Abstract
AIM
Hypoxic-ischemic encephalopathy (HIE) is associated with damage to deep gray matter; however, white matter involvement has become recognized. This study explored differences between patients and clinical controls on diffusion tensor imaging (DTI), and relationships between DTI and neurodevelopmental outcomes.
METHOD
DTI was obtained for 31 neonates afterHIE treated with therapeutic hypothermia and 10 clinical controls. A sub-group of patients with HIE (N=14) had neurodevelopmental outcomes correlated with DTI scalars.
RESULTS
Group differences in DTI scalars were observed in the putamen, anterior and posterior centrum semiovale, and the splenium of the corpus callosum. Differences in these ROIs were correlated with neurodevelopmental outcomes between ages 20–32 months.
CONCLUSION
Therapeutic hypothermia may not be a complete intervention for HIE, as neonatal white matter changes may continue to be evident, but further research is warranted. Patterns of white matter change on neonatal DTI correlated with neurodevelopmental outcomes in this exploratory pilot study.
Keywords: Hypoxic-ischemic encephalopathy, diffusion tensor imaging, infant, neurodevelopment, outcome
Perinatal hypoxic ischemic injury (HIE) remains a leading cause of neonatal mortality and morbidity1. Therapeutic hypothermia (i.e., cooling) is the standard treatment to mitigate secondary brain injury after neonatal HIE2; however, approximately 45% of neonates die or have significant neurodevelopmental impairment.3,4 Early prospective identification of neonates who will have unfavorable outcomes is important, but remains challenging given few reliable predictors.5
Prior to cooling era, conventional neuroimaging techniques used to evaluate acute HIE revealed predominant involvement of the deep gray matter (especially putamen and thalamus), peri-rolandic cortex and subcortical white matter, with brainstem tegmentum involvement in severe cases. 6 Correlations between neuroimaging findings and neurodevelopmental outcomes have been documented.7 Abnormal signal intensity in the bilateral posterior limb of the internal capsule (PLIC) is a predictor unfavorable neurodevelopmental outcomes.8 Quantitative neuroimaging techniques, such as diffusion tensor imaging (DTI), in children ages 12 to 18 months have demonstrated microstructural white matter abnormalities following HIE in the right centrum semiovale, splenium of the corpus callosum, anterior and posterior limbs of the internal capsules, external capsules, fornix, cingulum, cerebral peduncles, optic radiations, and inferior longitudinal fasciculus.9 White matter microstructure changes in these anatomical brain regions correlated with worse performance on measures of gross motor function and visual-motor integration.9
Cooling has decreased morbidity following moderate perinatal HIE and improved neurodevelopmental outcomes; however, neuroimaging findings prior to cooling may not be automatically transferred.10, 11 T2-hyperintense signal in the white matter is often seen as neuroradiological evidence of HIE during clinical imaging reads and its prognostic significance is unclear. Few studies have utilized neonatal DTI to evaluate the white matter following HIE post-cooling.9,12,13,14 Massaro et al. reported correlations between DTI scalars (i.e., fractional anisotropy or FA) and motor performance in term neonates following HIE post-cooling; however, there is a gap in the literature regarding DTI measurements and early domain-specific neurodevelopmental outcomes.14 Tursor et al. (2012) found lower FA values in the centrum semiovale, corpus callosum, PLIC, optic radiations, and inferior longitudinal fasciculus using DTI with tract-based spatial statistics. Additionally, these DTI findings were correlated with performance on all component subscales of the Griffiths Mental Development Scale, Revised between ages 12–24 months.
The goals of this exploratory pilot study were two-part. The first goal was to use DTI measurements to quantify white matter changes, including changes in the white matter tracts within deep gray matter structures, among term neonates following HIE post-cooling compared to clinical neonatal controls. Secondly, the relationship between quantitative DTI measurements and 1) qualitative neuroimaging evaluation of white matter, and 2) domain-specific neurodevelopmental outcomes were examined.
METHOD
The Johns Hopkins Institutional Review Board (IRB) approved this study and the parents/guardians of all participants provided written informed consent.
Study Population
Inclusion criteria for this pilot study were 1) moderate to severe perinatal HIE post-cooling and 2) availability of DTI data without artifacts for high-quality post-processing. The standard protocol for therapeutic hypothermia treatment included birth ≥ 35 weeks gestation, clinical evidence of HIE, and cooling within 6 hours of birth with body temperature maintained at 33.5 degrees C for 72 hours, followed by 6 hours of rewarming. Exclusion criteria for participation included 1) clinically determined diagnosis of partial prolonged HIE, 2) additional diagnoses of congenital anomalies, known genetic disorders, focal stroke, neoplasm, or maternal HIV exposure, and 3 ) birth weight of less than 1800 grams. The specific clinical factor used to determine partial prolonged HIE included an absence of a sentinel event during or immediately before labor and delivery (i.e., complete abruption, cord accident, shoulder dystocia), and a history of decreased fetal movement prior to onset of labor.
Patients were prospectively enrolled in an ongoing radiology clinical study that includes all neonates with HIE treated with hypothermia from January 1, 2010 to December 30, 2013. As such, the sample was a convenience sample with available data from this ongoing clinical research study. Age-matched neonatal clinical controls for this study were selected from a pediatric neuroimaging database based on: 1) MRI between 1 and 16 days of life with raw data without artifacts, 2) age at birth between 36 and 41 weeks gestation, 3) normal brain anatomy/normal brain imaging, 4) absence of neurological disorders.
Acquisition of MRI data
Brain MRI studies were performed on a 1.5 clinical MR scanner (Siemens, Erlangen, Germany) using standard departmental neonatal brain MRI protocol including isotropic three dimensional (3D)-T1-weighted images, axial T2-weighted images, axial susceptibility weighted imaging, and a single shot spin echo, echo planar axial DTI sequence with diffusion gradients along 20 non-collinear directions. An effective high b-value of 1000 s/mm2 was used for each of the 20 diffusion-encoding directions. An additional measurement without diffusion weighting (b=0 s/mm2) was performed. DTI data was acquired using: repetition time (TR)=8500 ms, echo time (TE)=86 ms, slice thickness=2.0 mm, field-of-view (FOV)=240×240 mm, and matrix size=192×192. Parallel imaging iPAT=2 with Generalized Auto-Calibrating Partial Parallel Acquisition (GRAPPA) reconstruction was used. Acquisition was repeated twice to enhance signal-to-noise ratio. Acquisition time for the DTI sequence was 6 minutes and 24 seconds and it was typically performed using an MRI compatible incubator (LMT, Germany). The DTI acquisition protocol was assessed weekly and was stable throughout the study period.
