Abstract
Electrospray Ionization (ESI) is often affected by corona discharge when spraying 100% aqueous solutions as the voltage that induces discharge can be well below the onset voltage of ESI. As a result, it is especially challenging to perform native mass spectrometry in negative ion mode where 100% aqueous solution is preferred. Here we report a simple instrumentation method to improve the performance of ESI in negative ion mode based on capillary vibrating sharp-edge spray ionization (cVSSI). By attaching a fused silica capillary emitter to a vibrating glass slide, improved signal quality is achieved for various analytes in aqueous solutions over applying ESI alone. Compared to commercial ESI sources using nebulization gas to reduce discharge, 10–100 fold enhancement in signal intensity and 3–10 fold improvement in S/N are achieved for various kinds of molecules including DNA, peptides, proteins, and oligosaccharides. Finally, the new method demonstrates utility for native mass spectrometry analysis of proteins and G-quadruplex DNA. The present method is expected to have great potential to be adopted by the scientific community because of its improved analytical performance, simplicity, and low cost.
Introduction
Electrospray ionization (ESI) mass spectrometry (MS) is a powerful tool for studying biomolecules and biological systems. It can allow for directly spraying biomolecules from their “native” solution conditions to study their assemblies, interactions, dynamics, and composition.1–6 Typically “native” solution conditions signify an aqueous media with an appropriate amount of ionic species that make up suitable pH and ionic strength conditions for stabilizing biomolecular structure. However, a 100% aqueous solution is generally difficult for ESI because the high surface tension of water leads to high ESI onset voltage.7 The high voltage increases the likelihood of corona discharge that degrades analyte signals. Especially under negative ion mode analysis, it has been well documented that corona discharge can occur well below the onset voltage of ESI.8,9 As a result, native mass spectrometry under negative ion mode often bares narrow voltage operating conditions, unstable signals, and low signal to noise (S/N) ratios.10 Given the importance of negative ion mode for studying acidic proteins and protein complexes, membrane proteins and various nucleic acids, the performance of negative ion mode ESI-MS must be improved.11–13
Existing methods for improving negative ion mode ESI can generally be divided into two categories, namely chemical additives and instrument modifications. Corona discharge can be effectively suppressed by adding organic solvents such as methanol and isopropanol to the spray solvents due to the reduced surface tension. Chlorosolvents and fluorosovlents have also been reported for improving negative ion ESI.14–16 A recent study showed that 0.2% trifluoroethanol (TFA) can suppress corona discharge of nano-ESI thereby improving the stability of nanoLC-MS experiments.17 Chemical additive-based methods are effective, easy to implement, and work for both nanoESI and ESI. However, these methods could complicate native mass spectrometry experiments as the native environment for most biomolecules is aqueous solution. Careful design of control experiments is required to determine the influence of organic additives. Compared to adding organic additives, instrumentation methods allow direct analysis of biomolecules in aqueous solutions, which is more suitable for native MS analysis. The most widely used instrumentation method is pneumatic assisted ESI, which employs a fast gas flow to nebulize liquid samples.18 Because liquid droplets are generated by the gas flow, the applied electric field around the capillary tip can be reduced. As a result, corona discharge is alleviated with the use of nebulization gas. Most current commercial ESI sources are equipped with nebulization gas to provide stable ESI performance. While using high-speed nebulization gas is an effective means of minimizing discharge for standard ESI, it is not an optimal setup to obtain high ion intensity. Moreover, the sheath gas is often not desirable for nano-ESI applications where the sample flow rate is below 1 μL/min as the high-speed gas could disrupt the narrow droplet plume associated with nano-ESI. Using gases with high dielectric constant such as SF6, and O2, can further improve the performance of negative ion ESI at the expense of higher operational cost.19,20 Chen et al. employed super atmospheric pressure to improve the performance of ESI and nanoESI for analyzing aqueous solutions.21,22 High pressures (2–7 bar) at the spray tip allow the use of higher spray voltage (e, g., 6 kv) without the concern of discharge, but it also requires a specialized source chamber to maintain the high pressure. In addition, while sharp, pulled-tip capillaries with ID <10 μm generally allow an ESI onset voltage below the discharge voltage, working with small pulled-tip capillaries requires extra care to prevent clogging and maintain a stable spray.23 The limiting of ion current via a high ohmic resistor or polarity reversing techniques have been reported to improve the performance of nanospray including for negative ion mode analyses.24–26 To date, although these instrumentation approaches have been demonstrated to be effective in improving negative ion ESI for aqueous solutions, the cost, complexity, and strict working conditions for most of these methods still limit their wide adoption by the research community. As a result, nebulization gas is still the most practical and widely used method for improving the signal quality of ESI in negative ion mode.
Here we report a simple instrumentation method that can effectively suppress corona discharge for aqueous solutions based on capillary vibrating sharp-edge spray ionization (cVSSI). cVSSI is a process that can generate a plume of liquid droplets at the outlet of a capillary by simply attaching it to a vibrating glass slide.27,28 By combining the cVSSI process with a bias voltage, negative ion signals of various analytes including DNA, proteins, peptides, and oligosaccharides from aqueous solutions are improved significantly. While several ultrasonic nebulization systems that can be coupled with ESI have been developed including an array of micromachined ultrasonic electrospray (AMUSE),29 ultrasonically assisted electrospray,30 single pulse nanoelectrospray,31 the capability of suppressing corona discharge for these methods has not been reported. Compared to existing ultrasonic based methods, cVSSI does not require a well-defined acoustic field or powerful acoustic transducers as it leverages the strong nebulization effect of vibrating sharp-edges.27 Therefore, the present method can be directly coupled to both pulled-tip and standard fused silica capillaries without special modification or fabrication. When coupled with a pulled-tip capillary, the method significantly extends the working range of applied voltage and thus produces a more stable signal in negative ion mode than using ESI alone. This method shows 10–100 fold enhancement in ion intensity and 3–10 fold enhancement in S/N over commercial ESI sources equipped with a nebulization gas system for a wide range of analytes in negative ion mode. Finally, we demonstrate the potential of this method for native MS analysis of G-quadruplex DNA allowing a significantly reduced flow rate and sample consumption compared to standard methods.
Experimental Section
Materials and Reagents
High performance liquid chromatography (HPLC) grade water, nuclease-free water, potassium chloride, and ammonium acetate (NH4OAc) were purchased from Fischer Scientific (NJ, USA). Trimethylammonium acetate (TMAA) was obtained by slowly adding 4.3 M acetic acid to 50 mL of 4.3 M trimethylamine (TCI chemical) solution until the pH was around 7.0, and this process was monitored by a pH meter (Mettler Toledo, OH). Acetic acid, fondaparinux sodium (C31H43N3Na10O49S8, m.w. 1728.1 g/mol) and chondroitin disaccharide Δdi-6S sodium salt (C14H19NO14SNa2, m.w. 503.34 g/mol) were purchased from Sigma-Aldrich (MO, USA). Insulin (human) and angiotensin 2 (human) were purchased from Alfa Aesar (MA, USA). Ubiquitin (~8.5 kDa) was purchased from Boston Biochem (MA, USA). Myoglobin (~17 kDa) was purchased from Sigma Aldrich (St. Louis, Mo, USA). All DNA oligonucleotides used in this work, d(CATATATG), d(ACGCGCGT), 56-mer ssDNA and human telomeric G-quadruplex sequence d(TT(GGGTTA)3GGGA) were purchased from Integrated DNA Technologies, Inc. (Coralville, IA, USA) All the chemical reagents and oligonucleotides were used without further purification.
