Abstract
Background
Advances in intracranial stereotactic radiosurgery (SRS) have led to dramatically reduced planning target volume (PTV) margins. However, tumor growth between planning and treatment may lead to treatment failure. Our purpose was to assess the kinetics of tumor growth before SRS for brain metastases.
Methods
This retrospective, monocentric study included all consecutive patients (pts) treated for brain metastases secondary to melanoma (ML) and non-small cell lung cancer (NSCLC) between June 2015 and May 2016. All pts underwent diagnostic brain imaging and a radiosurgery planning MRI, during which gross tumor volume (GTV) was delineated. Linear and exponential models were used to extrapolate a theoretical GTV at first day of treatment, and theoretical time to outgrow the PTV margins.
Results
Twenty-three ML and 31 NSCLC brain metastases (42 pts, 84 brain imaging scans) were analyzed. Comparison of GTV at diagnosis and planning showed increased tumor volume for 20 ML pts (96%) and 22 NSCLC pts (71%). The shortest time to outgrow a 1 mm margin was 6 days and 3 days for ML and 14 and 8 days for NSCLC with linear and exponential models, respectively.
Conclusions
Physicians should bear in mind the interval between SRS planning and treatment. A mathematical model could screen rapidly progressing tumors.
Keywords: brain metastases, interval, margins, stereotactic radiosurgery
Prognosis for non-small cell lung cancer (NSCLC) and metastatic melanoma (ML) has recently dramatically improved thanks to targeted therapy and immunotherapy.1 But the risk of spread to the CNS remains high, with occurrence of brain metastasis for 40% to 60% of patients (pts) with metastatic ML.2 On the other hand, more than 20% of lung cancers are associated with brain metastases at cancer diagnosis.3 Hypofractionated stereotactic radiosurgery (SRS) is considered to be a standard therapeutic option for pts with 1 to 5 brain metastases.4 SRS provides a high gradient dose between the tumor and surrounding tissue, and reduced planning target volume (PTV) margins are used to spare adjacent brain tissue. Therefore, to accurately delineate gross tumor volume (GTV), a fusion of MRI with the planning CT scan is recommended. However, tumor growth between the planning CT scan and the first fraction of SRS might be responsible for an inaccurate coverage of the tumor, which may lead to treatment failure. Our purpose was to assess the kinetics of tumor growth between diagnostic imaging and planning MRI for brain metastases secondary to ML and NSCLC. We also evaluated, using mathematical models, the extrapolated size of the tumor at first day of treatment and consequences in terms of inaccurate coverage of the tumor possibly leading to treatment failure. In addition, we estimated the theoretical interval for each tumor to outgrow the PTV margin.
Material and Methods
Patients
This retrospective, monocentric study included all consecutive pts treated with SRS for brain metastases secondary to ML or NSCLC between June 2015 and May 2016 at Bordeaux University Hospital, France. The eligibility criteria were age older than 18 years, histologically proved ML or NSCLC, and brain imaging data finding 1 to 5 brain metastases. Only nonresected metastases were analyzed. Pts with previous craniotomy were eligible if they had a new brain metastasis. Diagnosis of brain metastases was performed on CT or MRI and reviewed for confirmation by a multidisciplinary board, including radiation oncologists, medical oncologists, neurosurgeons, and radiologists. Imaging consisted of cerebral CT scan or MRI with a contrast-enhancement sequence. Pts with only one imaging scan available or with intratumoral bleeding were excluded. The institutional review board approved the study, and the need for written informed consent was waived.
Radiation Therapy
For SRS planning, all pts underwent a high-resolution, 3-dimensional, T1-weighted, gadolinium-enhanced MRI sequence reconstructed every 1.0 mm. A CT scan was carried out for radiation therapy planning using a slice reconstruction every 1.0 mm and a field of 35 cm. A thermoplastic mask was used for immobilization. The Eclipse treatment planning system (Varian Medical Systems, version 13.5) was used to coregister the MRI and CT scan and to segment optics, brainstem, spinal cord, retinas, brain, pituitary gland, GTV, and PTV. The margin from GTV to PTV used in our institution was 2 mm. Dose was prescribed to ensure a minimal coverage of 90% of the PTV before validation of the treatment planning. SRS doses were 1 fraction of 20 Gy, 3 fractions of 9, 10, or 11 Gy, or 5 fractions of 5 Gy depending on tumor size. Treatment was delivered using 6 MV photons by Elekta Versa HD associated with the Fraxion system for stereotaxy. The ExacTrac system was used to adapt the setting of the patient using a 6-dimensional rotation table for treatment.
