Abstract
Coronaviridae (CoV) is a large family of zoonotic viruses linked to a range of diseases from the common cold to severe acute and Middle East respiratory syndrome CoV epidemics. In 2019, a novel virus emerged from Wuhan, China, and resulted in a marked worldwide outbreak of respiratory illness. Prevention and containment became the prioritized intervention against COVID-19, coupled with a continued search for hallmarks of the disease that would allow early detection and provide insight into management and triage. Cutaneous findings associated with COVID-19 include diffuse maculopapular rashes, livedo reticularis, and acro-ischemic “COVID toes.” These skin findings occurred anywhere from days before respiratory symptom onset to weeks after recovery, and predominantly in child and adolescent populations. The role of dermatologists can be expanded during this COVID-19 pandemic to help identify disease through cutaneous presentations.
Keywords: Coronavirus, Pandemic, Cutaneous, Inflammation
Background
Members of the Coronaviridae (CoV) family are characterized by a single-stranded, positive sense RNA with a helical capsid. This enzoonotic virus can remain viably infectious even after capsid destruction through its ability to replicate within the cytoplasm of infected cells. Although this family of viruses is mostly associated with the common cold, there are much more virulent species within the taxa (Brian and Baric, 2005). Notably, the severe acute respiratory syndrome (SARS) CoV epidemic was thought to originate in bats, with the civet cat being the intermediate host prior to human infection (Guan et al., 2003). In 2007, Cheng et al. (2007) published an ominous report alluding to the potential for a catastrophic reemergence of a SARS-like disease, referencing the large reservoir of virus in mammals.
In December 2019, a cluster of patients in Wuhan, Hubei Province, China, presented with severe atypical pneumonia, and experts named the illness coronavirus disease 2019 (COVID-19; European Centre for Disease Prevention and Control, 2020). Although the source of this viral outbreak remains elusive, the original 27 cases were linked to Southern China’s Wuhan Seafood Wholesale Market (European Centre for Disease Prevention and Control, 2020). This market is known for selling various bushmeat and other wild animals to the public for consumption, which was speculated to include an unidentified intermediate host for COVID-19. As in SARS-CoV, bats are understood to be the main host of the COVID-19 virus.
On January 20, 2020, a 35-year old man residing in Washington state with recent travel exposure in Wuhan, China, was identified as the first case of COVID-19 in the United States (Holshue et al., 2020). The reproduction number of the virus is estimated to be an average of 3.28 (mean R0 = 3.28), indicating that each infected person is likely to pass the disease to >3 people (Liu et al., 2020). Fear of an increasing epidemic emerged with the identification of four cases in Thailand, Japan, and South Korea. An extended latency phase was postulated to occur, during which infected individuals remained asymptomatic, as two of four cases reported no contact with the Wuhan Central Hospital or the aforementioned market (Rothe et al., 2020).
Within 60 days, in response to the surge in medical need overloading medical care capacity in Wuhan, the Chinese government constructed and designated two new hospitals to care for the additional patients. In addition, reverse transcriptase polymerase chain reaction, and next-generation sequencing tests were made readily available to collect specimens of sputum, blood, stool, and bronchoalveolar lavage fluid cultures, which lead to the discovery of a novel coronavirus, SARS CoV-2, now known as COVID-19 (Wu et al., 2020a). Evolving epidemiologic evidence determined COVID-19′s capacity to spread through human-to-human droplet transmissions, with airborne transfer under investigation (Wu et al., 2020b). A subsequent study identified the presence of the virus in stool samples of an infected host, suggesting the potential for fecal-oral spread (Holshue et al., 2020).
A latency period of up to 2 weeks resulted in the rapid spread of COVID-19 through asymptomatic individuals harboring the disease, with international travel hubs playing a vital role in global transmission. Modern Internet infrastructure led to rapid generation of global index cases (Dong et al., 2020). In late January 2020, the first U.S. travel restrictions targeted travel from China, the source of the outbreak. Weeks later, additional travel bans included travel from Europe and the United Kingdom. The World Health Organization (WHO) declared COVID-19 a pandemic on March 11, 2020, with a global case total of >100,000. As of July 21, 2020, the worldwide total number of confirmed COVID-19 cases eclipsed 14.8 million with >600,000 deaths across six continents (Fig. 1). The United States surpassed China in the number of confirmed COVID-19 cases and deaths by March 31, 2020, and the current total number of cases as of June 21, 2020 exceeds 3.8 million with >140,000 deaths, with reports in all 50 states (Centers for Disease Control and Prevention [CDC], 2020a).
Fig. 1.
COVID-19 longitudinal global case tracker from January 2020 to July 21, 2020. Cases are stratified by world regions (World Health Organization COVID-19 dashboard; https://covid19.who.int/; License: CC BY-NC-SA 3.0 IGO).
