Abstract
Paraneoplastic neurological syndromes (PNSs) are rare complications of systemic cancers that can affect all parts of the central and/or peripheral nervous system. A body of experimental and clinical data has demonstrated that the pathogenesis of PNSs is immune-mediated. Nevertheless, the mechanisms leading to immune tolerance breakdown in these conditions remain to be elucidated. Despite their rarity, PNSs offer a unique perspective to understand the complex interplay between cancer immunity, effect of immune checkpoint inhibitors (ICIs), and mechanisms underlying the attack of neurons in antibody-mediated neurological disorders, with potentially relevant therapeutic implications. In particular, it is reported that ICI treatment can unleash PNSs and that the immunopathological features of PNS-related tumors are distinctive, showing prominent tumor-infiltrating lymphocytes and germinal center reactions. Intriguingly, similar pathological substrates have gained further attention as potential biomarkers of ICI-sensitivity and oncological prognosis. Moreover, the genetic analysis of PNS-associated tumors has revealed specific molecular signatures and mutations in genes encoding onconeural proteins, leading to the production of highly immunogenic neoantigens. Other than PNSs, autoimmune encephalitides (AEs) comprise a recently described group of disorders characterized by prominent neuropsychiatric symptoms, diverse antibody spectrum, and less tight association with cancer. Other triggering factors seem to be involved in AEs. Recent data have shed light on the importance of preceding infections (in particular, herpes simplex virus encephalitis) in inducing neurological autoimmune disorders in susceptible individuals (those with a selective deficiency in the innate immune system). In addition, in some AEs (e.g. LGI1-antibody encephalitis) an association with specific host-related factors [e.g., human leukocyte antigen (HLA)] was clearly demonstrated. We provide herein a comprehensive review of the most recent findings in the field of PNSs and AEs, with particular focus on their triggering factors and immunopathogenesis.
Keywords: autoimmune encephalitis, CTLA-4, HLA, immune checkpoint inhibitors, neurological adverse events, NMDAR, paraneoplastic neurological syndromes, PD-1
Introduction
In recent years, a large number of antibody (Ab)-associated neurological syndromes has been discovered, including a group of disorders showing tight association with cancer [paraneoplastic neurological syndromes (PNSs)] and emerging entities manifesting as encephalitis that demonstrate a less frequent pathogenic link with a tumor [autoimmune encephalitides (AEs)].1–4 In the latter group, several other environmental (e.g. infections)5–8 or host-related [e.g., human leukocyte antigen (HLA)]9–11 factors were shown to be relevant in inducing the autoimmune response. Very recently, it was shown that both PNSs and AEs can be triggered by cancer immunotherapy, in particular immune checkpoint inhibitor (ICI) treatment.12,13 Despite many advances in clinical characterization and Ab detection, the pathogenesis of immune tolerance breakdown in the setting of PNSs and AEs remains largely unknown.14,15 The importance of studying PNSs stems from the fact that they originate at the fulcrum between the patient’s immune system and the tumor, thus providing an exceptional model to decipher tumor immune surveillance and action of cancer immunotherapy, but also mechanisms underlying the attack of neurons in Ab-mediated neurological disorders, with potentially relevant therapeutic implications.14,15 In this regard, the analysis of triggering and predisposing factors of PNSs and AEs (tumors, cancer immunotherapy, genes, and infections) can provide further clues into the immunopathogenesis of these neurological conditions.1,4
We aimed herein to provide a comprehensive overview of the most recent findings in the field of PNSs and AEs, with particular focus on their triggering factors and immunopathogenesis.
PNS: definition, epidemiological and clinical aspects
PNSs are rare immune-related complications of a systemic malignancy, the incidence of which approximates 1/100,000 person-years and prevalence 4/100,000 persons (Table 1).16 Overall, 1 in every 334 patients with cancer is affected (especially those with lung, breast, and ovarian cancer), and their medical burden is higher than previously expected.16 In PNSs the target antigens (onconeural antigens, i.e., Hu, Ma2, Ri, CV2/CRMP5, Yo, Amphiphysin, Tr/DNER, and SOX-1) are proteins normally expressed by neurons, and ectopically by certain tumor cells.13,17 In the periphery, these tumor-associated antigens can be presented to T cells, generating a robust immune response against both the tumor and the nervous system.1,17,18 The direct consequence is that cancer growth is usually well controlled at the time the neurological syndrome ensues.19 Hence, it is not surprising that these patients are typically first seen by the neurologists due to the onset of neurological disturbances.1,17,18 Only later, tumor screening might reveal the presence of an underlying cancer, prompting oncological care. The only exception to this general rule is probably anti-Yo paraneoplastic cerebellar degeneration (PCD), in which the discovery of the associated tumor (usually an ovarian or breast cancer) often antedates the onset of PNS.20 In general, a diagnostic algorithm based on whole-body computed tomography (CT) followed, if negative, by fluorodeoxyglucose-positron emission tomography (FDG-PET) may reveal a tumor in up to 96% of patients with PNS at first screening, as in the case of small-cell lung cancer (SCLC) and Lambert-Eaton myasthenic syndrome.21,22 In other cases, the discovery of cancer can be more challenging. For example, it was demonstrated that young men with anti-Ma2 PNS and negative paraclinical investigations (including tumor markers, ultrasound, body CT, or FDG-PET) can harbor microscopic germ-cell neoplasm of the testis discovered only after orchiectomy.23 Onset of PNSs is usually subacute,18 and it likely reflects the time needed by the immune attack to damage neurons. Sometimes, progression can be very slow (i.e., chronic/progressive) and mimic neurodegenerative conditions, including degenerative dementias,24 motor neuron syndromes,25 and atypical Parkinsonisms or sporadic ataxias.26 Conversely, rare cases with hyper-acute onset have also been described, and almost 1/10 of patients with PCD shows a “stroke-like” onset.27 PNSs can involve all parts of the central or peripheral nervous system, and multifocal distribution of symptoms and signs is frequent.16,19,28 Overall, the most common syndromes in clinical practice are limbic encephalitis (LE) and PCD, which each account for approximately one-third of all PNSs.16 Accordingly, the most frequently associated onconeural Abs are anti-Hu and anti-Yo.16,29,30 Diagnosis of PNSs is aided by paraclinical tests, in particular brain and spine magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) analysis.16–18 Typical brain MRI alterations include hyperintensities restricted to limbic regions on T2-weighted imaging in cases of LE.1,16,17,31 Imaging can reveal normal/nonspecific changes in the initial stage of PCD, followed by cerebellar atrophy later over the course of the disease.27 Cases with isolated myelopathy can demonstrate symmetric, tract-specific or gray matter-specific, spinal cord abnormalities.32,33 Contrast-enhancing lesions are rare but possible in both brain (e.g., Ma2 encephalitis)25,34,35 and spinal cord PNS inflammatory lesions (e.g., CV2/CRMP5 myelopathy).36 CSF inflammatory alterations [including pleocytosis, increased protein content, and the presence of CSF-restricted oligoclonal bands (OCBs)] are not specific for PNSs, but are often present (particularly OCBs) and can direct the clinician in considering an autoimmune neurological disorder.37 However, sometimes the CSF can be normal.38 Clinical criteria for PNS diagnosis were provided in 2004.39 Since then, many new Ab specificities were discovered,40–42 and new challenges in Ab detection became apparent. In particular, we have recently shown that the detection of onconeural Abs using commercial immunoblots is limited by a high rate of false positive results.43 Therefore, clinical expertise and confirmation with other techniques in reference centers are required to avoid misdiagnosis.43
Table 1.