Assessment
Baseline patient characteristics included KPS, recursive partitioning analysis (RPA) status, steroid treatment, and number and location of brain metastases. Brain imaging consisted of MRI with gadolinium-enhanced sequences or CT scan with intravenous contrast. Time at brain metastasis diagnosis, radiosurgery planning imaging, and first day of treatment were recorded. The follow-up examinations consisted of an MRI scan every 3 months for 1 year and every 6 months thereafter. The primary endpoint was to assess the kinetics of tumor growth of brain metastases between diagnostic imaging and radiosurgery planning imaging, and to extrapolate the theoretical tumor volume at the time of SRS. Both types of imaging were implemented using the Eclipse system, version 13.5. GTV was delineated on the basis of contrast enhancement by a radiation oncologist experienced in the treatment of brain metastases by SRS. GTV1, GTV2, and GTV3 corresponded respectively to GTV at diagnosis, the planning imaging, and the estimated volume at first day of treatment with linear extrapolation or exponential extrapolation. The secondary endpoints were to assess tumor control and radiation-induced toxicities during follow-up. Toxicity was scored according to the Common Terminology Criteria for Adverse Events, version 4.0. Progressive disease was defined as a radiological progression without radionecrosis criteria in accordance with the Response Assessment in Neuro-Oncology Brain Metastases Working Group. Radionecrosis was diagnosed according to imaging features, such as increased contrast enhancement, nonprogression of lesion over 4 months, and reduced perfusion on dynamic MRI sequences.
Statistical Analysis
Linear extrapolation and exponential extrapolation were used to estimate the t minimum theoretical time allowing tumor diameter to increase by more than 4 mm (T-4mm) and more than 2 mm (T-2mm) from radiosurgery planning imaging. The linear model corresponds to the following equation and assumes that the tumor volume grows linearly over time. V’ = alpha; V(t = 0) = V0, where V’ denotes the time derivative of the volume V, alpha is a patient-specific parameter, and the origin of time is taken at the date of the first CT, with V0 denoting the corresponding tumor volume. Given 2 exams, alpha is uniquely determined for each patient (alpha = [V1–V0]/[t1–t0], where [t1, V1] is obtained from the planning scan). The exponential models correspond to the following equation and assume that the tumor volume is growing exponentially. V’ = alpha V; V(t = 0) = V0, where V’ denotes the time derivativee of the volume V, alpha is a patient-specific parameter, and the origin of time is taken at the date of the first CT, with V0 denoting the corresponding tumor volume. Given 2 exams, alpha is uniquely determined for each patient (alpha = [log(V1/V0)]/[t1–t0], where [t1, V1] is obtained from the planning imaging). Univariate analysis was carried out on patient characteristics to analyze the predictive parameters of radionecrosis.
Results
Patients
Out of 103 pts treated for brain metastases by SRS between June 2015 and May 2016, 21 were treated for ML and 21 for NSCLC. Two pts were excluded because of lack of imaging data. The median age was 71.4 years for ML pts (range, 25-92 years) and 67.7 years for NSCLC pts (range, 48-82 years). Only 1 patient with ML was RPA class 1. Other patients were RPA class 2 for 17 ML pts (81%) and 16 NSCLC pts (76%), and class 3 for 3 ML pts (14%) and 5 NSCLC pts (24%). A systemic treatment was given at diagnosis for 14 ML pts (67%) and 7 NSCLC pts (33%), including immunotherapy for 6 ML pts, targeted therapy for 5 ML pts and 1 NSCLC pt, and conventional chemotherapy for 3 ML pts and 6 NSCLC pts. Brain metastases in ML or NSCLC pts were synchronous with ML or NSCLC diagnosis respectively in 3 and 11 cases, or metachronous in 18 and 10 cases, with a median time since diagnosis of 4.8 years (1-40 years) and 2.1 years (1-9.5 years) for ML and NSCLC, respectively (Table 1).