All MRI studies of neonates were obtained while the neonates were in quiet sleep and none of the neonates received any form of sedation. Prior to MRI acquisition, neonates were fed and swaddled. Once the neonates were in quiet sleep, they were placed in an MRI compatible basinet and the MRI studies were performed.
Quantitative DTI
DTI raw data were post-processed using DtiStudio, DiffeoMap and RoiEditor software (free available at www.MriStudio.org). Raw diffusion-weighted imaging (DWI) images were co-registered to one of the DWI images and corrected for eddy current and subject motion using a 12-mode affine transformation. FA (fractional anisotropy), eigenvectors, color-coded FA, trace of diffusion, and axial (AD) and radial (RD) diffusivity maps were generated. After rigid transformation to a neonatal template for adjustment of position and rotation of images, regions of interest (ROIs) were manually drawn by E.N. as large as possible on the bilateral putamen, thalamus, anterior and posterior centrum semiovale, and the posterior limb of the internal capsule (PLIC) (Supplemental Figure 1). Proper placement of the ROIs was confirmed by A.P. and, axial trace of diffusion and axial FA maps were used to identify each structure and compared to the brain DTI atlas by Oishi et al.15 For each analyzed structure, three different ROIs on contiguous MR slices were placed and the average FA, trace of diffusion, AD and RD values were calculated. Mean diffusivity (MD) values were calculated using the following formula: MD=Trace of diffusion/3.
All available MRI sequences were systematically evaluated for focal and diffuse signal abnormalities. Left and right hemisphere focal abnormalities identified on single sequence were correlated with matching sequences to better characterize findings. White matter myelination/maturation was evaluated for T1 and T2 weighted signal and susceptibility weighted sequences were used to differentiate between possible hemorrhages and calcifications.
Neurodevelopmental outcome
All neonates with HIE post-cooling who were English speaking and not in the care of the state were recruited for participation as part of a larger IRB approved prospective longitudinal neurodevelopmental follow-up study at the Kennedy Krieger Institute. For the current study, only a subgroup of children who had already received formal research follow-up using the Mullen Scales of Early Learning were included. Other children enrolled in this longitudinal study only consented to the use of their clinical neurodevelopmental outcomes measured via the Capute Scales, and were not included given the psychometric differences between the Mullen Scales of Early Learning versus the Capute Scales (i.e., a norm-based neurodevelopmental assessment measure versus a criterion-based neurodevelopmental screening measure). Clinical controls were not available to be contacted for neurodevelopmental follow-up, as use of their imaging data was collected as part of the imaging study only.
Neurodevelopmental outcomes were assessed using the Mullen Scales of Early Learning (MSEL) between ages of 20–32 months in a subgroup of patients. The Mullen Scales of Early Learning provides a comprehensive norm-based measurement of neurodevelopment with an overall level of cognitive development based on four subscales: visual reception, fine motor, receptive language, and expressive language, and a supplementary scale of gross motor scale.16 Scores for each of the Mullen Scales of Early Learning subscales are T-scores (M=50, SD=10); therefore, scores between 40 and 60 are considered to fall within the average range. The Early Learning Composite Score, which is a summary of overall performance, is a standard score (M=100, SD=15); therefore, scores between 85 and 115 are considered to fall within the average range.
Data Analysis
Sample characteristics, including gestational age at birth, sex, and age at the time of MRI, were obtained using descriptive analyses. Umbilical cord pH and base deficit are reported for the patient group. Group differences were examined using Chi Square analysis, Fisher Exact Test, or independent group t-tests and significance set at p≤.05.
Differences between FA, MD, RD, and AD for the left and right ROIs were examined via a series of paired t-tests in both groups. Group differences in the selected ROIs were analyzed using multiple independent samples t-tests, and significance was set at p≤.05 given that this study was exploratory and pilot in nature. The relationships between DTI scalars and the ROIs selected for this study and neurodevelopmental outcomes were examined using a Spearman Rho correlation analysis, given the small size of the subsample with neurodevelopmental outcomes. Neurodevelopmental variables included the composite standard score, and all four subscales, and significance was again set at p≤.05 given the exploratory nature of this study.
RESULTS
Sample Characteristics
Thirty-one neonates with HIE post-cooling and ten controls were included in this study (Table 1). A total of 34 neonates were initially eligible for the study; however, 3 neonates did not have a sentinel event consistent with acute HIE and were excluded. There were no significant differences between patients and controls based on sex, χ2(1, N = 41)=1.39, p =.289; gestational age at birth, t(39)=−.380, p=.706; or age at MRI, t(40)=−.009, p=.993. Patients had significantly lower APGAR scores at 1, t(38)=−3.69, p=.001, and 5 minutes, t(38)=−3.54, p=.001. Most patients (N=26) and clinical controls (N=7) were treated prophylactically for suspected sepsis based on clinical protocol; however, treatment was discontinued upon receipt of negative blood culture. One patient and two clinical controls had confirmed positive cultures for sepsis. No clinical controls received anti-epileptic drugs (AEDs), and fifteen patients received AEDs. Brain MRIs were obtained for clinical controls to rule out central nervous system infections, central apnea or bradycardia, or neurocutaneous disorders (e.g., tuberous sclerosis).
Table 1.
Demographic and Clinical Characteristics of Neonatal Patients Diagnosed With HIE and Clinical Controls.a
| Patients diagnosed with HIE (n = 31) | Clinical controls (n = 10) | |
|---|---|---|
| Male, n | 19 | 4 |
| Gestational age, wk | 38.90 (1.71) | 39.13 (1.67) |
| Cord blood pH | 7.01 (.16) | n/a |
| Base deficit | 15.92 (6.08) | n/a |
| Apgar score at 1 min, median (IQR) | 2.00 (1.00) | 5.00 (5.00) |
| Apgar score at 5 min, median (IQR) | 5 (4.00) | 9.00 (2.50) |
| Age at MRI, d | 8.48 (3.01) | 8.50 (5.48) |
| Suspected sepsis, n | 26 | 7.78 (1.79) |
| Culture positive sepsis, n | 1 | 2 |
| AED administration | 15 | 0 |
Abbreviations: AED, antiepileptic drug: HIE, hypoxic-ischemic encephalopathy: IQR, interquartile range; MRI, magnetic resonance imaging.