Fabrication of pulled-tip capillary and cVSSI Device
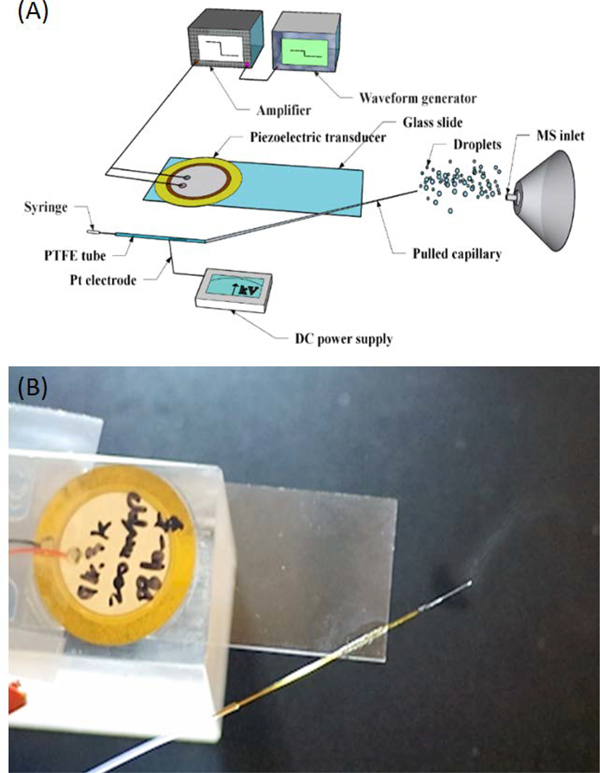
Pulled-tip capillaries were prepared using a Laser-Based Micropipette Puller Sutter P-2000 (CA, USA). Program settings for the pulled fused silica capillary were HEAT 750, FIL 4, VEL 60, DEL 200, PUL 175. Before being bonded to the No. 1 cover glass slide (24×60 mm, VWR), the pulled capillary was soaked in 30% hydrofluoric acid for ~2 mins until the end I.D of the pulled capillary is the desired ID (9–20 μm), and this process was monitored under the microscope. The fabrication procedures of each cVSSI device is similar to our previous report.27,28 Briefly, a piezoelectric transducer is first attached to a glass cover slide by epoxy glue, and the pulled capillary is bonded to the same glass slide by glass glue. The cVSSI device was activated using a function wave generator (RIGOL DG4102) and a power amplifier (Krohn-Hite 7500). The working frequency of each cVSSI device was determined by scanning frequencies from 90–105 kHz under a constant voltage input of 10 Vpp. The operational voltage is then determined to be the minimum voltage input that produces a stable plume under the selected working frequency. The normal working frequencies and amplitudes are 93–97 kHz, and 5–10 Vpp, respectively. A piece of 20 cm long PTFE tubing (#30 thin wall tubing, Cole-Parmer Instrument Company) was used to connect the sample syringe and the pulled capillary. A 5 cm long Pt wire (Diameter: 20 μm) was inserted into the PTFE tubing as an electrode, and the end of the Pt electrode was ~1 cm away from the end of the PTFE tube (Figure 1). During testing, the Pt electrode was connected with a DC power supply (HEOPS-10B2, MATSUSADA Precision Inc.) to provide bias voltage to the liquid samples (Figure 1). A Chemyx Syring pump (Model: Fusion 4000) was used to pump the sample solutions through the system lines, and the flow rate was set at 0.5–1 μL/min for the pulled capillary experiments and 10 μL/min for unmodified capillary experiments. Separate experiments reveal that the VSSI process can independently induce flow rates of 200 nL/min and thus the minimal flow rate that allows stable operation under current setup is ~200 nL/min. For flow rates higher than 200 nL/min, stable MS signals can be obtained (Figure S1). Additionally, using oil capture experiments and microscopy characterization,28 the droplet sizes for the cVSSI studies are estimated to be 3–9 μm in diameter (7.3 ± 2.4 μm, Figure S2).
Figure 1.
(A) Schematic of the cVSSI-MS experimental setup with a bias voltage. (B) Picture showing the liquid plume generated by the cVSSI process.
Preparation of the DNA Duplex and G-quadruplex
For all DNA samples, the stock solution concentrations were determined before an annealing process was employed using the Thermo Scientific Nanodrop 2000 Spectrophotometer. Stock solution of 100 μM ssDNA, d(CATATATG) was prepared in purified water and diluted to 10 μM in 100 mM NH4OAc.
The DNA duplex of self-complementary strand d(ACGCGCGT) was prepared by diluting the stock solution to reach 20 μM ssDNA and annealing was performed in 100 mM NH4OAc at 94 °C in an oil bath for 2 minutes. This was cooled to room temperature overnight to obtain 10 μM DNA duplex solution. Stock solutions of 1M TMAA and 1mM KCl were used for G-quadruplex DNA solutions. The G-quadruplex DNA stock solution was diluted to reach 10 μM ssDNA and quadruplex structure was prepared by mixing 100 μM ssDNA, 100 mM TMAA, and 100 or 500 μM KCl in water, annealing at 85 °C for 5 minutes then followed by cooling to room temperature overnight. Final concentration of G-quadruplex DNA is 10 μM as each single strand folds to create the quadruplex structure.
Mass Spectrometry Analysis
A Q-Exactive Hybrid Quadruple Orbitrap mass spectrometer and a LTQ-XL mass spectrometer (Thermo Fisher, San Jose, CA) were used for mass spectrometry measurements. Both mass spectrometers are equipped with Ion Max Ionization Sources with HESI probe for ESI ionization. For Q-Exactive mass spectrometric analysis, the resolving power was set at 70,000, and the inlet capillary temperature was maintained at 100 °C. For experiments using the HESI source, voltage and sheath gas flow rates were optimized for each analyte to achieve the highest ion intensities. Typical ranges for bias voltage and sheath gas rates were −3 to −4 kV, and 15 to 30 absolute unit (a.u.), respectively. For experiments using a cVSSI source plus voltage, the same instrument setting as HESI experiments was used except that the applied voltage was ~−1.1 to −1.4 kV and no sheath gas was applied. For ESI analysis with the LTQ-XL mass spectrometer, the capillary voltage, tube lens voltage, and the mass inlet capillary temperature were set at −10.00 V, −100.00 V and 275°C, respectively. Without special notice, the sample flow rates for both the Q-Exactive and LTQ-XL HESI were 10 μL/min, and sample flow rates for VSSI analysis were 1 μL/min. The S/N of features in mass spectra was calculated based on the following equation:
where I peak is the peak intensity of the analyte (above the average of the noise) and σ STD of the noise is the standard deviation of the noise. For each spectrum, the noise region was selected a few m/z before or after the analyte peak, and the noise region does not include any detectable ion peak.