To help curtail the spread of COVID-19, cruise ships were detained to hold passengers onboard in a 14-day quarantine. For example, a Diamond Princess cruise ship harboring infected persons was docked outside California (Saey, 2020). This Diamond Princess cruise ship had >700 infected crew and passengers onboard a tightly packed vessel harbouring >3700 individuals. Cruise lines create the perfect environment for infection to run rampant, as seen in the past with norovirus outbreaks causing widespread gastrointestinal distress. Unfortunately, passengers not infected had to wait through the same protocol until the ship was able to dock safely. For this reason, the CDC issued a public warning against traveling on any cruise ships for the time being, especially for high-risk individuals (CDC, 2020b). As of March 14, the CDC Director issued a no-sail order, and cruise ships remained voluntarily out of service from U.S. ports for the next 30 days (CDC, 2020c). The no-sail order was extended on April 9 to continue for a 100-day period unless the CDC Director or Secretary of Health and Human Services declares COVID-19 to no longer be a public health emergency, whereby the order can be terminated early.
Clinical presentation
Clinical symptoms of COVID-19 range from mild respiratory illness to acute respiratory distress syndrome. The original cohort of patients in Wuhan presented with fever, cough, and dyspnea along with corresponding chest computed tomography imaging illustrating bilateral ground glass opacities and a notable paucity of upper respiratory tract infection hallmarks (e.g., rhinorrhea, pharyngitis, or sinusitis; Assiri et al., 2013; Bernheim et al., 2020, Lee et al., 2020). This rapid progression to acute respiratory distress syndrome is believed to be a result of the virus homing in on the lower respiratory epithelium for attachment and infection (Huang et al., 2020). Researchers suggest that a relationship exists between this SARS CoV-2 and the previous SARS CoV-1 and Middle East respiratory syndrome (MERS) CoV epidemics. All three diseases originate from the genus Betacoronovirus and spread through respiratory droplets (Peeri et al., 2020). There is an 82% genomic sequence similarity between COVID-19 and SARS CoV-1 and 50% with MERS (Zhang et al., 2020). Laboratory findings for COVID-19 show lymphocytopenia, with a depletion of both CD4 + and CD8 + T cells in >80% of presenting patients (Guan et al., 2020). Liver injury is an emerging complication with COVID-19 and appears to be associated with severe disease phenotype (Zhang et al., 2020).
Published reported of skin findings have continued to evolve since the COVID-19 pandemic began (Table 1). A Chinese study claimed <1% incidence of cutaneous manifestations, whereas an Italian cohort reported >20%, suggesting presentational differences in subset populations (Recalcati, 2020, Sachdeva et al., 2020, Tang et al., 2020). The manifestations ranged from maculopapular exanthems, petechial rashes, and pernio-like findings to vesicular lesions (Bouaziz et al., 2020, Galván Casas et al., 2020, Manalo et al., 2020, Tammaro et al., 2020). Of note, many cases occurred in the child and adolescent populations, such as the pernio-like lesions of the feet dubbed “COVID toes” (Kolivras et al., 2020, Landa et al., 2020, Recalcati et al., 2020, Recalcati, 2020). These skin presentations have the potential to confound an early diagnosis of COVID-19 because they can simulate other infections (Lu et al., 2020). One such case was presented as a patient with a petechial rash who was given an original diagnosis of Dengue fever that was later confirmed to be COVID-19 (Joob and Wiwanitkit, 2020, Mungmungpuntipantip and Wiwanitkit, 2020).
Table 1.
Cutaneous manifestations of COVID-19.
| Presentation | Description | Literature |
|---|---|---|
| Morbilliform rash | Known viral exanthem; Italian and Spanish cohorts described various rates of eruption | Recalcati, 2020, Sachdeva et al., 2020, Galván Casas et al., 2020 |
| Urticaria | Cohorts in Spain and Italy presented with an urticarial rash; many presented prior to respiratory symptoms | Tang et al., 2020, Bouaziz et al., 2020 |
| Vesicular eruptions | Chickenpox-like rash observed in a diffuse pattern | Tammaro et al., 2020 |
| Acral pernio-like lesions | Acral pernio-like lesion identified in case reports; more common in children | Kolivras et al., 2020, Recalcati et al., 2020, Recalcati, 2020, Landa et al., 2020 |
| Livedo reticularis | Case reports in the United States of transient Livedo reticularis eruption unilaterally; mechanism may be related to thrombotic state | Manalo et al., 2020 |
| Petechial rash | Case report of petechial rash that can be mistaken for Dengue | Joob and Wiwanitkit, 2020 |
| Drug reaction | Drugs used to treat COVID suspected of causing exanthems are hydroxychloroquine (morbilliform) and azithromycin (morbilliform, urticarial) | Young and Fernandez, 2020 |
High variability is reported in the timing of skin manifestations, with some studies identifying skin findings days before respiratory signs and others weeks after recovery. Researchers speculate that COVID-19 skin findings are caused by an inflammatory response to the infection that results in complement-mediated microvascular injury (Magro et al., 2020). Some reports discuss the difficulty in confirming that cutaneous manifestations are specific to SARS-CoV-2; many critically ill patients receive multiple medication regimens that can yield similar cutaneous findings (Young and Fernandez, 2020). Although there is no necessary treatment for COVID-related cutaneous manifestations, dermatologists should follow American Academy of Dermatology guidance when prescribing immunosuppressive agents for autoimmune-mediated conditions normally treated with biologics (American Academy of Dermatology, 2020).
