Abstract
Objective:
To summarize the current understanding of anaphylaxis, with an emphasis on major findings that have been reported within the last 10 years.
Data Sources:
Queries relating to anaphylaxis, immunoglobulin E (IgE), and mast cells were conducted with PubMed and Google Scholar, searching for primary articles and review papers.
Study Selections:
We focused on articles written in English and which were reported in major allergy and immunology journals.
Results:
Anaphylaxis represents an extreme manifestation of a form of allergic immunity that appears to have evolved to protect against “toxic” threats that present at skin and mucosal barriers. The factors that have contributed to a rise in anaphylaxis are increasingly appreciated to relate to changes in hygiene and microbial ecology that have occurred with industrialization. Induction of allergen-specific IgG4 is often part of the allergic response and is associated with protection against anaphylaxis. The recognition of the α-Gal syndrome suggests that carbohydrates can be epitopes that are relevant to anaphylaxis and that IgE-mediated reactions do not always occur “immediately.”
Conclusion:
Our understanding of anaphylaxis has advanced significantly over the past 10 years. It is anticipated that ongoing research will build on this foundation to further advance our knowledge of anaphylaxis and also translate into clinically meaningful therapies.
Introduction
The term anaphylaxis was coined over a century ago; nonetheless many important questions remain unanswered with regard to mechanisms, pathogenesis, and also the apparent increase in prevalence/incidence.1 Over the last 10 years, many studies have been published that have influenced our understanding of anaphylaxis. Although much of this progress relates to immediate IgE-mediated anaphylaxis to food in children, such as peanut, egg, and milk, there are many other important developments. The best understood by us is the discovery and study of the α-Gal syndrome. Additional developments that influence our understanding of anaphylaxis include the recent evidence about a role for the microbiome, increasing appreciation of the interrelationship between IgG4 and IgE, and the hypothesis regarding the evolutionary significance of IgE and anaphylaxis as a protective mechanism. Our major focus here is on IgE-mediated forms of anaphylaxis; other mechanisms also contribute to a minority of cases of anaphylaxis, and we would refer the reader to several recent reviews of that topic.2–4 This article is not meant to be an exhaustive review of anaphylaxis, and for further reading we would direct the reader to a recent practice parameter on anaphylaxis5 and some excellent reviews on diagnosis and management.6,7
A Challenging Definition
Anaphylaxis is generally understood to be an immune-mediated reaction that occurs rapidly after exposure to a triggering substance and involves at least 2 body systems, but formulating a more precise definition has proved challenging.8 Mediators from mast cells such as histamine and tryptase often rise acutely in the setting of anaphylaxis, but no single biomarker that has both high sensitivity and high specificity to confirm the diagnosis. This has contributed in part to difficulties in understanding the true frequency of anaphylaxis in the population, and also hints at the varied pathophysiologic mechanisms that may underlie anaphylaxis.9–11 A Working Group of the American College of Allergy, Asthma, and Immunology in 2006 estimated that anaphylaxis prevalence (ie, the percentage of subjects who have experienced anaphylaxis at least once in their lifetime) was between 0.05% and 2.0%12; a more recent systematic review of anaphylaxis in Europe concluded a prevalence of 0.1% to 0.5%.13 A cross-sectional study of 1000 adults in the United States that was published in 2014 suggests the prevalence could be higher: 1.6% reported symptoms that were deemed “likely anaphylaxis,” but the number increased to 5.1% for a less stringent definition of “probable anaphylaxis.”14 In adults anaphylaxis is triggered most often by medications, whereas food is the dominant trigger in children.9 In the latter group the data are most compelling that rates have been increasing over the past 2 to 3 decades; despite these trends, one must realize that fatalities attributed to anaphylaxis are rare events, with estimates ranging from approximately 0.1 to 1 deaths per million person-years.15 Despite the fact that epinephrine remains the mainstay of treatment, many studies suggest that epinephrine is underprescribed and underused.16,17
Recent Insights into Food Allergy
The rise in the prevalence or recognition of children with allergies to peanut, milk, and egg in industrialized nations over the last 2 to 3 decades has been very striking.18,19 The causes that explain this rise are still very much up for debate. One of the most important advances in allergy and immunology over the last decade occurred in pediatric food allergy with the publication of the LEAP study.20 This study, for the first time, demonstrated definitive evidence in favor of early oral introduction of a highly allergenic food (eg, peanut) to prevent early childhood sensitization and clinical allergy related to that food.21 The results also support the “dual-allergen hypothesis,” which argues that allergen exposure via the skin favors sensitization, whereas exposure through the gut favors tolerance. Despite the promising results with peanut, that the results will extrapolate to all food allergens is not clear.22 Because multiple factors have likely contributed to the rise in food allergies, early introduction of foods alone is unlikely to stop this epidemic.19,23 Another example of a food allergy that may arise because of cutaneous exposure relates to wheat. Fukutomi et al in Japan reported 5 cases of wheat-related anaphylaxis in which sensitization is thought to have occurred through the application of facial soap containing hydrolyzed wheat protein.24 Curiously, but similar to other reports of wheat allergy, most of the cases only occurred in the setting of exercise. This is consistent with the important role of co-factors in what is often known as wheat-dependent exercise-induced anaphylaxis. Challenge studies have confirmed that exercise, but also alcohol and aspirin, are relevant co-factors in wheat-dependent exercise-induced anaphylaxis.25
The Microbiome and Food Allergy
