Abstract
Non-functioning pituitary adenomas (NFPAs) are benign pituitary tumours that constitute about one-third of all pituitary adenomas. They typically present with symptoms of mass effects resulting in hypopituitarism, visual symptoms, or headache. Most NFPAs are macroadenomas (>1 cm in diameter) at diagnosis that can occasionally grow quite large and invade the cavernous sinus causing acute nerve compression and some patients may develop acute haemorrhage due to pituitary apoplexy. The progression from benign to malignant pituitary tumours is not fully understood; however, genetic and epigenetic abnormalities may be involved. Non-functioning pituitary carcinoma is extremely rare accounting for only 0.1% to 0.5 % of all pituitary tumours and presents with cerebrospinal, meningeal, or distant metastasis along with the absence of features of hormonal hypersecretion. Pituitary surgery through trans-sphenoidal approach has been the treatment of choice for symptomatic NFPAs; however, total resection of large macroadenomas is not always possible. Recurrence of tumours is frequent and occurs in 51.5% during 10 years of follow-up and negatively affects the overall prognosis. Adjuvant radiotherapy can decrease and prevent tumour growth but at the cost of significant side effects. The presence of somatostatin receptor types 2 and 3 (SSTR3 and SSTR2) and D2-specific dopaminergic receptors (D2R) within NFPAs has opened a new perspective of medical treatment for such tumours. The effect of dopamine agonist from pooled results on patients with NFPAs has emerged as a very promising treatment modality as it has resulted in reduction of tumour size in 30% of patients and stabilization of the disease in about 58%. Despite the lack of long-term studies on the mortality, the available limited evidence indicates that patients with NFPA have higher standardized mortality ratios (SMR) than the general population, with women particularly having higher SMR than men. Older age at diagnosis and higher doses of glucocorticoid replacement therapy are the only known predictors for increased mortality.
Keywords: Non-functioning pituitary adenomas, hypopituitarism, pituitary apoplexy, immunohistochemistry, trans-sphenoidal surgery, perioperative management, postoperative outcomes, radiosurgery, pituitary carcinomas, temozolomide, mortality, quality of life
Introduction
Non-functioning pituitary adenomas (NFPAs) are benign pituitary tumours that arise from the adenohypophyseal cells, accounting for one-third of all pituitary adenomas.1 Widespread use of computed tomography (CT) and magnetic resonance imaging (MRI) for various clinical disorders has led to a surge in pituitary lesions being diagnosed incidentally, so-called pituitary incidentalomas.2 The clinical presentation of NFPAs varies from an incidental finding to life-threatening apoplexy. Non-functioning pituitary adenomas are diagnosed in the absence of clinical and biochemical evidence of tumour-related hormone hypersecretion.3 Typically, patients with NFPAs present with symptoms of mass effect more often than insidiously developing pituitary dysfunction.4-8 Central diabetes insipidus (CDI) at the time of diagnosis is rare and should alert the physician to consider other diagnoses such as craniopharyngioma and hypophysitis which are more likely to have CDI at the presentation.9 Non-functioning pituitary adenomas are classified according to their size as micro or macroadenomas. Surgery, radiotherapy (RT), pharmacological therapy, or careful monitoring is among the management options available at present.10,11 This article presents a review on the literature on the epidemiology, diagnosis, treatment, and follow-up of NFPAs.
Epidemiology
Pituitary adenomas are common brain lesions that are being diagnosed with increasing frequency. Based on radiologic and autopsy studies, the prevalence of pituitary adenomas in the general population is estimated to be around 15%.12 In fact, pituitary adenomas are the third most common central nervous system neoplasms following meningiomas and gliomas.13,14 Non-functioning pituitary adenomas represent around one-third of all pituitary adenomas and three quarters of all pituitary macroadenomas. NFPAs are the second most common type of pituitary adenomas after prolactinoma,1 with an estimated prevalence of 7 to 41.3 cases and standardized incidence rate of 0.65 to 2.34 cases per 100 000 population, respectively, in most of the epidemiological studies.15-17 Non-functioning pituitary adenomas are commonly diagnosed after the fourth decade of life with women presenting at an earlier age compared with men.16 Data regarding sex distributions are discordant.9,16,18
Most of pituitary adenomas arise sporadically19 but might also occur as part of hereditary endocrine syndromes. Among patients with multiple endocrine neoplasia type-1 (MEN 1), NFPAs constituted around 14.7% and 42.3% of all patients with pituitary adenomas, respectively, in 2 different studies.20,21 In a large retrospective multicentre study of 138 cases of familial isolated pituitary adenomas (FIPAs), 28 cases were NFPAs, representing almost a fifth of patients with FIPAs.22 Interestingly, a 30-year-old man with NFPA was diagnosed in the setting of germ-line SDHA mutation.23 Compared with the sporadic types, familial NFPAs were diagnosed at an average of 8 years earlier and were more invasive.22
Clinical Presentations
The clinical presentation of NFPAs varies across a spectrum spanning from asymptomatic patients at one end to life-threatening pituitary apoplexy at the other end. Diagnosis is usually incidental and often delayed by 1.96 ± 2.9 years due to the lack of hormonal over secretion features.4 Patients typically present with symptoms of mass effect such as headache, visual loss, ophthalmoplegias, or those related to hypopituitarism.1 Irrespective of the pituitary adenoma size, headache is common and is one of the most frequent reasons to perform imaging studies that has led to the detection of incidental NFPAs in around one-fifth of the cases.24,25 Increased intrasellar pressure, dural membrane stretching, and trigeminal pain pathways activation are among the proposed mechanisms of headache.26 Visual field defects are features of mass effect, caused by compression of optic chiasma by the tumour. The typical visual defect is bitemporal hemianopia but different patterns of visual field loss are seen with different sites of compression ranging from complete to partial and unilateral to bilateral. Chronic severe compression may lead to papilledema, optic atrophy, decreased visual acuity, and pupillary abnormalities which may be of insidious onset. Cavernous sinus invasion due to parasellar extension affects cranial nerves and may result in ophthalmoplegia, ptosis, diplopia, or strabismus. Most commonly affected cranial nerves are occulomotor followed by abducens, whereas trochlear or trigeminal nerve involvement is less common. Invasion of brain parenchyma, dural membranes, and other surrounding structures may cause temporal lobe epilepsy, intracranial hypertension, and obstructive hydrocephalus,27 whereas inferior extension of the tumour may cause erosion of sellar floor leading to cerebrospinal fluid (CSF) rhinorrhoea.28 Pituitary apoplexy complicating NFPA is a life-threatening clinical event resulting from acute haemorrhagic or an ischaemic event.29 Typically, patients present acutely with sudden severe headache, vomiting, and visual impairment with or without altered level of consciousness.30 Acute hormonal insufficiency is common, in particular, central adrenal insufficiency, which may occur in up to 70% of the patients.31 Non-functioning pituitary adenomas are the most common type of pre-existing adenomas in which apoplexy occurs and accounts for approximately 45% to 82% of the cases of apoplexy.32-34 It is possible that NFPAs are overrepresented in the pituitary apoplexy cohorts as functionality of the tumour is not evident at the time of evaluation due to the damage caused by pituitary apoplexy.30 While the pathophysiology of pituitary apoplexy is not completely understood, intrinsic factors with mismatch between tumour perfusion and metabolism and its blood supply could influence the occurrence of apoplexy.35 Surgery, RT, dynamic pituitary stimulation tests, dopamine agonist (DA) therapy, coagulopathy, and use of anticoagulation agents are recognized precipitating factors.29 However, most of the patients (around 75%) might present with apoplexy without any precipitating factors.36 Male sex, macroadenomas, and cavernous sinus invasion are observed more often in patients with pituitary apoplexy.37,38 Patients may have spontaneous resolution of the adenoma following apoplexy.29 However, long-term follow-up is essential as some patients may develop regrowth of the tumour, especially those with residual tumour or in the absence of RT.39 Among hormonal dysfunctions caused by NFPAs, mild hyperprolactinaemia is commonly encountered due to stalk effect as the growing mass blocks dopamine inhibition of lactotrophs but it rarely exceeds 95 ng/mL.40 Hypopituitarism develops insidiously and often goes undetected. Partial hypopituitarism develops in around 37% to 85% of the NFPA cases,4-6 whereas panhypopituitarism is reported in around 6% to 29% of the cases.7,8 Growth hormone (GH) axis is the most commonly affected one, with 61% to 100% of the patients showing biochemical evidence of GH deficiency. Gonadal axis is affected in 36% to 96% of the patients; central hypothyroidism is seen in 8% to 81% of the cases, whereas central adrenal insufficiency is observed in around 17% to 62%.41-44 Although most of the NFPAs are macroadenomas at diagnosis, CDI is rarely seen.9 The presence of CDI should alert the physician to consider other sellar pathologies such as craniopharyngiomas, hypophysitis, or malignancy. Gonadal axis is affected in 30%, central hypothyroidism occurs in 28%, adrenal insufficiency occurs in 18%, whereas GH axis is affected in 8% of the patients.45-47 Partial hypopituitarism is seen even with microadenomas as well, with 30% to 42% of the patients having at least one axis affected.48
Diagnosis and Evaluation
Pituitary adenomas are classified by their size as either macroadenomas (⩾ 10 mm) or microadenomas (<10 mm) in diameter. Magnetic resonance imaging is the gold standard modality of investigation for the evaluation of sellar and parasellar masses and its differential diagnosis. It is noteworthy that other lesions can be found in sellar area and may mimic a pituitary adenoma. In the case of erosion of the sella, CT scan is necessary to assess the bony anatomy of the sella. Patients with macroadenomas and those with symptoms of mass effects need careful eye and visual field examinations. The Congress of Neurological Surgeons has published guidelines for preoperative eye evaluation which may provide prognostic factors for recovery as well as helping in documenting postoperative changes. Older patients and those with visual loss for more than 4 months have less chances of postoperative visual recovery and they need to be counselled appropriately.49
NFPA is a diagnosis of exclusion. Therefore, it is of paramount important to exclude hormonal hypersecretion before labelling a pituitary mass as an NFPA (basic investigations included 8 am serum cortisol, IGF1, T4, thyroid-stimulating hormone). Full laboratory evaluation for both hormonal hypersecretion and hyposecretion for all patients with pituitary incidentaloma or NFPA is shown in Table 1. It is especially important to exclude prolactinoma beyond any degree of doubt as it is the only pituitary tumour that can be managed medically. Accordingly, prolactin measurement should be made in dilution when values are not as high as expected in a patient with a large pituitary macroadenoma, in which the case the serum prolactin levels should be re-measured after a 1:100 serum sample dilution to exclude the possibility of falsely low value due to the high dose hook effect (before providing a label of NFPA).51
Table 1.
