Hospital de Sant Joan Despí Moises Broggi, Sant Joan Despí, Spain
22Department of Neurology, St. Joseph Hospital Berlin-Weißensee, Gartenstr. 1, 13088 Berlin, Germany
23St. Michael’s Hospital, Keenan Research Centre of the Li Ka Shing Knowledge Institute, Division of Neurology, University of Toronto, Canada
24Royal Hallamshire Hospital, Sheffield and NIHR Sheffield Biomedical Research Centre (BRC), University of Sheffield, Sheffield, UK
25Department of Neurology with Institute of Translational Neurology, University Hospital Muenster, Albert-Schweitzer-Campus 1, 48149 Muenster, Germany
26Neurology and Multiple Sclerosis Center, Landeskrankenhaus Mistelbach-Gänserndorf, Liechtensteinstr. 67, 3120 Mistelbach, Austria
27Istanbul University Cerrahpaşa School of Medicine, Department of Neurology, Clinical Neuroimmunology Unit and MS Clinic, Istanbul, Turkey
281st Saint Petersburg Pavlov State Medical University, Neurology Department, 6-8 Lva Tolstogo Street, St Petersburg, 197022, Russia
29Center of Clinical Neuroscience, University Hospital Carl Gustav Carus, Dresden University of Technology, Germany
Footnotes
Conflict of interest statement: PSS has served on advisory boards for Biogen, Merck Healthcare KGaA, Novartis, Teva, MedDay Pharmaceuticals and GSK; on steering committees or independent data monitoring boards in trials sponsored by Merck Healthcare KGaA, Teva, GSK and Novartis; has received speaker honoraria from Biogen Idec, Merck Healthcare KGaA, Teva, Sanofi-Aventis, Genzyme, Celgene and Novartis. His department has received research support from Biogen, Merck Healthcare KGaA, Teva, Novartis, Roche and Genzyme.
DC is an Advisory Board member of Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva. He is also the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva.
GG has received speaker honoraria and consulting fees from Abbvie, Actelion, Atara Bio, Almirall, Bayer Schering Pharma, Biogen Idec, FivePrime, GlaxoSmithKline, GW Pharma, Merck & Co., Merck Healthcare KGaA, Pfizer Inc, Protein Discovery Laboratories, Teva Pharmaceutical Industries Ltd, Sanofi-Genzyme, UCB, Vertex Pharmaceuticals, Ironwood and Novartis; and has received research support unrelated to this study from Biogen Idec, Merck & Co., Novartis and Ironwood.
XM has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Actelion, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, Sanofi-Genzyme, Teva Pharmaceutical, Excemed, MSIF and NMSS
DS has received grants and/or personal fees from Teva, Merck Serono, Novartis, Roche, Genzyme, Sanofi-Genzyme, Biogen Inc. and Bayer HealthCare.
PV has received honoraria or consulting fees from Biogen, Sanofi-Genzyme, Servier, Novartis, Merck KGaA, Celgene, Roche, MedDay and Almirall; and research support from Biogen, Sanofi-Genzyme, Bayer and Merck KGaA.
HW is a member of Scientific Advisory Boards/Steering Committees for Bayer Healthcare, Biogen Idec, Sanofi Genzyme, Merck Serono, Novartis, Roche and Teva. He received speaker honoraria and travel support from Bayer Vital GmbH, Bayer Schering AG, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Genzyme, Merck Serono, Omniamed, Novartis, and Sanofi Aventis and Teva. He received compensation as a consultant from Biogen Idec, Merck Serono, Novartis, Omniamed, Roche and Sanofi Genzyme. He has received research supports from Bayer Healthcare, Bayer Vital, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Sanofi US, and Teva Pharma as well as German Ministry for Education and Research (BMBF), German Research Foundation (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, Merck Serono, Novartis, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (ISKF) Muenster and RE Children’s Foundation.
BY has received honoraria for lectures and advisory boards from Bayer, Biogen, Genpharm, Genzyme, Merck-Serono and Novartis; and has received research grants from Bayer, Biogen, Merck-Serono, Novartis and Pfizer.