Clinical features of PNS and AE.
| Syndromes | PNS | AE | References |
|---|---|---|---|
| Incidence (person-years) | 0.89/100,000 | 0.8/100,000 | Vogrig et al.16; Dubey et al.44 |
| Prevalence | 4.37/100,000 | 13.7/100,000 | |
| Onset | Usually subacute | Acute or subacute | Dalmau and Graus1; Honnorat and Antoine18 |
| Multifocal involvement | Frequent | Rare | Dalmau and Graus1; McKeon and Pittock19 |
| Most frequent associated Abs | Hu, Yo (adults) | NMDAR, LGI1 (adults) MOG, NMDAR (children) |
Vogrig et al.16; Dalmau et al.45; Armangue et al.46 |
| Role of Abs | Non-pathogenic | Pathogenic | Dalmau and Graus1; Dalmau et al.45 |
| Antigen location | Intracellular | Cell surface or synaptic | Dalmau et al.45 |
| Cancer association | Very strong | Low to mild (depends on Ab-type) | Dalmau and Graus1; Dalmau et al.45 |
| Typical cancer | SCLC, NSCLC, breast, ovary, lymphoma | Teratoma, thymoma, SCLC | Vogrig et al.16; Dalmau et al.45 |
| Immunopathology | T-cell infiltration, significant neuronal loss | Presence of B cells and plasma cells | Bien et al.47 |
| Genetic features | Somatic mutations within tumor cells (e.g. Yo-PNS) | Distinctive HLA haplotypes (e.g. LGI1 encephalitis) |
Muñiz-Castrillo et al.9; Small et al.14; de Pémille et al.15 |
| Response to immunotherapy | Usually unsatisfactory | Usually satisfactory | Dalmau and Graus1; Dalmau et al.45 |
Ab, antibody; AE, autoimmune encephalitides; HLA, human leukocyte antigen; LGI1, leucine-rich glioma-inactivated 1; MOG, myelin oligodendrocyte glycoprotein; NMDAR, N-methyl-D-aspartate receptor; NSCLC, non-small-cell lung cancer; PNS, paraneoplastic neurological syndrome; SCLC, small-cell lung cancer.
Tumors as triggers of PNSs
In classical PNSs, Abs are produced mostly against onconeural antigens located intracellularly (at the level of the nucleus, nucleolus, or cytoplasm of the neurons). This means that onconeural Abs have no direct access to their targets, and there is no evidence that they are able to inactivate or change the protein function and lead to cell death.45,48,49 Nevertheless, in the few neuropathological studies available on PNS patients, clear neuronal loss was documented, signifying that alternative mechanisms are responsible for the immune attack. In particular, the most likely pathogenesis appears to be T-cell-mediated. Accordingly, prominent infiltrates of cytotoxic T cells surrounding neurons were found in anti-Yo, Hu and Ma2 cases.19,47,49–51 In addition, cytotoxic T cells were shown to express membranolytic enzymes such as perforin or granzyme B, and they were found in close apposition to neurons, providing clear clues on their active role as final effectors in killing neurons.13,47,49 Conversely, in a mouse model of PCD, pathological studies revealed neither immunoglobulin binding nor complement deposition on Purkinje cells, suggesting that humoral immune response does not play a major role.13 Pathological findings in PNSs may be confined to specific brain regions, correlating with neurological symptoms. For example, a selective loss of hypocretinergic neurons in the hypothalamus is the pathological correlate of anti-Ma2-associated narcolepsy-cataplexy,52 whereas CD8+ T lymphocyte infiltration and gliosis in the brainstem and descending spinal cord tract characterizes jaw dystonia and laryngospasm in anti-Ri PNS.53
The clinical repercussions of the T-cell immunopathology of PNSs are resistance to immunotherapies aimed at removing Abs or Ab-producing cells; the need of a combined approach with early tumor treatment followed by a more aggressive immunosuppression; and the gradual development of irreversible neuronal loss and brain atrophy in the affected regions in treatment-resistant cases.19,45 Although onconeural Abs do not have a pathogenic role in PNSs, they are excellent biomarkers, and they should direct the search of the underlying tumor, whose spectrum is different according to the detected auto-Ab.17,45
The immunological mechanisms of PNSs are closely linked to those of anti-tumor immunity. In particular, cancer-derived antigens – including neoantigens resulting from mutations in the tumor genes encoding onconeural proteins – are released upon apoptosis from tumors under attack by innate immune cells.14,15,17,54 These peptides are then processed and presented by antigen presenting cells (APCs) to CD4+ helper T cells via major histocompatibility complex class II (MHC II), which in turn will activate antigen-specific B cells into Ab-producing plasma cells, leading to the appearance of onconeural Abs in serum (first) and in CSF (later); but also CD8+ cytotoxic T lymphocytes via MHC class I molecules, which will cause the neurological dysfunction.17,54,55
It should be considered that many tumors express onconeural proteins (e.g., constant expression of HuD by SCLC) but only a minority of patients harboring such cancers develop PNS.17,56 Moreover, 10–15% of patients with SCLC harbor low circulating anti-Hu Abs in the absence of neurological symptoms.17,56 Intriguingly, patients with PNS have a better prognosis than those with histologically identical tumors not linked to PNS.57 The latter observation is probably explained by the higher number of tumor-infiltrating lymphocytes and germinal centers in patients with PNS,14,28 which are known oncological prognostic factors.58
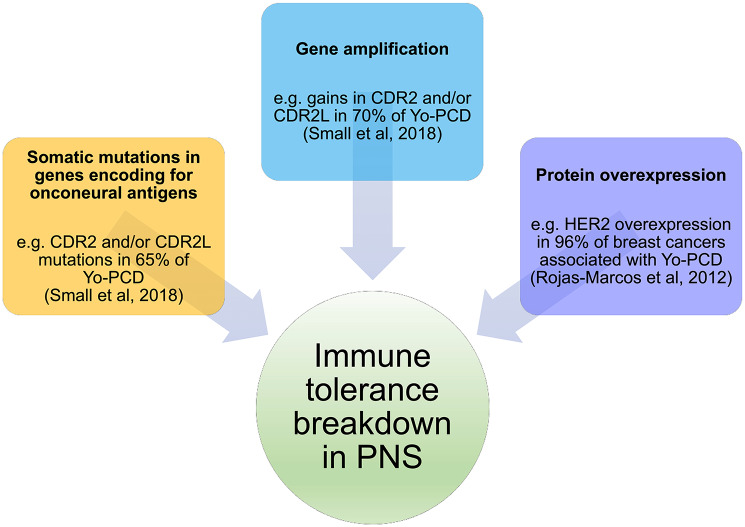
The first event in the pathogenesis of PNSs, which leads to the immune tolerance break, is not well understood yet. However, several lines of evidence suggest that this is not related to a particular histological tumor type, but rather to a specific molecular signature of cancer cells. For instance, a number of recent studies have reported that tumor genetic alterations are the initial triggers.14,15 These abnormalities may result from somatic mutations in genes encoding for onconeural antigens: this is the case of somatic Yo-antigens (CDR2L and CDR2) mutations in ovarian tumor cells, which are present in two-thirds of patients with Yo-PCD; they may also result from gene amplification: for example, gains in CDR2, CDR2L, or both are detectable in 70% of the patients with Yo-PCD; and also protein overexpression: HER2 overexpression was reported in 96% of patients with breast cancer associated with Yo-PCD as compared with 15–25% in breast cancer unrelated to PCD (Figure 1).14,15,59 Other PNS with the same clinical presentation (PCD) and same cancer association (breast) but different Ab specificity (Ri) did not show HER2 overexpression, pinpointing that this pathway can be relevant for some, but not all, PNSs.26 Interestingly, a prominent transcriptomic overrepresentation of CD8+ and Treg cells was found in anti-Yo PCD related to ovary cancer, as compared with a cohort of patients with the same tumor type not associated with PCD.15 Additionally, an up-regulation of the autoimmune-regulator (AIRE) gene, responsible for the negative selection of self-recognizing T cells, was found in the same study, thus providing a clear link with development of autoimmune diseases.15
Figure 1.