Evaluation of pituitary function in intrasellar masses.
|
Measurements:
Serum IGF1, cortisol (09:00 am), prolactin, FSH/LH, oestradiol (females)/testosterone (males), TSH, and FT4 |
|
Check for hormone hypersecretion:
• If IGF1 is elevated, further evaluation for GH excess • Screening for glucocorticoid excess (overnight dexamethasone suppression test, 24-hour urinary free cortisol, midnight salivary cortisol) may be considered, regardless of clinical suspicion |
|
Check for hypopituitarism:
• In case of suspected GH deficiency, GH stimulation testing is recommended. Biochemical testing for GHD can be avoided in patients with clear-cut features of GHD and 3 other documented pituitary hormone deficits. Insulin tolerance test/GHRH + arginine/glucagon tests may be performed. • Adrenal insufficiency (AI) is indicated by a basal cortisol level < 3 μg/dL and a cortisol level > 15 μg/dL likely excludes an (AI) diagnosis. One of the following dynamic tests (ACTH stimulation test/ITT/low dose ACTH stimulation test) may be performed to check for central adrenal insufficiency. Peak cortisol levels less than 18.1 μg/dL (500 nmol/L) at 30 or 60 minutes indicates adrenal insufficiency. |
| Additional appropriate screening and follow-up investigations are needed in patients with personal or family history of multiple endocrine neoplasia. |
Furthermore, laboratory findings can indicate high prolactin levels in the absence of typical symptoms of hyperprolactinaemia. Therefore, it is important to assess for the presence of macroprolactin to avoid clinical confusion and inappropriate management.52
Physiological and pathological causes of hyperprolactinaemia and their implications for interpreting prolactin results should not be confused with true tumorous hyperprolactinaemia.
Workup for familial subtypes such as FIPA with germ-line aryl hydrocarbon receptor interacting protein mutations should be considered with the occurrence of large pituitary adenomas at a younger age, in patients who lack clinical evidence of MEN1 syndrome or Carney complex.53
Pathogenesis
Most of pituitary adenomas are sporadic.19 The pathological mechanisms involved in adenohypophyseal cell tumorigenesis include activation of oncogene, inactivation of tumour suppressor gene, epigenetic changes, and deregulation of microRNAs.54 It is estimated that 80% of pituitary adenomas demonstrate alterations in at least one cell cycle regulator.55 Mammalian sterile-20-like kinase (MST4), which has been shown to be upregulated in NFPAs of gonadotroph origin, stimulates p38, AKT, and HIF1, which are known tumorigenic factors in pituitary adenomas.56 Non-functioning/silent somatotroph adenomas lack sporadic mutations of the GNAS gene coding for the Gsα protein occurring in around 40% of functioning somatotroph adenomas.57 While the ubiquitin-specific protease 8 (USP8) was known to be frequently mutated in functioning corticotroph adenomas, nonetheless this has not been the case with not in non-functioning/silent corticotroph adenomas.58 Germ-line mutations are rare in NFPAs and mutations in the succinate dehydrogenase genes (SDHx) cause a group of syndromes in which NFPAs can occur.59
Histopathological Classification
The principles of the classification and grading of NFPAs are outlined in the recently published fourth edition of the World Health Organization (WHO) classification of endocrine tumours which established a more precise and cell lineage–specific system.50,60 This novel approach is based on the expression of anterior pituitary (adenohypophyseal) hormones and pituitary-specific transcription factors.50 Another change in the latest WHO classification is the elimination of the term ‘atypical adenoma’ and introduction of ‘high-risk pituitary adenomas’ including tumours with specific histological subtypes, elevated cellular proliferation assessed by mitotic rate, and Ki-67 labelling index and evidence of invasive growth evaluated by imaging and/or histology60 (Table 2).
Table 2.
Classification of non-functioning pituitary adenomas, transcription factor, hormonal content, and tumour behaviour.
| Tumour type | Transcription factor | Hormone | Behaviour |
|---|---|---|---|
| Gonadotroph | SF1 | β-LH, β-FSH, α-SU | — |
| Corticotroph | T-Pit | ACTH | High-risk |
| Somatotroph | Pit-1 | GH | High-risk |
| Lactotroph | Pit-1 | PRL | — |
| Pleurihormonal | Pit-1 | GH, PRL, TSH, α-SU | High-risk |
| Double/Triple NFPA | Variable | Variable | — |
| Null-cell | None | None | — |
Abbreviations: ACTH, adrenocorticotropic hormone; PRL, prolactin; TSH, thyroid-stimulating hormone.
Management
Treatment options available for NFPAs include observation, surgery, RT, and medical therapy.10,11 Non-functioning pituitary adenomas can be complex and should be discussed in a multidisciplinary team (MDT) ideally in specialized centres with extensive experience in the management of pituitary adenomas. The most appropriate option needs to be considered rationally for each patient on individual basis (Table 3).
Table 3.
Management options/strategies and rationales (indications) for each choices.
| Management option/Strategy | Rationale/Indications |
|---|---|
| Conservative (no treatment) | Microadenomas, no pressure effects, low probability of tumour growth over time |
| Surgery | Pituitary surgery has been the treatment of choice for NFPAs |
| Medical therapy | Total resection of tumour was not possible leaving ample room for medical therapy |
| Radiotherapy | Symptomatic patients with incomplete resection or recurrence of tumour after surgery should be managed with second surgery and/or radiotherapy |
Abbreviation: NFPAs, non-functioning pituitary adenomas.
Observation
As NFPAs are diagnosed in symptomatic as well as asymptomatic patients, the probability of tumour growth over time is the main rationale for watchful monitoring; therefore, understanding the natural history of the tumour is important because it gives insight into which tumour can be observed over time. In a review of 14 observational series including 648 patients with NFPAs, 65% had macroadenomas and 35% had microadenomas.18,45,61,62 Tumour size enlargement on MRI during follow-up over 1 to 8 years was related to initial tumour size (96 out of 419 patients with macroadenomas, 96 [23%] had increase in tumour size, while 272 patients [65%] had stable tumour in size and 51 patients [12%] showed decrease in tumour size). Whereas out of the 229 patients with microadenomas, 23 (10%) had an increase in tumour size, 189 (83%) had no change and 17(7%) showed reduction in tumour size18,45-47,63. Solid tumours showed greater growth tendency than cystic tumours. Seven out of the 8 cases of pituitary apoplexy occurred in macroadenomas group, whereas only 1 case was reported in a microadenoma. New pituitary hormone deficiency occurred at a rate of 2.8 per 100 patient-years and was detected exclusively in enlarging macroadenomas.11 Enormous variability was reported in the rate of tumour enlargement as tumour size doubling duration ranged from 0.8 to 27.2 years. No correlation was seen between tumour size doubling and initial tumour size.63 Of prospective randomized controlled studies, the observational approach, and its recommendations are based on expert opinions.
Surgery
The main goal in managing NFPAs is to decompress the mass effect caused by the enlarging adenoma making surgery the first choice of treatment, however, preserving the normal pituitary gland is a very important outcome as well. Although gross tumour resection is a goal, the benefits of complete resection must be balanced with the risks associated with aggressive surgical approach. Even when surgery is not curative, it leads to considerable debulking and volume reduction in nearly all patients.64 Generally, indications to operate include symptomatic patients presenting with visual loss, neurological deficits, and hormonal deficiencies and in lately presenting p cases an obstructive hydrocephalus. In asymptomatic patients, surgery will be considered if signs of impending visual loss are visible anatomically. In acute pituitary apoplexy, specific indications of surgery in pituitary apoplexy have changed in the current practice such as unlikley previously indicated, surgical intervention is not recommended in all cases, but only in those patients with visual deficits and a deteriorating level of consciousness.65,66
Preservation of normal pituitary function could be achieved intra-operatively by careful extra-capsular dissection of tumours from normal pituitary gland and surrounding dura matter with minimal manipulation of the gland, thus avoiding damage to neurovascular structures. Many considerations are taken intra-operatively as well to minimize the likelihood of CSF leak including CSF diversion methods and dura closure using autologous fat or fascia lata graft placement.67
For many years, microscopic trans-sphenoidal surgery (TSS) has been the standard of care. However, with the recent on-going advances in endoscopic visualization, endoscopic technology has been considered a major alternative.