Another novel phenomenon associated with COVID-19 is a Kawasaki-like syndrome currently termed “hyperinflammatory shock” or more specifically “pediatric inflammatory multisystem syndrome” (Morand et al., 2020). A cluster of reported cases appeared in previously healthy children who presented with high fevers, conjunctivitis, rash, and refractory shock with minimal respiratory symptoms (Riphagen et al., 2020). Similar to classic Kawasaki’s disease, the children may have enlarged and aneurysmal coronary vessels on echocardiogram. Test results in the majority of children were positive for the serology for SARS CoV-2 but negative on polymerase chain reaction for active infection, suggesting that this presentation is a delayed immune-mediated response in children with a history of infection (Viner and Whittaker, 2020).
Risk factors for adverse consequences
The patients with the highest risk of mortality are those who have underlying health conditions that affect their lung health (e.g., asthma, emphysema, and smoking) and those with comorbidities that lower their overall health status (e.g., low vitamin D level, cancer, renal disease, diabetes, and hypertension). Fang et al. (2020) suggest that these comorbidities stem from current angiotensin II receptor blocker medication therapy.
The current case fatality rate is calculated at approximately 2% (Fauci et al., 2020). COVID-19 is already responsible for more fatalities than SARS CoV-1 and MERS combined (Mahase, 2020). Although COVID-19 has a significantly lower case fatality rate than SARS CoV-1 (10%) and MERS (30%), this novel virus has the ability to spread undetected through a prolonged asymptomatic incubation period, which increases transmission and thus increases the relative mortality rate. When comparing mortality across the affected countries, Italy has one of the highest death rates related to COVID-19. The advanced age of the population in Italy was speculated early on to represent a unique factor with regard to susceptibility to severe respiratory illness; Italy is known to have the oldest European population, with more than a quarter of people over the age of 65 years (Rettner, 2020).
Data on the risk of vertical transmission in pregnant women continue to emerge (Schwartz and Graham, 2020). A review of pregnancies and deliveries for women who tested positive for COVID-19 suggested low morbidity risk, with the most common complication being preterm delivery (Mullins et al., 2020). Despite this, it is recommended that neonates and pregnant women take precautions and avoid public places. The CDC currently recommends continuing breastfeeding because evidence does not show that the virus can be transferred through breastmilk. Breastfeeding mothers with a suspected infection should continue to practice proper hygiene and wear a mask while breastfeeding to avoid transference of respiratory particles (CDC, 2020d). Children and adolescents are susceptible to infection, but little is known regarding their risk status (Lee et al., 2020). Similar precautions are in place for the adolescent population to protect against infection and spread (Schmitt, 2020).
Avoidance of ibuprofen and other nonsteroidal anti-inflammatory drugs (NSAIDs) has been suggested by French researchers because studies show that patients who took NSAIDs before becoming ill experienced more severe pneumonia than those who had not (Day, 2020). However, multiple studies have responded by stating not enough data exist to suggest poor outcomes based on NSAID use due to the complexity of the targeted cyclooxygenase pathway (FitzGerald, 2020, Giollo et al., 2020). Switching from NSAIDs to acetaminophen is a reasonable alternative in healthy individuals who require antipyretics until more data are available.
International collaborative scientific response
Scientific collaborations have revealed that cytopathic effects and poor prognosis are associated with increased release of cytokines such as interleukin-6 and discovered that the angiotensin converting enzyme 2 receptor (ACE2) serves as the infective portal of entry (Cai, 2020, Letko and Munster, 2020, Zhu et al., 2020). The host virus uses its CoV S protein receptor binding motif to bind ACE2 in the target cell (Millet and Whittaker, 2015). Injury is thought to occur by the downregulation of ACE2 in alveolar cells, which normally provide a protective function against lung injury (Xu et al., 2020). Downregulation of ACE2 leads to downstream effects, including upregulating type 1A ACE2 receptors, and results in increased pulmonary vascular permeability (Imai et al., 2005).