HS is an employee of Ares Trading S.A., and affiliate of Merck Serono S.A., Eysins, Switzerland.
PR has received honoraria for lectures/steering committee meetings from Merck, Biogen Idec, Bayer Schering Pharma, Boehringer-Ingelheim, Sanofi-Aventis, Genzyme, Novartis, Teva Pharmaceutical Industries, and Serono Symposia International Foundation.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Merck KGaA, Darmstadt, Germany who provided funding for the project. The SC members received financial compensation for attendance at two advisory board meetings as part of this programme. No payments were made to the authors for the writing of this manuscript. The Extended Faculty received no financial compensation for the completion of the on-line questionnaire, or the consensus voting.
ORCID iD: Diego Centonze  https://orcid.org/0000-0002-8390-8545
https://orcid.org/0000-0002-8390-8545
Contributor Information
Per Soelberg Sørensen, Department of Neurology, Danish Multiple Sclerosis Center, University of Copenhagen, 2082, Rigshospitalet, 9, Blegdamsvej, Copenhagen, 2100, Denmark.
Diego Centonze, Unit of Neurology and Neurorehabilitation, IRCCS Neuromed, Pozzilli (IS), Italy.
Gavin Giovannoni, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Xavier Montalban, Department of Neurology, Neuroimmunology, Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron University Hospital, Barcelona, Spain; Division of Neurology, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Daniel Selchen, Division of Neurology, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Patrick Vermersch, Univ. Lille, INSERM-U1172, CHU Lille, FHU Imminent, Lille, France.
Heinz Wiendl, Department of Neurology, Institute of Translational Neurology, University of Münster, Münster, Germany.
Bassem Yamout, Nehme and Therese Tohme Multiple Sclerosis Center, American University of Beirut, Beirut, Lebanon.
Hashem Salloukh, Ares Trading S.A., and affiliate of Merck Serono S.A., Eysins, Switzerland.
Peter Rieckmann, Department of Neurology, Center for Clinical Neuroplasticity, Medical Park Loipl, Bischofswiesen, University of Erlangen, Germany.
References
- 1. Cree BAC, Mares J, Hartung HP. Current therapeutic landscape in multiple sclerosis: an evolving treatment paradigm. Curr Opin Neurol 2019; 32: 365–377. [DOI] [PubMed] [Google Scholar]
- 2. Saposnik G, Montalban X. Therapeutic inertia in the new landscape of multiple sclerosis care. Front Neurol 2018; 9: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Linker RA, Chan A. Navigating choice in multiple sclerosis management. Neurol Res Pract 2019; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwong JS, Chen H, Sun X. Development of evidence-based recommendations: implications for preparing expert consensus statements. Chin Med J (Engl) 2016; 129: 2998–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Medicines Agency. MAVENCLAD. Assessment Report, https://www.ema.europa.eu/en/documents/assessment-report/mavenclad-epar-public-assessment-report_en.pdf (2017, Accessed August 2018).
- 6. Deeks ED. Cladribine tablets: a review in relapsing MS. CNS Drugs 2018; 32: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 8. Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler 2018; 24: 1594–1604. [DOI] [PubMed] [Google Scholar]
- 9. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 10. Boguniewicz M, Alexis AF, Beck LA, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract 2017; 5: 1519–1531. [DOI] [PubMed] [Google Scholar]
- 11. Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis 2015; 60: 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrante M, Karmiris K, Newnham E, et al. Physician perspectives on unresolved issues in the use of conventional therapy in Crohn’s disease: results from an international survey and discussion programme. J Crohns Colitis 2012; 6: 116–131. [DOI] [PubMed] [Google Scholar]
- 13. Mrowietz U, de Jong EM, Kragballe K, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol 2014; 28: 438–453. [DOI] [PubMed] [Google Scholar]
- 14. Papay P, Ignjatovic A, Karmiris K, et al. Optimising monitoring in the management of Crohn’s disease: a physician’s perspective. J Crohns Colitis 2013; 7: 653–669. [DOI] [PubMed] [Google Scholar]
- 15. Fernandez O. Is there a change of paradigm towards more effective treatment early in the course of apparent high-risk MS? Mult Scler Relat Disord 2017; 17: 75–83. [DOI] [PubMed] [Google Scholar]
- 16. Cleveland Clinic. Mellen center approaches: highly active multiple sclerosis, https://my.clevelandclinic.org/-/scassets/files/org/neurological/multiple-sclerosis/14-neu-528-highly-active-ms.ashx?la=en (accessed August 2018).