Tumor genetic alterations leading to immune tolerance breakdown in PNS.
The model of PCD is shown since it is the only one in which genetic studies on PNS-related tumors have been performed. Three main mechanisms are presented: somatic mutations, gene amplification, and protein overexpression. These processes are not mutually exclusive.
HER2, human epidermal growth factor receptor 2; PCD, paraneoplastic cerebellar degeneration; PNS, paraneoplastic neurological syndrome.
Like PNSs, AEs and other neurological syndromes with Abs targeting neuronal surface and synaptic receptors can be triggered by cancer, although the frequency of this association is clearly lower. Examples include the presence of SCLC in 66% of patients with anti-gamma aminobutyric acid B receptor (GABABR) limbic encephalitis,41 ovarian teratoma in 36% of patients with anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis,60 and malignant thymoma in 15–20% of patients with anti-contactin-associated protein-like 2 (CASPR2)-syndromes.61,62 In these settings, some clues can suggest a higher risk of an underlying cancer. Older age, history of smoking, and presence of additional Abs [e.g., potassium channel tetramerization domain-containing (KCTD)16-Abs] suggest that anti-GABABR encephalitis is associated with cancer.41 The same applies for the presence of distinctive clinical phenotypes (e.g., Morvan’s syndrome, characterized by peripheral nerve hyperexcitability, autonomic instability, and encephalopathy) in anti-CASPR2 syndromes.61,62 Female sex, black ethnicity, and age between 12 and 45 years were associated with the presence of ovarian teratoma in patients with anti-NMDAR encephalitis.60,63 The study of ovarian teratomas in this setting is of significant interest as it provides a model of PNS in which Abs are directed towards synaptic antigens. As for patients with SCLC and associated HuD protein, the presence of a teratoma expressing NMDAR is not sufficient per se to induce an AE, suggesting the presence of other tumor specificities in anti-NMDAR encephalitis patients.64 In particular, the expression of GluN1 (the subunit targeted by NMDAR-Abs) by a particular cell type within the teratoma (glial cell) seems to be involved in the outbreak of this AE.64 Moreover, ovarian teratomas in patients with anti-NMDAR encephalitis contain more frequently a nervous tissue component, and the latter can demonstrate histological features of a brain neuroglial tumor (resembling oligodendroglioma, ganglioma, or malignant glioma).64,65 Importantly, teratomas linked to AE also demonstrate prominent infiltration by immune cells (T- and B-cells, and dendritic cells), sometimes organized in tertiary lymphoid structures (TLS).64–66 The latter are ectopic lymphoid organs that develop at sites of chronic inflammation and are important to provide a local source for Ab production (in this model, NMDAR-Abs are initially produced in the periphery, like classic PNSs).64
ICIs as triggers of PNSs
The activation of T cells requires a two-step process. An antigen needs to be presented via MHC class I or II molecules to the T cell receptor and, additionally, a co-stimulatory signal is needed to up-regulate the immune response (e.g., binding of CD80/86 on APC to the CD28 receptor on the CD4+ T cell). Alternatively, it is also possible to inhibit the immune response if certain receptors [such as the cytotoxic T-lymphocyte antigen 4 (CTLA4), or the programmed cell death protein 1 (PD-1)] bind to their specific ligands (CD80 and PD-L1, respectively).67–69 Tumor cells can escape the immune control by over-expressing checkpoint inhibitors ligands (e.g., PD-L1 receptors), thus down-regulating the immune response.67–69 Pharmacological blocking of these interactions using monoclonal Abs represents the major advance in clinical oncology over the last 30 years.67 These drugs (collectively known as ICIs) are classified on the basis of their target: anti-PD1 (nivolumab, pembrolizumab, cemiplimab), anti-PDL1 (atezolizumab, avelumab, durvalumab), and anti-CTLA4 (ipilimumab). Their benefit in terms of increasing survival has been demonstrated in many cancer types, including melanoma, lung and kidney cancers, and their use is increasing dramatically.67
Immune-related adverse events (ir-AEs) can be unleashed by the use of ICIs. Severe neurological ir-AEs are rare (approximately 1% of patients) and appear to be more common in cases treated with anti-CTLA4, especially if in combination with anti-PD1/PD-L1, where the frequency of neurological complications approximates 3%.70,71 Most (90%) of the neurological toxicities develop within the first six cycles of ICI treatment, or even sooner (within four cycles) after change of ICI.71 Hyperacute (fulminant) presentation seems to correlate with combination therapy, and these cases usually manifest a myasthenia gravis-like phenotype.72 This disorder has several similarities with its idiopathic counterpart, but it is more often associated with myocarditis, myositis, and myasthenic crisis.73 These factors explain the high fatality rate of ICI-triggered myasthenia gravis (20%).72,73 Importantly, class-specific associations in the phenotypic spectrum were observed. Myasthenia gravis and encephalitis were more frequently observed in patients treated with anti-PD-1, whereas Guillain-Barre syndrome and meningitis appear to be more common after anti-CTLA4 therapy.72