Data presented in the patients diagnosed with HIE and controls include the mean (standard deviation) unless otherwise specified
Developmentally, the mean composite score on the Mullen Scales of Early Learning (i.e., Early Learning Composite) was within the average range for the overall sample (M = 95.29, SD = 19.03); however, there was a great deal of variability in scores with a minimum standard score of 67 and a maximum standard score of 123. The T-scores across subscales of the Mullen Scales of Early Learning were also within the average range for the sub-sample of patients who had neurodevelopmental testing (i.e., Gross Motor T = 48.71, SD = 10.83; Fine Motor T = 45.86, SD = 10.91; Visual Reception T = 44.71, SD = 15.01; Receptive Language T = 52.50, SD = 8.74; Expressive Language T = 46.50, SD = 10.57). One patient had a diagnosis of cerebral palsy at the time the neurodevelopmental outcomes were collected.
The mean values for most DTI scalars were calculated and used in analyses. DTI scalars in the left and right putamen and PLIC were significantly different; therefore, left and right scalars for these ROIs were used in all analyses.
Group Differences in DTI Scalars by ROIs
The DTI scalars for each group in selected ROIs are summarized in Table 2. No correction for multiple comparisons was performed given the exploratory nature of this pilot study. There were no significant differences between patients and controls for age at time of MRI (t = .009, p=.993). FA values in the right (t=1.994, p=.053) and left putamen (t=2.303, p=.027) were significantly lower in patients with HIE compared to clinical controls, but there were not any statistically significant differences for other DTI scalars between patients and clinical controls in the putamen. Statistically significant differences in FA were also observed in the anterior CSO (t=2.011, p=.051), such that patients with HIE had lower FA values. FA values in the posterior CSO were lower and statistically significant (t=−3.003, p=.012) and MD values were higher and statistically significant (t=2.592, p=.015) in patients compared to clinical controls. RD in the posterior centrum semiovale was also significantly higher in patients (t=2.951, p=.005). There were no statistically significant group differences for AD in the posterior centrum semiovale (t=1.712, p=.095). In the splenium of the corpus callosum, FA was lower in patients compared to clinical controls, and statistically significant (t=−2.374, p=.023). RD was significantly higher in patients versus clinical controls in the splenium of the corpus callosum (t=3.168, p=.003); however, there was no statistically significant difference between patients and controls with regards to MD (t=1.748, p=.089) and AD (t=1.020, p=.314).
Table 2.
Differences in Diffusion Tensor Imaging Scalars Between 31 Neonates With Perinatal HIE Following Therapeutic Hypothermia and 10 Clinical Controls.a
| FA |
MD (× 10−3 mm/s2) |
AD (× 10−3 mm/s2) |
RD (× 10−3 mm/s2) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regions of interest | HIE | Control | Significance | HIE | Control | Sig. | HIE | Control | Sig. | HIE | Control | Sig. |
| Right putamen | 0.129 (0.031) | 0.150 (0.027) | .05* | 1.114 (0.068) | 1.113 (0.052) | .97 | 1.260 (0.073) | 1.270 (0.57) | .72 | 1.041 (0.071) | 1.019 (0.044) | .28 |
| Left putamen | 0.134 (0.026) | 0.156 (0.027) | .03* | 1.108 (0.064) | 1.097 (0.066) | .66 | 1.256 (0.065) | 1.247 (0.059) | .73 | 1.034 (0.068) | 1.002 (0.058) | .20 |
| Thalamus | 0.168 (0.031) | 0.180 (0.026) | .25 | 1.032 (0.072) | 1.046 (0.070) | .62 | 1.199 (0.076) | 1.213 (0.054) | .58 | 0.948 (0.075) | 0.954 (0.072) | .82 |
| Right PLIC | 0.466 (0.054) | 0.476 (0.040) | .59 | 1.056 (0.076) | 1.044 (0.056) | .65 | 1.659 (0.089) | 1.654 (0.092) | .88 | 0.754 (0.093) | 0.739 (0.059) | .62 |
| Left PLIC | 0.470 (0.061) | 0.467 (0.036) | .88 | 1.043 (0.080) | 1.033 (0.062) | .73 | 1.643 (0.088) | 1.623 (0.087) | .53 | 0.742 (0.104) | 0.738 (0.062) | .90 |
| Genu CC | 0.541 (0.085) | 0.544 (0.090) | .92 | 1.285 (0.130) | 1.228 (0.081) | .27 | 2.176 (0.207) | 2.120 (0.112) | .42 | 0.840 (0.146) | 0.783 (0.117) | .27 |
| Splenium CC | 0.608 (0.078) | 0.649 (0.033) | .02* | 1.198 (0.131) | 1.145 (0.059) | .23 | 2.168 (0.166) | 2.133 (0.054) | .31 | 0.713 (0.147) | 0.617 (0.047) | .00** |
| Anterior CSO | 0.152 (0.290) | 0.199 (0.047) | .05* | 1.517 (0.202) | 1.440 (0.148) | .28 | 1.747 (0.194) | 1.704 (0.130) | .52 | 1.402 (0.220) | 1.286 (0.170) | .13 |
| Posterior CSO | 0.154 (0.029) | 0.204 (0.051) | .01* | 1.608 (0.139) | 1.471 (0.166) | .01* | 1.859 (0.135) | 1.772 (0.155) | .1 | 1.483 (0.145) | 1.316 (0.188) | .01** |
Abbreviations: AD, axial diffusivity; CC, corpus callosum; CSO, centrum semiovale; FA, fractional anisotropy; HIE, hypoxic-ischemic encephalopathy; MD, mean diffusivity; PLIC, posterior limb of the internal capsule; RD, radial diffusivity; Sig., significance.
Data presented In the HIE and control columns include the mean (standard deviation). Boldface Indicates statistical significance.
P < .05
P < .01.