Given the fact that human somatic and germ cells are outnumbered by the microbiota present in our gut and on our skin, it should be no surprise that disruptions of the microbiome could have profound effects on human health and disease.26 Major research initiatives over the past decade have shown that the microbiome is integral to food allergy, and by extension, anaphylaxis.27–29 One of the reasons that this work is compelling is that “microbial dysbiosis” provides a mechanistic and perhaps unifying framework for thinking about how industrialization, with accompanying changes in hygiene, could make a major contribution to the food allergy epidemic. The following have been shown to impact or would be expected to impact the diversity of the gut and skin microbiome: 1) dietary changes relating to consumption of highly processed foods30; 2) antibiotic use31; 3) changes in delivery practices and infant feeding (eg, increases in Cesarean-section deliveries and decrease in breastfeeding)32; and 4) industrial-scale application of chemicals and pesticides.33 Thus, it is not surprising that several investigators have reported an association between decreased gut microbial diversity, and in some cases specific microbial signatures, and food allergy.34 Two recent studies employed fecal microbial transplants (FMT) from humans into germ-free mice and compared outcomes when the FMT was derived from food-allergic or healthy subjects. Feehley et al28 found that FMT from healthy infants, but not from infants with cow’s milk allergy, could suppress immunoglobulin E (IgE) induction and anaphylaxis in a model using β-lactoglobulin sensitization and challenge.35 A similar result was reported by Abdel-Gadir and colleagues,29 who used the food allergy-prone IL4raF709 mouse in an ovalbumin sensitization model and demonstrated that induction of specific IgE and severity of anaphylaxis were mitigated by FMT from healthy human infants, but not from infants with food allergy.36,37 A strength of both studies was the identification of specific commensals that had protective effects on the development of the allergic responses. Although attempts to prevent or treat food allergy with interventions such as prebiotics and probiotics have not proven successful in humans to date, the recent microbiome work is promising and suggests that tailored manipulation of the microbiota may play a role in the future.
The α-Gal Syndrome as a Major “New” Cause of Anaphylaxis
As articulated in a recent publication by Pattanaik and colleagues,31 the “face of anaphylaxis” has changed over the past decade with the appreciation of a new form of food allergy with several unusual features—the α-Gal syndrome.38 Sensitization to the oligosaccharide galactose-α−1, 3-galactose (α-Gal) was first recognized as a cause of immediate or anaphylactic reactions to the monoclonal antibody cetuximab.39,40 Those reactions occurred during the first infusion and varied in severity from hives that were easy to treat to severe anaphylaxis that developed within 10 minutes. In contrast to the reactions to cetuximab, everything else about the α-Gal syndrome was contrary to standard knowledge about allergic disease and particularly food allergy. This related to the age of disease onset, the timing of symptom onset in relation to ingestion of the relevant antigen, and the nature of the epitope and symptoms41–44 (Table 1).
Table 1.
Features of the α-Gal Syndrome that Depart From Traditional Food Allergies
|
The fact that α-Gal can contribute to severe anaphylaxis is not in question, as we have consulted on 20 or more patients with the syndrome who presented to hospital with elevated serum tryptase at the time of an attack. During challenge studies using 150 g pork sausage, Commins et al. reported symptoms as well as the activation of CD63 on basophils and serum tryptase.43 The results in patients included itching or hives starting 2 to 4 hours after eating mammalian meat, which occurred in parallel with activation of CD63 in vivo (documented ex vivo) and also at the same time as symptom onset. In addition, 3 of the 13 patients had a significant elevation of tryptase, again with timing that matched symptom onset. At a simple level, those results confirmed the timing reported by most patients,42 because we know that basophils from these patients that have been incubated in vitro with α-Gal-expressing glycoprotein (eg, α-Gal-HSA, cetuximab, or beef thyroglobulin) will activate CD63 within 20 minutes.43,45 The results argue strongly that the delay reflects the time taken for a relevant form of the antigen to appear in the circulation. Given the clear evidence about a delay in the response to eating red meat, we have considered many possible explanations. Perhaps the best understood digestive process that takes as long as 4 to 6 hours is the absorption and metabolism of dietary lipids.46 Because α-Gal is an oligosaccharide that can be present in glycolipids as much as glycoproteins,47 this in turn was an important reason for considering the “glycolipid hypothesis” (which was actually proposed to the senior author by Rob Aalberse in 2014).48 The absorption of fat occurs in the small intestines and involves the production of chylomicrons in lacteals that then pass up the thoracic duct and into the superior vena cava. The particles initially are relatively large, possibly 400 nm in diameter, but over the next 2 to 4 hours they decrease in size, to approximately 20 nm in the case of low-density lipoproteins. These particles are small enough to pass out of the vasculature into the interstitial tissue of the skin or arterial walls; if these particles still carried the α-Gal epitope they would be ideally placed to activate mast cells in the tissues. In support of this hypothesis, Roman-Carrasco et al42 recently reported that glycolipid forms of α-Gal, but not glycoproteins, passed through an epithelial barrier model intact and were packaged into chylomicrons.49 As an extension of this glycolipid hypothesis we have also been interested in the possibility that IgE to α-Gal could be relevant to inflammatory diseases that are not traditionally considered “allergic.” In a cohort of adults who were enrolled without regard to allergic history, we recently reported that the severity of coronary artery disease was greater in subjects who were sensitized to α-Gal.50 This finding prompts several questions but raises the possibility that IgE to glycolipid food allergens could have severe long-term consequences in addition to their contribution to anaphylaxis. Our working hypothesis is that chronic consumption of mammalian products could act in an antigen-dependent manner to stimulate tissue-resident mast cells, including those in the walls of coronary arteries, in subjects who make IgE to α-Gal. Of course this would only be true for subjects who have not had severe allergic reactions and continue to consume red meat or dairy; however, this fits with epidemiological data showing that some 90% of high-risk forest workers who were sensitized to α-Gal denied any history of allergic reactions to red meat.51
What Role Do IgG and Particularly IgG4 Antibodies Play in Controlling Anaphylaxis?