Results and Discussion
Enhancing ion signal levels in negative ion mode with cVSSI
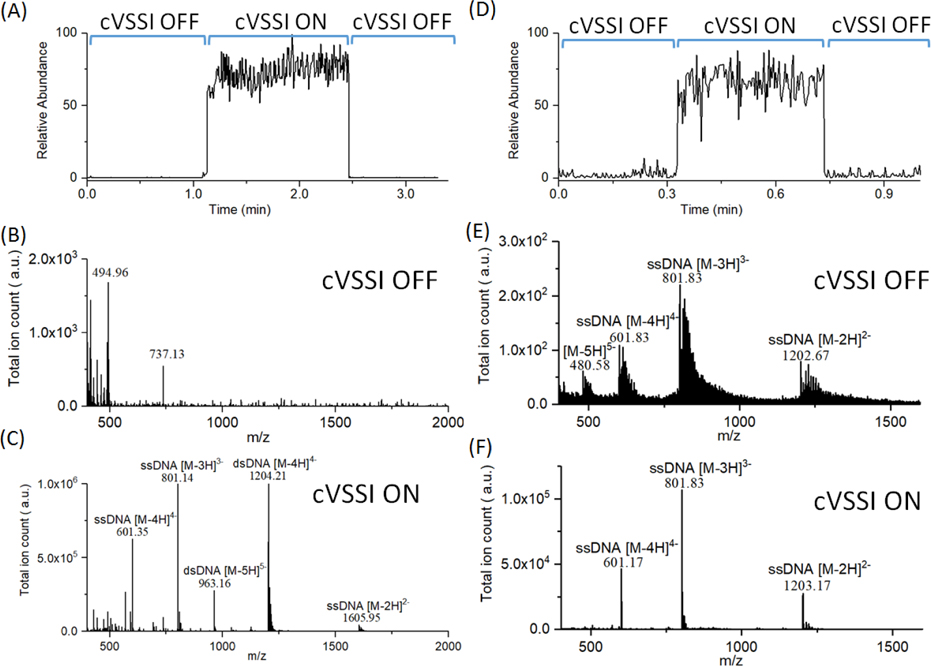
Because the cVSSI process allows the generation of a plume independent of the electrical properties of the solvent, it is speculated that this process should provide benefits to ESI experiments when the required voltage for ESI onset is high. We therefore first examined if applying cVSSI to a pulled-tip capillary with a negative bias voltage can enhance signal levels of DNA molecules in negative ion mode analyses. DNA mass spectrometry is primarily performed in negative ion mode because DNA contains a negatively charged phosphodiester backbone which facilitates negative ion formation by ESI.32–34 This highly polar nature of DNA presents significant challenges with regard to native MS analysis requiring significant bath gas usage to aid the desolvation process. Nano-ESI circumvents this requirement;35,36 however, it provides unique challenges for stable and efficient ion production.37 For example, the larger influence of corona discharge (see discussion above) has a significant effect on the overall stability of the process and thus affects the reproducibility of the measurement.17 This is unfortunate especially in cases where only limited sample is available. To examine the potential role of cVSSI in DNA analysis, experiments were designed to compare ion production of a DNA duplex d(ACGCGCGT)2 sample for conditions in which voltage biasing (−1.27 kV) of the emitter tip (ID: ~15 μm) was employed alone with that of the addition of cVSSI. The total ion chronogram is presented in Figure 2A and shows the enhancement of total ion production as a function of time obtained with the Q-exactive mass spectrometer. Initially, when no cVSSI is employed at the emitter tip, the overall level of produced ions is low. Immediately after the application of VSSI over the time frame of ~1.15 min to 2.45 min., this level rises dramatically. With the subsequent removal of VSSI (~2.5 min.), the ion signal returns to its previous level. This result clearly shows the influence of cVSSI on signal levels of ESI in negative ion mode. It is instructive to consider the types of ions that are produced under both ion production operational modes. During the initial data collection period in which only −1.27 kV was applied to the sample emitter tip (cVSSI off), the mass spectrum (Figure 2B) is dominated by low molecular weight ions with a dataset feature at m/z ~494 comprising the base peak of the spectrum. Notably, none of the dominant dataset features corresponds to DNA ions. With the addition of cVSSI to the capillary device at time 1.15 min., the dominant peaks observed in the mass spectrum (Figure 2C) were m/z ~ 801.14 and 1204.21 which corresponded to ssDNA [M-3H]3− ions and dsDNA [M-4H]4− ions, respectively. Here, the signal enhancement of the DNA ions cannot be overstated. With the application of VSSI, the signal level for DNA ions shifts from essentially 0 to >106. That is, although ions are produced with the application of emitter tip bias voltage, essentially no DNA ions are observed until cVSSI is utilized. Notably, the duplex nature of DNA can be preserved with this ionization process as evidenced by the abundant −3 to −5 charge state distribution for dsDNA.
Figure 2.
(A)-(C) Total ion chronogram and full scan mass spectra of DNA duplex d(ACGCGCGT)2 with a capillary bias voltage of −1.27 kV in the presence and absence of cVSSI. Mass spectra were recorded in negative ion mode with 10 μM DNA duplex with 100 mM ammonium acetate using the Q-Exactive Orbitrap mass spectrometer. (A) Total ion chronogram for DNA duplex d(ACGCGCGT)2. Labels show the cVSSI status for 0 min to ~1.14 min, 1.14 min to ~2.45 min, and ~2.45 min to 3.30 min. (B) Mass spectrum in the presence of applied voltage and the absence of cVSSI. (C) Mass spectrum in the presence of both voltage and cVSSI. (D)-(F) Total ion chronogram and full scan mass spectra of single strand DNA d(CATATATG) with a capillary bias voltage of −1.19 kV in the presence and absence of cVSSI. Spectra were recorded in negative ion mode 10 μM DNA single strand with 100 mM ammonium acetate using the LTQ-XL mass spectrometer. (D) Total ion chronogram for single strand DNA d(CATATATG). While the voltage was continuously applied, labels show the VSSI status for 0 min to 0.33 min, 0.33 min to 0.73 min, and 0.73 min to 1.00 min. (E) Mass spectrum in the presence of applied voltage and the absence of cVSSI. (F) Mass spectrum in the presence of both voltage and cVSSI. Mass spectra dataset features are identified with labels.
Figure 2 shows that remarkable ion production can be achieved with the addition of cVSSI even under conditions in which the applied field alone is not sufficient to yield biomolecular ion signals. A question arises as to whether or not such an advantage remains under more optimal electrospray conditions for the employed emitter tip upon application of cVSSI. Figure 2 shows data for ssDNA d(CATATATG) as a function of time in which results for optimized electrospray ionization (−1.19 kV) alone are compared with those employing both cVSSI and emitter tip voltage application using a LTQ-XL mass spectrometer. From the ion chronogram shown in Figure 2D, a clear enhancement in ion production is observed over the time frame of 0.33 to 0.73 min, corresponding with the addition of VSSI to optimal conditions of the emitter tip voltage application.
As before, it is instructive to compare the types of ions produced for the different experimental conditions. In the mass spectrum recorded without VSSI (Figure 2E), a clear charge state distribution is evident with a dominant dataset feature at m/z ~801.83. This feature corresponds to ssDNA [M-3H]3− ions. Overall the distribution is comprised of −2 to −5 ions. The spectrum shows that the DNA ions exist as a wide variety of ion adduct species (primarily Na and NH3) leading to very broad features associated with each charge state. When cVSSI is applied to the device containing the voltage bias, for the most part the same charge state distribution is produced; notably, relatively fewer −5 ions are produced (Figure 2F). The adduct peaks are suppressed and the spectrum is dominated by [M-nH]n− ions. It is important to note that neither the spray voltage nor the mass spectrometer acquisition parameters were changed with the application and removal of VSSI. Therefore, the enhancement/favoring of the deprotonated ions is associated entirely with the vibration of the emitter tip.