Correlation between DTI findings and neurodevelopmental outcome
The relationship between DTI scalars in the putamen, splenium of the corpus callosum, anterior centrum semiovale, and posterior semiovale and neurodevelopmental outcomes were examined for a subgroup of patients who returned for research evaluations (N=14; Table 4). The characteristics of these patients and differences between those returning for neurodevelopmental follow-up versus those who did not return are presented in Table 3. The relationship between ROIs not found to be significantly different from clinical controls and the neurodevelopmental outcomes were also examined, and these results are summarized in Supplementary Table 1.
Table 4.
Spearman Rho Correlations between the MSEL Domains and Diffusion Tensor Imaging Scalars in the Putamen, Anterior Centrum Semiovale, Posterior Centrum Semiovale, and Splenium of the Corpus Callosum (n = 14).
| MSEL Domain |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM |
FM |
VR |
RL |
EL |
Composite |
|||||||
| Regions of interest | ρ | Sig. | ρ | Sig. | ρ | Sig. | ρ | Sig. | ρ | Sig. | ρ | Sig. |
| Right putamen FA | −0.044 | .88 | −0.089 | .76 | 0.127 | .67 | 0.023 | .94 | 0.032 | .91 | −0.046 | .88 |
| Right putamen MD | −0.405 | .15 | 0.205 | .48 | 0.101 | .73 | 0.236 | .42 | 0.294 | .31 | 0.246 | .40 |
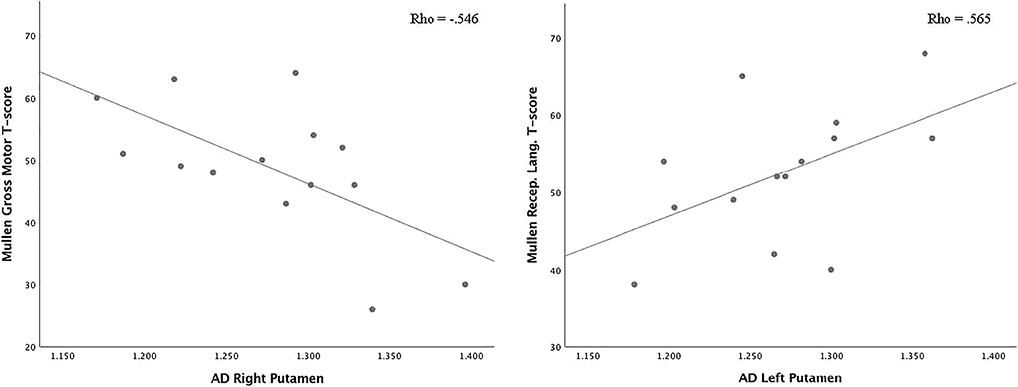
| Right putamen AD | −0.546 | .04* | 0.077 | .79 | 0.079 | .79 | 0.302 | .29 | 0.206 | .48 | 0.13 | .66 |
| Right putamen RD | −0.339 | .24 | 0.209 | .47 | 0.084 | .78 | 0.218 | .45 | 0.25 | .39 | 0.231 | .43 |
| Left putamen FA | 0.024 | .94 | −0.121 | .68 | 0.009 | .98 | 0.013 | .96 | −0.12 | .68 | −0.143 | .63 |
| Left putamen MD | −0.455 | .10 | 0.275 | .34 | 0.159 | .59 | 0.448 | .11 | 0.427 | .13 | 0.354 | .21 |