Table 1.
Characteristics of Patients Treated With Stereotactic Radiation for Brain Metastases Secondary to Melanoma or Non-Small Cell Lung Carcinoma
| Melanoma Patients (N = 21) |
Non-Small Cell Lung Carcinoma Patients (N = 21) |
|
|---|---|---|
| Median age at brain metastasis diagnosis, y | 71.4 (25-92) | 67.7 (48-82) |
| RPA status | ||
| 1 | 1 (5%) | 0 |
| 2 | 17 (81%) | 16 (76%) |
| 3 | 3 (14%) | 5 (24%) |
| Metastasis concurrent to melanoma diagnosis | 3 | 11 |
| Metachronous metastasis (No. of patients) | 18 | 10 |
| Median time postdiagnosis, y | 4.8 (1-40) | 2.1 (1-9.5) |
| Metastases (N = 23) | Metastases (N = 30) | |
| Delay between diagnosis and planning imaging, d | 24 (7-70) | 29 (8-107) |
| Delay between planning imaging and first radiation, d | 19 (7-57) | 19 (11-77) |
| Irradiation dose prescription (Gy on isodose 80%) | ||
| -Monofractionated (1 × 20 Gy) | 5 (22%) | 3 (10%) |
| -Trifractionated (3 × 9, 10 or 11 Gy) | 15 (65%) | 25 (84%) |
| -Pentafractionated (5 × 5 Gy) | 3 (13%) | 2 (6%) |
| Healthy brain irradiated volume, PTV, cm3 | 9.65 (2.8-19.7) | 7.7 (1.1-29.8) |
| Follow-up | ||
| Metastasis postradiation control | 20/22 | 22/22 |
| Radiologic necrosis occurrence (N metastasis) | 8/22 (38%) | 8/22 (36%) |
| New metastasis on follow-up (N patients) | 5 (22%) | 6 (29%) |
| Median time follow-up (months) | 12 (1.6-23) | 17 (9-23) |
Abbreviations: PTV, planning target volume; RPA, recursive partitioning analysis.
Metastases
A total of 23 ML and 31 NSCLC metastases were analyzed from 42 pts. One NSCLC patient with 1 metastasis was excluded because of tumor bleeding. Location was supratentorial in 38 cases, cerebellar in 3 cases, and mesencephalic in 1 case. Six pts had already received SRS for previous other brain metastases. One patient received previous whole-brain radiotherapy (30 Gy/10 fractions). All metastases analyzed had not been previously irradiated. Radiotherapy was delivered according to a monofractionated schedule for 5 ML and 3 NSCLC metastases, a 3-fraction schedule for 15 ML and 25 NSCLC metastases, or a 5-fraction schedule for 3 ML and 2 NSCLC metastases (Table 1).
Assessment
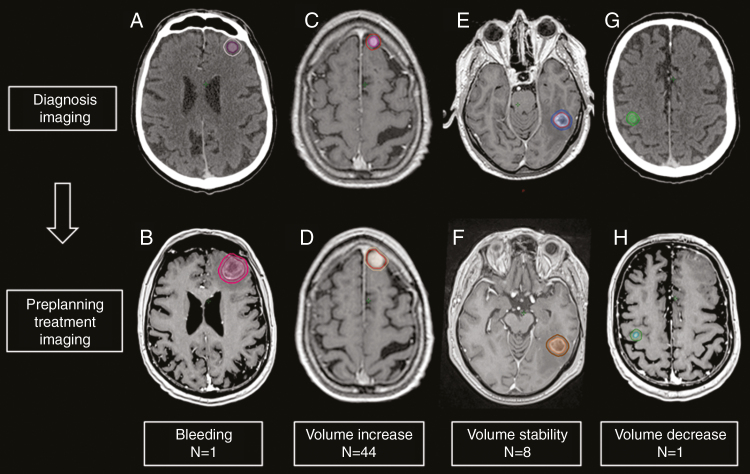
Eighty-four brain imaging scans (67 MRI, 17 CT scan) were analyzed. Comparison of imaging between diagnosis and radiosurgery planning showed increased tumor volume for 22 ML and 22 NSCLC metastases; stability for 1 ML and 7 NSCLC metastases, bleeding inside 1 NSCLC metastasis (excluded), and 1 metastasis volume decrease (Fig. 1).