Limited illumination and smaller field of vision are minor disadvantages of the microscopic approach, whereas better illumination and visualization with a panoramic view with endoscopic approach yet at the cost of two-dimensional images instead of three-dimensional; the latter approach allows a better extent of resection and bony exposure by endoscopic approach in the theory.68 Nonetheless both approaches are equally safe and effective regarding outcomes and extent of resection. Moreover, a personalized approach based on multiple factors is crucial in determining the optimal treatment strategy.69
In all surgical approaches, tumour size and cavernous sinus invasion were found to be the factors adversely impacting the rate of gross total resection. Transcranial approach is less frequently used such as when the trans-sphenoidal approach is not appropriate; however, in selected case with an important suprasellar extension, transcranial surgery becomes mandatory.70 A combined transcranial and trans-sphenoidal approach is recommended in selected patients.71,72
Penn et al have recently reviewed the outcomes of surgical management and reported improvement in vision in 56.4% to 90.0% of the patients presenting initially with visual fields and acuity deficits, whereas headache improved in around 89.7% of the patients.65 Although studies specific to NFPAs are limited, recovery of hypopituitarism has been reported in 16% to 48% of the patients with all types of adenomas. Interestingly, Alfio et al reported the surgical outcomes of PAs in the elderly patients, who have been considered at a higher risk compared with younger counterparts, found no statistical differences in surgical outcomes compared with younger patients, demonstrating that TSS is a safe and effective treatment option for PAs in elderly patients. Furthermore, they found no difference in visual outcomes after surgery in older patients.74
The probability of tumour recurrence during 5 years of follow-up was directly affected by the extent of the tumour resection as patients with subtotal tumour resection showed radiological and symptomatic progression in 61% and 17%, respectively, compared with patients with complete resection of tumour where only 12% had tumour recurrence.65 Loss of vision, hypopituitarism, CDI, and rarely injury to carotid vasculature were the commonest complications with overall rate of 9.1% as reported by Penn et al. Persistent postoperative CSF leak can lead to meningitis, pneumocephalus, and intracranial bleedings. Epistaxis, crusting, and septal perforations are some other complications.62 Recurrence of tumours after surgery is an issue and there might be a need to manage symptomatic patients with a second surgery and/or RT.75
Medical Treatment
Unfortunately, total resection of tumour is not always possible leaving ample room for medical therapy (Table 4) and RT (Table 5). Residual as well as recurrent tumours are frequent and this affects the overall prognosis. During 5 and 10 years of follow-up, the recurrence rates reported are as high as 24.4% and 51.5%, respectively.106 Adjuvant RT can decrease and prevent tumour growth but at the cost of significant side effects.107
Table 4.
Drugs and classes of medical agents used for NFPAs.
| Classes of medications | References |
| Dopamine agonists (DA) | 62, 76-80 |
| Somatostatin receptor ligands (SRLs) | 81-85 |
| Combination treatment with SRLs plus DAs | 85 |
| Temozolomide (TMZ) | 86-89 |
| Peptide receptor radionuclide therapy (PRRT) | 86, 90-93 |
| Gonadotrophin-releasing hormone (GnRH) receptor agonists and antagonists | 62 |
| Octreotide-mediated tumour-targeted cytotoxic drug delivery | 94, 95 |
| Folate receptor-mediated drug targeting | 32, 96-98 |
| Targeting of PI3K/AKT/mTOR pathway | 99, 100-104 |
Table 5.
Summary of the clinical indications and radiotherapy techniques in the context NFPAs.
| Indications for radiotherapy for NFPAs | Contemporary techniques for radiotherapy |
|---|---|
| 1. Residual or recurrent disease postsurgery or postmedical treatment | 1. Three-dimensional conformal radiotherapy (3DCRT) |
| 2. Intensity-modulated radiotherapy (IMRT) | |
| 2. Patient declined or not eligible for surgery or medical treatment | 3. Volumetric-modulated arc therapy (VMAT) |
| 4. Stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) | |
| 5. Fractionated stereotactic radiotherapy (FSRT) |
Source: Minniti et al.105
Abbreviation: NFPA, non-functioning pituitary adenoma.
The demonstration of somatostatin receptor types 2 and 3 (SSTR3 and SSTR2) and D2-specific dopaminergic receptors (D2R) within NFPAs has opened a new perspective of medical treatment for such tumours.76,108 Several case reports and small prospective studies using agents that modulate these receptors have been published. Although large placebo-controlled clinical trials are lacking, these studies have shown the efficacy of medications indicated for functional pituitary tumours in NFPAs as well after incomplete resection.
Dopamine agonists
The role of DA arises from the fact that irrespective of their cell lineage of origin, most of pituitary adenomas express dopamine receptors and over the past 3 decades, several studies have documented the presence and functionality of dopamine receptors in NFPAs.76 Bromocriptine and quinagolide were the agents of choice in earlier studies. However, cabergoline, the more potent and specific D2R agonist, has been used in recent studies. The outcome of a small study using bromocriptine with mean follow-up of 40 months showed tumour control achieved in about 78.8% of patients, in comparison with 33.3% in the untreated patients. Of note, control rate reached 90% when DA was initiated before evidence of enlargement of remnant tumour in comparison with 61.5% if DA treatment was initiated later in the course of the disease.77
The clinical experience with DA use in NFPAs was analysed by Colao et al62 where the cumulative evidence for tumour size reduction in patients treated with DA was reported in 27.6% of cases and the rate of tumour stabilization was reported in more than 90% of the cases, whereas the tumour regrowth was only seen in 8.5% of the patients. In another larger study, 55 patients received cabergoline as preventive therapy for tumour regrowth, whereas 24 patients were given cabergoline only when tumour regrowth was evident and this study showed that preventive therapy was effective in tumour control in 87.3% of patients compared with 46.7% in the control group.78 Factors associated with poor response to treatment were male sex, younger age, and large preoperative tumour size.79 However, we cannot predict at this point which NFPA will respond to therapy based on analysis of D2R expression in the surgical specimen.80
Based on the available data, DAs could be used in patients with NFPAs for prevention of regrowth of postoperative residual tumour which subsequently can reduce the requirement of a second surgery or RT. However, further research in this area is needed to confirm their effectiveness.
Somatostatin receptor ligands
Anti-proliferative and anti-secretory effects of somatostatin are mediated by binding to 5 different subtypes of somatostatin receptors (SSTR). They are broadly expressed on NFPAs, particularly SSTR3 being the most abundant subtype, followed by SSTR2.81 For this reason, somatostatin receptor ligands (SRLs), such as octreotide and lanreotide, have been studied and used over the past 3 decades for treatment of patient with NFPAs. Octreotide LAR showed stability of postsurgical tumour remnant in 81% of patients with NFPA while having limited effect on tumour shrinkage and without any effect on visual field deficits or pituitary functions.82 Another study on patients with NFPA not cured by surgery reported an improvement of visual field defects with use of high-dose octreotide (1200 μg/daily) for 3 to 6 months in 3 out of 4 patients without any significant change in tumour remnant volume.83 Similar data were obtained from another study which showed 50% improvement of visual field defects in the absence of change in tumour volume after 1 month of subcutaneous octreotide treatment.84 Findings from a longer follow-up study with up to 12 months’ octreotide therapy in patients with NFPAs described a significant increase in tumour volume in only 2 out of 9 patients (22%).85 Pasireotide has not been studied so far in patients with NFPA. Therefore, it should not be used till further studies are done to explore its safety and efficacy. In summary, SRL therapy has limited effect on tumour shrinkage in NFPAs; nonetheless, it has the potential effect in stabilizing and preventing tumour remnant regrowth in most of the patients, but long-term studies are needed to assess its place in the management algorithm of patients with NFPA.
Combination treatment with SRLs plus DAs
Colao et al85 reported remarkable decrease of residual tumour volume and improvement in mean defects at visual perimetry among 10 patients, who previously had unsuccessful pituitary surgery, when they were treated with combination of lanreotide plus cabergoline for 6 months. However, clinical experience is very limited. At present, the use of such combination therapy is not evidence based due to paucity of data and studies. Therefore, further studies are needed to confirm the role of combination treatment in patients with NFPAs.
Temozolomide
Temozolomide (TMZ) is an oral alkylating agent which has been used mainly in the treatment of glioblastoma multiforme but also for the treatment of pituitary carcinoma and aggressive pituitary adenomas since 2006.86 The effectiveness of TMZ in aggressive pituitary adenomas and pituitary carcinomas is about 55% and 58%, respectively.87 Recently, data on the efficacy of TMZ have been reported in about 100 patients with pituitary adenomas and the response in NFPAs was 22% (6/27) with a further 48% (13/27) reported to have stable disease, but it has to be noted that the response rate was somewhat lower for NFPA as compared with other secreting pituitary adenomas with higher rate of recurrence after TMZ treatment in the range of 50%.88,89
Temozolomide remains an important treatment option for aggressive and recurrent pituitary adenomas and carcinomas, but large-scale studies are needed to determine the effectiveness, proper doses, and duration of treatment.
Peptide receptor radionuclide therapy
Peptide receptor radionuclide therapy with radio-labelled somatostatin analogues represents an alternative therapeutic option by which focused and precise therapy is given by specifically targeting tumours that express these receptors and is used routinely for metastatic neuroendocrine tumours of gut.86 However, the clinical experience with this modality in pituitary adenomas is limited to few case reports.90-93
Gonadotrophin-releasing hormone receptor agonists and antagonists
The clinical experience with gonadotrophin-releasing hormone (GnRH) analogues’ uses in NFPAs was analysed by Colao et al62 where no beneficial effects on tumour volume as well as on visual field defects have been reported.