| Left putamen AD | −0.444 | .11 | 0.289 | .32 | 0.185 | .53 | 0.565 | .04* | 0.413 | .14 | 0.348 | .22 |
| Left putamen RD | −0.495 | .07 | 0.229 | .43 | 0.101 | .73 | 0.401 | .16 | 0.382 | .18 | 0.312 | .28 |
| SCC FA | 0.108 | .71 | −0.26 | .37 | −0.009 | .98 | −0.082 | .78 | −0.22 | .45 | −0.141 | .63 |
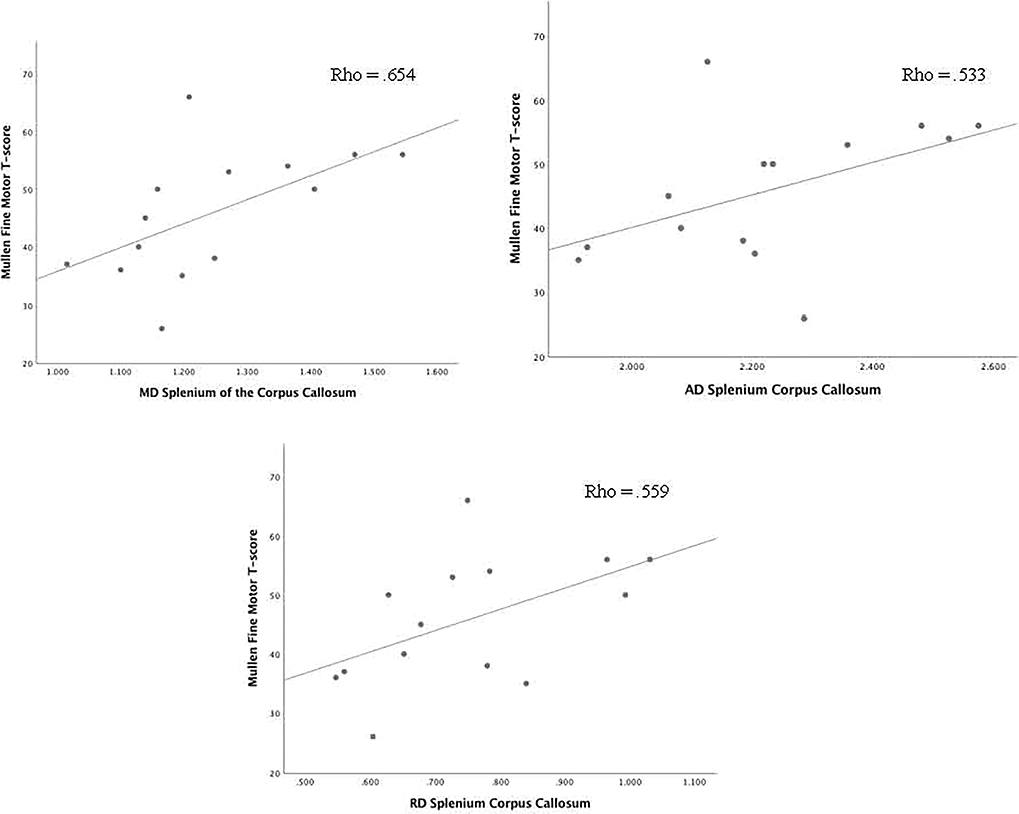
| SCC MD | 0.062 | .83 | 0.654 | .01* | 0.317 | .27 | 0.362 | .20 | 0.444 | .11 | 0.473 | .09 |
| SCC AD | 0.117 | .69 | 0.533 | .05* | 0.308 | .28 | 0.505 | .07 | 0.292 | .31 | 0.425 | .13 |
| SCC RD | −0.035 | .91 | 0.559 | .04* | 0.194 | .51 | 0.163 | .58 | 0.336 | .24 | 0.339 | .24 |
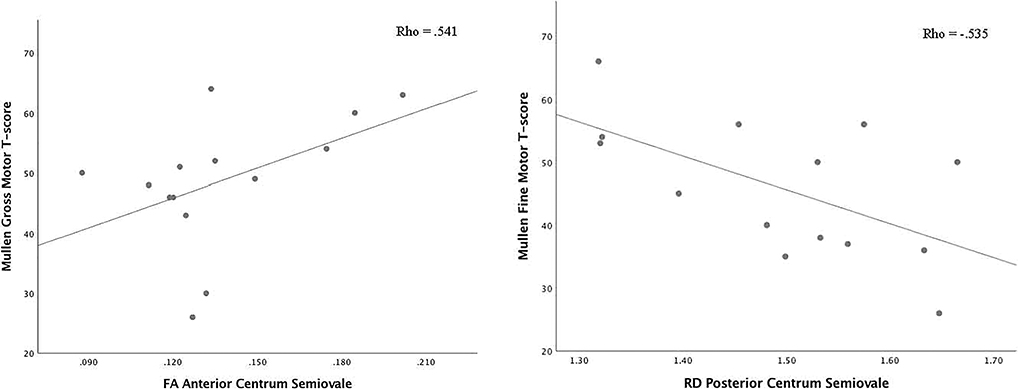
| Anterior CSO FA | 0.541 | .05* | 0.319 | .27 | 0.515 | .06 | −0.181 | .54 | 0.272 | .35 | 0.271 | .35 |
| Anterior CSO MD | −0.286 | .32 | −0.227 | .44 | −0.229 | .43 | 0.337 | .24 | −0.07 | .81 | −0.057 | .85 |
| Anterior CSO AD | −0.218 | .45 | −0.156 | .59 | −0.081 | .78 | 0.262 | .37 | 0.009 | .98 | 0.02 | .95 |
| Anterior CSO RD | −0.293 | .31 | −0.187 | .52 | −0.207 | .49 | 0.37 | .19 | −0.03 | .92 | −0.011 | .97 |
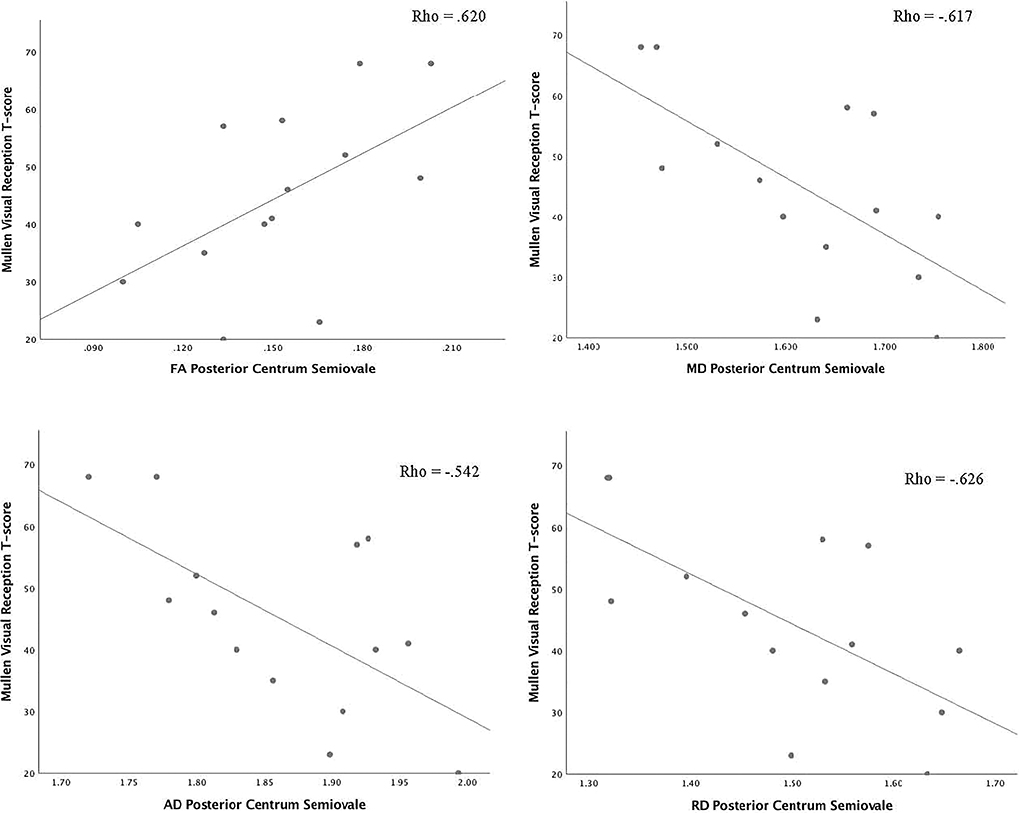
| Posterior CSO FA | 0.417 | .14 | 0.51 | .06 | 0.620 | .02* | −0.216 | .46 | 0.419 | .14 | 0.452 | .11 |
| Posterior CSO MD | −0.354 | .21 | −0.531 | .05 | −0.617 | .02* | 0.161 | .58 | −0.45 | .10 | −0.453 | .10 |
| Posterior CSO AD | −0.42 | .14 | −0.509 | .06 | −0.542 | .05* | 0.141 | .63 | −0.41 | .15 | −0.414 | .14 |
| Posterior CSO RD | −0.392 | .17 | −.535* | .05* | −0.626 | .02* | 0.196 | .50 | −0.47 | .09 | −0.464 | .09 |
Abbreviations: AD, axial diffusivity; CSO, centrum semiovale; EL, expressive language; FA, fractional anisotropy; FM, fine motor; GM, gross motor; MD, mean diffusivity; MSEL, Mullen Scales of Early Learning; RD, radial diffusivity; RL, receptive language; SCC, splenium of the corpus callosum; VR, visual reception.