PNSs tend to be worsened or revealed by ICIs, and cases of limbic encephalitis are of particular concern, given their severity.12,74 We previously observed an increased frequency of anti-Ma2 PNS in France after the use of ICIs became widespread in clinical practice.12 Most anti-Ma2 cases of this series developed in the context of a non-small-cell lung cancer, whereas the absence of the typical germ cell tumor was explained by the lack of indication for immunotherapy in this type of cancer.12 Following this observation, we and others speculated an analogous increase in anti-Hu and anti-CV2/CRMP5-associated syndromes with ICI treatment of SCLC [US Food and Drug Administration (FDA) approval in 2018] or anti-Ri syndromes in breast cancer (FDA approval in 2019).12,17 In agreement with this hypothesis, the first reports of these associations were recently described.75,76
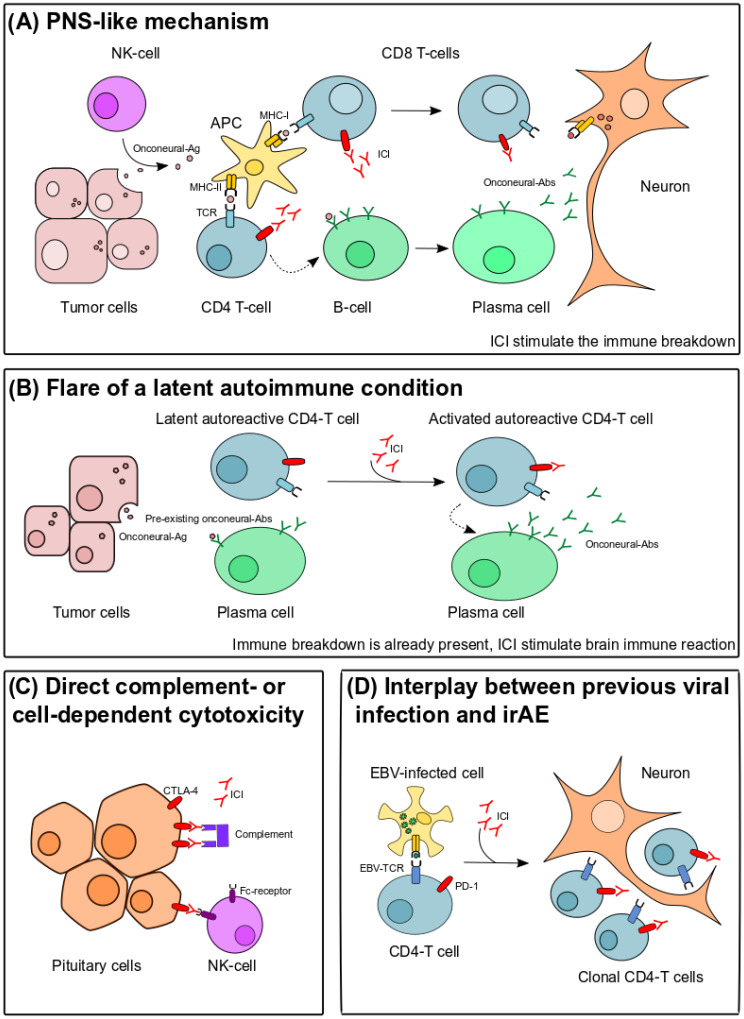
The mechanisms underlying neurological ir-AEs still need to be fully elucidated. The first to be proposed was an immune-response against an auto-antigen shared between the tumor and neural tissue (Figure 2A), as in classical PNSs.77 Proof of concept was brought recently in an experimental study that adopted a mouse model that expressed a neo-self-antigen in both Purkinje cells and in implanted breast cancer cells.13 In this model, ICI treatment with anti-CTLA4 was essential to develop significant cerebellar inflammation and neuronal loss consistent with those observed in PCD.13 Interestingly, some authors have suggested that cases induced by ICIs respond better to steroids and do not always require long-term treatment as compared with classical PNSs.71 Our experience is similar in seronegative cases, but we observed a severe course and the need of prolonged treatment (often with second-line immunosuppression) in cases of ICI-triggered anti-Ma2 PNS.12 The second possibility is that patients with ICI-related toxicities have an underlying autoimmune predisposition or a latent autoimmune condition present before the onset of immunotherapy. In this context, treatment with ICIs is able to flare the autoimmune disorder, which becomes then clinically apparent (Figure 2B).77 An important argument for this hypothesis is the presence of neural Abs before the onset of cancer immunotherapy in the few cases in which a pre-treatment sample was stored and analyzed.12,78 Is it therefore tempting to speculate that patients with pre-existing Abs are at increased risk of developing irAEs, but further prospective studies are needed to verify the existence of an increased susceptibility. A third possibility is that the target molecules (PD-1, PD-L1, or CTLA-4) are expressed on resident cells of the nervous system and therefore direct complement-dependent or cell-dependent cytotoxicity may take place in the presence of ICIs (Figure 2C).77 This model seems to be particularly adapted to explain ICI-triggered hypophysitis, where histological analysis in one case revealed local deposition of monoclonal Abs targeting CTLA-4, as well as complement deposition.79 In agreement with this model, hypophysitis is a frequent complication of ipilimumab (10% of patients), and does not increase in frequency if anti-PD1/PD-L1 are added, suggesting a drug-specific effect.77 Finally, activation of clonal Epstein-Barr virus (EBV) positive T-cells was demonstrated in a case of encephalitis developing after melanoma treatment with ipilimumab and pembrolizumab (Figure 2D).80 This finding seems particularly interesting since EBV was shown to have the ability to subvert host immune surveillance by modulating PD-L1 expression in EBV-associated tumors (e.g., diffuse large B-cell lymphomas).81 Given that EBV is highly prevalent worldwide, it is reasonable to think that its role in PD-L1 expression extends to other cancer types, including solid tumors.82
Figure 2.
Proposed mechanisms of neurological irAEs triggered by ICIs. (A) PNS-like mechanism. An immune-response against a shared auto-antigen between the tumor and neural tissue is triggered by ICIs. In the periphery, tumor-related antigens (including mutant forms of onconeural proteins) are released after immune attack by NK-cells. Onconeural antigens are then phagocytosed and presented by APCs to cells of the adaptive immune system. CD4+ helper T cells play a key role in activating B cells, which are then able to differentiate into antibody-producing plasma cells. Antigen-specific CD8+ cytotoxic T cells are also primed by APCs and represent the main effectors of neuronal damage. (B) Flare of a latent autoimmune condition. In this hypothesis, immune breakdown is already present and ICI stimulate the brain immune-reaction. (C) Direct complement-dependent or cell-dependent cytotoxicity. CTLA-4 is expressed on pituitary cells and ICI treatment can trigger complement-mediated direct damage, explaining the high rate (approximately 10%) of hypophysitis in patients treated with anti-CTLA4 Abs (ipililumab). (D) Interplay between previous viral infection and irAE. Oligoclonal activation of CD4+ T cells specific for a viral pathogen (e.g., EBV) in the presence of ICIs.
Abs, antibodies; Ag, antigen; APC, antigen-presenting cell; CTLA4, cytotoxic T-lymphocyte antigen 4; EBV, Epstein-Barr virus; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; MHC, major histocompatibility complex; NK-cell, natural killer cell; PD-1, programmed cell death protein 1; PNS, paraneoplastic neurological syndrome; TCR, T-cell receptor.