Fig. 1.
Brain Metastasis Evolution From (A, C, E, and G) Diagnostic Imaging to (B, D, F, and H) Treatment Planning Imaging
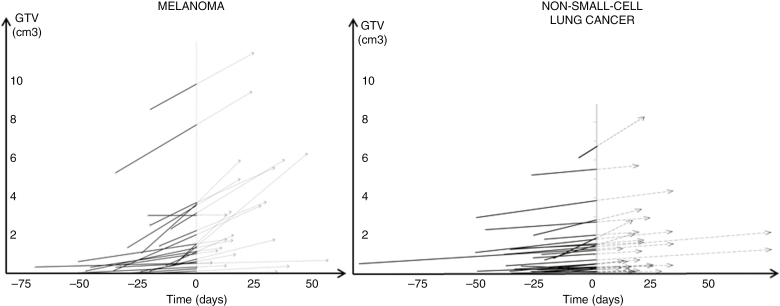
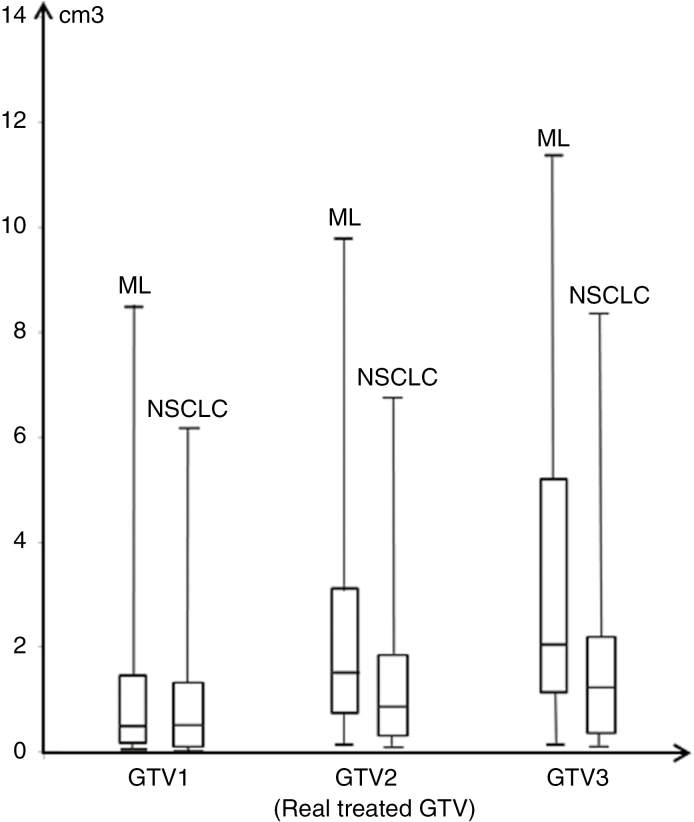
Median time between brain imaging at diagnosis and radiosurgery planning imaging was 24 days for ML and 29 days for NSCLC. Median time between radiosurgery planning imaging and first day of radiosurgery was 19 days for ML and for NSCLC. Median time between brain imaging at diagnosis and first day of treatment was similar for ML and NSCLC (Table 2). For each patient, tumor growth was calculated between diagnosis and treatment planning, and extrapolated between planning and SRS (Fig. 2). Median GTV1 was 0.5 cm3 for ML and 0.4 cm3 for NSCLC; median GTV2 was 1.5 cm3 for ML and 0.8 cm3 for NSCLC. GTV3 at first day of treatment for ML was significantly increased compared with NSCLC with the linear and exponential models (2.1 cm3 vs 1.2 cm3 and 4.3 cm3 vs 1.6 cm3, respectively, P = .05) (Fig. 3).
Table 2.