Immunoglobulin G4 is an antibody subclass that is often considered to be part of a type 2 immune response, but it has several features that suggest anti-inflammatory activity.52 Clear evidence suggests that levels of allergen-specific IgG4 increase with subcutaneous immunotherapy and oral immunotherapy. Accordingly, specific IgG4 could play a role in reducing allergic reactions mediated by IgE. Milk and peanut represent 2 major foods that are an important source of foreign antigens but induce strikingly different antibody responses during the first 5 to 7 years of life. Cow’s milk proteins give rise to transient IgE responses in early childhood, but they are increasingly recognized as a dominant antigen associated with a completely different form of food allergy.53 Patients with eosinophilic esophagitis often have low levels of IgE to those foods that are important triggers of the disease, such as milk and wheat, but they experience no immediate symptoms.54,55 Evidence for the relevance of IgG4 in eosinophilic esophagitis first appeared in 2014.56 Since then, several studies have confirmed the presence of food-specific IgG4 in the serum.57,58 In our recent work, the calculated quantitative ratios of IgG4 ab: IgE ab specific to Bos d 4 and Bos d 5 were greater than 1000:1.58 The implication is that this level of IgG4 can both inhibit IgE assays in vitro and prevent immediate symptoms in vivo. We have also asked a different question: “What is the normal level of IgG4 to common antigens in the population?” Using the Viva birth cohort in Boston, we have reported IgG4 levels at age 7 and age 13.58,59 At age 7, the pattern of IgG4 and IgE ab to milk and peanut allergens was already dramatically different (Fig 1). At ages 12 to 13, high IgG4 levels to cow’s milk proteins are relatively common; by contrast, comparably high levels of IgG4 to peanut proteins are rare. Extending the question of IgG4 further, the antibody response to tick bites can involve dramatic increases in IgE to α-Gal, but includes little or no specific IgG4.60 Thus, the fact that patients with the α-Gal syndrome can get severe anaphylaxis to cetuximab and also to red meat is in keeping with a high ratio of IgE to IgG4. Other examples of recent work support a protective role for IgG4 in allergic disease. At least 2 groups have reported that using the ratio of specific IgG4/IgE to egg had better performance characteristics than specific IgE alone for discriminating children with hen’s egg allergy from those who were asymptomatically sensitized.61,62 The same has not always been found with peanut,63 but perhaps this goes back to the observation that IgG4 responses to peanut proteins are modest on a quantitative basis when compared with IgG4 specific for milk or egg. Venom-specific IgG4 levels have recently been reported to be associated with protection from recurrent stings in subjects undergoing venom immunotherapy.64 Finally, a recent clinical study using a novel recombinant IgG4 antibody specific to Fel d 1 has provided additional evidence that specific IgG4 can protect against IgE-mediated allergy and is some of the best in vivo evidence that specific IgG4 can directly exert anti-inflammatory effects in humans.65
Figure 1.
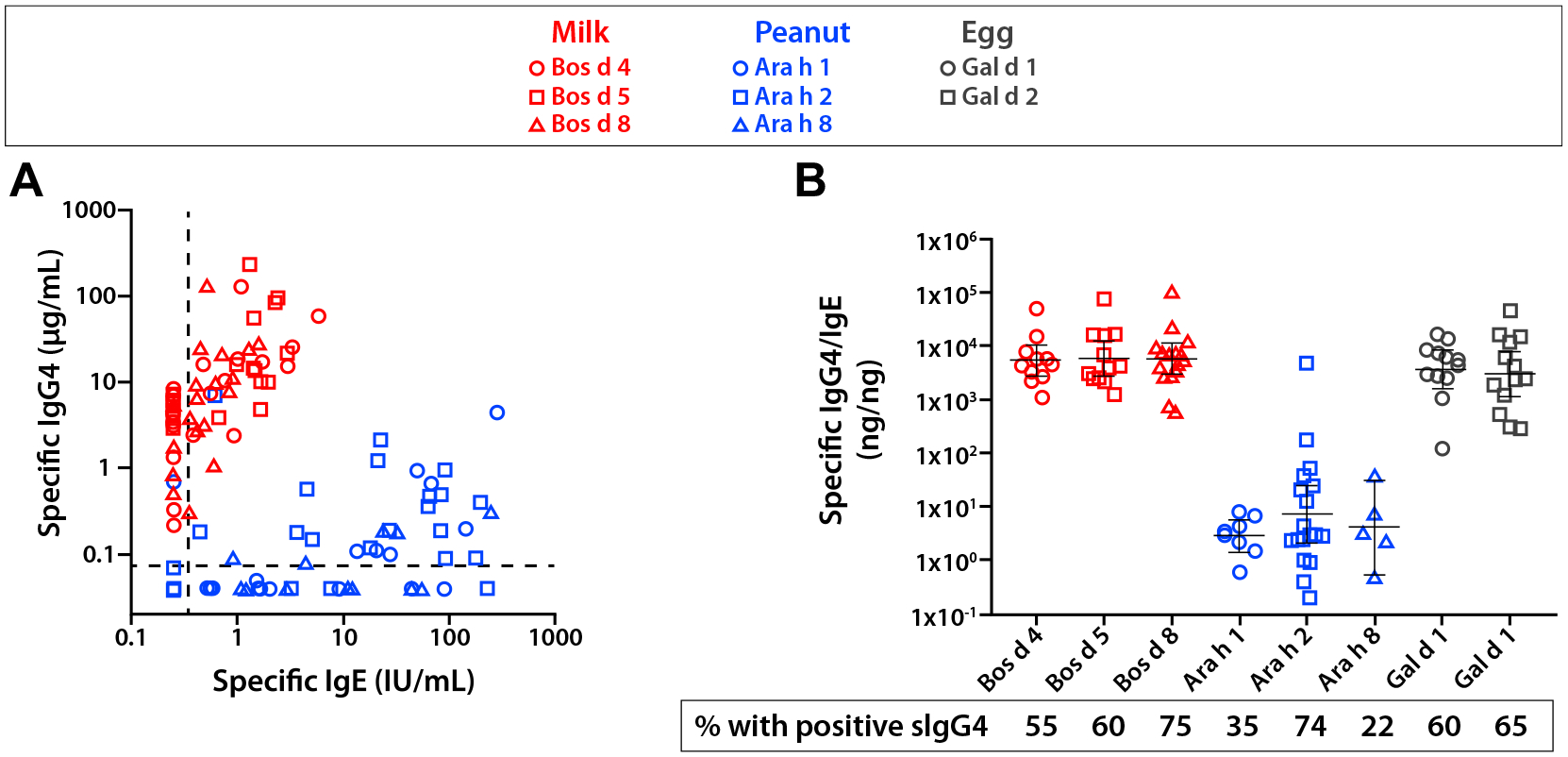
(A) Specific IgE and IgG4 responses to food components among 7-year-old children who were sensitized to milk or peanut. Where sIgE and sIgG4 were less than the detection limit, they were depicted with the values 0.25 IU/mL and 0.04 μg/mL, respectively. (B) Component-specific IgG4 to IgE ratio in sera that were positive. Figures adapted from Wilson et al, J Allergy Clin Immunol, 2018.59
Is There a Biological Rationale for Anaphylaxis Related to IgE Antibodies?