AE: definition, epidemiological and clinical aspects
In adults, AE is clinically defined by the subacute onset of memory deficits, altered mental status, and psychiatric symptoms in association with one or more of the following: new focal central nervous system findings, seizures, CSF pleocytosis, or brain MRI features consistent with encephalitis.37 In children, a new set of criteria have been proposed recently, accounting for the differences in the presentation of AE in the pediatric population.83 Importantly, it was underlined that the onset of AE in children can be acute or subacute, many patients do not present with well-defined syndromes, and the clinical picture can include focal or diffuse neurological deficits, sometimes in the form of a developmental regression.83 In addition, the Ab-spectrum is different between adults and children (Table 1). In adults with AE, the most common Ab detected is anti-NMDAR (especially in young female cases), followed by anti-leucine-rich glioma-inactivated 1 (LGI1; typically in middle-aged/elderly males).1,45 In children, it was recently reported that the most frequent specificity is myelin oligodendrocyte glycoprotein (MOG), followed by NMDAR.46 Anti-MOG encephalitis should be considered in young patients showing extensive cortical encephalitis, isolated basal ganglia and/or thalamus involvement, or with refractory status epilepticus and mild MRI changes.46 Seizures are a cardinal symptom in both adult and pediatric AE cases, they are often resistant to anti-seizure medications, and have different semiology and outcome according to the sex, age of the patient, and Ab-association.84 Some patients with AE may manifest prominent or isolated psychiatric features (i.e., autoimmune psychosis).85 In this context, red flags pointing to an autoimmune etiology include rapid progression, adverse response to antipsychotics (including neuroleptic malignant syndrome), appearance of aphasia/mutism, or other additional neurological findings.85
Very few epidemiological studies have been performed in AE. The annual incidence per million person-years has been estimated at 8 for AE (both Ab-positive and negative) in Olmstead County, MN, USA,44 2.2 for pediatric anti-NMDAR encephalitis in Hong Kong,86 and 0.83 for anti-LGI1 encephalitis in the Netherlands.87
Most AEs are associated with Abs targeting epitopes exposed to the neuronal cell surface.1,2,45 Differently from PNSs, the role of these auto-Abs seems to be pathogenic, therefore AEs often respond favourably to treatments aimed to remove Abs or depleting Ab-producing cells.1,2,45 Brain pathology findings of cases with anti-NMDAR encephalitis confirmed the absence of dominant T cell infiltrate, whereas B cells and plasma cells were detected frequently in the perivascular spaces.47,49 Interestingly, complement activation and neuronal loss were absent in anti-NMDAR encephalitis. Conversely, a modest degree of complement-dependent cell loss was observed in anti-LGI1 and anti-CASPR2-associated syndromes,47,49 which could account for the more prolonged clinical course of these patients, and the frequent detection of hippocampal atrophy in the advanced stages of the disease.61 AEs have a peculiar spectrum of disease triggers and predisposing factors, including distinctive HLA haplotypes,9 infectious triggers,5 and the reciprocal interactions between these two factors. In addition, as with PNS, AE can also be induced by ICI treatment (Figure 3).88
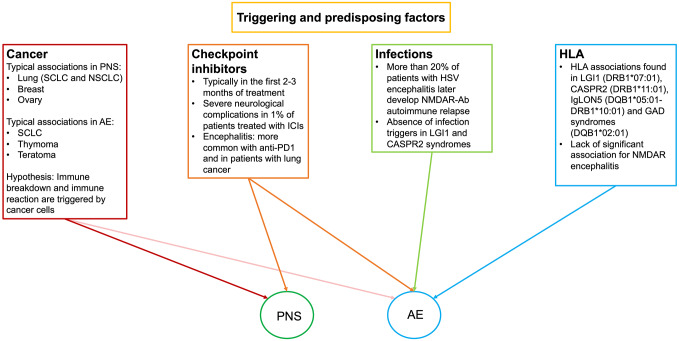
Figure 3.
Schematic representation of the four known triggering factors in PNS and AE.
AE, autoimmune encephalitides; CASPR2, contactin-associated protein-like 2; GAD, glutamic-acid decarboxylase; HLA, human leukocyte antigen; HSV, herpes simplex virus; IgLON5, immunoglobulin-like cell adhesion molecule 5; LGI1, leucine-rich glioma-inactivated 1; NMDAR, N-methyl-D-aspartate receptor; NSCLC, non-small-cell lung cancer; PNS, paraneoplastic neurological syndromes; SCLC, small-cell lung cancer.
Infections as triggers of AEs
Patients with AEs often report an infectious prodrome antedating the development of the neurological symptoms by days or a few weeks5; typically, these mild “flu-like” episodes are characterized by upper respiratory tract infections or self-resolving gastroenteritis. The same applies for other autoimmune neurological syndromes involving the peripheral (e.g., Guillain-Barré syndrome after Campylobacter jejuni infection) as well as central nervous system (e.g., Sydenham chorea associated with streptococcal infection).4,5 In the case of AE, the most common associated pathogen is herpes simplex virus (HSV) causing viral encephalitis.4–7 The resulting autoimmune relapse usually manifests itself as anti-NMDAR syndrome, which accounts for the pediatric disorder previously known as “choreoathetosis post-HSV encephalitis”.5,7 The reasons why HSV seems to be particularly prone to induce anti-NMDAR encephalitis among all infectious pathogens are unknown. One possibility is linked to the high destructive potential of HSV, which is able to release antigens (including cell surface or synaptic receptors) from the damaged brain tissue, and subsequent self-immunization against the GluN1 subunit of NMDAR.7,45 The fact that these patients commonly develop Abs against multiple neuronal proteins (e.g., GABAAR) renders the possibility of molecular mimicry less likely to explain the pathogenesis of this immune-mediated complication.4,5,7 Overall, 27% of patients with HSV encephalitis subsequently develop AE (mostly anti-NMDAR encephalitis, but other auto-Abs have been detected in up to 36% of the cases).7 The clinical presentation and the outcome of the relapse differ according to the age of onset. Patients younger than 4 years tend to have shorter intervals between viral encephalitis and AE (typically less than 1 month) as compared with adult cases, more frequently present with choreoathetosis (whereas older patients demonstrate more commonly psychosis), and show a worse outcome (the response to immunotherapy is better in adult cases).7 The interplay between genetic factors and infective pathogens is of paramount importance in this context, as it was demonstrated that 5% of patients who develop HSV encephalitis harbor a particular deficiency in the gene encoding Toll-like receptor 3 (TLR3), an important pathway for innate immunity. Despite the rarity of this genetic defect among patients with HSV encephalitis, it is very interesting to note that 66% of those who harbor TLR3 deficiency later develop AE.89 A mouse model of post-HSV anti-NMDAR encephalitis has shown that intranasal inoculation of HSV-1 in mice was able to induce the production of NMDAR-Abs in more than half of the animals, along with reduced levels of hippocampal NMDAR.8 Few other infectious pathogens have been linked to NMDAR encephalitis, including varicella zoster virus (VZV), mycoplasma, and human immunodeficiency virus (HIV).5,90 In contrast to NMDAR encephalitis, no infectious prodrome was observed in anti-LGI1 and anti-CASPR2 encephalitis,61 suggesting that other factors may be relevant.
Association between HLA and AEs
HLA is the main genetic factor related to autoimmunity, and several associations have been described in autoimmune neurological diseases, especially in those presenting with auto-Abs.9 Despite the modest associations initially reported in classic PNSs,91,92 the study of genetic predisposition in AEs has recently led to highly interesting and promising findings.
Patients with neurological syndromes with anti-glutamic-acid decarboxylase (GAD) Abs and their relatives usually present with other organ-specific autoimmune diseases, suggesting a strong genetic predisposition to autoimmunity. In agreement with this observation, anti-GAD neurological syndromes were the first to be associated with HLA, although initial studies only included patients with stiff-person syndrome and genotyping was incomplete.93,94 We have recently confirmed the association with the extended haplotype DQA1*05:01-DQB1*02:01-DRB1*03:01 in a large series.95 This haplotype was already known to be linked to several organ-specific autoimmune diseases, and, therefore, constitutes a shared genetic predisposing factor between them and anti-GAD neurological syndromes. However, other HLA may be involved in particular familial cases.96
Encephalitis with anti-immunoglobulin-like cell adhesion molecule 5 (IgLON5) Abs is strongly associated with the class II HLA allele DRB1*10:01, which was carried by 86.6% of the patients in one of the first large samples.97 Moreover, in silico studies predicted strong binding between IgLON5 peptides and DRB1*10:01.98 Intriguingly, some clinical and immunological differences were observed between DRB1*10:01 carriers and non-carriers. The detection of anti-IgLON5 Abs in CSF, as well as the typical sleep and bulbar disturbances, were more frequent among the DRB1*10:01 carriers. Conversely, non-carriers had more commonly a negative CSF, cognitive impairment, and PSP-like presentation.97,98 It is therefore unclear whether anti-IgLON5 encephalitis represents a heterogeneous disease, or, alternatively, two different entities with distinct genetic, immunological, and clinical characteristics are associated with anti-IgLON5 Abs.