P < .05
P < .01.
Table 3.
Demographic and Clinical Differences Between Patients Who Participated and Did Not Participate in Neurodevelopmental Testing.a
| Neonates diagnosed with HIE who did not receive ND testing (n = 17) | Neonates diagnosed with HIE who received in ND testing (n = 14) | P | |
|---|---|---|---|
| Male, n | 12 | 7 | .870 |
| Gestational age, wk | 39.50 (1.30) | 38.16 (1.90) | .027* |
| Apgar score, 1 min | 2.53 (2.24) | 2.07 (2.17) | .568 |
| Apgar score, 5 min | 5.47 (2.29) | 3.93 (2.20) | .068 |
| Cord blood pH | 7.01 (0.19) | 7.01 (0.11) | .947 |
| Base deficit | 15.56 (6.30) | 16.59 (5.97) | .691 |
| AED administration, n | 8 | 7 | .870 |
Abbreviations: AED, antiepileptic drug; HIE, hypoxic-ischemic encephalopathy; ND, neurodevelopmental.
Data presented include the mean (SD) unless otherwise specified.
Correlations between DTI scalars in the ROIs that were significantly different from clinical controls and the Mullen Scales of Early Learning composite and subscale scores are presented in Table 4. There was one significant correlations between AD in the right putamen and performance on the Gross Motor subscale of the Mullen Scales of Early Learning (Figure 1). A correlation between AD in the left putamen and performance on the Receptive Language subscale was observed (spearman rho = .565, p=.035), such that higher AD was correlated with better performance on this subscale (Figure 1). Lower FA in the anterior centrum semiovale was significantly correlated with worse performance on the Gross Motor (spearman rho=.541, p=.046) subscale of the Mullen Scales of Early Learning (Figure 2). Significant correlations between FA (spearman rho=.620, p=.018), MD (spearman rho=−.617, p=.019), AD (spearman rho=−.542, p=.045), and RD (spearman rho=−.626, p=.017) in the posterior centrum semiovale and Mullen Scales of Early Learning Visual Reception subscale were observed, such that lower FA, and higher MD, AD, and RD were correlated with worse performance (Figure 3). Higher RD in the posterior centrum semiovale was also significantly correlated with worse performance on the Mullen Scales of Early Learning Fine Motor subscale (spearman rho=−.535, p=.049; Figure 2) While there were no significant correlations between FA in the SCC and scores on the Mullen Scales of Early Learning, higher MD (spearman rho=.654, p=.011), AD (spearman rho=.533, p=.050), and RD (spearman rho=.559, p=.038) were related to better fine motor performance (Figure 4). There were no statistically significant correlations between DTI scalars in the posterior centrum semiovale or splenium of the corpus callosum and the Mullen Scales of Early Learning composite score or Gross Motor, Receptive Language, or Expressive Language subscales.
Figure 1.
Axial diffusivity in right and left putamen and correlation with the Mullen Scales of Early Learning gross motor and receptive language subscale T scores. (Abbreviations: AD, axial diffusivity; Mullen, Mullen Scales of Early Learning; Recep. Lang., receptive language.)
Figure 2.
Correlations between Mullen Scales of Early Learning gross motor and fine motor subscales and diffusion tensor imaging scalars in the anterior and posterior centrum semiovale. (Abbreviations: FA, fractional anisotropy; Mullen, Mullen Scales of Early Learning; RD, radial diffusivity.)
Figure 3.
Correlations between Mullen Scales of Early Learning visual reception subscale and diffusion tensor imaging scalars in the posterior centrum semiovale. (Abbreviations: AD, axial diffusivity; FA, fractional anisotropy; MD, mean diffusivity; Mullen, Mullen Scales of Early Learning; RD, radial diffusivity.)
Figure 4.
Correlations between diffusion tensor imaging scalars in the splenium of the corpus callosum and the fine motor subscale of the Mullen Scales of Early Learning. (Abbreviations: AD, axial diffusivity; MD, mean diffusivity; Mullen, Mullen Scales of Early Learning; RD, radial diffusivity.)
DISCUSSION
This study is limited by the small number of participants and the large number of potential associations of interest, making for a high number of statistical tests and the possibility that some results are due to type one error and are unlikely to be replicated. For a given hypothesis with every 100 tests we expect that several results may be due to an artifact of multiple testing, and interpretation of the findings within the context of this small exploratory pilot study should be cautious. Bearing this in mind, the goals of this pilot study were two-fold. First, differences in neonatal white matter microstructure following HIE post-cooling compared to age-matched clinical controls were examined, as previous research suggests cooling may be less protective of white matter following perinatal HIE.17 Differences in DTI scalars were observed in the putamen, anterior and posterior centrum semiovale, and the splenium of the corpus callosum. Lower FA values in the putamen bilaterally for patients with HIE who had received cooling compared to controls may suggest that there was less white matter integrity in the small white matter tracks running through this gray matter structure in patients compared to clinical controls, even during the neonatal period when myelination is just beginning. Similarly, lower FA was observed in the anterior centrum semiovale in patients compared to clinical controls and lower FA and higher MD and RD in the posterior centrum semiovale were observed, suggesting microstructural differences in subcortical white matter in patients with HIE compared to clinical controls. Changes in subcortical white matter microstructure among patients with histories of HIE have been previously reported among patients who were diagnosed with neonatal hypoxic-ischemic encephalopathy.18 Lower FA and higher RD in the splenium of the corpus callosum were also observed in the present study. Taken together, the observed microstructural white matter changes after neonatal HIE and treatment with cooling, are consistent with other DTI methodologies (i.e., tract-based DTI) and may suggest incomplete neuroprotection.9 Additionally, higher MD in the posterior centrum semiovale and splenium of the corpus callosum prior to the time period of complete pseudo normalization (i.e., ≥12 days),19 may suggest an overall less severe injury pattern in these ROIs 20; however, further investigation is warranted as these particular regions of white matter are fundamentally different in terms of architecture. White matter fibers of the splenium of the corpus callosum are unidirectional or singular, while the centrum semiovale consists of multiple crossing white matter fiber tracts. Research involving adolescents born preterm has previously demonstrated that patterns of DTI scalars vary based on such white matter architectural differences when examining single fiber white matter tracts versus those with crossing fibers.21 Single white matter fiber tracts tend to generate a pattern of decreased FA and increased RD, but ROIs with crossing white matter fibers yield increased FA. Our findings include decreases in FA with accompanying elevations in RD in the splenium of the corpus callosum following neonatal HIE treated with hypothermia; however, decreased FA was observed in the posterior centrum semiovale within the context of crossing white matter fibers.22 Therefore, our preliminary findings suggest white matter microstructural changes in ROIs that align with other studies using tract-based DTI methods,9 but remain somewhat difficult to interpret until this type of imaging methodology is employed.