Containment and quarantine
The use of quarantine dates back to the 14th century when Italian officials required ships to stay off port for 40 days in an effort to prevent the plague from entering mainland (CDC, 2020e). The bubonic plague of the 14th century, also referred to as the black death pandemic, accounted for an estimated 50 to 100 million deaths across Eurasia (Frith, 2012). To put this into perspective, approximately 60% of Europe’s population was decimated by the disease (McEvedy, 1988). The responsible bacterium, Yersinia pestis, causes a prodrome stage of myalgias, fever, and shortness of breath, along with characteristic buboes, or swollen and tender lymph nodes (Cui and Song, 2016). The most lethal form of the plague is spread through respiratory droplets from human-to-human transmission, resulting in a pneumonic plague that reaches a near 100% fatality rate (CDC, 2018).
An equally lethal pandemic known as the Spanish flu occurred in 1918. This influenza spread across the entire globe within 6 months and resulted in an estimated 30 million deaths between 1918 and 1919 (Patterson and Pyle, 1991). The lethality of the flu was due in part to three distinct waves of disease (Grabowski et al., 2017). The first wave took place in the U.S. Midwest in the spring of 1918 and reached the Far East and Europe by July. The second wave originated within France in August 1918 and was known as the fatal wave because of the associated high virulence and fatality. The final wave is not well defined and occurred sporadically in the spring of 1919.
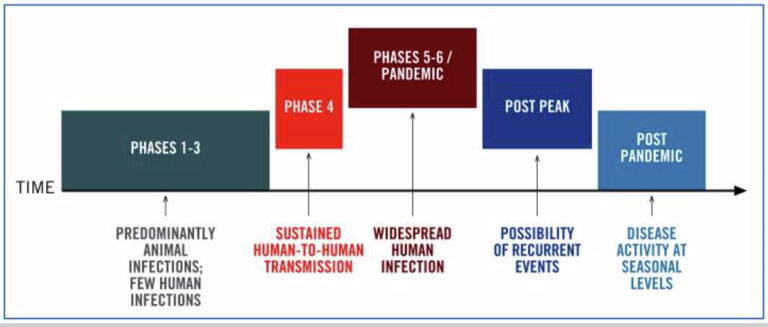
The WHO defines a pandemic as an infection that is able to sustain infectivity and spread across a global region and toward which the human population has no immunity (Fig. 2; Doshi, 2011). Because of COVID-19′s accelerated spread across multiple continents, the global response for rapid intervention has focused on containment (WHO, 2020). Infectious diseases start out as endemics, meaning they infect a particular subpopulation at a stable and predictable rate (Grennan, 2019). When the infection progresses to spread at an unpredictable rate, the disease is classified as an outbreak. Outbreaks evolve into epidemics once the virus spreads over a larger geographic region, and eventually into pandemics when the spread is at a global scale.
Fig. 2.
Six phases of a pandemic timeline (World Health Organization [WHO] pandemic phases; pandemic influenza preparedness and response: A WHO guidance document; License: CC BY-NC-SA 3.0 IGO; https://www.ncbi.nlm.nih.gov/books/NBK143061/figure/ch4.f1/?report=objectonly).
Pandemics can be broken down into six phases, starting with phases 1 through 3, which are defined as mostly animal infections with a few human cases (WHO, 2020). Phase 4 is reached after sustained human-to-human transmission. Phases 5 and 6 are defined as meeting the pandemic criteria with progressive global transmission and represent the current global phase of COVID-19, with some regions, including the US, experiencing post-peak transmission rates.
The United States Government has placed level 3 travel precautions on multiple countries, including China, Iran, South Korea, the United Kingdom, and many European nations, and effectively banned nonessential travel. Level 3 travel advisories are issued to discourage travel due to significant risks toward safety, health, and security. U.S. citizens looking to return to the United States are asked to self-quarantine at home for a 14-day period (CDC, 2020f). The government is not allowing foreign nationals who have visited Iran or China in the last 14 days to enter the country at this time. In an unprecedented move, the CDC also issued a 14-day domestic travel advisory for New York, New Jersey, and Connecticut on March 30, asking residents of these states to refrain from any travel (CDC, 2020g).
Tourism and travel (e.g., trains, airplanes, and cruise ships) are associated with the highest risk of infection because they take place in generally highly concentrated areas with people who have visited multiple countries. Abstaining from visiting airports and cruise ships is recommended by the CDC (2020g). In addition, the CDC recommends maintaining household emergency plans. Families should consider alternative options for work and school in case local and federal governments call for a shutdown. A two-headed approach of prevention and containment offers the greatest intervention point with COVID-19. Individuals who believe they have contracted COVID-19 but are clinically stable should remain at home and self-quarantine for 14 days minimum and make a telephone visit to determine if further medical care is required (CDC, 2020h). This reduces the risk of infecting the individuals at hospitals with chronic illness who are the most susceptible to severe respiratory failure and alleviates the burden of overwhelming hospital personnel.