Anti-LGI1 encephalitis is strongly associated with another class II allele, DRB1*07:01, which was carried by nearly 90% of the patients in several studies.10,11,99 So far, no significant clinical or immunological difference has been reported between DRB1*07:01 carriers and non-carriers.10,11,99 Regarding its binding properties, in silico studies identified several LGI1-derived peptides that may potentially bind with high affinity DRB1*07:01, but the binding specificity remains to be clarified.10,99 The allele DRB1*11:01 was detected in approximately 50% of the patients presenting various neurological diseases with CASPR2-Abs.99 In addition, in silico studies also identified several CASPR2-derived peptides as strong binders to DRB1*11:01.99 Taken together, these findings suggest that an altered peptide presentation by the particular associated HLA for each AE may be involved in their pathogenesis.
In contrast to the aforementioned diseases, no consistent genetic predisposition has been reported in anti-NMDAR encephalitis, with only two weak and infrequent HLA associations (DRB1*16:01 and B*07:02) described.100,101 Nevertheless, larger and more comprehensive studies should investigate the possible role of other non-HLA loci in the pathogenesis of this disease.
Conclusion
The mechanisms underlying immune tolerance breakdown in PNSs and AEs remain largely unknown. Important clues come from the study of triggering and predisposing factors, namely tumors, cancer immunotherapy, infections and specific HLA haplotypes. In PNSs, studies addressing genetic and immunopathological characterization of tumors, including those treated with ICIs, have provided exceptional models for understanding the pathophysiology of these conditions. In AEs, the complex interplay between genetic (HLA) and environmental (infections) factors was found to be of crucial importance. Despite the rarity of PNSs and AEs, the repercussions of these findings will likely extend far beyond the field of neuroimmunology, with major impact in several other areas of medicine.
Acknowledgments
We gratefully acknowledge Philip Robinson for help in manuscript preparation (Direction de la Recherche Clinique, Hospices Civils de Lyon).
Footnotes
Author contributions: AV, SMC, and JH designed the review. AV and SMC performed the literature search and drafted the first version of the article. AV, SMC, VD, BJ, and JH revised the article critically for important intellectual content. JH supervised the study. All authors agreed on the final submitted version.
Conflict of interest statement: AV reported receiving a fellowship grant from the European Academy of Neurology (EAN). No other disclosures were reported.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by research grants from the French national research agency (ANR; ANR-14-CE15-0001-MECANO), and FRM (Fondation pour la recherche médicale, DQ20170336751). This work has been developed within the BETPSY project, which is supported by a public grant overseen by the French national research agency (ANR), as part of the second “Investissements d’Avenir” program (ANR-18-RHUS-0012).
ORCID iDs: Alberto Vogrig  https://orcid.org/0000-0002-3652-7061
https://orcid.org/0000-0002-3652-7061
Bastien Joubert  https://orcid.org/0000-0003-4631-3056
https://orcid.org/0000-0003-4631-3056
Contributor Information
Alberto Vogrig, French Reference Center for Paraneoplastic Neurological Syndromes, Hospices Civils de Lyon, Hospital for Neurology and Neurosurgery Pierre Wertheimer, Lyon, France; SynatAc Team, NeuroMyoGene Institute, INSERM U1217/CNRS UMR5310, Lyon, France; University Claude Bernard Lyon 1, Université de Lyon, Lyon, France.
Sergio Muñiz-Castrillo, French Reference Center for Paraneoplastic Neurological Syndromes, Hospices Civils de Lyon, Hospital for Neurology and Neurosurgery Pierre Wertheimer, Lyon, France; SynatAc Team, NeuroMyoGene Institute, INSERM U1217/CNRS UMR5310, Lyon, France; University Claude Bernard Lyon 1, Université de Lyon, Lyon, France.
Virginie Desestret, French Reference Center for Paraneoplastic Neurological Syndromes, Hospices Civils de Lyon, Hospital for Neurology and Neurosurgery Pierre Wertheimer, Lyon, France; SynatAc Team, NeuroMyoGene Institute, INSERM U1217/CNRS UMR5310, Lyon, France; University Claude Bernard Lyon 1, Université de Lyon, Lyon, France.
Bastien Joubert, French Reference Center for Paraneoplastic Neurological Syndromes, Hospices Civils de Lyon, Hospital for Neurology and Neurosurgery Pierre Wertheimer, Lyon, France; SynatAc Team, NeuroMyoGene Institute, INSERM U1217/CNRS UMR5310, Lyon, France; University Claude Bernard Lyon 1, Université de Lyon, Lyon, France.
Jérôme Honnorat, Centre de Référence National pour les Syndromes Neurologiques Paranéoplasiques, Hôpital Neurologique, 59 Boulevard Pinel, Bron Cedex, 69677, France; SynatAc Team, NeuroMyoGene Institute, INSERM U1217/CNRS UMR5310, Lyon, France; University Claude Bernard Lyon 1, Université de Lyon, Lyon, France.
References
- 1. Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med 2018; 378: 840–851. [DOI] [PubMed] [Google Scholar]
- 2. Ramanathan S, Al-Diwani A, Waters P, et al. The autoantibody-mediated encephalitides: from clinical observations to molecular pathogenesis. J Neurol. Epub ahead of print 26 October 2019. DOI: 10.1007/s00415-019-09590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chefdeville A, Honnorat J, Hampe CS, et al. Neuronal central nervous system syndromes probably mediated by autoantibodies. Eur J Neurosci 2016; 43: 1535–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexopoulos H, Dalakas MC. The immunobiology of autoimmune encephalitides. J Autoimmun 2019; 104: 102339. [DOI] [PubMed] [Google Scholar]
- 5. Joubert B, Dalmau J. The role of infections in autoimmune encephalitides. Rev Neurol (Paris) 2019; 175: 420–426. [DOI] [PubMed] [Google Scholar]
- 6. Alexopoulos H, Akrivou S, Mastroyanni S, et al. Postherpes simplex encephalitis: a case series of viral-triggered autoimmunity, synaptic autoantibodies and response to therapy. Ther Adv Neurol Disord 2018; 11: 1756286418768778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018; 17: 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linnoila J, Pulli B, Armangué T, et al. Mouse model of anti-NMDA receptor post-herpes simplex encephalitis. Neurol Neuroimmunol Neuroinflamm 2018; 6: e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muñiz-Castrillo S, Vogrig A, Honnorat J. Associations between HLA and autoimmune neurological diseases with autoantibodies. Auto Immun Highlights 2020; 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim TJ, Lee ST, Moon J, et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann Neurol 2017; 81: 183–192. [DOI] [PubMed] [Google Scholar]
- 11. van Sonderen A, Roelen DL, Stoop JA, et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann Neurol 2017; 81: 193–198. [DOI] [PubMed] [Google Scholar]
- 12. Vogrig A, Fouret M, Joubert B, et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm 2019; 6: e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yshii LM, Gebauer CM, Pignolet B, et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain 2016; 139: 2923–2934. [DOI] [PubMed] [Google Scholar]
- 14. Small M, Treilleux I, Couillault C, et al. Genetic alterations and tumor immune attack in Yo paraneoplastic cerebellar degeneration. Acta Neuropathol 2018; 135: 569–579. [DOI] [PubMed] [Google Scholar]