Characteristics of Melanoma and Non-Small Cell Lung Carcinoma Metastases Time to Treatment and Evolution of Tumor Volume
| Melanoma | Non-Small Cell Lung Carcinoma | |
|---|---|---|
| Median time in days | ||
| Diagnosis to planning imaging, d (range) | 24 (7-70) | 29 (8-107) |
| Planning imaging to first day radiotherapy, d (range) | 19 (7-57) | 19 (11-77) |
| T-4 mm with linear model, d | 15 | 32 |
| T-4 mm with exponential model, d | 6 | 15 |
| T-2 mm with linear model, d | 6 | 14 |
| T-2 mm with exponential model, d | 3 | 8 |
| Tumor volume evolution real and predictive | ||
| Median GTV1 in cm3 (range) | 0.5 (0.05-8.6) | 0.4 (0.05-6.1) |
| Median GTV2 in cm3 (range) corresponding to actual treated GTV | 1.5 (0.1-9.9) | 0.8 (0.1-6.7) |
| Median GTV3 in cm3 (range) with linear model | 2.1 (0.15-11.5) | 1.2 (0.1-8.3) |
| Median GTV3 in cm3 (range) with exponential model | 4.3 (0.2-13) | 1.6 (0.1-8.6) |
| Median PTV2 in cm3 (range) corresponding to real treated PTV | 3.9 (0.9-23.6) | 2.35 (0.5-21) |
Abbreviations: GTV, gross tumor volume; PTV, planning target volume.
Fig. 2.
Evolution of Brain Metastases in Melanoma and Non-Small Cell Lung Cancer
J0 corresponds to planning cerebral imaging, with corresponding gross tumor volume (GTV) (GTV2) on the Y axis. Negative part was time at diagnostic imaging for each brain metastasis with assessment of corresponding GTV (GTV1) on the Y axis. Positive part was time interval to stereotactic radiosurgery, associated for each metastasis with the predictive GTV (GTV3) at time to treatment (with linear extrapolation).
Fig. 3.
Box Plots Illustrative of GTV Evolution at Diagnosis (GTV1), at Planning Treatment (GTV2), and Predictive of First-Day Radiation (GTV3) for ML and Lung Cancer Brain Metastases With Linear Extrapolation Model in cm3
GTV indicates gross tumor volume; ML, melanoma; NSCLC, non-small cell lung cancer.
The shorter T-4mm, meaning the shorter time to outgrow a 2 mm diameter margin from the real GTV (GTV2) to the real PTV, was 15 days or 6 days for ML with the linear or exponential models, respectively. The shorter T-4mm was 32 or 15 days for NSCLC with the linear or exponential models, respectively. Real time from planning imaging to first day of treatment was then compared with the theoretical time T-4mm. Linear and exponential models showed respectively 2 ML and no NSCLC and 11 ML and 1 NSCLC expected to outgrow the PTV margins the first day of treatment. These metastases were potentially undertreated.
The shortest T-2mm was 6 days or 3 days for ML and 14 or 8 days for NSCLC with the linear or exponential models, respectively. Twelve ML and 3 NSCLC were expected to outgrow the PTV margins during the first day of treatment according to the linear model, whereas the exponential model predicted the same concern for 14 ML and 7 NSCLC.
Follow-Up
Median follow-up was 12 and 17 months for ML and NSCLC pts, respectively. Brain metastases were controlled at the end of the follow-up for all except 2 ML cases. One brain metastasis had a tumor bleeding 16 months after SRS. Four pts died from extracranial cancer progression (ML: 2 pts, NSCLC: 2 pts), and 3 ML pts died from new brain metastases. At 3 months, out of 39 patients alive, 11 had at least 1 new brain metastasis (ML: 5 pts, NSCLC: 6 pts). Acute toxicity included only one Grade 1 asthenia during treatment. Radionecrosis was observed for 8 of 21 (36%) ML and 8 of 22 (36%) NSCLC pts, asymptomatic for 15 metastases, and symptomatic for only 1 pt. Out of 8 (6 ML, 2 NSCLC) pts with steroids prescribed because of brain metastasis neurological symptoms, 3 (1 ML, 2 NSCLC) experienced radionecrosis. Five additional pts required steroids after radiation for symptomatic radionecrosis. No effect of steroids on tumor volume was observed. None of the patients with radionecrosis received bevacizumab. No predictive parameter for radionecrosis was found in univariate analysis.