Octreotide-mediated tumour-targeted cytotoxic drug delivery
A recent method of targeted anticancer drug delivery is by coupling potent chemotherapeutic agents with SSTR2-preferential somatostatin analogues. This allows drug delivery at receptor specific sites, reduces systemic side effects, and increases their anti-tumour efficacy. Doxorubicin, methotrexate, and paclitaxel are being used in this way.94 Although effective in vitro, their clinical use is limited due to hydrolysis of the delicate ester bond linking cytotoxic drug to the SRL, in vivo. Using a cleavable disulfide-intercalating linker, a doxorubicin-octreotide bioconjugate has been developed and was found to suppress adrenocorticotropic hormone (ACTH) secretion in AtT20 mouse pituitary cells. Cytotoxicity to pituitary, pancreatic, and breast cancer cell lines was also reported.95 Although being apparently promising, further studies are needed to establish the clinical value of this modality of treatment for patient with cancer and with aggressive pituitary tumours.
Folate receptor–mediated drug targeting
Exploration of FRα-targeted therapeutic approaches has led to emergence of FRα-specific monoclonal antibodies and conjugates of folic acid with anticancer agents, which are in early stages of clinical trials for lung and ovarian cancer. FRα overexpression is seen in NFPAs, both at the messenger RNA (mRNA) and protein levels but not in normal pituitary tissue or secretory pituitary adenomas.96 Invasive and large tumours with high Ki-67 proliferation index have shown much higher expression.97 In primary cell cultures derived from patients with NFPA, anti-proliferative, anti-invasive, and pro-apoptotic activity shown by FRα-targeted liposomal doxorubicin was encouraging and is of potential value in management of aggressive pituitary tumours.32 Detection of FRα-expressing NFPA with 99mTc-Folate SPECT-CT helps in identifying tumours amenable to FRα-targeted therapy as demanded by the concept of personalized/precision cancer therapy which requires selection of patients for a specific targeted therapy based on molecular characterization of the tumour and its microenvironment.98
Targeting of PI3K/AKT/mTOR pathway
Non-functioning pituitary adenomas have high AKT and cyclin D1 mRNA expression levels compared with other pituitary adenomas and this is associated with early recurrence.99,100,109 Positive correlation has been seen between tumour size and invasiveness and expression level of RAPTOR and RICTOR, which are regulatory proteins of the mTOR pathway. Thus, inhibition of PI3K signalling came naturally as a valuable therapeutic target for pituitary tumours and NFPA.101 Significant reduction in cell viability along with enhanced apoptosis in NFPA primary culture is reported with everolimus.100 In patients with NFPA, anti-proliferative effects were reported with NVP-BEZ235, a dual inhibitor of PI3K-AKT-mTOR,102 and in cells resistant to rapamycin monotherapy, decreased protein kinase B (AKT) phosphorylation by octreotide combination regimen restored sensitivity and enhanced the anti-proliferative effect in rapamycin-sensitive cells.103 Despite all this optimism, the results of everolimus-octreotide combination therapy were discouraging in ACTH-secreting pituitary carcinoma,104 and its role in management of NFPA needs further research.
Radiotherapy
Routine use of RT postoperatively is no longer indicated in the management of NFPAs and should only be considered if there is cavernous sinus involvement not amenable to surgical excision and/or if significant postoperative residual tumour is present.75 As per the Congress of Neurological surgeon guidelines, symptomatic patients with incomplete resection or recurrence of tumour after surgery should be managed with a second surgery and/or RT.75 Despite good efficacy, RT had limited use in past due to potential risk of long-term radiation adverse effects such as radiation-induced hypopituitarism and neurological damages. Recent advancement in imaging technologies, software systems, and radiation dose delivery has markedly improved the overall outcome.110 Clinical contexts and techniques for RT in the context of NFPAs are summarized in Table 5. Treatment outcomes are similar among all the techniques listed in Table 5. However, exposure of healthy tissue around the pituitary gland to RT and duration of treatment may be different, which may impact on treatment-related side effects and patient convenience. Three-dimensional conformal radiotherapy requires simpler treatment planning, whereas intensity-modulated radiotherapy (Figure 1), volumetric-modulated arc therapy, and stereotactic radiosurgery/stereotactic radiotherapy (SRS/SRT) need relatively advanced treatment planning. Linear accelerator unit is the most commonly used way to deliver the above-mentioned RT techniques. Other treatment units used are as follows: TomoTherapy, CyberKnife, Gamma Knife, Proton, and other charged particles. Cone beam CT, megavoltage voltage CT, and orthogonal films are often used to verify the position of patient during treatment.105
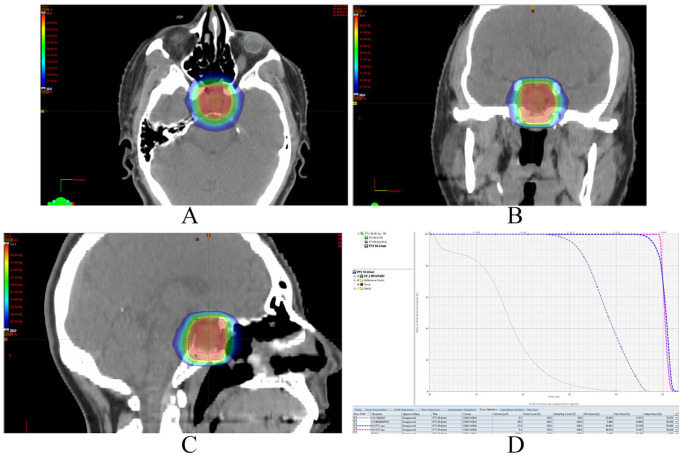
Figure 1.
Pituitary adenoma IMRT planning pictures: (A) axial image, (B) coronal image, (C) sagittal image, (D) dose volume histogram (DVH) showing dose received per volume by targets and organs at risk.
Pink continuous line represents gross target volume (GTV) and dark blue line shows planning target volume (PTV).
Red radiation dose cloud shows 100%, whereas dark blue cloud at edges shows 50% of prescribed dose.
RT doses
Standard dose fractionations commonly use doses between 45 and 55 Gy, in 1.8 to 2.0 Gy daily fractions. Thus, 25 to 28 fractions are required to deliver the full dose which is completed over 5 to 6 weeks with 5 daily fractions per week. Sometimes, twice daily fraction sizes of 1.2 Gy with 6 hours apart are used to deliver total dose of 55.2 Gy. The SRS/SRT dose fractionations use a single fraction of 12 to 20 Gy. Alternatively, 25 Gy can be delivered in 5 daily sessions. Caution should be used for lesions >2.5 to 3 cm in size and/or involving the anterior optic pathways. Fractionated stereotactic radiotherapy dose fractionations are 25 to 28 daily fractions of 1.8 to 2.0 Gy with a total dose of 45 to 50 Gy.111
Conventional RT
Minniti et al105 reviewed and analysed efficacy of conventional RT in NFPAs from 13 large series, where 10- and 20-year’s tumour control rates were 80% to 90% and 75% to 90%, respectively. Hypopituitarism was the most common long-term complication occurring in around 20% to 30% cases at 5 years and the rate increases with time. Risk of optic pathway and cranial nerve injury were reported in 1% to 5% cases.112,113 Regarding the risk of secondary brain tumours, cumulative risk was found to be 2.0% and 2.4% at 10 and 20 years, respectively.105 Higher incidence of cognitive decline and cerebrovascular disease has been observed but the role and contribution of radiation is not clear so far.114,115
Stereotactic Radiosurgery
Results from several large retrospective series on SRS for NFPAs had revealed tumour control of 90% to 95% at 5 years as analysed by Minniti et al. Tumour shrinkage was observed in 20% to 60% of the cases along with improvement in ocular motility and visual functions in around 25% patients. The most commonly used technique was Gamma Knife.103 A margin dose of 12 to 20 Gy was found to be as effective as a dose more than 20 Gy; however, a dose less than 12 Gy was found to be associated with worse outcomes. Larger tumour volume, suprasellar extension, and low radiation doses were associated with poor tumour control outcomes.116 Hypopituitarism again is the most common long-term side effect observed with figures of 10% to 40% at 5 years.105 Visual disturbances and cranial nerve dysfunctions were seen in 1% to 4% of the patients, whereas radiation-induced necrosis of the brain and other cranial nerve neuropathies were rarely observed. Compared with conventional RT, the risk of secondary brain tumours has not been precisely determined with SRS.117 For NFPAs located close to optic pathways, alternative practice is to deliver SRS dose of 18 to 25 Gy over 2 to 5 fractions. A tumour control rate of 98% was reported at 3 years with side effects observed in 5% of the cases.118
Fractionated stereotactic RT
In a recent review of several large series with fractionated stereotactic radiotherapy (FSRT), Minniti et al reported tumour control rate of 95% at 5 years. Reduction in tumour size was found in 20% to 50% with improvement in visual symptoms in around 30% cases. Hypopituitarism was the most commonly observed complications with new onset pituitary hormone deficiency in 10% to 48% over mean follow-up duration of 30 to 75 months, whereas optic neuropathy occurred in 1% of the patients. Other complications observed with conventional RT were rarely seen with FSRT but larger studies with long-term follow-up are needed before reaching to final conclusion.105
Health-Related Quality of Life
Quality of life (QoL) might be affected by a number of factors such as effects of surgery, RT, deficiency of one or multiple pituitary hormones along with various comorbidities.119 The published data regarding the health-related QoL in patients with NFPAs are diverse and conflicting. Fewer studies have shown that compared with normal population, patients with NFPAs were found to have impaired QoL with significant impairment in mental and physical measures during active disease which remained unchanged even after successful treatment,120,121 whereas others did not find any significant difference in QoL between patients with NFPAs and control populations.122,123 Recent advances in the management of NFPAs such as centralization of pituitary surgery to specialized centres, availability of SRS and FSRT, and hormonal replacement therapies for hypopituitarism have improved the treatment outcome in general and have been reflected in QoL outcome studies as well. A recent study from Finland found that the overall QoL was near-normal over a mean follow-up period of more than 7 years, with mean QoL scores for patients with NFPAs that were only marginally lower than controls with lack of statistical significance. The patients with NFPA had significantly poor scores on sexual activity and vision, depression and distress with speech, vitality, usual activities, discomfort, and symptoms, whereas no difference was observed regarding dimensions of hearing, movement, sleeping, eating, excretion, and mental function. Hypopituitarism has been found to be an independent predictor of impaired QoL. Patients on hydrocortisone and testosterone replacement had a higher mean score, whereas those with repeat surgery and on thyroxine replacement had poorer scores.119 Furthermore, surgical intervention can be an effective way of restoring visual defects as well as improving the QoL to older patients.74 Impairment of body pain, mental health, and general health perception was reported more frequently in patients with NFPAs with GH deficiency compared with GH-sufficient subjects, which improved after optimum replacement with GH. Patients treated with craniotomy reported more impairment in QoL compared with those who underwent TSS. It was also observed that compared with male patients, female patients with NFPAs had more physical and emotional problems, lower energy levels, and poorer health perception.122 Although the health-related QoL in patients with NFPA is improving as shown in recent studies, disease-specific questionnaire is needed to identify more sensitive or specific impairments in this population.