Corona Discharge Suppression by cVSSI
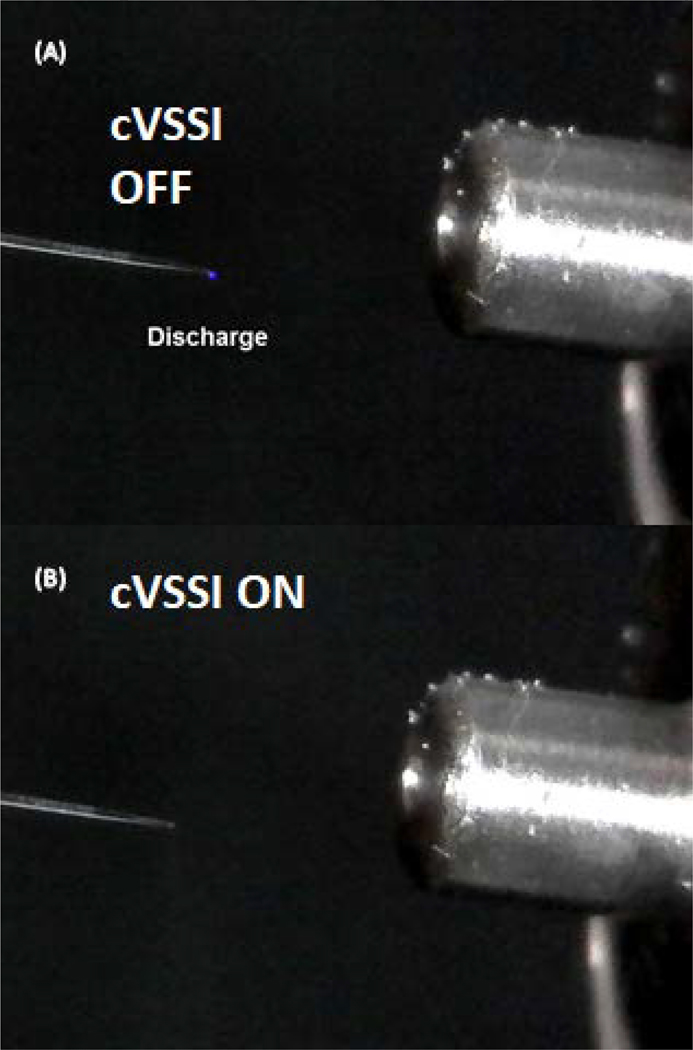
Part of the enhanced signal levels in negative ion ESI could be attributed to discharge suppression by cVSSI. To test this hypothesis, we employed a digital microscope to monitor the emitter tip (ID: ~18 μm) during ESI experiments. As shown in Fig. 3A, a blue glow was seen at the emitter tip when a voltage of −1.27 kV was applied, which indicates the onset of corona discharge. When an AC signal (94.3 kHz, 10 Vpp) was applied to the piezoelectric transducer to initiate cVSSI, the blue glow disappeared indicating that the discharge was suppressed at the onset of cVSSI. Notably, the onset and suppression of corona discharge also corresponds to the ion production of ssDNA (d(CATATATG)) species. When −1.27 kV was applied to the emitter tip, a low molecular weight ion m/z 403.67 is the major peak, and none of the major peaks in this spectrum corresponds to ssDNA ions (Fig. S3). With cVSSI, ssDNA peaks are clearly detected with a dominant peak at m/z 801.83 for [M-3H]3− ions, and the overall detected charge state distribution extends from −2 to −5 ions (Fig. S3). The mechanism of discharge suppression could be explained by a reduced charge build up at the emitter tip during the cVSSI process. As demonstrated in our previous work, cVSSI can generate a liquid plume at the emitter tip independent of the applied voltage, which prevents the buildup of excessive charge at the tip. In addition, because of the cVSSI operation, the emitter tip has a vibration amplitude of ~10 μm that can further disperse the charges. Collectively, these effects lead to a much lower level of charge accumulation directly at the tip when cVSSI is on, which results in the discharge suppression observed in Fig. 3.
Figure 3.
Corona discharge behavior without cVSSI and with cVSSI. (A) A picture of the corona discharge glow without cVSSI. (B) A picture showing corona discharge suppression with cVSSI.
cVSSI stabilizes the nanoESI in negative ion mode
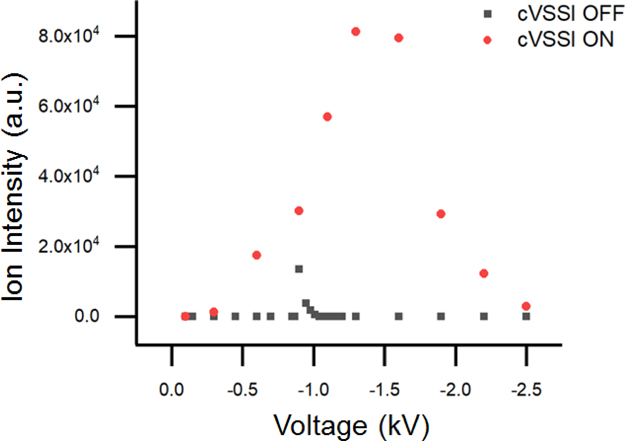
The above results demonstrate the role of cVSSI in suppressing corona discharge and enhancing ion signals in negative ion mode analyses of DNA in aqueous solutions. In many cases, cVSSI salvages the signal from corona discharge as no detectable DNA peaks are obtained by ESI alone. The emitter tips used in these experiments have an ID ranging in size from 15–20 μm. For these larger capillaries, their onset voltage of ESI is often higher than the onset voltage of discharge. As a result, no detectable signals can be seen with the voltage alone. Previous studies report that nano-ESI using emitter tips with ID <10 μm allow the ESI onset voltage to be smaller than the discharge voltage.38 Here, we examine whether cVSSI can impart any benefits to smaller emitter tips in negative ion mode. 10 μM ssDNA d(CATATATG) in 100 mM NH4OAc aqueous solution was sprayed with and without cVSSI using an emitter tip of ~9 μm (Figure S4). With the smaller tip, direct electrospray under the optimal voltage setting of −0.9 kV is able to produce reasonable spectra for the ssDNA sample. While the peak performance of direct electrospray is acceptable without cVSSI, the benefits of applying cVSSI to the biased tip is shown in Fig. 4. Without cVSSI, direct electrospray only has a very narrow working voltage range from −0.85 to −1.01 kV. The performance degrades rapidly as the applied voltage deviates from the optimal value (−0.9 kV). Applying cVSSI allows a much wider range of working voltages from −0.3 to −2.5 kV with the best peak performance observed at −1.3 kV. There are two major advantages associated with the extended voltage range. First, a higher optimal voltage is achieved with cVSSI compared to direct electrospray alone, which results in ~6-fold improvement in ion intensity (Figure S5). Second, a larger working voltage range implies better signal stability over time. The narrow working range of direct electrospray makes ion signals very sensitive to field fluctuations at the emitter tip which can occur in nanoESI experiments in which the tips degrade.39,40 During the experiments, we frequently observed signal disruption every several minutes, which requires re-adjusting the optimal voltage to restore the signal. When cVSSI is applied with the negative voltage, stable and continuous analyte signals are maintained for 1 hr using a flow rate of 500 nL/min without any intervention until we stopped the experiment. Long-term stable signals are especially advantageous for kinetics studies and quantification experiments.
Figure 4.
Relationship between ion intensity and different bias voltages with cVSSI and without cVSSI. 10 μM ssDNA d(CATATATG) in 100 mM NH4Ac aqueous solution was sprayed with and without cVSSI under different voltages. When the cVSSI is on, the working range of voltages is extended significantly.
In addition to pulled-tip capillaries, cVSSI can also be applied to unmodified fused silica capillaries with IDs ranging from 30 to 100 μm to salvage analyte signals in negative ion mode. In this regime, the ESI onset voltage (~−4 to −5 kV) for aqueous solution is much larger than the optimal spray voltage (~−2 to −3 kV). No analyte signals can be observed with voltage alone, so cVSSI is indispensable.