When IgE was first identified as a result of the work of Dr. Ishizaka in Denver and Johannson and Benich in Sweden,66 many theories already existed about the biological relevance of a molecule with these properties. As early as 1972, Dr. Ishizaka and colleagues spelt out a “gatekeeper hypothesis” in which IgE antibodies on mast cells in the skin or on epithelial surfaces were able to rapidly mobilize serum and cellular elements of an immune response to reduce the access of schistosomes, but also other “foreign hordes invading across skin and mucosal barriers.”67 Early investigations tried to prove that IgE antibodies were the central element of control of helminth infection. However, that may have been naïve, because the helminths that cause severe infection are the ones that have adapted to survive our attempts to control them, and it has not been possible to show that 1 element alone (such as IgE) is protective. In 1974, James Stebbings, Jr. proposed a role for IgE in relation to arthropods: “The immediate hypersensitivity reactions would seem to be proper defenses against.flying insects, the mosquitoes, midges, black flies, and also ticks, chiggers, and mites.”68 In 1991, Dr. Margie Profet proposed a “toxin hypothesis of allergy,” which stated that a shared feature of allergens is their capacity to be recognized by the immune system either directly as toxins or as antigens which are often associated with toxins.69 Thus, she proposed that the mast cell system including IgE played a major role in mitigating the effect of toxin exposure by releasing a wide variety of proteases and other mediators. The theory related to venoms of many types, including stinging insects and snake venom, but also more broadly other environmental toxins to which we are exposed at the skin and mucosal interface. In many ways this could be understood to fit within the danger model of immunity that was also put forth in the early 1990s by Polly Matzinger.70 The allergy toxin hypothesis was appealing in that the “weep and sweep” reflex that underlies many allergic symptoms (eg, mucus production, tearing, cough, sneeze, vomit, diarrhea) could be a biological response that evolved to expunge exogenous toxic substances.
Over the past decade increasing evidence from both experimental models and human studies supports many of these early ideas. Steve Galli and his colleagues62 have presented extensive evidence, primarily from animal models, that mast cells and IgE can play an important protective role against a variety of toxins from bees, vipers, gila monsters, and so forth.71 This includes IgE specific to toxins such as Russell viper venom and phospholipase A2 in bee venom, as also reported by Palm et al.72–74 The emergence of the α-Gal syndrome has helped to highlight the link between tick bites and IgE induction,75,76 although research from the 1980s and 1990s had already shown that IgE and mast cells were part of the immune response that protected guinea pigs or mice from repeat tick infestation.77 The significance of IgE in evolutionary terms is further highlighted by the fact that comparative studies of mammals have revealed that, despite significant variation in other antibody isotypes, IgE is always present as a single functional gene across the class mammalia.78 A last point relates to the idea that an additional feature of the allergic response could be the acquisition of learned avoidance behavior.79 A subject who experiences a “toxic” exposure that leads to IgE sensitization might also be expected to learn to avoid that type of exposure in the future. Data from animal models support the idea that interleukin (IL)-4 and IL-13, which are also the cytokines that are critical to promoting IgE class-switch, play a role in memory acquisition.80,81 This at least raises the question of whether novel drugs that intervene in allergic pathways (eg, the monoclonal antibody dupilumab, which recognizes IL-4Rα), although welcome additions to the armamentarium used to treated severe allergic disease, could have unforeseen consequences.