Malignant Transformations
Patients with NFPAs have higher rates of various types of neoplasms, the reason for which remains unknown; the hypothesis of genetic and/or epigenetic predisposition is still being studied.124
Although most of NFPAs are benign, around 45% to 55% are locally invasive and may show aggressive features.17 Proliferative markers such as Ki-67, mitotic rate, and p53 immunoreactivity are commonly used for assessment for aggressive behaviour potential; at the same time, higher SSTR3 and low MGMT expression are also associated with more aggressive NFPAs.125,126 Non-functioning pituitary carcinomas (NFPCs) are very rare entity defined as tumours of adenohypophyseal origin with cerebrospinal, meningeal, or distant metastasis along with the absence of features of hormonal hypersecretion.127 Fortunately, it is very rare and accounts for only 0.1% to 0.5% of all pituitary adenomas.128,129 To date, approximately 38 cases of NFPC have been reported.130
Ki-67 is the most reliable marker of proliferation with values between 1.3% and 10% and more than 10% being associated with tumour recurrence and malignancy, respectively, whereas values more than 3% are a reliable prognostic marker. Increased p53 immunoreactivity is another proliferation marker; however, the lack of reliable methods of quantification has led to its removal from WHO 2017 classification. Similarly, a mitotic rate more than 2 per 10 high power fields is seen commonly with pituitary carcinoma and is associated with increased recurrence rate.58,131
The progression from benign to malignant pituitary tumours is not fully understood and largely remains unknown; however, there is general acceptance that it is associated with genetic and epigenetic abnormalities.127,132 Activation of classical oncogenes such as Ras and mutation of p53 are a common event in other cancer types but are rarely seen in pituitary carcinomas.127,133 The benign nature of most pituitary tumours, according to Lenders and McCormack,127 is thought to result from oncogene-induced senescence, with upregulation of cell cycle regulator genes such as p53, p21, and p16. Other factors that may play a role in the malignant transformation of benign NFPA include chromosomal instability with chromosomal gains involving 14q, 5p, and 7p.134 Furthermore, increased nuclear expression of pituitary tumour transformation gene (PTTG) has been linked with tumours aggressiveness135; upregulation of certain microRNAs has been shown to play a role in NFPCs, with no evidence of overexpression in the primary tumour.136
Non-functioning pituitary carcinomas cannot be reliably differentiated from benign tumours by any clinical, biochemical, or radiological features.128 As reviewed by Lenders et al,127 out of 38 cases of NFPC seen, 23 of them were females with median age at diagnosis being 48 years. Non-functioning pituitary carcinoma usually evolves from aggressive macroadenomas with mean latency period of 6.6 years between primary tumour diagnosis and metastasis and presents initially with features of mass effects128; however, 5 cases showed extremely rapid progression where metastasis occurred within 1 month only from the initial presentation.127 Cerebrospinal metastases were commoner than systemic ones with intracranial metastasis being the commonest.137
Mortality
Long-term survival of patients with NFPAs is compromised due to morbidities related to the tumour itself, the selected treatment modality, or its related side effects.58 Although limited data are available, studies do suggest mortality rates higher than general population. A study from Denmark and Sweden population showed a significantly increased mortality rate for women only (standardized mortality ratio [SMR]: 1.97 and 1.37, respectively).138 Furthermore, RT was found to be associated with increased mortality rate (SMR: 1.62) which appears to be secondary to hypopituitarism.139 Higher mortality rate was also reported by another study done in United States on 663 patients.140 The SMR of 1.70 was observed by Tomlinson et al where causes of death were respiratory, cardiovascular, and cerebrovascular, and the mortality was again higher in women compared with men (SMR: 2.23; 99% confidence interval [CI]: 1.60-3.11 vs SMR 1.37; 99% CI: 0.98-1.91, P = .006).141 The prognosis of NFPC is very poor with a median survival of only 8 months, although long-term survivors have been described.142
Conclusions
Clinically, non-functional pituitary adenomas are benign tumours of the pituitary gland that commonly present with symptoms related to mass effect. Although multiple pathological subtypes of NFPAs exist the first-line treatment for symptomatical lesions is surgical after detailed hormonal, ophthalmologic, and radiographic assessment. Surgical resection, most commonly through a transnasal trans-sphenoidal approach, is the preferred treatment option with adjunctive RT used sometimes for symptomatic patients with incompletely resected tumours or those with recurrence of the tumour after surgery. Perioperative care should include neurological evaluation and monitoring for symptoms and signs of diabetes insipidus and syndrome of inappropriate antidiuretic hormone. Although surgical complications can be devastating, surgery can be performed safely with low morbidity and mortality in experienced hands. Therefore, multidisciplinary team approach including endocrinologists, neurosurgeons, and ophthalmologists, is mandatory for the comprehensive management of patients with NFPAs and for long-term follow-up.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: The authors were assigned specific sections to draft; these were developed into a single manuscript which was reviewed and approved by all the authors.
ORCID iD: Mussa H AlMalki  https://orcid.org/0000-0003-4068-5465
https://orcid.org/0000-0003-4068-5465
References
- 1. Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317:516-524. [DOI] [PubMed] [Google Scholar]
- 2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:894-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greenman Y, Stern N. Non-functioning pituitary adenomas. Best Prac Res Clin Endocrinol Metab. 2009;23:625-638. [DOI] [PubMed] [Google Scholar]
- 4. Drange MR, Fram NR, Herman-Bonert V, et al. Pituitary tumor registry: a novel clinical resource. J Clin Endocrinol Metab. 2000;85:168-174. [DOI] [PubMed] [Google Scholar]
- 5. Fatemi N, Dusick JR, Mattozo C, et al. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008;63:709-718; discussion 718-719. [DOI] [PubMed] [Google Scholar]
- 6. Webb SM, Rigla M, Wägner A, et al. Recovery of hypopituitarism after neurosurgical treatment of pituitary adenomas. J Clin Endocrinol Metab. 1999;84:3696-3700. [DOI] [PubMed] [Google Scholar]
- 7. Dekkers OM, Pereira AM, Romijn JA. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 2008;93:3717-3726. [DOI] [PubMed] [Google Scholar]
- 8. Del Monte P, Foppiani L, Ruelle A, et al. Clinically nonfunctioning pituitary macroadenomas in the elderly. Aging Clin Exp Res. 2007;19:34-40. [DOI] [PubMed] [Google Scholar]
- 9. Al-Dahmani K, Mohammad S, Imran F, et al. Sellar masses: an epidemiological study. Can J Neurol Sci. 2016;43:291-297. [DOI] [PubMed] [Google Scholar]
- 10. Molitch ME. Management of incidentally found nonfunctional pituitary tumors. Neurosurg Clin N Am. 2012;23:543-553. [DOI] [PubMed] [Google Scholar]
- 11. Molitch ME. Nonfunctioning pituitary tumors. Handb Clin Neurol. 2014;124:167-184. [DOI] [PubMed] [Google Scholar]
- 12. Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613-619. [DOI] [PubMed] [Google Scholar]
- 13. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14:1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shibui S. The present status and trend of brain tumors based on the data of the Brain Tumor Registry of Japan. Brain Nerve. 2012;64:286-290. [PubMed] [Google Scholar]
- 15. Agustsson TT, Baldvinsdottir T, Jonasson JG, et al. The epidemiology of pituitary adenomas in Iceland, 1955–2012: a nationwide population-based study. Eur J Endocrinol. 2015;173:655-664. [DOI] [PubMed] [Google Scholar]
- 16. Tjörnstrand A, Gunnarsson K, Evert M, et al. The incidence rate of pituitary adenomas in western Sweden for the period 2001–2011. Eur J Endocrinol. 2014;171:519-526. [DOI] [PubMed] [Google Scholar]