Prevention
The greatest intervention in the COVID-19 pandemic is the application of effective preventative measures. Officials in Wuhan, China, engaged in rapid preventative action and placed suspected patients on airborne precautions and issued fit-tested N-95 mask precautions to health care personnel (Huang et al., 2020). These respirators are named for their ability to filter >95% of particles up to 0.3 microns in size, which covers SARS CoV-2 (Kirby, 2020). Health care personnel have been directed to use appropriate personal protective equipment, such as N-95 respirator masks, when caring for infected patients and triaging suspected cases.
The CDC has issued guidelines for handwashing as well as household and workplace sanitation (Table 2). The recommendation calls for handwashing using soap and warm water while scrubbing for a minimum of 20 seconds (U.S. Food and Drug Administration, 2019). Alternatively, hand sanitizer can be effective in the absence of soap and water, such as in public forums. All household and workplace areas are recommended to be frequently disinfected using 75% alcohol products or disinfecting wipes. Examples of places to clean include doorknobs, tables, counters, and computer keyboards. Persons are recommended to avoid touching their face (mouth, nose, or eyes) to prevent fomites from reaching high-risk areas of the body. Additionally, unnecessary travel to overcrowded locations (e.g., amusement parks, sports events, movie theatres, and hospitals) should be avoided to reduce potential transmission burden. Self-awareness and handwashing are critical; studies show that the average human touches their face >200 times per day (Kwok et al., 2015). Similarly, social distancing practices should be implemented throughout the day (Table 3). The CDC defines social distancing as keeping 6 feet (or two arm-lengths) away from the nearest person.
Table 2.
CDC-recommended hygienic steps to avoid spreading COVID-19 (adapted from CDC, 2020).
| CDC hygienic steps to prevent spread |
|---|
| Keep your hands clean by washing with soap and water for at least 20 seconds or using a hand sanitizer with at least 60% alcohol |
| Avoid touching your eyes, nose, and mouth |
| Cover your coughs and sneezes with tissues or your arm/sleeve and dispose of tissues in the trash |
| Avoid people who are sick |
| Avoid sharing items such as dishes and bedding and keep surfaces clean with disinfecting wipes |
| Wear a homemade mask whenever outside of home if risk of contact with others (e.g., groceries, gas, work) |
CDC, Centers for Disease Control and Prevention.
Table 3.
Social distancing protocol to reduce transmission of COVID-19 (adapted from CDC, 2020).
| Situation | Solution |
|---|---|
| 1. Social gatherings | When feasible, limit all gatherings to under 10 people. If not possible, keep 6 feet distance from nearest person. Wear a homemade mask whenever outside. |
| 2. Dining | Avoid dine-in areas when possible. If necessary, use take-out/delivery options. Always wear a homemade mask covering mouth and nose when out in public. |
| 3. Work and school meetings | Convert all physical meetings to virtual through video chat applications. |
| 4. Confined areas (e.g., elevators) | Reduce exposure to confined areas such as elevators by using alternative measures such as stairways when possible. |
| 5. Encountering sick individuals | Maintain 6-feet social distancing whenever encountering an individual that appears ill. Always wear a mask when taking care of sick persons. |
| 6. Unable to maintain social distancing | Maintain proper hygiene by using disposable tissues or upper arm to cover sneeze or cough. Avoid touching unnecessary surfaces and use soap and water or hand sanitizer often. |
CDC, Centers for Disease Control and Prevention.
Fear of contracting COVID-19 has led to large public demand for personal protective equipment, resulting in sparse supplies of masks and gloves. Standard surgical masks have been shown to be similarly effective in preventing infection in lower-risk situations, such as outdoor areas, because they provide a direct barrier of the nose and mouth and prevent incidental fomite contact (Derrick and Gomersall, 2005). Studies from the original SARS CoV-1 epidemic recommend using absorbent (e.g., cotton) over disposable gowns because disposable gowns allowed for longer virus survival (Cheng et al., 2007). Although the CDC’s and WHO’s original stance did not call for masks for the general public, the recommendation came later from the state level for citizens to wear homemade versions of masks in public to spare medical-grade surgical masks and N95 respirators for health care personnel. These masks should cover the mouth and nose entirely and be washed daily. A tutorial is provided on the CDC website with several creative methods to create a mask with cloth materials at home (CDC, 2020i).
Treatment and management
Supportive care is the mainstay treatment for respiratory distress in COVID-19, encompassing ventilation support and extracorporeal membrane oxygenation, while information is obtained regarding effective treatment for the COVID-19 infection. Researchers have looked to the treatment strategies gained by the analysis of the MERS and SARS outbreaks to get a better understanding of possible therapeutic regimens.