- 17. Diaz C, Zarco LA, Rivera DM. Highly active multiple sclerosis: an update. Mult Scler Relat Disord 2019; 30: 215–224. [DOI] [PubMed] [Google Scholar]
- 18. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 2018; 24: 96–120. [DOI] [PubMed] [Google Scholar]
- 19. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology 2018; 90: 777–788. [DOI] [PubMed] [Google Scholar]
- 20. Freedman MS, Selchen D, Arnold DL, et al. Treatment optimization in MS: Canadian MS working group updated recommendations. Can J Neurol Sci 2013; 40: 307–323. [DOI] [PubMed] [Google Scholar]
- 21. Stangel M, Penner IK, Kallmann BA, et al. Towards the implementation of ‘no evidence of disease activity’ in multiple sclerosis treatment: the multiple sclerosis decision model. Ther Adv Neurol Disord 2015; 8: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giovannoni G, Tomic D, Bright JR, et al. “No evident disease activity”: The use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 2017; 23: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giovannoni G, Turner B, Gnanapavan S, et al. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 2015; 4: 329–333. [DOI] [PubMed] [Google Scholar]
- 24. Medicines.org.uk. MAVENCLAD 10 mg tablets. EMC, https://www.medicines.org.uk/emc/product/8435/smpc (2018, accessed August 2018).
- 25. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol 2011; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- 26. Ali S, Paracha N, Cook S, et al. Reduction in healthcare and societal resource utilization associated with cladribine tablets in patients with relapsing-remitting multiple sclerosis: analysis of economic data from the CLARITY Study. Clin Drug Investig 2012; 32: 15–27. [DOI] [PubMed] [Google Scholar]
- 27. Soelberg Soerensen P, Rieckmann P, Comi G, et al. Relapses and lymphocyte counts before and after rescue therapy in the phase III, 96-week, double-blind, placebo-controlled CLARITY study of cladribine tablets for relapsing–remitting multiple sclerosis (Poster P917). Presented at 5th Joint Triennial Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 19–22 October 2011, Amsterdam, The Netherlands. [Google Scholar]
- 28. Cook S, Comi G, Giovannoni G, et al. Rates of lymphopenia year-by-year in patients with relapsing multiple sclerosis treated and retreated with cladribine tablets 3.5 mg/kg (Poster P666). Presented at ECTRIMS/ACTRIMS, 25–28 October 2017, Paris, France. [Google Scholar]
- 29. Novakovic AM, Thorsted A, Schindler E, et al. Pharmacometric analysis of the relationship between absolute lymphocyte count and expanded disability status scale and relapse rate, efficacy end points, in multiple sclerosis trials. J Clin Pharmacol 2018; 58: 1284–1294. [DOI] [PubMed] [Google Scholar]
- 30. Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord 2019; 29: 157–167. [DOI] [PubMed] [Google Scholar]
- 31. Competence Network. Multiple sclerosis (KKNMS) cladribin therapieempfehling, https://www.kompetenznetz-multiplesklerose.de/wp-content/uploads/2018/03/KKNMS_Therapieempfehlung_Cladribin_20180320_webfrei.pdf (2018, accessed August 2018).
- 32. Galazka A, Nolting A, Cook S, et al. An analysis of malignancy risk in the clinical development programme of cladribine tablets in patients with relapsing multiple sclerosis (ePoster 1083). Presented at EAN, 16-19 June 2018, Lisbon, Portugal. [Google Scholar]
- 33. Pakpoor J, Disanto G, Altmann DR, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflamm 2015; 2: e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harty G, Damian D, Budhia S, et al. Subsequent disease modifying drug treatment of patients treated with cladribine tablets (Poster 191). Presented at Association of British Neurologists Annual Meeting, 9-11 May 2018, Birmingham. [Google Scholar]