Ion production enhancement from VSSI compared with commercial ESI sources
The experiments described above demonstrate the benefits of applying cVSSI to the ESI emitter tip over using ESI alone in negative ion mode when spraying DNA from aqueous solutions. Here we will compare the performance of using cVSSI plus voltage with commercial ESI sources equipped with nebulization gas. Nebulization gas is the most widely used and amenable instrumentation method to alleviate the discharge issue in negative ion mode. Due to the challenging working conditions of nano-ESI for negative ion production of molecules in aqueous solutions as shown above, many native MS experiments are performed using commercial ESI sources equipped with nebulization gas capabilities.41–43 It is instructive to compare the ionization efficiency of cVSSI plus voltage with that obtained from a well-engineered commercial ESI source. For the following comparisons, HESI sources on the Q-Exactive mass spectrometer and the LTQ-XL mass spectrometer were optimized and compared with the device that couples cVSSI with electrospray voltage. In brief, ion signal levels are again dramatically enhanced with the combined approach.
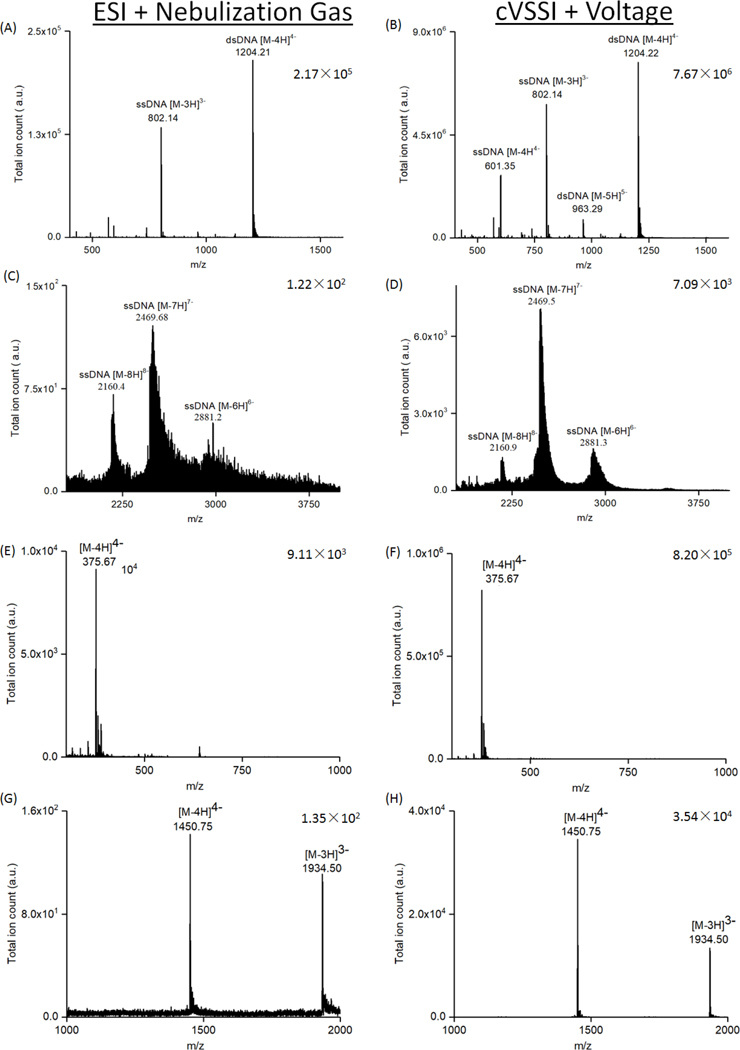
We tested a series of solutions including nucleic acids, oligosaccharides, peptides, and proteins in negative ion mode. Figures 5 and 6 as well as Figures S6A and S6B in the Supporting Information section show the comparison between experiments conducted with the HESI and the VSSI plus bias voltage ion sources on the Q-Exactive mass spectrometer. Experiments show that ion production is significantly enhanced for 10 μM ssDNA d(CATATATG) with the VSSI device compared with the commercial source. For the commercial ion source, the base peak in the mass spectrum (Figure S6A) corresponds to [M-3H]3− ions. The [M-2H]2− ions comprise ~16% of the base peak in the mass spectrum. Usage of the VSSI based ion source also produces a base peak (Figure S6B) corresponding to [M-3H]3− ions. The [M-2H]2− ions now comprise ~23% of the base peak. Additionally, [M-4H]4− ions are observed at ~55% of the base peak height. For the base peak, the overall improvement in ion signal level is ~150 fold. The improvement in S/N is ~110 fold. For experiments with dsDNA, similar enhancements in ion signal levels are observed. Figure 5A and B shows mass spectra obtained for dsDNA d(ACGCGCGT)2. Experiments with the commercial ionization source show that the base peak (Figure 5A) is comprised of [M-4H]4− dsDNA ions. Also observed is the [M-3H]3− ssDNA ion at ~60% relative intensity, compared with the base peak. In comparison, the VSSI based ion source also produces [M-4H]4− dsDNA ions in the greatest abundance (Figure 5B). The [M-3H]3− and [M-4H]4− ssDNA ions are observed at respective intensities of 75% and 35%. For this DNA sample, improvements in base peak intensity level and S/N are ~35 fold and ~3 fold, respectively.
Figure 5.
Comparison of ionization performance between a commercial ESI sources with nebulization gas and the cVSSI plus voltage system. Applied voltages and nebulization gas flowrates in the commercial sources were optimized for the highest ion intensity. (A) and (B): Comparison of 10 μM duplex DNA d(ACGCGCGT)2 in 100 mM NH4OAc solution. Mass spectra were recorded in negative ion mode using 10 μM duplex DNA with 100 mM NH4OAc. (C) and (D): Comparison of 1 μM 56mer ssDNA in 100 mM NH4OAc solution. (E) and (F): Comparison of 10 μM fondaparinux sodium with 0.1% HAc. (G) and (H): Comparison of 10 μM insulin in 100 mM NH4OAc. Mass spectra dataset features are identified with labels. Base peak intensities are provided for each spectrum.
Figure 6.
Comparison of charge state distributions for 10 μM ubiquitin in 100 mM NH4OAc between a commercial ESI source and a cVSSI plus voltage source. Full scan mass spectra of ubiquitin in both positive and negative ion mode. Mass spectra dataset features are labeled. (A) shows data collected using a commercial ESI source with a bias voltage of +4.1 kV and a 20 a.u. gas flow rate, and (B) shows data collected using a cVSSI plus +1.08 kV. (C) shows data collected using a commercial ESI source with a bias voltage of −3.5 kV and a 30 a.u. gas flow rate, and (D) shows data collected using a cVSSI plus −1.07 kV.
Having demonstrated improved ionization for small oligonucleotides including double stranded molecules, it is worthwhile to determine whether or not the ionization enhancement effect of cVSSI extends to much larger oligonucleotides. For these studies, 1 μM of a 56-mer DNA oligonucleotide in 100 mM NH4OAc solution was examined by ESI as well as the VSSI plus spray voltage. Figure 5 shows the mass spectra collected using the commercial ionization source (Figure 5C) as well as the prototype cVSSI source (Figure 5D). ESI of the large oligonucleotide produces the −6 to −8 charge states. For the prototype ionization source, the same charge states are produced. A comparison of the relative intensities of the dataset features shows that the order of charge state intensities is −7 > −8 > −6 and −7 > −6 > −8 for the commercial and prototype ionization sources, respectively. Additionally, significantly narrower (~3 fold for FWHM −7 ions) peaks are observed for the cVSSI source indicating a favoring of ions with fewer adducts. Remarkably, the overall improvement in ion signal level and S/N for the −7 ions is observed to be ~60 and ~10 fold, respectively.