Treatment horizons
Two previous trials of systemic corticosteroids to address the high cytokine load did not improve mortality and instead caused a delay in viral clearance (Arabi et al., 2018, Stockman et al., 2006). That said, a new clinical trial of methylprednisolone has recently been approved to determine whether COVID-19 cases respond (Harrison, 2020). Switching to short-term, moderate-dose regimens of corticosteroids, combined with immunoglobins, improves oxygenation (Zhou et al., 2020).
Many researchers suggest a trial of antiviral medications to help suppress viral replication, and both ritonavir and lopinavir are being studied in clinical trials (Shionogi, Toyama Chemical; Clinical trials, 2020). The use of these protease inhibitors was proven to be efficacious during the original SARS epidemic (Chu et al., 2004). Remdesivir (Gilead Pharmaceuticals Inc, Foster City, CA) is an investigational drug that was considered as a treatment for Ebola but never approved by the U.S. Food and Drug Administration. Remdesivir is currently being tested in clinical trials for efficacy against COVID-19. Select cases have shown that remdesivir is capable of shortening the time until clinical improvement in patients with COVID-19 infection (Grein et al., 2020).
Hydroxychloroquine, a disease-modifying antirheumatic drug, has been considered as a potential option to treat patients infected with COVID-19 who demonstrate cytokine storm because of its ability to sequester both innate and adaptive immunity, making it a powerful anti-inflammatory agent (Schrezenmeier and Dörner, 2020). This agent provides a similar benefit in systemic lupus erythematous by attenuating the proinflammatory cascade (Tang et al., 2012). A nonrandomized clinical trial combining azithromycin and hydroxychloroquine initially showed promising therapeutic potential (Gautret et al., 2020). The authors recommended risk stratification because the combination of drugs can potentiate cardiac arrythmias. Most recently, hydroxychloroquine has fallen out of favor as a therapy of choice because of its significant side effect profile and lack of clear clinical benefit, followed by the retraction of a Lancet article supporting hydroxychloroquine (Chorin et al., 2020, Mehra et al., 2020).
Additionally, the interleukin-6 human monoclonal antibody tocilizumab is being studied in a clinical trial for COVID-19 treatment (Chugai Pharmaceutical, Zhejiang Hisun Pharmaceutical, Jiangsu Qyun Bio-Pharmaceutical, Jiangsu, China). This novel use of a monoclonal antibody as an antiviral agent is suggested to work through its immunosuppression of acute-phase reactants, which is associated with inflammation and leukocyte recruitment and could potentially reduce respiratory distress. Another promising and emerging area of investigation is the use of convalescent plasma in patients with severe COVID-19 presentations. Early results suggest that this therapy may clear viremia by way of neutralizing antibodies from recovered donors (Duan et al., 2020).
The utilization of zinc, a heavy metal, has been postulated to improve outcomes in patients with COVID-19 (Zhang and Liu, 2020). The mechanism of action works through the interference of RNA viral replication (te Velthuis et al., 2010).
No vaccine to date has been created to prevent COVID-19 infection. Most recently, the National Institutes of Health announced on March 16 the start of phase 1 trials to evaluate an investigational vaccine of COVID-19 at the Kaiser Permanente Washington Health Research Institute (Routh, 2020). As of April 8, there were >70 vaccine candidates in various stages of testing, with several trials entering phase 2 (Thanh Le et al., 2020). With a new public–private partnership between the National Institutes of Health and multiple pharmaceutical companies, all parties hope to accelerate the speed at which a vaccine is developed and tested for efficacy and safety to be utilized globally (Corey et al., 2020).
Conclusion
COVID-19 remains a devastating pandemic that continues to threaten the health infrastructure. Therapeutic agents continue to remain elusive as no gold standard therapy has emerged as the singular frontrunner in the treatment of symptomatic patients. Supportive care remains the mainstay of treatment as the total case number continues to increase. Reports on cutaneous manifestations of COVID-19, ranging from maculopapular exanthems to acral pernio-like lesions termed COVID toes, are emerging, offering additional diagnostic clues for earlier diagnosis and intervention.
Conflict of Interest
None.
Funding
None.
Study Approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
References
- American Academy of Dermatology. Guidance on the use of immunosuppressive agents [Internet]. 2020 [cited 2020 June 9]. Available from: https://www.aad.org/member/practice/coronavirus/clinical-guidance/biologics.