Conclusion
Anaphylaxis is best understood as an overexuberant manifestation of a form of immunity that evolved as an adaptive response to rapidly mobilize proteases and inflammatory mediators to contend with immediate threats from the environment. In this system, which is integral to allergic defense, IgE acts as a sensor for environmental toxins that helps to confer sensitivity, specificity, and memory to the responses of the mast cell, an innate immune cell that resides in the skin in mucosal tissue and long predates the development of adaptive immunity (Fig 2). The apparent increase in the prevalence of anaphylaxis in industrialized societies strongly suggests that alterations in hygiene or disruptions in microbial ecology have led to a dysregulation in the mast cell-IgE axis, particularly in regard to food allergy. Ongoing investigations into the connection with microbial flora are expected to reveal novel insights into the pathophysiology of anaphylaxis and could well lead to new approaches for prevention and treatment. Equally, further study into the dynamic interplay between IgG4 and IgE will have implications for understanding factors that favor tolerance and possibly could also have therapeutic implications. The recognition of the α-Gal syndrome is important for several reasons: first, it provides an explanation for a large number of anaphylaxis cases that were previously regarded as idiopathic, and second, it serves to establish that severe reactions related to IgE do not always involve an immediate time course or protein epitope. Over the last few years, several major developments have also established the principle that the skin is a major site of IgE sensitization (Fig 3) and that symptoms related to oral food exposure can result from a primary exposure through a different route. Good examples include 1) exposure of skin that is slightly or severely inflamed to peanut, milk, or egg in early childhood; 2) tick bites, which can penetrate normal skin at any age; and 3) pollen exposure at the nasal passages, which can form the basis of the oral allergy syndrome. Finally, emerging work suggests that IgE sensitization that occurs through the skin may have ramifications that extend beyond diseases and symptoms that are traditionally described as “allergic.”
Figure 2.
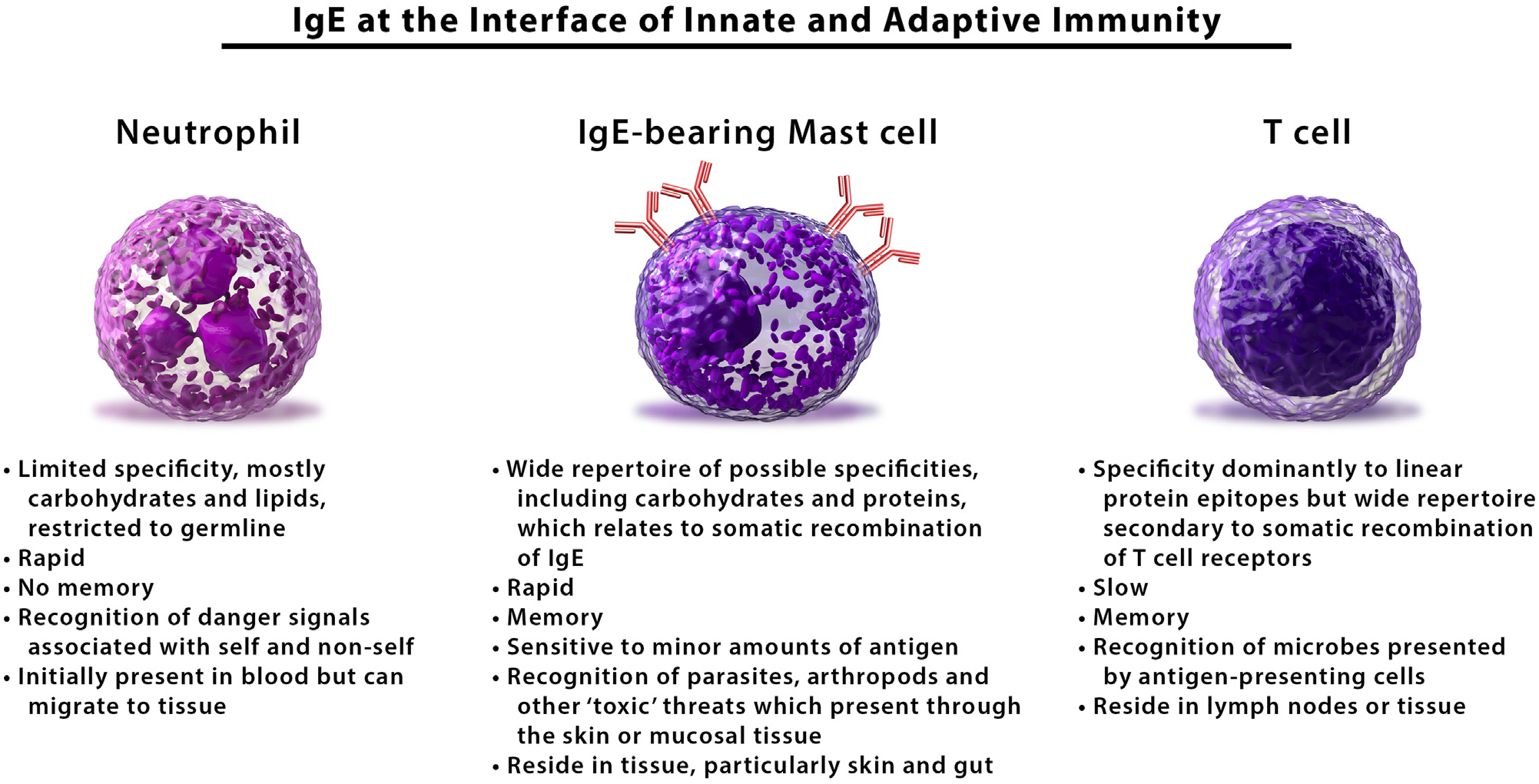
The IgE-mast cell axis is a central feature of allergic immunity: mast cells that are “armed” with IgE share features with both innate (eg, neutrophil) and adaptive (eg, T cell) immune cells. This form of immunity represents an adaptive response to combat parasites, arthropods, or other exogenous “toxic” substances that present in the skin or mucosal tissue. Anaphylaxis, including anaphylactic shock, represents an extreme manifestation of this response.