In addition to the DNA samples, a similar level of improvement was observed for oligosaccharides, peptides, and proteins, when comparing the HESI and the cVSSI plus bias voltage ion sources on the LTQ-XL mass spectrometer. For oligosaccharides, 10 μM of chondroitin disaccharide Δdi-6S sodium (Figure S6C and D) and fondaparinux sodium (Figure 5E and F) solutions were tested, respectively. Chondroitin disaccharide was detected at a m/z 458.08 which corresponds to the peak of [M-H]− ions; and fondaparinux was detected at a m/z 375.67 which corresponds to the peak of [M-4H]4− ions. As shown in Figure S6C and D, Figure 5E and F, the cVSSI plus voltage system shows ~240 and ~90 fold improvements in ion intensity and ~2 fold and ~3 fold improvements in S/N, for the chondroitin disaccharide and fondaparinux samples, respectively.
Angiotensin 2 (Figure S6E and F) and insulin (Figure 5G and H) in 100 mM NH4OAc were also tested to evaluate the performance of the cVSSI plus voltage ion source for peptide and protein ion generation from aqueous media. Although angiotensin and insulin are often analyzed in positive ion mode, they also show clear deprotonated peaks under negative ion mode settings so they can be used in this comparison. Significant improvements in both total ion counts (~100 fold) and S/N (~10 fold) were observed for VSSI coupled with voltage.
These early experiments clearly demonstrate that the cVSSI plus voltage source provides better ion intensity and S/N than the commercial HESI source does. Replacing the nebulization gas with cVSSI to suppress the discharge enables an ionization source setup that has a significant improvement in total ion intensity detected. Part of the enhancement could come from the use of a pulled fused silica capillary tip instead of the stainless-steel capillary used in the HESI system. Replacing the nebulization gas with cVSSI to suppress the discharge could also contribute to the enhancement. This contrast between the nebulization and cVSSI process is rendered more striking by the fact that the flow rates (and thus sample consumption) in HESI experiments are 5–10 fold higher.
The improvements in ion signal level and S/N have very clear implications with regard to different analyses. For example, S/N improvements would impact the ability to distinguish precursor ions in complex mixtures such as those encountered in ‘omics analyses. That is, S/N improvements allow the detection of lower-signal species in the presence of higher-signal ions.45–47 In the case of ion signal level enhancements, a clear advantage is provided to tandem MS and multi-stage tandem MS (MSn).48–52 Here, the ability to detect fragment ions from isolated precursor ions is very dependent on ion numbers in the precursor ion selection step. This is especially true for species that may not undergo facile fragmentation or when attempting to observe a low-frequency ion fragment. Finally, enhancements in S/N levels of ions from aqueous media enable the coupling of separation techniques that rely on such media. Thus, the enhancements in ion signal level and S/N realized here with cVSSI provide immediate advantages for a number of fields employing MS analysis.
Potential applications in native MS analysis
An increasing area of interest in biological mass spectrometry is the characterization of biomolecular structure.53–58 To evaluate the potential of cVSSI plus voltage for structure characterization, experiments have been conducted for molecules for which it has been proposed that native MS allows the preservation of solution structure in the gas-phase.59 The first study involves the examination of the globular protein ubiquitin which was examined in both positive and negative ion mode. The second study involves the examination of the formation of G-quadruplex DNA structure in solution. Each is described in greater detail below.
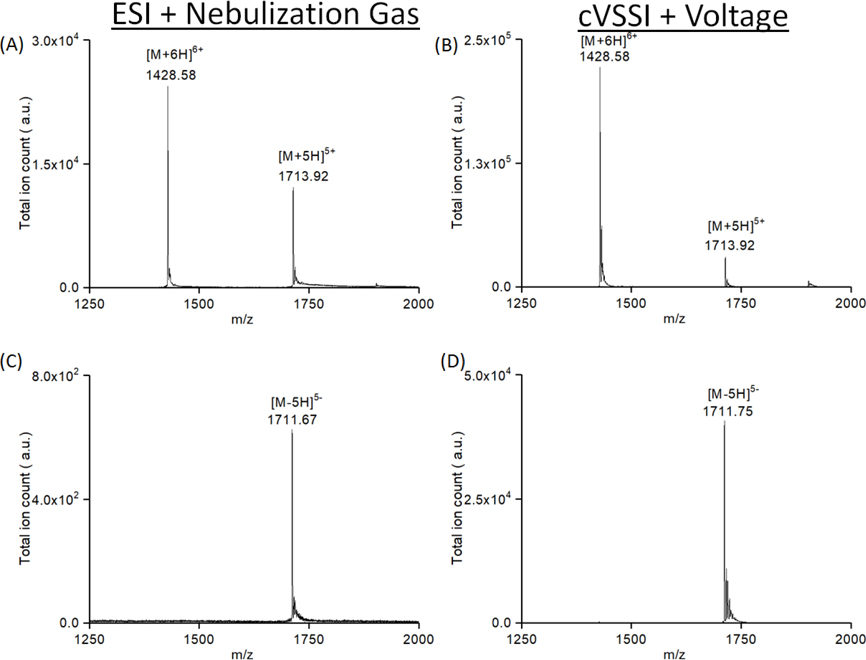
Positive and negative ion mode experiments have been conducted for a solution of the globular protein ubiquitin. Ubiquitin is a well-studied protein for native MS analysis, and the relationship between charge state distribution and solution structures has been reported before.60 Therefore, it is a good indicator for whether or not the cVSSI process disrupts the native structure of biomolecules. For these experiments, we also tested positive ion mode conditions in addition to those for negative ion mode analysis as most of the existing studies for ubiquitin are performed in positive ion mode. Ion production using a commercial ESI source was compared to that using a cVSSI source plus voltage. In positive ion mode, ESI produces two charge states comprised of [M+6H]6+ (m/z 1428.58) and [M+5H]5+ (m/z 1713.92) ions (Figure 6A) for 10 μM ubiquitin in 100 mM NH4AC solution. Here the +5 charge state intensity is nearly half that of the base peak, +6 charge state. When the cVSSI plus voltage is used (Figure 6B), the base peak ion signal and S/N levels increased by values of ~10 and ~35 fold, respectively. The same two charge states dominate the mass spectrum. A difference is that the +5 charge state comprises a slightly lower level (~10% of the base peak) of the total ion population. The notable similarity in the two mass spectra shown in Figure 6 is that the charge state distribution is “pinched off” at the +6 charge state. Previous experiments have shown that, for native MS, such a phenomenon is indicative of the preservation of solution structure into the gas phase.59 Notably, a direct comparison to ESI with the same pulled-tip capillary (10 μm) also shows ~5 fold improvement for the usage of cVSSI plus voltage as demonstrated in Figure S7 in the Supporting Information section. The observation of similar charge state production also holds in the comparison conducted for negative ion mode analysis of ubiquitin as shown in Figure 6C and 6D. Here a notable difference is that the “pinch off” occurs at an even lower charge state (−5) for both the ESI and cVSSI plus voltage experiments. Consistent with the DNA analysis described above, for negative ion mode experiments of ubiquitin, ion signal level and S/N enhancements are also dramatic (~60 and ~15 fold, respectively). Here we note that further evidence for preservation of solution structure is provided in Figure S8 in the Supporting Information section where the preservation of holo-myoglobin is observed with the usage of cVSSI in both negative ion mode and positive ion mode. In addition, we also tested long-term operation of cVSSI and its impact on protein structures using myoglobin in 100 mM NH4OAc. Results show that with cVSSI on, the spray was continuous and stable for 1 h with a flow rate of 1 μL/min. Comparing the mass spectrum at 0 h and 1 h shows no difference in the ratio between the holo and the apo (barely present) forms of myoglobin (Figure S9).