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz JD, Duong T, Jachiet M, Velter C, Lestang P, Cassius C, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatol Venereol 2020 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Brian D.A., Baric R.S. Coronavirus genome structure and replication. Curr Top Microbiol Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. An insight of comparison between COVID-19 (2019-nCoV disease) and SARS in pathology and pathogenesis [Internet]. 2020 [cited 2020 March 1]. Available from: https://www.genemedi.net/i/comparison-between-covid-19-and-sars-ncov-in-pathology-pathogenesis
- Centers for Disease Control and Prevention. Coronavirus disease 2019 situation summary [Internet]. 2020a [cited 2020 March 1]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html
- Centers for Disease Control and Prevention. Interim guidance for ships on managing suspected coronavirus disease 2019 [Internet]. 2020b [cited 2020 March 13]. Available from: https://www.cdc.gov/quarantine/maritime/recommendations-for-ships.html
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) guidance for ships [Internet]. 2020c [cited 2020 March 16]. Available from: https://www.cdc.gov/quarantine/cruise/index.html
- Centers for Disease Control and Prevention. If you are pregnant, breastfeeding, or caring for your children [Internet]. 2020d [cited 2020 July 21]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnancy-breastfeeding.html
- Centers for Disease Control and Prevention. Quarantine and isolation [Internet]. 2020e [cited 2020 March 8, 2020]. Available from: https://www.cdc.gov/quarantine/historyquarantine.html
- Centers for Disease Control and Prevention. Quarantine and isolation [Internet]. 2018 [cited 2020 March 17]. Available from: https://www.cdc.gov/plague/symptoms/index.html
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID19): Travel [Internet]. 2020f [cited 2020 April 11]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/travelers/index.html
- Centers for Disease Control and Prevention. Coronavirus and travel in the United States [Internet]. 2020g [cited 2020 April 11]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/travelers/travel-in-the-us.html
- Centers for Disease Control and Prevention. Social distancing, quarantine and isolation [Internet]. 2020h [cited 2020 April 11]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
- Centers for Disease Control and Prevention. Use of cloth face coverings to help slow the spread of COVID-19 [Internet]. 2020i [cited 2020 April 11]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html
- Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorin E., Wadhwani L., Magnani S., Dai M., Shulman E., Nadeau-Routhier C. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;S1547–5271(20):30435. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C.C., Hung F.N., Wong M.M.L., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368(6494):948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- Cui Y., Song Y. Genome and evolution of yersinia pestis. Adv Exp Med Biol. 2016;918:171–192. doi: 10.1007/978-94-024-0890-4_6. [DOI] [PubMed] [Google Scholar]
- Day M. Covid-19: Ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- Derrick J.L., Gomersall C.D. Protecting healthcare staff from severe acute respiratory syndrome: filtration capacity of multiple surgical masks. J Hosp Infect. 2005;59(4):365–368. doi: 10.1016/j.jhin.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;S1473–3099(20):30120–30121. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi P. The elusive definition of pandemic influenza. Bull World Health Organ. 2011;89(7):532–538. doi: 10.2471/BLT.11.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020:202004168. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control. Risk assessment: Outbreak of acute respiratory syndrome associated with a novel coronavirus, Wuhan, China; first update. Stockholm: ECDC; January 22, 2020.
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;S2213–2600(20):30116–30118. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - Navigating the Uncharted. N Engl J Med. 2020;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G.A. Misguided drug advice for COVID-19. Science. 2020;367(6485):1434. doi: 10.1126/science.abb8034. [DOI] [PubMed] [Google Scholar]
- Frith J. The history of plague-part 1: The three great pandemics. J Mil Vet health. 2012;20:11–16. [Google Scholar]
- Galván Casas C., Català A., Carretero Hernández G., Rodríguez-Jiménez P., Fernández-Nieto D., Rodríguez-Villa Lario A. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Giollo A, Adami G, Gatti D, Idolazzi L, Rossini M. Coronavirus disease 19 (Covid-19) and non-steroidal anti-inflammatory drugs (NSAID). Ann Rheum Dis 2020 [Epub ahead of publication]. [DOI] [PubMed]
- Grabowski M.L., Kosińska B., Knap J.P., Brydak L.B. The lethal Spanish influenza pandemic in Poland. Med Sci Monit. 2017;23:4880–4884. doi: 10.12659/MSM.906280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grennan D. What is a pandemic? JAMA. 2019;321(9):910. doi: 10.1001/jama.2019.0700. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38(4):379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joob B., Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T. Australian government releases face masks to protect against coronavirus. Lancet Respir Med. 2020;S2213–2600(20):30064–30065. doi: 10.1016/S2213-2600(20)30064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolivras A., Dehavay F., Delplace D., Francesco Feoli F., Meiers I., Milone L. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok Y.L., Gralton J., McLaws M.L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43(2):112–114. doi: 10.1016/j.ajic.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa N., Mendieta-Eckert M., Fonda-Pascual P., Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 Pandemic. Int J Dermatol. 2020;59(6):739–743. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.I., Hu Y.L., Chen P.Y., Huang Y.C., Hsueh P.R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;S1684–1182(20):30039–30046. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko MC, Munster V. Functional assessment of cell entry and receptor usage for lineage B β-coronaviruses, including 2019-nCoV. bioRxiv 2020;2020.01.22.915660.