Discussion
SRS is now considered to be the standard treatment for 1-3 nonresectable brain metastases and has dramatically changed the management of brain metastases.5 Major advances in imaging and radiotherapy techniques enable high doses to be delivered with reduced margins to focal cancers.6 Current trends continue to focus on improvement of SRS accuracy. Nevertheless, concerns may be raised about potential risks of treatment failure due to tumor growth during the interval between treatment planning and initiation of SRS. This is why we assessed tumor growth before treatment of ML and NSCLC brain metastases and the risk of inaccurate coverage of the tumor relating to time before SRS.
In our study, all patients had a 2 mm GTV to PTV margin. So T-4mm corresponded to the shorter time to outgrow a 2 mm diameter margin from the real GTV (GTV2) to the real PTV and reflects the PTV time to outgrow in our experience. However, many current publications and centers today use a 1 mm GTV to PTV margin. For this reason, we decided also to provide the T2 mm data corresponding to the time to outgrow a 1 mm GTV to PTV margin. A 1 mm margin could be recommended for the risk of radionecrosis related to the margin. However, we wanted to highlight the risk of marginal recurrence in case of too tight a margin.7
Our study showed a tumor progression for 82% of brain metastases. Garcia et al quantified brain metastasis growth before SRS on preradiosurgical imaging.8 They previously found and confirmed our results that metastasis growth was associated with time. As expected, time to outgrow a 1 mm PTV margin (= T-2mm) was short, and clinicians should be careful with intervals when using a 1 mm margin from GTV to PTV. Garcia and colleagues showed a 1.35-fold volume increase at 14 days.8 With a 1 mm PTV margin, we also found that 15 days corresponds to an acceptable time to treatment for NSCLC. For ML brain metastasis, time should be reduced as much as possible. The choice of extrapolation model may lead to over- or underestimation of the size of the tumor on the first day of treatment. No patient underwent brain imaging on the first day of treatment to validate our models. A prospective study with imaging on the first day of treatment would help statisticians and physicians to identify the best model for prediction of metastasis evolution. Interestingly, among the 2 patients who experienced a local failure, one was considered at risk of undertreatment according to our models. Time from planning imaging to treatment may lead to underestimation of the real GTV.9 The present series highlights the need to reconsider our current management. To overcome this interval, one option could be to adapt the margin to the time to treatment. However, this may be considered unethical.9 The other option is to reconsider the planning of the radiotherapy process.
Two series reported the interval between treatment planning and radiosurgery for brain metastases. Seymour and colleagues9 reported 82 pts with heterogeneous brain metastases. Time from radiation oncologist consultation to radiosurgery planning MRI was 28 days. Time from planning MRI to first day of treatment was 11 days. In most cases, diagnosis of brain metastases was established before the radiation oncologist consultation. Hence, our delays were roughly similar. In previous publications, local failure-free progression dramatically decreased when time from planning MRI to treatment was less than 14 days.10 Garcia et al reported a second series of 256 pts treated for brain metastases with fixed-frame SRS. The mean time between diagnosis of metastases and MRI on day of treatment was 25 days. In this technique of fixed-frame SRS, the treatment planning MRI was carried out the same day as the treatment. Growth of brain metastases was observed in 30% of patients, particularly for melanoma. However, local failure-free progression was not compromised when growth of brain metastases was observed before treatment planning.11 Time from diagnosis to planning may result from the requirements of systemic treatments. This interval may also be explained by the poor availability of the SRS technique in some centers, and to the period before referring the patient to a stereotactic radiation unit. To our knowledge, the interval between diagnosis and initiation of SRS has not yet been assessed. In addition, the interval between radiosurgery planning and first day of treatment may differ among centers because of specific local protocols.9,10
Interestingly, we found a difference in tumor growth between ML and NSCLC metastases. Initial median volumes were similar (0.50 cm3 and 0.45 cm3 respectively for ML and NSCLC), whereas median GTV2 was higher in melanoma (1.5 cm3) than in lung cancer (0.8 cm3). Garcia and colleagues also found that metastasis growth was associated with melanoma histology.8 The slower tumor growth in lung cancer explained the 1 NSCLC with a long interval between diagnosis and treatment planning because of diagnostic doubt. This study highlighted that the heterogeneity between cancer types needs to be considered in brain metastases. Other studies will be necessary to identify rapid-progression brain metastases that should be treated earlier with SRS or surgery. On the other hand, brain metastases predicted to be slowly progressive could be treated with local or systemic treatment.12
Radionecrosis was observed in 8 of 21 irradiated MLs and was symptomatic in only 1 case. Incidence of radionecrosis is estimated from 10% to 25%.13,14 Using imaging-based diagnosis, Minniti et al reported a 24% incidence of radionecrosis (14% symptomatic, 10% asymptomatic).11 Renal carcinoma, lung adenocarcinoma (anaplastic lymphoma kinase rearrangement specifically), HER2-amplied breast cancer, and BRAF V600 wild-type melanoma are suggested to present a higher risk of radionecrosis.13 These considerations could explain the present high rate of radionecrosis. No predictive parameter was found in our study.