- 17. Day PF, Loto MG, Glerean M, et al. Incidence and prevalence of clinically relevant pituitary adenomas: retrospective cohort study in a Health Management Organization in Buenos Aires, Argentina. Arch Endocrinol Metab. 2016;60:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iglesias P, Arcano K, Triviño V, et al. Prevalence, clinical features, and natural history of incidental clinically non-functioning pituitary adenomas. Horm Metab Res. 2017;49:654-659. [DOI] [PubMed] [Google Scholar]
- 19. Gadelha MR, Diem M, Hernández-Ramírez LC, Korbonits M. Genetics of pituitary adenomas. In: Stratakis CA. (ed.) Endocrine tumor syndromes and their genetics. Basel, Switzerland; 2013:111-140. [Google Scholar]
- 20. Vergès B, Boureille F, Goudet P, et al. Pituitary disease in MEN type 1 (MEN1): data from the France–Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87:457-465. [DOI] [PubMed] [Google Scholar]
- 21. de Laat JM, Dekkers OM, Pieterman CR, et al. Long-term natural course of pituitary tumors in patients with MEN1: results from the Dutch MEN1 Study Group (DMSG). J Clin Endocrinol Metab. 2015;100:3288-3296. [DOI] [PubMed] [Google Scholar]
- 22. Daly AF, Jaffrain-Rea ML, Ciccarelli A, et al. Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab. 2006;91:3316-3323. [DOI] [PubMed] [Google Scholar]
- 23. Dwight T, Mann K, Benn DE, et al. Familial SDHA mutation associated with pituitary adenoma and pheochromocytoma/paraganglioma. J Clin Endocrinol Metab. 2013;98:E1103-E1108. [DOI] [PubMed] [Google Scholar]
- 24. Rizzoli P, Iuliano S, Weizenbaum E, et al. Headache in patients with pituitary lesions: a longitudinal cohort study. Neurosurgery. 2016;78:316-323. [DOI] [PubMed] [Google Scholar]
- 25. Jahangiri A, Wagner JR, Chin AT, et al. Incidence of headache as a presenting complaint in over 1000 patients with sellar lesions and factors predicting postoperative improvement. Clin Neurol Neurosurg. 2015;132:16-20. [DOI] [PubMed] [Google Scholar]
- 26. Greenman Y, Melmed S. Diagnosis and management of nonfunctioning pituitary tumors. Annu Rev Med. 1996;47:95-106. [DOI] [PubMed] [Google Scholar]
- 27. Verhelst J, Berwaerts J, Abs R. Obstructive hydrocephalus as complication of a giant nonfunctioning pituitary adenoma: therapeutical approach. Acta Clin Belg. 1998;53:47-52. [DOI] [PubMed] [Google Scholar]
- 28. Landeiro JA, Fonseca EO, Monnerat AL, et al. Nonfunctioning giant pituitary adenomas: invasiveness and recurrence. Surg Neurol Int. 2015;6:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pearce JMS. On the origins of pituitary apoplexy. Eur Neurol. 2015;74:18-21. doi: 10.1159/000431090. [DOI] [PubMed] [Google Scholar]
- 30. Capatina C, Inder W, Karavitaki N, Wass JA. Management of endocrine disease: pituitary tumour apoplexy. Eur J Endocrinol. 2015;172:R179-R190. [DOI] [PubMed] [Google Scholar]
- 31. Leyer C, Castinetti F, Morange I, et al. conservative management is preferable in milder forms of pituitary tumor apoplexy. J Endocrinol Invest. 2011;34:502-509. [DOI] [PubMed] [Google Scholar]
- 32. Möller-Goede DL, Brändle M, Landau K, et al. Pituitary apoplexy: re-evaluation of risk factors for bleeding into pituitary adenomas and impact on outcome. Eur J Endocrinol. 2011;164:37-43. [DOI] [PubMed] [Google Scholar]
- 33. Lubina A, Olchovsky D, Berezin M, et al. Management of pituitary apoplexy: clinical experience with 40 patients. Acta Neurochir (Wien). 2005;147:151-157; discussion 157. [DOI] [PubMed] [Google Scholar]
- 34. da Motta LA, de Mello PA, de Lacerda CM, et al. Pituitary apoplexy. Clinical course, endocrine evaluations and treatment analysis. J Neurosurg Sci. 1999;43:25-36. [PubMed] [Google Scholar]
- 35. Oldfield EH, Merrill MJ. Apoplexy of pituitary adenomas: the perfect storm. J Neurosurg. 2015;122:1444-1449. [DOI] [PubMed] [Google Scholar]
- 36. Semple PL, Jane JA, laws ER. Clinical relevance of precipitating factors in pituitary apoplexy. Neurosurgery. 2007;61:956-961. [DOI] [PubMed] [Google Scholar]
- 37. Zhu X, Wang Y, Zhao X, et al. Incidence of pituitary apoplexy and its risk factors in Chinese people: a database study of patients with pituitary adenoma. PLoS ONE. 2015;10:e0139088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cinar N, Tekinel Y, Dagdelen S, Oruckaptan H, Soylemezoglu F, Erbas T. Cavernous sinus invasion might be a risk factor for apoplexy. Pituitary. 2013;16:483-489. [DOI] [PubMed] [Google Scholar]
- 39. Pal A, Capatina C, Tenreiro AP, et al. Pituitary apoplexy in non-functioning pituitary adenomas: long term follow up is important because of significant numbers of tumour recurrences. Clin Endocrinol. 2011;75:501-504. [DOI] [PubMed] [Google Scholar]
- 40. Behan LA, O’Sullivan EP, Glynn N, et al. Serum prolactin concentration at presentation of non-functioning pituitary macroadenomas. J Endocrinol Invest. 2013;36:508-514. [DOI] [PubMed] [Google Scholar]
- 41. Arafah BM. Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 1986;62:1173-1179. [DOI] [PubMed] [Google Scholar]
- 42. Cury ML, Fernandes JC, Machado HR, et al. Non-functioning pituitary adenomas: clinical feature, laboratorial and imaging assessment, therapeutic management and outcome. Arq Bras Endocrinol Metabol. 2009;53:31-39. [DOI] [PubMed] [Google Scholar]
- 43. Chen L, White WL, Spetzler RF, et al. A prospective study of nonfunctioning pituitary adenomas: presentation, management and clinical outcome. J Neurooncol. 2011;102(1):129-138. [DOI] [PubMed] [Google Scholar]
- 44. Wichers-Rother M, Hoven S, Kristof RA, et al. Non-functioning pituitary adenomas: endocrinological and clinical outcome after transsphenoidal and transcranial surgery. Exp Clin Endocrinol Diabetes. 2004;112:323-327. [DOI] [PubMed] [Google Scholar]
- 45. Feldkamp J, Santen R, Harms E, et al. Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas – results of a prospective study. Clin Endocrinol (Oxf). 1999;51:109-113. [DOI] [PubMed] [Google Scholar]
- 46. Day PF, Guitelman M, Artese R, et al. Retrospective multicentric study of pituitary incidentalomas. Pituitary. 2004;7:145-148. [DOI] [PubMed] [Google Scholar]
- 47. Reincke M, Allolio B, Saeger W, et al. The ‘incidentaloma’ of the pituitary gland. Is neurosurgery required? JAMA. 1990;263:2772-2776. [PubMed] [Google Scholar]
- 48. Carosi G, Malchiodi E, Ferrante E, et al. Hypothalamic–pituitary axis in non-functioning pituitary adenomas: focus on the prevalence of isolated central hypoadrenalism. Neuroendocrinology. 2015;102:267-273. [DOI] [PubMed] [Google Scholar]
- 49. Newman SA, Turbin RE, Bodach ME, et al. Congress of Neurological Surgeons systematic review and evidence-based guideline on pretreatment ophthalmology evaluation in patients with suspected nonfunctioning pituitary adenomas. Neurosurgery. 2016;79:E530-E532. [DOI] [PubMed] [Google Scholar]
- 50. Lloyd RV, Osamura YR, Kloppel G, Rosai J. WHO Classification of Tumors of Endocrine Organs. Geneva, Switzerland: WHO Press; 2017:78-80. [Google Scholar]
- 51. Petersenn S. Biochemical diagnosis in prolactinomas: some caveats. Pituitary. 2020;23:9-15. doi: 10.1007/s11102-019-01024-z. [DOI] [PubMed] [Google Scholar]
- 52. Fahie-Wilson MN, John R, Ellis AR. Macroprolactin: high molecular mass forms of circulating prolactin. Ann Clin Biochem. 2005;42:175-192. [DOI] [PubMed] [Google Scholar]
- 53. Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocrine Rev. 2013;34:239-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sapochnik M, Nieto LE, Fuertes M, Arzt E. Molecular mechanisms underlying pituitary pathogenesis. Biochem Genet. 2016;54:107-119. [DOI] [PubMed] [Google Scholar]
- 55. Caimari F, Korbonits M. Novel genetic causes of pituitary adenomas. Clin Cancer Res. 2016;22:5030-5042. [DOI] [PubMed] [Google Scholar]
- 56. Xiong W, Knox AJ, Xu M, et al. Mammalian Ste20-like kinase 4 promotes pituitary cell proliferation and survival under hypoxia. Mol Endocrinol. 2015;29:460-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marques P, Korbonits M. Genetic aspects of pituitary adenomas. Endocrinol Metab Clin North Am. 2017;46:335-374. [DOI] [PubMed] [Google Scholar]
- 58. Perez-Rivas LG, Theodoropoulou M, Ferraù F, et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J Clin Endocrinol Metab. 2015;100:E997-E1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dénes J, Swords F, Rattenberry E, et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: results from a large patient cohort. J Clin Endocrinol Metab. 2015;100:E531-E541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huan W, Molitch ME. Management of nonfunctioning pituitary adenomas (NFAs): observation. Pituitary. 2018;21:162-167. [DOI] [PubMed] [Google Scholar]
