Abstract
Background
The coronavirus disease 2019 pandemic has resulted in unprecedented challenges for the oncology community. For people living with cancer, treatments are interrupted, surgeries cancelled, and regular oncology evaluations rescheduled. People with cancer and their physicians must balance plausible fears of coronavirus disease 2019 and cancer treatment with the consequences of delaying cancer care.
Objective
We aim to evaluate the experience of women with ovarian cancer during the coronavirus disease 2019 pandemic.
Study Design
Women with a current or previous diagnosis of ovarian cancer completed an online survey focusing on treatment interruptions and quality of life. The quality of life was measured with the Cancer Worry Scale and Hospital Anxiety and Depression Scale. The survey was distributed through survivor networks and social media. Univariate and multivariable linear regression analysis were used to evaluate the effect of participant characteristics on quality of life survey scores.
Results
A total of 603 women, from 41 states, visited the survey website between March 30, 2020, and April 13, 2020, and 555 (92.0%) completed the survey. The median age was 58 years (range, 20–85). At the time of survey completion, 217 participants (43.3%) were in active treatment. A total of 175 participants (33%) experienced a delay in some component of their cancer care. Ten (26.3%) of the 38 participants scheduled for surgery experienced a delay, as did 18 (8.3%) of the 217 participants scheduled for nonsurgical cancer treatment. A total of 133 participants (24.0%) had a delayed physician appointment, 84 (15.1%) laboratory tests, and 53 (9.6%) cancer-related imaging. Among the cohort, 88.6% (489) reported significant cancer worry, 51.4% (285) borderline or abnormal anxiety, and 26.5% (147) borderline or abnormal depression. On univariate analysis, age less than 65 years, being scheduled for cancer treatment or cancer surgery, delay in oncology care, being self-described as immunocompromised, and use of telemedicine were all associated with higher levels of cancer worry. Higher anxiety scores were associated with age less than 65 years and being self-described as immunocompromised. Higher depression scores were associated with age less than 65 years, being scheduled for cancer surgery, delay in oncology care, being self-described as immunocompromised, and use of telemedicine. On multivariable linear regression analysis, age less than 65 and being self-described as immunocompromised were independently predictive of greater cancer worry, anxiety, and depression, and delay in cancer care was predictive of anxiety and depression.
Conclusion
The coronavirus disease 2019 crisis is affecting care of patients with ovarian cancer; surgeries, treatments, scheduled physician appointments, laboratory tests, and imaging are cancelled or delayed. Younger age, presumed immunocompromise, and delay in cancer care were associated with significantly higher levels of cancer worry, anxiety, and depression. Providers must work with patients to balance competing risks of coronavirus disease 2019 and cancer, recognizing that communication is a critical clinical tool to improve quality of life in these times.
Key words: anxiety, cancer worry, coronavirus, COVID-19, depression, ovarian cancer
In the United States, there has been a rapid growth in the number of cases and coronavirus disease 2019 (COVID-19)–related deaths with a tremendous impact on the organization and function of medical care. Healthcare systems, including cancer centers, are attempting to find balance, minimizing viral transmission and protecting staff and patients without compromising the delivery of patient care.1 , 2 The literature on COVID-19 and cancer is quickly growing as authors attempt to educate the oncologic community with their experience in an area in which data are limited. Early reports suggest that people with cancer may experience worse outcomes from COVID-19, including higher risk of admission to intensive care units, requirement for invasive ventilation, and death.3 , 4 However, these studies are limited by small sample size, heterogeneous cancer types, and confounding variables including other medical comorbidities.
AJOG at a Glance.
Why was this study conducted?
The goal of this survey was to evaluate the experience of women with ovarian cancer during the coronavirus disease 2019 (COVID-19) pandemic, assessing the effects of the pandemic on cancer-directed treatment and quality of life (QOL).
Key findings
Of women with current or prior ovarian cancer, 33% experienced a delay in cancer care during the COVID-19 pandemic. During the pandemic, 89% reported significant cancer worry. Younger age, presumed immunocompromise, and delay in care were associated with a significant increase in cancer worry, anxiety, and depression.
What does this add to what is known?