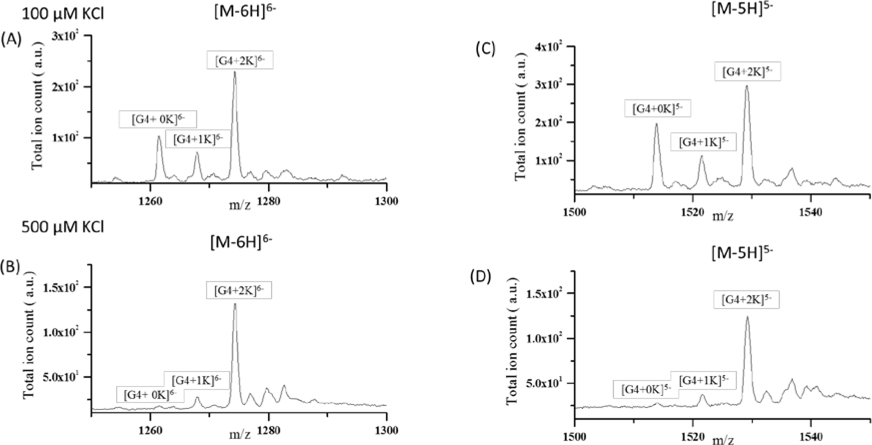
Nucleic acids are another important class of biomolecules that can assume 3D structures in solution. While nucleic acids can be studied in both positive and negative ion mode, studies suggest that ions produced in negative ion mode better reflect their solution structure61 Therefore, native DNA/RNA mass spectrometry experiments are usually performed in negative ion mode. Some biomolecular structures require solutions of significant ionic strength in order to form. An example is the G-quadruplex DNA which can form in solutions containing potassium due to charge-dipole stabilization of the G-quartets. To demonstrate that the new VSSI source can preserve G-qaudruplex structure similar to ESI,62 experiments were conducted with the human telomeric G-quadrplex DNA (TTGGGTTAGGGTTAGGGTTAGGGA) sequence (G4). As above, experiments were conducted using the cVSSI plus voltage device. Samples containing two different concentrations (100 and 500 μM) of KCl were examined by MS. Figure 7 shows expanded regions of the MS spectra highlighting the −6 and −5 charge states of the DNA ions. For discussion purposes, oligonucleotide peaks are described (and labeled in Figure 7) as the form [G4+nK]m− where n and m correspond to the number of associated K+ ions and net charge, respectively. For solutions containing 100 μM KCl, for the [M-6H]6− ions (Figure 7A), dataset features corresponding to [G4+0K]6-, [G4+1K]6− and [G4+2K]6− appeared at m/z ~1261.50, 1268.00 and 1274.25, respectively. Similarly, for [M-5H]5− ions (Figure 7C), [G4+0K]5−, [G4+1K]5− and [G4+2K]5− peaks were observed at m/z ~1514.00, 1521.67 and 1529.25, respectively. Notably the relative intensity levels of the respective ions produced by the new VSSI ion source are very similar to those produced by ESI experiments previously.62 Additionally, when the KCl concentration is increased to 500 μM, the dominant species observed for both charge states are the [G4+2K]m− ions (Figure 7B and D). This is also consistent with the prior ESI experiments.62 This limiting effect is consistent with the G4-quadruplex structure containing 3 G-quartets stabilized by 2 K+ ions. Taken together, this data suggests that the new ionization source employing cVSSI plus voltage can preserve the G-quadruplex structure to a similar degree as ESI. Notably, the present system achieved similar results to the previous study62 with 3–6 fold lower flow rates, which can be a much desired characteristic for studying biomolecules.
Figure 7.
Mass spectra for different charge states – [M-6H]6− (A, B) and [M-5H]5− (C,D) of human telomeric G-quadruplex DNA (TTGGGTTAGGGTTAGGGTTAGGGA) sequence (G4) in 100 and 500 mM KCl solutions. Peaks are labeled in the form of [G4+nK] where n and m represents the number of potassium adducts and total charge, respectively. High signal intensities of [G4+2K]5- and 6− were observed in each spectrum as there are 3 guanine quartets present in the G-quadruplex structure. See reference 50 for comparison to ESI results.
Conclusion
Combining the cVSSI process with voltage can effectively suppress discharge in conventional ESI experiments thereby improving the signal quality significantly. Overall, cVSSI is easy and cost effective to implement with a wide range of working flow rates. When coupled to a pulled-tip capillary, it can handle flow rates of 0.2–1 μL/min for small volume samples. cVSSI can also work with normal capillaries (ID ~100 μm) to accommodate high flow rates. All the components of the cVSSI system can be purchased directly with a cost of less than $10 per device. The device can also be easily fabricated without the need of special equipment or processes. Therefore, it has great potential to be adopted widely by laboratories studying native mass spectrometry in negative ion mode.
In the work presented here, little attention is given to cVSSI usage in positive ion mode. The impetus for the focus on negative ion mode analyses is because such studies are significantly hindered in native MS as evidenced by the fact that, for the most part, they require auxiliary methods/techniques to obtain useful analyses. Additionally, the signal enhancement provided by cVSSI is significantly greater for negative ion mode studies. That said, Figure 6 and Figure S6 suggest that significant signal enhancements may also be made available to native MS using positive ion mode. Although beyond the scope of the work presented here, detailed, exhaustive studies of the overall impact of cVSSI as a tool for stable and robust native MS in positive ion mode are worthy of future exploration.
Supplementary Material
Acknowledgement
This work is supported by West Virginia University Start-up fund and Deans’ Instrumentation Seed Program for Innovative Research (InSPIRe). Partial support was provided by the National Science Foundation (CHE-1553021) and the National Institutes of Health (R01GM114494 and R01GM135432). We acknowledge use of the WVU Shared Research Facilities for mass spectrometry analysis. We thank Dr. Callee Walsh and Sandra Majuta in the WVU-BNRF research facility for assisting in mass spectrometry experiments.