- 61. Esteves C, Neves C, Augusto L, et al. Pituitary incidentalomas: analysis of a neuroradiological cohort. Pituitary. 2015;18:777-781. [DOI] [PubMed] [Google Scholar]
- 62. Colao A, Di Somma C, Pivonello R, Faggiano A, Lombardi G, Savastano S. Medical therapy for clinically non-functioning pituitary adenomas. Endocrine Related Cancer. 2008;15:905-915. [DOI] [PubMed] [Google Scholar]
- 63. Fernandez-Balsells MM, Murad MH, Barwise A, et al. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2011;96:905-912. [DOI] [PubMed] [Google Scholar]
- 64. Lucas JW, Bodach ME, Tumialan LM, et al. Congress of neurological surgeon’s systematic review and evidence-based guideline on primary management of patients with nonfunctioning pituitary adenomas. Neurosurgery. 2016;79:E533-E535. [DOI] [PubMed] [Google Scholar]
- 65. Penn DL, Burke WT, Laws ER. Management of non-functioning pituitary adenomas: surgery. Pituitary. 2018;21:145-153. [DOI] [PubMed] [Google Scholar]
- 66. Nomikos P, Ladar C, Fahlbusch R, et al. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas – a study on 721 patients. Acta Neurochir (Wien). 2004;146:27-35. [DOI] [PubMed] [Google Scholar]
- 67. Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882-1886. [DOI] [PubMed] [Google Scholar]
- 68. Laws ER, Barkhoudarian G. The transition from microscopic to endoscopic transsphenoidal surgery: the experience at Brigham and Women’s Hospital. World Neurosurg. 2014;82:S152-S154. [DOI] [PubMed] [Google Scholar]
- 69. Karppinen A, Kivipelto L, Vehkavaara S, et al. Transition from microscopic to endoscopic transsphenoidal surgery for nonfunctional pituitary adenomas. World Neurosurg. 2015;84:48-57. [DOI] [PubMed] [Google Scholar]
- 70. Mortini P, Barzaghi R, Losa M, et al. Surgical treatment of giant pituitary adenomas: strategies and results in a series of 95 consecutive patients. Neurosurgery. 2007;60:993-1004. [DOI] [PubMed] [Google Scholar]
- 71. Aghi MK, Chen CC, Fleseriu M, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas: executive summary. Neurosurgery. 2016;79:521-523. [DOI] [PubMed] [Google Scholar]
- 72. Kuo JS, Barkhoudarian G, Farrell CJ, et al. Congress of neurological surgeons systematic review and evidence-based guideline on surgical techniques and technologies for the management of patients with nonfunctioning pituitary adenomas. Neurosurgery. 2016;79:E536-E538. [DOI] [PubMed] [Google Scholar]
- 73. Halvorsen H, Ramm-Pettersen J, Josefsen R, et al. Surgical complications after transsphenoidal microscopic and endoscopic surgery for pituitary adenoma: a consecutive series of 506 procedures. Acta Neurochir (Wien). 2014;156:441-449. [DOI] [PubMed] [Google Scholar]
- 74. Spina A, Losa M, Mortini P. Pituitary adenomas in elderly patients: clinical and surgical outcome analysis in a large series. Endocrine. 2019;65:637-645. [DOI] [PubMed] [Google Scholar]
- 75. Sheehan J, Lee CC, Bodach ME, et al. Congress of neurological surgeon’s systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery. 2016;79:E539-E540. [DOI] [PubMed] [Google Scholar]
- 76. Hofland LJ, Lamberts SW. Somatostatin receptors in pituitary function, diagnosis and therapy. Front Hor Res. 2004;32:235-252. [DOI] [PubMed] [Google Scholar]
- 77. Greenman Y, Tordjman K, Osher E, et al. Postoperative treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists decreases tumour remnant growth. Clin Endocrinol. 2005;63:39-44. [DOI] [PubMed] [Google Scholar]
- 78. Greenman Y, Cooper O, Yaish I, et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur J Endocrinol. 2016;175:63-72. [DOI] [PubMed] [Google Scholar]
- 79. Verde G, Oppizzi G, Chiodini PG, Dallabonzana D, Luccarelli G, Liuzzi A. Effect of chronic bromocriptine administration on tumor size in patients with ‘nonsecreting’ pituitary adenomas. J Endocrinol Investig. 1985;8:113-115. [DOI] [PubMed] [Google Scholar]
- 80. Even-Zohar N, Greenman Y. Management of NFAs: medical treatment. Pituitary. 2018;21:168-175. [DOI] [PubMed] [Google Scholar]
- 81. Taboada GF, Luque RM, Bastos W, et al. Quantitative analysis of somatostatin receptors subtype (SSTR1–5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol. 2007;156:65-74. [DOI] [PubMed] [Google Scholar]
- 82. Fusco A, Giampietro A, Bianchi A, et al. Treatment with octreotide LAR in clinically non-functioning pituitary adenoma: results from a case-control study. Pituitary. 2012;15:571-578. [DOI] [PubMed] [Google Scholar]
- 83. De Bruin T, Kwekkeboom D, Van’t Verlaat J, et al. Clinically nonfunctioning pituitary adenoma and octreotide response to long term high dose treatment and studies in vitro. J Clin Endocrinol Metab. 1992;75:1310-1317. [DOI] [PubMed] [Google Scholar]
- 84. Borson-Chazot F, Houzard C, Ajzenberg C, et al. Somatostatin receptor imaging in somatotroph and non-functioning pituitary adenomas: correlation with hormonal and visual responses to octreotide. Clin Endocrinol (Oxf). 1997;47:589-598. [DOI] [PubMed] [Google Scholar]
- 85. Colao A, Filippella MD, di Somma C, et al. Somatostatin analogs in treatment of non-growth hormone-secreting pituitary adenomas. Endocrine. 2003;20:279-283. [DOI] [PubMed] [Google Scholar]
- 86. Raverot G, Sturm N, de Fraipont F, et al. Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. J Clin Endocrinol Metab. 2010;95:4592-4599. [DOI] [PubMed] [Google Scholar]
- 87. Bruno OD, Juárez-Allen L, Christiansen SB, et al. Gómeztemozolomide therapy for aggressive pituitary tumors: results in a small series of patients from Argentina. Int J Endocrinol. 2015;2015:587893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hirohata T, Asano K, Ogawa Y, et al. DNA mismatch repair protein (MSH6) correlated with the responses of atypical pituitary adenomas and pituitary carcinomas to temozolomide: the national cooperative study by the Japan Society for Hypothalamic and Pituitary Tumors. J Clin Endocrinol Metab. 2013;98:1130-1136. [DOI] [PubMed] [Google Scholar]
- 89. Halevy C, Whitelaw BC. How effective is temozolomide for treating pituitary tumours and when should it be used? Pituitary. 2017;20:261-266. [DOI] [PubMed] [Google Scholar]
- 90. Priola SM, Esposito F, Cannavo S, et al. Aggressive pituitary adenomas: the dark side of the moon. World Neurosurg 2017;97:140-155. [DOI] [PubMed] [Google Scholar]
- 91. Komor J, Reubi JC, Christ ER. Peptide receptor radionuclide therapy in a patient with disabling non-functioning pituitary adenoma. Pituitary. 2014;17:227-231. [DOI] [PubMed] [Google Scholar]
- 92. Baldari S, Ferrau F, Alafaci C, et al. First demonstration of the effectiveness of peptide receptor radionuclide therapy (PRRT) with 111In-DTPA-octreotide in a giant PRL-secreting pituitary adenoma resistant to conventional treatment. Pituitary. 2012;15(Suppl. 1):S57-S60. [DOI] [PubMed] [Google Scholar]
- 93. Maclean J, Aldridge M, Bomanji J, Short S, Fersht N. Peptide receptor radionuclide therapy for aggressive atypical pituitary adenoma/carcinoma: variable clinical response in preliminary evaluation. Pituitary. 2014;17:530-538. [DOI] [PubMed] [Google Scholar]
- 94. Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26:2034-2043. [DOI] [PubMed] [Google Scholar]
- 95. Lelle M, Kaloyanova S, Freidel C, et al. Octreotide-mediated tumor targeted drug delivery via a cleavable doxorubicin-peptide conjugate. Mol Pharmacol. 2015;12:4290-4300. [DOI] [PubMed] [Google Scholar]
- 96. Evans CO, Reddy P, Brat DJ, et al. Differential expression of folate receptor in pituitary adenomas. Cancer Res. 2003;63:4218-1424. [PubMed] [Google Scholar]
- 97. Liu X, Ma S, Yao Y, et al. Differential expression of folate receptor alpha in pituitary adenomas and its relationship to tumor behavior. Neurosurgery. 2012;70:1274-1280. [DOI] [PubMed] [Google Scholar]
- 98. Galt JR, Halkar RK, Evans CO, et al. In vivo assay of folate receptors in nonfunctional pituitary adenomas with 99mTcfolate SPECT/CT. J Nucl Med. 2010;51:1716-1723. [DOI] [PubMed] [Google Scholar]
- 99. Dworakowska D, Wlodek E, Leontiou CA, et al. Activation of RAF/MEK/ERK and PI3K/AKT/mTOR pathways in pituitary adenomas and their effects on downstream effectors. Endocr Relat Cancer. 2009;16:1329-1338. [DOI] [PubMed] [Google Scholar]
- 100. Jia W, Sanders AJ, Jia G, Liu X, Lu R, Jiang WG. Expression of the mTOR pathway regulators in human pituitary adenomas indicates the clinical course. Anticancer Res. 2013;33:3123-3131. [PubMed] [Google Scholar]