- 15. de Pémille CV, Berzero G, Small M, et al. Transcriptomic immune profiling of ovarian cancers in paraneoplastic cerebellar degeneration associated with anti-Yo antibodies. Br J Cancer 2018; 119: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vogrig A, Gigli GL, Segatti S, et al. Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol. Epub ahead of print 24 September 2019. DOI: 10.1007/s00415-019-09544-1. [DOI] [PubMed] [Google Scholar]
- 17. Graus F, Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol. Epub ahead of print 12 March 2019. DOI: 10.1038/s41571-019-0194-4. [DOI] [PubMed] [Google Scholar]
- 18. Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis 2007; 2: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKeon A, Pittock SJ. Paraneoplastic encephalomyelopathies: pathology and mechanisms. Acta Neuropathol 2011; 122: 381–400. [DOI] [PubMed] [Google Scholar]
- 20. Shams’ili S, Grefkens J, de Leeuw B, et al. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain 2003; 126: 1409–1418. [DOI] [PubMed] [Google Scholar]
- 21. Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol 2011; 18: 19-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Titulaer MJ, Wirtz PW, Willems LNA, et al. Screening for small-cell lung cancer: a follow-up study of patients with Lambert-Eaton myasthenic syndrome. J Clin Oncol 2008; 26: 4276–4281. [DOI] [PubMed] [Google Scholar]
- 23. Mathew RM, Vandenberghe R, Garcia-Merino A, et al. Orchiectomy for suspected microscopic tumor in patients with anti-Ma2-associated encephalitis. Neurology 2007; 68: 900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wesley SF, Ferguson D. Autoimmune encephalitides and rapidly progressive dementias. Semin Neurol 2019; 39: 283–292. [DOI] [PubMed] [Google Scholar]
- 25. Vogrig A, Joubert B, Maureille A, et al. Motor neuron involvement in anti-Ma2-associated paraneoplastic neurological syndrome. J Neurol. Epub ahead of print 29 November 2018. DOI: 10.1007/s00415-018-9143-x. [DOI] [PubMed] [Google Scholar]
- 26. Simard C, Vogrig A, Joubert B, et al. Clinical spectrum and diagnostic pitfalls of neurologic syndromes with Ri antibodies. Neurol Neuroimmunol Neuroinflamm 2020; 7: e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vogrig A, Bernardini A, Gigli GL, et al. Stroke-like presentation of paraneoplastic cerebellar degeneration: a single-center experience and review of the literature. Cerebellum 2019; 18: 976–982. [DOI] [PubMed] [Google Scholar]
- 28. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003; 349: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 29. Giometto B, Grisold W, Vitaliani R, et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol 2010; 67: 330–335. [DOI] [PubMed] [Google Scholar]
- 30. Chan AM, Baehring JM. Paraneoplastic neurological syndromes: a single institution 10-year case series. J Neurooncol 2019; 141: 431–439. [DOI] [PubMed] [Google Scholar]
- 31. Vogrig A, Joubert B, Ducray F, et al. Glioblastoma as differential diagnosis of autoimmune encephalitis. J Neurol 2018; 265: 669–677. [DOI] [PubMed] [Google Scholar]
- 32. Flanagan EP, McKeon A, Lennon VA, et al. Paraneoplastic isolated myelopathy: clinical course and neuroimaging clues. Neurology 2011; 76: 2089–2095. [DOI] [PubMed] [Google Scholar]
- 33. Liu Z, Jiao L, Qiu Z, et al. Clinical characteristics of patients with paraneoplastic myelopathy. J Neuroimmunol 2019; 330: 136–142. [DOI] [PubMed] [Google Scholar]
- 34. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain 2004; 127: 1831–1844. [DOI] [PubMed] [Google Scholar]
- 35. Vogrig A, Ferrari S, Tinazzi M, et al. Anti-Ma-associated encephalomyeloradiculopathy in a patient with pleural mesothelioma. J Neurol Sci 2015; 350: 105–106. [DOI] [PubMed] [Google Scholar]
- 36. Kunchok A, Zekeridou A, Pittock S. CRMP5-IgG-associated paraneoplastic myelopathy with PD-L1 inhibitor therapy. JAMA Neurol. Epub ahead of print 20 December 2019. DOI: 10.1001/jamaneurol.2019.4379. [DOI] [PubMed] [Google Scholar]
- 37. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Escudero D, Guasp M, Ariño H, et al. Antibody-associated CNS syndromes without signs of inflammation in the elderly. Neurology 2017; 89: 1471–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004; 75: 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mandel-Brehm C, Dubey D, Kryzer TJ, et al. Kelch-like protein 11 antibodies in seminoma-associated paraneoplastic encephalitis. N Engl J Med 2019; 381: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Coevorden-Hameete MH, de Bruijn MAAM, de Graaff E, et al. The expanded clinical spectrum of anti-GABABR encephalitis and added value of KCTD16 autoantibodies. Brain 2019; 142: 1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Do LD, Gupton SL, Tanji K, et al. TRIM9 and TRIM67 are new targets in paraneoplastic cerebellar degeneration. Cerebellum 2019; 18: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Déchelotte B, Muñiz-Castrillo S, Joubert B, et al. Diagnostic yield of commercial immunodots to diagnose paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm 2020; 7: e701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 2018; 83: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev 2017; 97: 839–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Armangue T, Olivé-Cirera G, Martínez-Hernandez E, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol 2020; 19: 234–246. [DOI] [PubMed] [Google Scholar]
- 47. Bien CG, Vincent A, Barnett MH, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 2012; 135: 1622–1638. [DOI] [PubMed] [Google Scholar]
- 48. Tanaka K, Ding X, Tanaka M. Effects of antineuronal antibodies from patients with paraneoplastic neurological syndrome on primary-cultured neurons. J Neurol Sci 2004; 217: 25–30. [DOI] [PubMed] [Google Scholar]
- 49. Bauer J, Bien CG. Neuropathology of autoimmune encephalitides. Handb Clin Neurol 2016; 133: 107–120. [DOI] [PubMed] [Google Scholar]
- 50. Roberts WK, Deluca IJ, Thomas A, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest 2009; 119: 2042–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Albert ML, Darnell JC, Bender A, et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med 1998; 4: 1321–1324. [DOI] [PubMed] [Google Scholar]
- 52. Dauvilliers Y, Bauer J, Rigau V, et al. Hypothalamic immunopathology in anti-Ma-associated diencephalitis with narcolepsy-cataplexy. JAMA Neurol 2013; 70: 1305–1310. [DOI] [PubMed] [Google Scholar]
- 53. Pittock SJ, Parisi JE, McKeon A, et al. Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri). Arch Neurol 2010; 67: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 54. Zekeridou A, Lennon VA. Neurologic autoimmunity in the era of checkpoint inhibitor cancer immunotherapy. Mayo Clin Proc 2019; 94: 1865–1878. [DOI] [PubMed] [Google Scholar]
- 55. Iorio R, Spagni G, Masi G. Paraneoplastic neurological syndromes. Semin Diagn Pathol 2019; 36: 279–292. [DOI] [PubMed] [Google Scholar]
- 56. Pignolet BS, Gebauer CM, Liblau RS. Immunopathogenesis of paraneoplastic neurological syndromes associated with anti-Hu antibodies: a beneficial antitumor immune response going awry. Oncoimmunology 2013; 2: e27384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maddison P, Newsom-Davis J, Mills KR, et al. Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma. Lancet 1999; 353: 117–118. [DOI] [PubMed] [Google Scholar]