Conclusion
Physicians should bear in mind the interval between SRS planning and treatment, especially using a 1 mm margin. Growth of brain metastases is highly heterogenic between cancer types. A mathematical model could help to screen rapidly progressive tumors to individualize treatment.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement. None declared.
References
- 1. Schulze AB, Schmidt LH. PD-1 targeted immunotherapy as first-line therapy for advanced non-small-cell lung cancer patients. J Thorac Dis. 2017;9(4):E384–E386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen JV, Tawbi H, Margolin KA, et al. Melanoma central nervous system metastases: current approaches, challenges, and opportunities. Pigment Cell Melanoma Res. 2016;29(6):627–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, Davis FG. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 2015;17(1):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zindler JD, Rodrigues G, Haasbeek CJ, et al. The clinical utility of prognostic scoring systems in patients with brain metastases treated with radiosurgery. Radiother Oncol. 2013;106(3):370–374. [DOI] [PubMed] [Google Scholar]
- 5. Kibbi N, Kluger H. The treatment of melanoma brain metastases. Curr Oncol Rep. 2016;18(12):73. [DOI] [PubMed] [Google Scholar]
- 6. Wiggenraad R, Verbeek-de Kanter A, Kal HB, Taphoorn M, Vissers T, Struikmans H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol. 2011;98(3):292–297. [DOI] [PubMed] [Google Scholar]
- 7. Noël G, Simon JM, Valéry CA, et al. Radiosurgery for brain metastasis: impact of CTV on local control. Radiother Oncol. 2003;68(1):15–21. [DOI] [PubMed] [Google Scholar]
- 8. Garcia MA, Anwar M, Yu Y, et al. Brain metastasis growth on preradiosurgical magnetic resonance imaging. Pract Radiat Oncol. 2018;8(6):e369–e376. [DOI] [PubMed] [Google Scholar]
- 9. Seymour ZA, Fogh SE, Westcott SK, et al. Interval from imaging to treatment delivery in the radiation surgery age: how long is too long? Int J Radiat Oncol Biol Phys. 2015;93(1):126–132. [DOI] [PubMed] [Google Scholar]
- 10. Garcia M, Duriseti S, Fogh S, et al. RTRB-06 Time between diagnostic MRI and frame-fixed stereotactic radiosurgery planning MRI is associated with brain metastasis growth but not worse local control. Neuro Oncol. 2015;17(5):v196. [Google Scholar]
- 11. Minniti G, Scaringi C, Clarke E, Valeriani M, Osti M,Enrici RM. Frameless linac-based stereotactic radiosurgery (SRS) for brain metastases: analysis of patient repositioning using a mask fixation system and clinical outcomes. Radiat Oncol. 2011;6:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller JA, Bennett EE, Xiao R, et al. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol Biol Phys. 2016;96(5):1060–1069. [DOI] [PubMed] [Google Scholar]
- 14. Bohoudi O, Bruynzeel AM, Lagerwaard FJ, Cuijpers JP, Slotman BJ, Palacios MA. Isotoxic radiosurgery planning for brain metastases. Radiother Oncol. 2016;120(2):253–257. [DOI] [PubMed] [Google Scholar]