- 101. Zatelli MC, Minoia M, Filieri C, et al. Effect of everolimus on cell viability in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2010;95:968-976. [DOI] [PubMed] [Google Scholar]
- 102. Lee M, Wiedemann T, Gross C, et al. Targeting PI3K/mTOR signaling displays potent antitumor efficacy against nonfunctioning pituitary adenomas. Clin Cancer Res. 2015;21:3204-3215. [DOI] [PubMed] [Google Scholar]
- 103. Cerovac V, Monteserin-Garcia J, Rubinfeld H, et al. The somatostatin analogue octreotide confers sensitivity to rapamycin treatment on pituitary tumor cells. Cancer Res 2010;70:666-674. [DOI] [PubMed] [Google Scholar]
- 104. Jouanneau E, Wierinckx A, Ducray F, et al. New targeted therapies in pituitary carcinoma resistant to temozolomide. Pituitary. 2012;15:37-43. [DOI] [PubMed] [Google Scholar]
- 105. Minniti G, Flickinger J, Tolu B, Paolini S. Management of nonfunctioning pituitary tumors: radiotherapy. Pituitary. 2018;21:154-161. [DOI] [PubMed] [Google Scholar]
- 106. O’Sullivan EP, Woods C, Glynn N, et al. The natural history of surgically treated but radiotherapy-naive nonfunctioning pituitary adenomas. Clin Endocrinol. 2009;71:709-714. [DOI] [PubMed] [Google Scholar]
- 107. Woollons AC, Hunn MK, Rajapakse YR, et al. Non-functioning pituitary adenomas: indications for postoperative radiotherapy. Clin Endocrinol (Oxf). 2000;53:713-717. [DOI] [PubMed] [Google Scholar]
- 108. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological Reviews. 1998;78:189-225. [DOI] [PubMed] [Google Scholar]
- 109. Noh TW, Jeong HJ, Lee MK, Kim TS, Kim SH, Lee EJ. Predicting recurrence of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2009;94:4406-4413. [DOI] [PubMed] [Google Scholar]
- 110. Minniti G, Gilbert DC, Brada M. Modern techniques for pituitary radiotherapy. Rev Endocr Metab Disord. 2009;10:135-144. [DOI] [PubMed] [Google Scholar]
- 111. Minniti M, Osti M, Niyazi M. Target delineation and optimal radiosurgical dose for pituitary tumors. Radiat Oncol. 2016;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Al-Mefty O, Kersh JE, Routh A, Smith RR. The longterm side effects of radiation therapy for benign brain tumours in adults. J Neurosurgery. 1990;73:502-512. [DOI] [PubMed] [Google Scholar]
- 113. McCord MW, Buatti JM, Fennell EM, et al. Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biol Phys. 1997;39:437-444. [DOI] [PubMed] [Google Scholar]
- 114. Peace KA, Orme SM, Padayatty SJ, Godfrey HP, Belchetz PE. Cognitive dysfunction in patients with pituitary tumour who have been treated with transfrontal or transsphenoidal surgery or medication. Clin Endocrinol. 1998;49:391-396. [DOI] [PubMed] [Google Scholar]
- 115. van Varsseveld NC, van Bunderen CC, Ubachs DH, et al. Cerebrovascular events, secondary intracranial tumors, and mortality after radiotherapy for nonfunctioning pituitary adenomas: a subanalysis from the Dutch National Registry of Growth Hormone Treatment in Adults. J Clin Endocrinol Metab. 2015;100:1104-1112. [DOI] [PubMed] [Google Scholar]
- 116. Starke RM, Williams BJ, Jane JA, Sheehan JP. Gamma Knife surgery for patients with nonfunctioning pituitary macroadenomas: predictors of tumor control, neurological deficits, and hypopituitarism. J Neurosurg. 2012;117:129-135. [DOI] [PubMed] [Google Scholar]
- 117. Ecemis GC, Atmaca A, Meydan D. Radiation-associated secondary brain tumors after conventional radiotherapy and radiosurgery. Expert Rev Neurotherapeut. 2013;13:557-565. [DOI] [PubMed] [Google Scholar]
- 118. Iwata H, Sato K, Tatewaki K, et al. Hypofractionated stereotactic radiotherapy with Cyber Knife for nonfunctioning pituitary adenoma: high local control with low toxicity. Neuro Oncol. 2011;13:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Karppinen A, Ritvonen E, Roine R, et al. Health-related quality of life in patients treated for nonfunctioning pituitary adenomas during the years 2000–2010. Clin Endocrinol. 2016;84:532-539. [DOI] [PubMed] [Google Scholar]
- 120. Dekkers OM, van der Klaauw AA, Pereira AM, et al. Quality of life is decreased after treatment for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2006;91:3364-3369. [DOI] [PubMed] [Google Scholar]
- 121. Biermasz NR, Joustra SD, Donga E, et al. Patients previously treated for nonfunctioning pituitary macroadenomas have disturbed sleep characteristics, circadian movement rhythm, and subjective sleep quality. J Clin Endocrinol Metab. 2011;96:1524-1532. [DOI] [PubMed] [Google Scholar]
- 122. Capatina C, Christodoulides C, Fernandez A, et al. Current treatment protocols can offer a normal or near-normal quality of life in the majority of patients with non-functioning pituitary adenomas. Clin Endocrinol (Oxf). 2013;78:86-93. [DOI] [PubMed] [Google Scholar]
- 123. Nielsen EH, Lindholm J, Laurberg P, et al. Nonfunctioning pituitary adenoma: incidence, causes of death and quality of life in relation to pituitary function. Pituitary. 2007;10:67-73. [DOI] [PubMed] [Google Scholar]
- 124. Olsson DS, Hammarstrand C, Bryngelsson L, et al. Incidence of malignant tumours in patients with a non-functioning pituitary adenoma. Endocr Relat Cancer. 2017;24:227-235. [DOI] [PubMed] [Google Scholar]
- 125. Lee M, Lupp A, Mendoza N, et al. SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. Endocr Relat Cancer. 2015;22:111-119. [DOI] [PubMed] [Google Scholar]
- 126. Dai C, Sun B, Liu X, et al. O-6-Methylguanine-DNA methyltransferase expression is associated with pituitary adenoma tumor recurrence: a systematic meta-analysis. Oncotarget. 2017;8:19674-19683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lenders N, McCormack A. Malignant transformation in non-functioning pituitary adenomas (pituitary carcinoma). Pituitary. 2018;21:217-229. [DOI] [PubMed] [Google Scholar]
- 128. Pernicone PJ, Scheithauer BW, Sebo TJ, et al. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79:804-812. [DOI] [PubMed] [Google Scholar]
- 129. Hansen TM, Batra S, Lim M, et al. Invasive adenoma and pituitary carcinoma: a SEER database analysis. Neurosurg Rev. 2014;37:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. van der Zwan JM, Mallone S, van Dijk B, et al. Carcinoma of endocrine organs: results of the RARECARE project. Eur J Cancer. 2012;48:1923-1931. [DOI] [PubMed] [Google Scholar]
- 131. Lopes MBS. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathologica. 2017;134:521-535. [DOI] [PubMed] [Google Scholar]
- 132. Daly AF, Tichomirowa MA, Beckers A. The epidemiology and genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab. 2009;23:543-554. [DOI] [PubMed] [Google Scholar]
- 133. Pei L, Melmed S, Scheithauer B, Kovacs K, Prager D. H-ras mutations in human pituitary carcinoma metastases. J Clin Endocrinol Metab. 1994;78:842-846. [DOI] [PubMed] [Google Scholar]
- 134. Rickert CH, Scheithauer BW, Paulus W. Chromosomal aberrations in pituitary carcinoma metastases. Acta Neuropathol. 2001;102:117-120. [DOI] [PubMed] [Google Scholar]
- 135. Vandeva S, Tichomirowa MA, Zacharieva S, Daly AF, Beckers A. Genetic factors in the development of pituitary adenomas. Endocr Dev. 2010;17:121-133. [DOI] [PubMed] [Google Scholar]
- 136. Wei Z, Zhou C, Liu M, et al. MicroRNA involvement in a metastatic non-functioning pituitary carcinoma. Pituitary. 2015;18:710-721. [DOI] [PubMed] [Google Scholar]
- 137. Kaltsas GA, Grossman AB. Malignant pituitary tumours. Pituitary. 1998;1:69-81. [DOI] [PubMed] [Google Scholar]
- 138. Lindholm J, Nielsen H, Bjerre P, et al. Hypopituitarism and mortality in pituitary adenomas. Clin Endocrinol. 2006;65:51-58. doi: 10.1111/j.1365-2265.2006. 02545.x. [DOI] [PubMed] [Google Scholar]
- 139. O’Reilly MW, Reulen RC, Gupta S, et al. ACTH and gonadotropin deficiencies predict mortality in patients treated for nonfunctioning pituitary adenoma: long-term follow-up of 519 patients in two large European centres. Clin Endocrinol (Oxf). 2016;85:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chang EF, Zada G, Kim S, et al. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J Neurosurg. 2008;108:736-745. doi: 10.3171/JNS/2008/108/4/0736. [DOI] [PubMed] [Google Scholar]
- 141. Tomlinson JW, Holden N, Hills RK, et al. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet. 2001;357:425-431. [DOI] [PubMed] [Google Scholar]
- 142. Lenders N, McCormack A. Malignant transformation in non-functioning pituitary adenomas (pituitary carcinoma). Pituitary. 2018;21(2): 217–229. [DOI] [PubMed] [Google Scholar]