Figure 3.
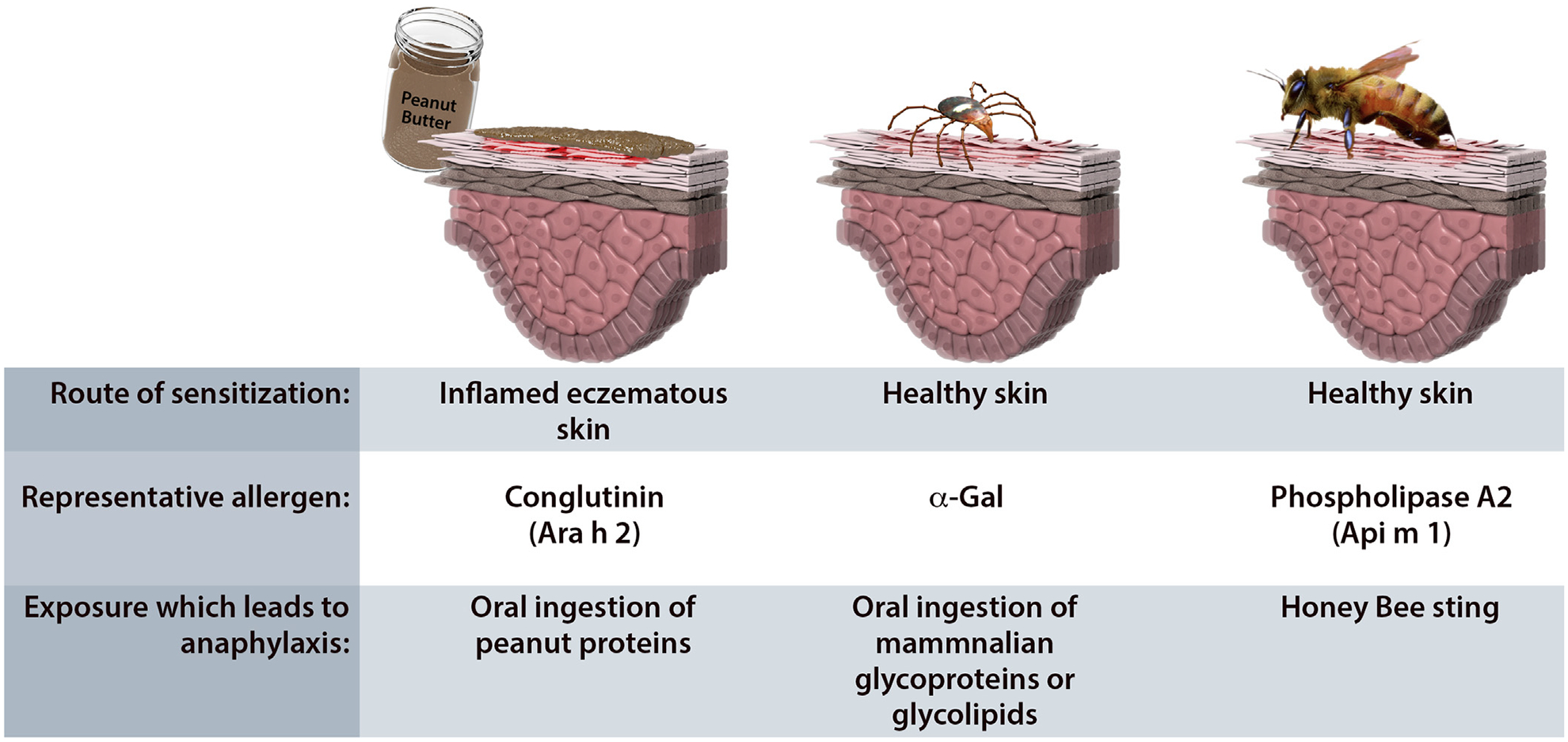
Three forms of allergic sensitization that occur via the skin. (A) Peanut allergy often develops in children with underlying eczema who are exposed to peanut allergens via the skin. Subsequent oral exposure can lead to allergic reactions. (B) Sensitization to α-Gal is caused by tick bites and generally occurs in subjects without eczema or other inflammatory skin diseases. This reflects that ticks can have a prolonged feed while attached to skin and also have salivary factors that act as Th-2-promoting adjuvants. (C) Venomous insect stings lead to sensitization and also can lead to allergic reactions on subsequent exposures via the skin.
Disclosures:
TPM has a patent on an IgE assay to α-Gal and has received assay support from Phadia/Thermo-Fisher; JW has received research support from Phadia/Thermo-Fisher.
Funding Sources: TPM is supported by NIH grant R37-AI-20565.