The second goal of this study was to examine relationships between the DTI ROIs in which significant differences were found between patients with HIE who were treated with cooling and clinical controls and later neurodevelopmental outcomes. A subgroup of patients with HIE (N= 14) participated in neurodevelopmental follow-up testing between ages 20–32 months. Examination of relationships between neonatal DTI findings post-cooling and specific domains of neurodevelopmental outcomes during early childhood is more limited.14,23,24
There were no associations between lower FA in the putamen bilaterally and the composite score or any of the subscales on the Mullen Scales of Early Learning, but there was a significant association between decreased AD in the left putamen and worse performance on the Receptive Language subscale. Other studies have appreciated lower AD in other ROIs (corpus callosum and corticospinal tract) and neurodevelopmental outcomes (i.e., motor function and overall cognitive development),14 but to our knowledge there has not been an observed relationship between the microstructure of white matter tracts through the putamen during the neonatal period and receptive language skills at ages 20–32 months. A morphometric imaging study of healthy/typically children at school age has previously revealed that there is an association between the putamen and other gray matter structures in the striatum and perception of vocal pitch.25 Another morphometric studies found that children with developmental dyslexia had smaller left putamen volumes compared to typically developing peers, and the smaller left putamen volume was associated with deficits in phonological processing, a component of language development.26 Furthermore, a recent meta-analysis examining the relationship between the putamen and language using connectivity modeling methodology highlighted relationships between the left putamen and other regions of interest in the left hemisphere (i.e., inferior frontal lobe, superior temporal gyrus, middle frontal gyrus, and inferior parietal lobe) associated with language comprehension and production.27
The present study also revealed relationships between white matter microstructural changes in the centrum semiovale and neurodevelopment. More specifically, lower FA in the anterior centrum semiovale in the neonatal period following HIE treated with cooling was associated with worse performance on gross motor and visual-motor integration and visual perceptual tasks (i.e., Visual Reception subtest of the Mullen Scales of Early Learning) between ages 20–32 months. Lower FA, and higher MD, AD, and RD were associated with worse performance on visual-motor integration and visual perceptual tasks (i.e., Visual Reception subtest of the Mullen Scales of Early Learning), while only higher RD in the posterior centrum semiovale was associated with worse fine motor performance between ages 20–32 months. These findings are consistent with those of Tusor et al., who found WM changes in the CSO were associated with performance on visual-motor integration tasks.9 Research in other clinical populations, such as those born preterm, also demonstrates worse performance on motor and visual motor integration tasks with increasing burden of white matter microstructural abnormalities.28,29 Further investigation of white matter changes following HIE post-cooling and the relationship with neurodevelopmental outcomes is warranted using diffusion metrics that do not rely on a tensor model, given the complexity of the WM fiber tracts in the centrum semiovale.
Unexpected findings included positive correlations between MD, AD, and RD, in the SCC and fine motor performance between ages 20 and 32 months. While these relationship cannot be fully explained within the bounds of this study, better fine motor function among children at ages 20 to 32 months who have white matter microstructural changes in the splenium of the corpus callosum during the neonatal period may suggest that this structure is particularly plastic and critical for fine motor development following HIE. In a review of the functional mapping of the corpus callosum, Fabri et al. highlighted how the splenium of the corpus callosum is unique in its fiber composition and chemical specificity compared to more anterior regions of the corpus callosum.30 The splenium of the corpus callosum is particularly important in the functional recovery of transfer of multisensory information after callosotomy or other neurologic insults in which part of the corpus callosum remained. Given the proximity of the splenium of the corpus callosum to the posterior centrum semiovale, a potential future hypothesis that warrants exploration is whether the splenium of the corpus callosum is important for later compensation on tasks that require the integration of fine motor skills and visual information in this clinical population.
Limitations
Limitations of this study included a small sample size and use of a clinical control group. Only 14 neonates with HIE post-cooling returned for neurodevelopmental follow-up, introducing a possible selection bias. Additionally, there was no opportunity for collection of neurodevelopmental outcomes on the clinical control group. Taken together, these factors limit the generalizability of these findings.
In terms of imaging methodology, manual drawing of ROIs may also be a limitation to accuracy; however, when there is structural injury present, this method can also be a potential strength. Thegiven the pilot nature of this study, DTI without tractography was utilized, but this may not provide highly specific information about white matter integrity in ROIs that have crossing white matter tracts in brain regions such as the centrum semiovale.31 Finally, the day of life on which MRI was collected was also a limitation, as this was prior to the pseudonormalization period. Given that imaging needed to be collected according to clinical protocol and prior to discharge or transfer, it was not possible for most neonates in this study to have imaging after the pseudonormalization period.
Considerations for Future Research
Further examination of white matter development and neurodevelopmental correlates in neonates and children with after HIE and cooling and after pseudonormalization is warranted. Future approaches emphasizing the use of tractography may provide more specific information about how white matter connections develop. Finally, evaluating the predictive value of DTI in conjunction with other neonatal biomarkers for later neurodevelopmental outcomes will be important for prognosticating who is at higher risk for disabilities and will benefit from early interventions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors have stated that they have no interests which might be perceived as a posing a conflict or bias.
This article is dedicated to the memory of our dear colleague and the senior author of this article, Dr. Andrea Poretti (1977–2017), a physician, scientist, and friend. He gave all of himself to making the lives of others better through his clinical and scientific endeavors.
We extend many thanks to the extraordinary support and guidance from Dr. Marilee Allen on this study and all ongoing work in this area.