- 58. Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019; 19: 307–325. [DOI] [PubMed] [Google Scholar]
- 59. Rojas-Marcos I, Picard G, Chinchón D, et al. Human epidermal growth factor receptor 2 overexpression in breast cancer of patients with anti-Yo–associated paraneoplastic cerebellar degeneration. Neuro Oncol 2012; 14: 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Sonderen A, Petit-Pedrol M, Dalmau J, et al. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat Rev Neurol 2017; 13: 290–301. [DOI] [PubMed] [Google Scholar]
- 62. Joubert B, Saint-Martin M, Noraz N, et al. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol 2016; 73: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 63. Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chefdeville A, Treilleux I, Mayeur ME, et al. Immunopathological characterization of ovarian teratomas associated with anti-N-methyl-D-aspartate receptor encephalitis. Acta Neuropathol Commun 2019; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Day GS, Laiq S, Tang-Wai DF, et al. Abnormal neurons in teratomas in NMDAR encephalitis. JAMA Neurol 2014; 71: 717–724. [DOI] [PubMed] [Google Scholar]
- 66. Makuch M, Wilson R, Al-Diwani A, et al. N-methyl-D-aspartate receptor antibody production from germinal center reactions: therapeutic implications. Ann Neurol 2018; 83: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Haugh AM, Probasco JC, Johnson DB. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf 2020; 19: 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford) 2019; 58(Suppl. 7): vii59–vii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cuzzubbo S, Javeri F, Tissier M, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer 2017; 73: 1–8. [DOI] [PubMed] [Google Scholar]
- 71. Dubey D, David WS, Reynolds KL, et al. Severe neurological toxicity of immune checkpoint inhibitors: growing spectrum. Ann Neurol. Epub ahead of print 22 February 2020. DOI: 10.1002/ana.25708. [DOI] [PubMed] [Google Scholar]
- 72. Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer 2019; 7: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017; 89: 1127–1134. [DOI] [PubMed] [Google Scholar]
- 74. Manson G, Maria ATJ, Poizeau F, et al. Worsening and newly diagnosed paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 immunotherapies, a descriptive study. J Immunother Cancer 2019; 7: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gill A, Perez MA, Perrone CM, et al. A case series of PD-1 inhibitor-associated paraneoplastic neurologic syndromes. J Neuroimmunol 2019; 334: 576980. [DOI] [PubMed] [Google Scholar]
- 76. Raibagkar P, Ho D, Gunturu KS, et al. Worsening of anti-Hu paraneoplastic neurological syndrome related to anti-PD-1 treatment: case report and review of literature. J Neuroimmunol 2020; 341: 577184. [DOI] [PubMed] [Google Scholar]
- 77. Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol 2017; 13: 755–763. [DOI] [PubMed] [Google Scholar]
- 78. Mammen AL, Rajan A, Pak K, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 2019; 78: 150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Iwama S, De Remigis A, Callahan MK, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014; 6: 230ra45. [DOI] [PubMed] [Google Scholar]
- 80. Johnson DB, McDonnell WJ, Gonzalez-Ericsson PI, et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat Med 2019; 25: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Anastasiadou E, Stroopinsky D, Alimperti S, et al. Epstein−Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas. Leukemia 2019; 33: 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nakayama A, Abe H, Kunita A, et al. Viral loads correlate with upregulation of PD-L1 and worse patient prognosis in Epstein-Barr virus-associated gastric carcinoma. PLoS One 2019; 14: e0211358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cellucci T, Van Mater H, Graus F, et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm 2020; 7: e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vogrig A, Joubert B, André-Obadia N, et al. Seizure specificities in patients with antibody-mediated autoimmune encephalitis. Epilepsia. Epub ahead of print 8 July 2019. DOI: 10.1111/epi.16282. [DOI] [PubMed] [Google Scholar]
- 85. Pollak TA, Lennox BR, Müller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry 2020; 7: 93–108. [DOI] [PubMed] [Google Scholar]
- 86. Ho ACC, Chan SHS, Chan E, et al. Anti-N-methyl-d-aspartate receptor encephalitis in children: incidence and experience in Hong Kong. Brain Dev 2018; 40: 473–479. [DOI] [PubMed] [Google Scholar]
- 87. van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 2016; 87: 1449–1456. [DOI] [PubMed] [Google Scholar]
- 88. Williams TJ, Benavides DR, Patrice KA, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 2016; 73: 928–933. [DOI] [PubMed] [Google Scholar]
- 89. Lim HK, Seppänen M, Hautala T, et al. TLR3 deficiency in herpes simplex encephalitis: high allelic heterogeneity and recurrence risk. Neurology 2014; 83: 1888–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moloney PB, Hutchinson S, Heskin J, et al. Possible N-methyl-D-aspartate receptor antibody-mediated encephalitis in the setting of HIV cerebrospinal fluid escape. J Neurol. Epub ahead of print 20 January 2020. DOI: 10.1007/s00415-019-09693-3. [DOI] [PubMed] [Google Scholar]
- 91. de Graaf MT, de Beukelaar JWK, Haasnoot GW, et al. HLA-DQ2+ individuals are susceptible to Hu-Ab associated paraneoplastic neurological syndromes. J Neuroimmunol 2010; 226: 147–149. [DOI] [PubMed] [Google Scholar]
- 92. Hillary RP, Ollila HM, Lin L, et al. Complex HLA association in paraneoplastic cerebellar ataxia with anti-Yo antibodies. J Neuroimmunol 2018; 315: 28–32. [DOI] [PubMed] [Google Scholar]
- 93. Pugliese A, Solimena M, Awdeh ZL, et al. Association of HLA-DQB1*0201 with stiff-man syndrome. J Clin Endocrinol Metab 1993; 77: 1550–1553. [DOI] [PubMed] [Google Scholar]
- 94. Dalakas MC, Fujii M, Li M, et al. The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology 2000; 55: 1531–1535. [DOI] [PubMed] [Google Scholar]
- 95. Muñiz-Castrillo S, Ambati A, Dubois V, et al. Primary DQ effect in the association between HLA and neurological syndromes with anti-GAD65 antibodies. J Neurol. Epub ahead of print 9 March 2020. DOI: 10.1007/s00415-020-09782-8. [DOI] [PubMed] [Google Scholar]
- 96. Belbezier A, Joubert B, Montero-Martin G, et al. Multiplex family with GAD65-Abs neurologic syndromes. Neurol Neuroimmunol Neuroinflamm 2017; 5: e416. eCollection 2018 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gaig C, Graus F, Compta Y, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology 2017; 88: 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gaig C, Ercilla G, Daura X, et al. HLA and microtubule-associated protein tau H1 haplotype associations in anti-IgLON5 disease. Neurol Neuroimmunol Neuroinflamm 2019; 6: e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Binks S, Varley J, Lee W, et al. Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain 2018; 141: 2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mueller SH, Färber A, Prüss H, et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann Neurol 2018; 83: 863–869. [DOI] [PubMed] [Google Scholar]
- 101. Shu Y, Qiu W, Zheng J, et al. HLA class II allele DRB1*16:02 is associated with anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry 2019; 90: 652–658. [DOI] [PubMed] [Google Scholar]