References
- 1.Cohen SG, Zelaya-Quesada M. Portier, Richet, and the discovery of anaphylaxis: a centennial. J Allergy Clin Immunol. 2002;110(2):331–336. [DOI] [PubMed] [Google Scholar]
- 2.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016;137(6):1674–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman P Mechanisms of anaphylaxis beyond classically mediated antigen-and IgE-induced events. Ann Allergy Asthma Immunol. 2017;118(3):246–248. [DOI] [PubMed] [Google Scholar]
- 4.Munoz-Cano R, Picado C, Valero A, Bartra J. Mechanisms of anaphylaxis beyond IgE. J Investig Allergol Clin Immunol. 2016;26(2):73–82. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis–a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115(5):341–384. [DOI] [PubMed] [Google Scholar]
- 6.Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. 2017;140(2):321–333. [DOI] [PubMed] [Google Scholar]
- 7.Greenhawt M, Gupta RS, Meadows JA, et al. Guiding principles for the recognition, diagnosis, and management of infants with anaphylaxis: An expert panel consensus. J Allergy Clin Immunol Pract. 2019;7(4):1148–1156.e5. [DOI] [PubMed] [Google Scholar]
- 8.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. [DOI] [PubMed] [Google Scholar]
- 9.Yu JE, Lin RY. The epidemiology of anaphylaxis. Clin Rev Allergy Immunol. 2018; 54(3):366–374. [DOI] [PubMed] [Google Scholar]
- 10.Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140(2):335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez-Rodriguez TW, Garcia-Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. 2018;11:121–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman P, Camargo CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006; 97(5):596–602. [DOI] [PubMed] [Google Scholar]
- 13.Panesar SS, Javad S, de Silva D, et al. The epidemiology of anaphylaxis in Europe: a systematic review. Allergy. 2013;68(11):1353–1361. [DOI] [PubMed] [Google Scholar]
- 14.Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461–467. [DOI] [PubMed] [Google Scholar]
- 15.Tejedor Alonso MA, Moro Moro M, Mugica Garcia MV. Epidemiology of anaphylaxis. Clin Exp Allergy. 2015;45(6):1027–1039. [DOI] [PubMed] [Google Scholar]
- 16.Sicherer SH, Simons FER, Section on Allergy and Immunology. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017;139(3). [DOI] [PubMed] [Google Scholar]
- 17.Chooniedass R, Temple B, Becker A. Epinephrine use for anaphylaxis: Too seldom, too late: Current practices and guidelines in health care. Ann Allergy Asthma Immunol. 2017;119(2):108–110. [DOI] [PubMed] [Google Scholar]
- 18.Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58. [DOI] [PubMed] [Google Scholar]
- 19.Platts-Mills TA. The allergy epidemics: 1870–2010. J Allergy Clin Immunol. 2015;136(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du Toit G, Sayre PH, Roberts G, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med. 2016;374(15):1435–1443. [DOI] [PubMed] [Google Scholar]
- 21.Lack G Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008; 121(6):1331–1336. [DOI] [PubMed] [Google Scholar]
- 22.Palmer DJ, Sullivan TR, Gold MS, Prescott SL, Makrides M. Randomized controlled trial of early regular egg intake to prevent egg allergy. J Allergy Clin Immunol. 2017;139(5):1600–1607. [DOI] [PubMed] [Google Scholar]
- 23.Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18(10):1076–1083. [DOI] [PubMed] [Google Scholar]
- 24.Fukutomi Y, Itagaki Y, Taniguchi M, et al. Rhinoconjunctival sensitization to hydrolyzed wheat protein in facial soap can induce wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2011;127(2):531–533. e1–3. [DOI] [PubMed] [Google Scholar]
- 25.Brockow K, Kneissl D, Valentini L, et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2015;135(4):977–984.e4. [DOI] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finding diversity in the microbiome. Nat Med. 2019;25(6):863–863. [DOI] [PubMed] [Google Scholar]
- 28.Stephen-Victor E, Chatila TA. Regulation of oral immune tolerance by the microbiome in food allergy. Curr Opin Immunol. 2019;60:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iweala OI, Nagler CR. The microbiome and food allergy. Annu Rev Immunol. 2019;37:377–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilg H, Moschen AR. Food, immunity, and the microbiome. Gastroenterology. 2015;148(6):1107–1119. [DOI] [PubMed] [Google Scholar]
- 31.Wypych TP, Marsland BJ. Antibiotics as instigators of microbial dysbiosis: implications for asthma and allergy. Trends Immunol. 2018;39(9):697–711. [DOI] [PubMed] [Google Scholar]
- 32.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713. [DOI] [PubMed] [Google Scholar]
- 33.Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W, Ho HE, Bunyavanich S. The gut microbiome in food allergy. Ann Allergy Asthma Immunol. 2019;122(3):276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feehley T, Plunkett CH, Bao R, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med. 2019;25(3):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel-Gadir A, Stephen-Victor E, Gerber GK, et al. Microbiota therapy acts via a regulatory T cell MyD88/RORgammat pathway to suppress food allergy. Nat Med. 2019;25(7):1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noval Rivas M, Burton OT, Wise P, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131(1):201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Ann Allergy Asthma Immunol. 2018; 121(5):594–597. [DOI] [PubMed] [Google Scholar]
- 39.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364(1):8–18. [DOI] [PubMed] [Google Scholar]
- 40.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2): 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson JM, Schuyler AJ, Workman L, et al. Investigation into the alpha-Gal syndrome: characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract. 2019;7(7):2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Commins SP, James HR, Stevens W, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014;134(1):108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galili U Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehlich J, Fischer J, Hilger C, et al. Basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization. J Allergy Clin Immunol. 2019;143(1):182–189. [DOI] [PubMed] [Google Scholar]