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Lin J, Zhang Z, Xiao L, Jiang Z, Chen J, et al. Alert for non-respiratory symptoms of Coronavirus Disease 2019 (COVID-19) patients in epidemic period: A case report of familial cluster with three asymptomatic COVID-19 patients. J Med Virol 2020 [Epub ahead of publication]. [DOI] [PubMed]
- Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- Manalo I.F., Smith M.K., Cheeley J., Jacobs R. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J Am Acad Dermatol. 2020;S0190–9622(20):30558–30562. doi: 10.1016/j.jaad.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvedy C. The bubonic plague. Sci Am. 1988;258(2):118–123. doi: 10.1038/scientificamerican0288-118. [DOI] [PubMed] [Google Scholar]
- Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Retracted: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020;S0140–6736(20):31180–31186. doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Millet J.K., Whittaker G.R. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand A, Urbina D, Fabre A. COVID-19 and Kawasaki-like disease: the known-known, the unknown-known and the unknown-unknown. Preprints 2020;2020050160.
- Mullins E., Evans D., Viner R.M., O'Brien P., Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–592. doi: 10.1002/uog.22014. [DOI] [PubMed] [Google Scholar]
- Mungmungpuntipantip R., Wiwanitkit V. COVID-19 and cutaneous manifestations. J Eur Acad Dermatol Venereol. 2020;34(6):e246. doi: 10.1111/jdv.16483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K., Pyle G. The geography and mortality of the 1918 influenza pandemic. Bull Hist Med. 1991;65(1):4–21. [PubMed] [Google Scholar]
- Peeri NC, Shrestha N, Rahman MS, Rafdzah Zaki R, Tan Z, Bibi S,et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol 2020;dyaa033. [DOI] [PMC free article] [PubMed]
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- Recalcati S, Barbagallo T, Frasin LA, Prestinari F, Cogliardi A, Provero MC, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol 2020 [Epub ahead of publication]. [DOI] [PMC free article] [PubMed]
- Rettner R. Why deaths from coronavirus are so high in Italy. Sci Am 2020 [Epub ahead of publication].
- Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh J. National Institutes of Health clinical trial of investigational vaccine for COVID-19 begins [Internet]. 2020 [cited 2020 March 17]. Available from: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saey TH. Cruise ship outbreak helps pin down how deadly the new coronavirus is [Internet]. 2020 [cited 2020 March 13]. Available from: https://www.sciencenews.org/article/coronavirus-outbreak-diamond-princess-cruise-ship-death-rate
- Sachdeva M., Gianotti R., Shah M., Bradanini L., Tosi D., Veraldi S. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98(2):75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt B. Coronavirus (COVID-19) exposure. Pediatric 2020 [Epub ahead of publication].
- Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):E194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammaro A, Adebanjo GAR, Parisella FR, Pezzuto A, Rello J. Cutaneous manifestations in COVID-19: The experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol 2020 [Epub ahead of publication]. [DOI] [PMC free article] [PubMed]
- Tang C., Godfrey T., Stawell R., Nikpour M. Hydroxychloroquine in lupus: emerging evidence supporting multiple beneficial effects. Intern Med J. 2012;42(9):968–978. doi: 10.1111/j.1445-5994.2012.02886.x. [DOI] [PubMed] [Google Scholar]
- Tang K, Wang Y, Zhang H, Zheng Q, Fang R, Sun Q. Cutaneous manifestations of the Coronavirus Disease 2019 (COVID-19): A brief review. Dermatol Ther 2020 [Epub ahead of publication]. [DOI] [PMC free article] [PubMed]
- te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLOS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh Le T., Andreadakis Z., Kumar A., Gómez Román R., Tollefsen S., Saville M. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- U.S Food and Drug Administration. Coronavirus Disease 2019 (COVID-19) [Internet]. 2020 [cited 2020 March 8]. Available from: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/coronavirus-disease-2019-covid-19
- Viner R.M., Whittaker E. Kawasaki-like disease: Emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Coronavirus disease (COVID-19) travel advice [Internet]. 2020 [cited 2020 March 8]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Hao X., Lau E.H.Y., Wong J.Y., Leung K.S.M., Wu J.T. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25(3):2000044. doi: 10.2807/1560-7917.ES.2020.25.3.2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):E244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S, Fernandez AP. Skin manifestations of COVID-19. Cleve Clin J Med 2020 [Epub ahead of publication]. [DOI] [PubMed]
- Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Xie S., Zhang J., Zheng F., Jiang D., Li K. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020;2020:030065. [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]