References
- (1).Leney AC; Heck AJ J Am Soc Mass Spectrom 2017, 28, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Yewdall NA; Allison TM; Pearce FG; Robinson CV; Gerrard JA Chem Sci 2018, 9, 6099–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Martin EM; Kondrat FDL; Stewart AJ; Scrivens JH; Sadler PJ; Blindauer CA Sci Rep 2018, 8, 8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ishii K; Zhou M; Uchiyama S Biochim Biophys Acta Gen Subj 2018, 1862, 275–286. [DOI] [PubMed] [Google Scholar]
- (5).Patterson A; Tokmina-Lukaszewska M; Bothner B Methods Enzymol 2019, 616, 87–116. [DOI] [PubMed] [Google Scholar]
- (6).Poltash ML; McCabe JW; Shirzadeh M; Laganowsky A; Clowers BH; Russell DH Anal Chem 2018, 90, 10472–10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Wampler FM; Blades AT; Kebarle P J Am Soc Mass Spectrom 1992, 4, 289–295. [DOI] [PubMed] [Google Scholar]
- (8).Kebarle P; Verkerk UH Mass Spectrom Rev 2009, 28, 898–917. [DOI] [PubMed] [Google Scholar]
- (9).Cole RB; Harrata AK J Am Soc Mass Spectrom 1993, 4, 546–556. [DOI] [PubMed] [Google Scholar]
- (10).Gapeev A; Berton A; Fabris D J Am Soc Mass Spectrom 2009, 20, 1334–1341. [DOI] [PubMed] [Google Scholar]
- (11).Liko I; Hopper JT; Allison TM; Benesch JL; Robinson CV J Am Soc Mass Spectrom 2016, 27, 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Greer SM; Cannon JR; Brodbelt JS Anal Chem 2014, 86, 12285–12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Fabris D J Am Soc Mass Spectrom 2010, 21, 1–13. [DOI] [PubMed] [Google Scholar]
- (14).Cole RB; Harrata AK Rapid Commun Mass Spectrom 1992, 6, 536–539. [Google Scholar]
- (15).Zhu J; Cole RB J Am Soc Mass Spectrom 2000, 11, 932–941. [DOI] [PubMed] [Google Scholar]
- (16).Erb R; Oberacher H Electrophoresis 2014, 35, 1226–1235. [DOI] [PubMed] [Google Scholar]
- (17).McClory PJ; Hakansson K Anal Chem 2017, 89, 10188–10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kruve A; Leito I; Herodes K; Laaniste A; Lohmus R J Am Soc Mass Spectrom 2012, 23, 2051–2054. [DOI] [PubMed] [Google Scholar]
- (19).Ikonomou MG; Blades AT; Kebarle P J Am Soc Mass Spectram 1991, 2, 497–505. [DOI] [PubMed] [Google Scholar]
- (20).Yamashita M; Fenn JB J. Phys. Chem. 1984, 88, 4671–4675. [Google Scholar]
- (21).Chen LC; Mandal MK; Hiraoka K J Am Soc Mass Spectrom 2011, 22, 2108–2114. [DOI] [PubMed] [Google Scholar]
- (22).Chen LC; Mandal MK; Hiraoka K J Am Soc Mass Spectrom 2011, 22, 539–544. [DOI] [PubMed] [Google Scholar]
- (23).Gibson GT; Mugo SM; Oleschuk RD Mass Spectrom Rev 2009, 28, 918–936. [DOI] [PubMed] [Google Scholar]
- (24).Rahman MM; Chingin K Analytical Methods 2019, 11, 205–212. [Google Scholar]
- (25).Wei Z; Han S; Gong X; Zhao Y; Yang C; Zhang S; Zhang X Angew Chem Int Ed Engl 2013, 52, 11025–11028. [DOI] [PubMed] [Google Scholar]
- (26).Gong X; Xiong X; Zhao Y; Ye S; Fang X Anal Chem 2017, 89, 7009–7016. [DOI] [PubMed] [Google Scholar]
- (27).Ranganathan N; Li C; Suder T; Karanji AK; Li X; He Z; Valentine SJ; Li P J Am Soc Mass Spectrom 2019, 30, 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Li X; Attanayake K; Valentine SJ; Li P Rapid Commun Mass Spectrom 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Forbes TP; Dixon RB; Muddiman DC; Degertekin FL; Fedorov AG J Am Soc Mass Spectrom 2009, 20, 1684–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Shiea J; Chang D-Y; Lin C-H; Jiang S-J Anal. Chem. 2001, 73, 4983–4987. [DOI] [PubMed] [Google Scholar]
- (31).Berggren WT; Westphall MS; Smith LM Anal. Chem. 2002, 74, 3443–3448. [DOI] [PubMed] [Google Scholar]
- (32).Potier N; Dorsselaer AV; Cordier Y; Roch O; Bischoff R Nucleic Acids Research 1994, 22, 3895–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Tretyakova N; Villalta PW; Kotapati S Chem Rev 2013, 113, 2395–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hua Y; Wainhaus SB; Yang Y; Shen L; Xiong Y; Xu X; Zhang F; Bolton JL; Breemen RB v. J Am Soc Mass Spectrom 2000, 12, 80–87. [DOI] [PubMed] [Google Scholar]
- (35).Banerjee S; Mazumdar S Int J Anal Chem 2012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Banoub JH; Newton RP; Esmans E; Ewing DF; Mackenzie G Chem. Rev. 2005, 105, 1869–1915. [DOI] [PubMed] [Google Scholar]
- (37).Rahman MM; Mandal MK; Hiraoka K; Chen LC Analyst 2013, 138, 6316–6322. [DOI] [PubMed] [Google Scholar]
- (38).Chowdhury SK; Chait BT Anal Chem 1991, 63, 1661–1664. [DOI] [PubMed] [Google Scholar]
- (39).Kirby AE; Jebrail MJ; Yang H; Wheeler AR Rapid Commun Mass Spectrom 2010, 24, 3425–3431. [DOI] [PubMed] [Google Scholar]
- (40).Wetterhall M; Klett O; Markides KE; Nyholm L; Bergquist J Analyst 2003, 128, 728–733. [DOI] [PubMed] [Google Scholar]
- (41).Khristenko N; Amato J; Livet S; Pagano B; Randazzo A; Gabelica V J Am Soc Mass Spectrom 2019, 30, 1069–1081. [DOI] [PubMed] [Google Scholar]
- (42).Porrini M; Rosu F; Rabin C; Darre L; Gomez H; Orozco M; Gabelica V ACS Cent Sci 2017, 3, 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Gabelica V; Livet S; Rosu F J Am Soc Mass Spectrom 2018, 29, 2189–2198. [DOI] [PubMed] [Google Scholar]
- (44).Counterman AE; Hilderbrand AE; Barnes CAS; Clemmer DE J Am Soc Mass Spectrom 2001, 12, 1020–1035. [Google Scholar]
- (45).Faktor J; Sucha R; Paralova V; Liu Y; Bouchal P Proteomics 2017, 17. [DOI] [PubMed] [Google Scholar]
- (46).Bantscheff M; Schirle M; Sweetman G; Rick J; Kuster B Anal Bioanal Chem 2007, 389, 1017–1031. [DOI] [PubMed] [Google Scholar]
- (47).Yates JR; Ruse CI; Nakorchevsky A Annu Rev Biomed Eng 2009, 11, 49–79. [DOI] [PubMed] [Google Scholar]
- (48).McLuckey SA; Berkel GJV; Glish GL J Am Soc Mass Spectrom 1992, 3, 60–70. [DOI] [PubMed] [Google Scholar]
- (49).McLuckey SA; Habibi-Goudarzi S J Am Soc Mass Spectrom 1994, 5, 740–747. [DOI] [PubMed] [Google Scholar]
- (50).Hengel SM; Goodlett DR Int J Mass Spectrom 2012, 312, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Heller M; Schlappritzi E; Stalder D; Nuoffer JM; Haeberli A Mol Cell Proteomics 2007, 6, 1059–1072. [DOI] [PubMed] [Google Scholar]
- (52).Kang H; Pasa-Tolic L; Smith RD J Am Soc Mass Spectrom 2007, 18, 1332–1343. [DOI] [PubMed] [Google Scholar]
- (53).Cheatham TE 3rd; Brooks BR; Kollman PA Curr Protoc Nucleic Acid Chem 2001, Chapter 7, Unit 7 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Baker ES; Bernstein SL; Bowers MT J Am Soc Mass Spectrom 2005, 16, 989–997. [DOI] [PubMed] [Google Scholar]
- (55).Zucker SM; Lee S; Webber N; Valentine SJ; Reilly JP; Clemmer DE J Am Soc Mass Spectrom 2011, 22, 1477–1485. [DOI] [PubMed] [Google Scholar]
- (56).Kondalaji SG; Khakinejad M; Valentine SJ J Am Soc Mass Spectrom 2018, 29, 1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Zhang W; Zhang D; Chen Q; Wu J; Ouyang Z; Xia Y Nat Commun 2019, 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Zhang W; Ren Y; Lin Z; Ouyang Z Anal Chem 2019, 91, 6986–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Wyttenbach T; Bowers MT J Phys Chem B 2011, 115, 12266–12275. [DOI] [PubMed] [Google Scholar]
- (60).El-Baba TJ; Woodall DW; Raab SA; Fuller DR; Laganowsky A; Russell DH; Clemmer DE J Am Chem Soc 2017, 139, 6306–6309. [DOI] [PubMed] [Google Scholar]
- (61).Rosu F; Pirotte S; Pauw ED; Gabelica V International Journal of Mass Spectrometry 2006, 253, 156–171. [Google Scholar]
- (62).Marchand A; Gabelica V J Am Soc Mass Spectrom 2014, 25, 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.