- 46.Labbe SM, Grenier-Larouche T, Croteau E, et al. Organ-specific dietary fatty acid uptake in humans using positron emission tomography coupled to computed tomography. Am J Physiol Endocrinol Metab. 2011;300(3):E445–E453. [DOI] [PubMed] [Google Scholar]
- 47.Galili U, Basbaum CB, Shohet SB, Buehler J, Macher BA. Identification of erythrocyte Gal alpha 1–3Gal glycosphingolipids with a mouse monoclonal antibody, Gal-13. J Biol Chem. 1987;262(10):4683–4688. [PubMed] [Google Scholar]
- 48.Wilson JM, Platts-Mills TAE. The oligosaccharide galactose-α−1,3-galactose and the α-Gal syndrome: insights from an epitope that is causal in IgE-mediated immediate and delayed anaphylaxis. EMJ Allergy Immunol. 2018;3(1):89–98. [Google Scholar]
- 49.Roman-Carrasco P, Lieder B, Somoza V, et al. Only aGal bound to lipids, but not to proteins, is transported across enterocytes as an IgE reactive molecule that can induce effector cell activation. Allergy. 2019;74(10):1956–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson JM, Nguyen AT, Schuyler AJ, et al. IgE to the mammalian oligosaccharide galactose-α−1,3-galactose is associated with increased atheroma volume and plaques with unstable characteristics. Arterioscler Thromb Vasc Biol. 2018; 38(7):1665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer J, Lupberger E, Hebsaker J, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy. 2017;72(10): 1540–1547. [DOI] [PubMed] [Google Scholar]
- 52.Aalberse RC, Platts-Mills TA, Rispens T. The developmental history of IgE and IgG4 antibodies in relation to atopy, eosinophilic esophagitis, and the modified TH2 response. Curr Allergy Asthma Rep. 2016;16(6):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103(6):1173–1179. [DOI] [PubMed] [Google Scholar]
- 54.Erwin EA, Tripathi A, Ogbogu PU, et al. IgE antibody detection and component analysis in patients with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2015;3(6):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cianferoni A, Shuker M, Brown-Whitehorn T, Hunter H, Venter C, Spergel JM. Food avoidance strategies in eosinophilic oesophagitis. Clin Exp Allergy. 2019; 49(3):269–284. [DOI] [PubMed] [Google Scholar]
- 56.Clayton F, Fang JC, Gleich GJ, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3): 602–609. [DOI] [PubMed] [Google Scholar]
- 57.Wright BL, Kulis M, Guo R, et al. Food-specific IgG4 is associated with eosinophilic esophagitis. J Allergy Clin Immunol. 2016;138(4):1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuyler AJ, Wilson JM, Tripathi A, et al. Specific IgG4 antibodies to cow’s milk proteins in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson JM, Workman L, Schuyler AJ, et al. Allergen sensitization in a birth cohort at midchildhood: focus on food component IgE and IgG4 responses. J Allergy Clin Immunol. 2018;141(1):419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One. 2013;8(2), e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caubet JC, Bencharitiwong R, Moshier E, Godbold JH, Sampson HA, Nowak-Wegrzyn A. Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy. J Allergy Clin Immunol. 2012;129(3):739–747. [DOI] [PubMed] [Google Scholar]
- 62.Okamoto S, Taniuchi S, Sudo K, et al. Predictive value of IgE/IgG4 antibody ratio in children with egg allergy. Allergy Asthma Clin Immunol. 2012;8(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Datema MR, Eller E, Zwinderman AH, et al. Ratios of specific IgG4 over IgE antibodies do not improve prediction of peanut allergy nor of its severity compared to specific IgE alone. Clin Exp Allergy. 2019;49(2): 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jarkvist J, Salehi C, Akin C, Gulen T. Venom immunotherapy in patients with clonal mast cell disorders: IgG4 correlates with protection. Allergy. 2019. [DOI] [PubMed] [Google Scholar]
- 65.Orengo JM, Radin AR, Kamat V, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun. 2018; 9(1):1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Platts-Mills TA, Heymann PW, Commins SP, Woodfolk JA. The discovery of IgE 50 years later. Ann Allergy Asthma Immunol. 2016;116(3):179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinberg P, Ishizaka K, Norman PS. Possible role of Ige-mediated reaction in immunity. J Allergy Clin Immun. 1974;54(6):359–366. [Google Scholar]
- 68.Stebbings JH Jr. Immediate hypersensitivity: a defense against arthropods? Perspect Biol Med. 1974;17(2):233–239. [DOI] [PubMed] [Google Scholar]
- 69.Profet M The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66(1):23–62. [DOI] [PubMed] [Google Scholar]
- 70.Matzinger P The danger model: a renewed sense of self. Science. 2002; 296(5566):301–305. [DOI] [PubMed] [Google Scholar]
- 71.Galli SJ, Starkl P, Marichal T, Tsai M. Mast cells and IgE in defense against venoms: possible “good side” of allergy? Allergol Int. 2016;65(1):3–15. [DOI] [PubMed] [Google Scholar]
- 72.Marichal T, Starkl P, Reber LL, et al. A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity. 2013;39(5):963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39(5): 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Starkl P, Marichal T, Gaudenzio N, et al. IgE antibodies, FcepsilonRIalpha, and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol. 2016;137(1):246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chandrasekhar JL, Cox KM, Loo WM, Qiao H, Tung KS, Erickson LD. Cutaneous exposure to clinically relevant lone star ticks promotes IgE production and hypersensitivity through CD4(+) T cell- and MyD88-dependent pathways in mice. J Immunol. 2019;203(4):813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuda H, Watanabe N, Kiso Y, et al. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornis ticks in mice. J Immunol. 1990;144(1):259–262. [PubMed] [Google Scholar]
- 78.Hellman LT, Akula S, Thorpe M, Fu Z. Tracing the origins of IgE, mast cells, and allergies by studies of wild animals. Front Immunol. 2017;8:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012; 484(7395):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Derecki NC, Cardani AN, Yang CH, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brombacher TM, Nono JK, De Gouveia KS, et al. IL-13-mediated regulation of learning and memory. J Immunol. 2017;198(7):2681–2688. [DOI] [PubMed] [Google Scholar]