We very grateful to Dr. Susumu Mori for his review of the imaging methodology for this study.
We also extend many thanks to Dr. Jamie Perin for her review of the statistical analysis and ongoing support for this study.
FUNDING ACKNOWLEDGEMENTS
This work was supported by the Cerebral Palsy Foundation and the National Institute of Child Health and Development (NICHD R01 HD086058-01A1).
ETHICAL APPROVAL
This study was reviewed by the Johns Hopkins University School of Medicine Institutional Review Board. Approval for this study (NA_00086831) was granted via a waiver of consent.
Footnotes
DECLARATION OF CONFLICTING INTERESTS
The authors do not have any conflicts of interest in reference to this research.
DISCLOSURES
The authors do not have any disclosures in reference to this research.
REFERENCES
- (1).Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Elsevier; 2008. [Google Scholar]
- (2).Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013. January 31;1:CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 2014. July 10;371(2):140–149. [DOI] [PubMed] [Google Scholar]
- (4).Pappas A, Shankaran S, McDonald SA, Vohr BR, Hintz SR, Ehrenkranz RA, et al. Cognitive outcomes after neonatal encephalopathy. Pediatrics 2015. March;135(3):e624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ferriero DM, Bonifacio SL. The search continues for the elusive biomarkers of neonatal brain injury. J Pediatr 2014. March;164(3):438–440. [DOI] [PubMed] [Google Scholar]
- (6).Volpe JJ. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol 2012. August;72(2):156–166. [DOI] [PubMed] [Google Scholar]
- (7).Marlow N, Rose AS, Rands CE, Draper ES. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed 2005. September;90(5):F380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Rutherford MA, Ramenghi LA, Cowan FM. Neonatal stroke. Arch Dis Child Fetal Neonatal Ed 2012. September;97(5):F377–84. [DOI] [PubMed] [Google Scholar]
- (9).Tusor N, Wusthoff C, Smee N, Merchant N, Arichi T, Allsop JM, et al. Prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr Res 2012. July;72(1):63–69. [DOI] [PubMed] [Google Scholar]
- (10).Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010. January;9(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sabir H, Cowan FM. Prediction of outcome methods assessing short- and long-term outcome after therapeutic hypothermia. Semin Fetal Neonatal Med 2015. April;20(2):115–121. [DOI] [PubMed] [Google Scholar]
- (12).Ly MT, Nanavati TU, Frum CA, Pergami P. Comparing tract-based spatial statistics and manual region-of-Interest labeling as diffusion analysis methods to detect white matter abnormalities in infants with hypoxic-Ischemic encephalopathy. J Magn Reson Imaging 2015. December;42(6):1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Gano D, Sargent MA, Miller SP, Connolly MB, Wong P, Glass HC, et al. MRI findings in infants with infantile spasms after neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol 2013. December;49(6):401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Massaro AN, Evangelou I, Brown J, Fatemi A, Vezina G, McCarter R, et al. Neonatal neurobehavior after therapeutic hypothermia for hypoxic ischemic encephalopathy. Early Hum Dev 2015. October;91(10):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 2008. November 15;43(3):447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mullen EM. Mullen Scales of Early Learning. San Antonio, TX: Pearson Clinical Assessments; 1995. [Google Scholar]
- (17).Davidson JO, Yuill CA, Zhang FG, Wassink G, Bennet L, Gunn AJ. Extending the duration of hypothermia does not further improve white matter protection after ischemia in term-equivalent fetal sheep. Sci Rep 2016. April 28;6:25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Seo Y, Kim GT, Choi JW. Early detection of neonatal hypoxic-ischemic white matter injury: an MR diffusion tensor imaging study. Neuroreport 2017. September 6;28(13):845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J, Shimony J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 2012. May 1;78(18):1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Alderliesten T, de Vries LS, Khalil Y, van Haastert IC, Benders MJ, Koopman-Esseboom C, et al. Therapeutic hypothermia modifies perinatal asphyxia-induced changes of the corpus callosum and outcome in neonates. PLoS One 2015. April 29;10(4):e0123230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Groeschel S, Tournier JD, Northam GB, Baldeweg T, Wyatt J, Vollmer B, et al. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. Neuroimage 2014. February 15;87:209–219. [DOI] [PubMed] [Google Scholar]
- (22).Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage 2012. February 1;59(3):2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Brissaud O, Amirault M, Villega F, Periot O, Chateil JF, Allard M. Efficiency of fractional anisotropy and apparent diffusion coefficient on diffusion tensor imaging in prognosis of neonates with hypoxic-ischemic encephalopathy: a methodologic prospective pilot study. AJNR Am J Neuroradiol 2010. February;31(2):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ferrari F, Todeschini A, Guidotti I, Martinez-Biarge M, Roversi MF, Berardi A, et al. General movements in full-term infants with perinatal asphyxia are related to Basal Ganglia and thalamic lesions. J Pediatr 2011. June;158(6):904–911. [DOI] [PubMed] [Google Scholar]
- (25).Tang X, Chen N, Zhang S, Jones JA, Zhang B, Li J, et al. Predicting auditory feedback control of speech production from subregional shape of subcortical structures. Hum Brain Mapp 2018. January;39(1):459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wang Z, Yan X, Liu Y, Spray GJ, Deng Y, Cao F. Structural and functional abnormality of the putamen in children with developmental dyslexia. Neuropsychologia 2018. July 17. [DOI] [PubMed] [Google Scholar]
- (27).Vinas-Guasch N, Wu YJ. The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct Funct 2017. December;222(9):3991–4004. [DOI] [PubMed] [Google Scholar]
- (28).Tusor N, Benders MJ, Counsell SJ, Nongena P, Ederies MA, Falconer S, et al. Punctate White Matter Lesions Associated With Altered Brain Development And Adverse Motor Outcome In Preterm Infants. Sci Rep 2017. October 16;7(1):13250–017-13753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Hollund IMH, Olsen A, Skranes J, Brubakk AM, Haberg AK, Eikenes L, et al. White matter alterations and their associations with motor function in young adults born preterm with very low birth weight. Neuroimage Clin 2017. October 4;17:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Fabri M, Pierpaoli C, Barbaresi P, Polonara G. Functional topography of the corpus callosum investigated by DTI and fMRI. World J Radiol 2014. December 28;6(12):895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage 2012. February 1;59(3):2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.