Literature on the experience of patients with cancer during COVID-19 is limited; this study provides the perspective of ovarian cancer survivors on cancer-directed care and QOL.
The provision of relevant, understandable, and realistic information is considered a dimension of quality in cancer care. We have shown previously that women with ovarian cancer find communication between survivors and physicians to be an essential component of care.5, 6, 7, 8 As oncology providers and survivors struggle to balance plausible fears of COVID-19 and cancer treatment with the consequences of delaying cancer care, communication between patients and physicians will likely become only more critical.10, 11, 12, 9 Physicians treating women with ovarian cancer must not only remain well informed on the relationship between COVID-19 and cancer but also appreciate the unique challenges experienced by these survivors during this unprecedented time. The goal of this survey was to evaluate the experience of women with ovarian cancer during the COVID-19 pandemic, assessing the effects of the pandemic on cancer-directed treatment and quality of life (QOL).
Methods
This study was approved by the Weill Cornell Medical College and Icahn School of Medicine at Mount Sinai institutional review boards. The COVID-19 Concern Survey combined questions to assess participant demographics and cancer history, cancer-directed treatment during the COVID-19 pandemic, and QOL. The validated QOL survey instruments included the Hospital Anxiety and Depression Scale (HADS)13 and the Cancer Worry Scale (CWS).14 , 15 The HADS is a questionnaire investigating anxiety (HADS-A) and depression (HADS-D). Both HADS subscales consist of 7 items answered on a 4-point Likert scale, resulting in scores from 0 to 21. A score of 0 to 7 is considered normal anxiety or depression; 8 to 10, borderline abnormal; and 11 and 21, abnormal. The CWS is a scale to assess cancer-specific distress, consisting of 6 items answered on a 4-point Likert scale, resulting in scores ranging from 6 to 24. The higher the score, the higher the level of cancer worry, with a cutoff of 10 or greater for significant cancer worry.
The COVID-19 Concern Survey consisted of 65 questions and was distributed online via survivor networks, including the Ovarian Cancer Research Alliance and Community Partners, National Ovarian Cancer Coalition, SHARE Cancer Support, Sandy Rollman Foundation, Woman to Woman Program and Tina’s Wish, and social media via Twitter and Facebook groups, including #gyncsm (Gynecologic Cancer Social Media Community), Ovarian Cancer 101, Rare Ovarian Cancer Subtypes Team, Sisterhood of Ovarian Cancer Survivors, and Teal Life – Ovarian Cancer Awareness. The survey was created by the advocates of ovarian cancer and oncology providers to capture the experience of the COVID-19 pandemic among women with current or prior ovarian cancer. The survey was available for 14 days, from March 30, 2020, to April 13, 2020. This 2-week period was selected on the basis of prior experience with response volume to online survivor network surveys, with the goal of achieving a high level of participation and rapid dissemination of findings to women with ovarian cancer and providers.7 , 16 The survey was completed online by self-identified ovarian cancer survivors, including any woman with a current or prior diagnosis of ovarian cancer regardless of treatment status. Participation was anonymous and consent was provided electronically. Participants provided their state of residence, and states were coded based on the Centers for Disease Control and Prevention (CDC) designation as high, intermediate, and low case volume on April 17, 2020.17
Statistical methods
The distribution of continuous variables was tested for normality via the Shapiro-Wilk normality test. Univariate tests were applied based on whether the variable of interest was distributed normally (ie, t-test and analysis of variance) or not normally (ie, Mann-Whitney U test and Kruskal-Wallis test). Associations between categorical variables were evaluated using the chi-square test or Fisher exact tests, as appropriate for category size. Kappa coefficients were used to examine collinearity between variables. For assessment of QOL, validated surveys were scored per the survey protocol. Multivariable linear regression analysis was explored to evaluate the independent effect of age less than 65 years, living alone, COVID-19 case volume by state, cancer stage, scheduled for cancer treatment or surgery, delay in oncology care, medical comorbidities, access to counseling, self-described as immunocompromised, and the use of telemedicine for oncology care on the QOL survey scores (CWS, HADS-A, and HADS-D). Statistical significance was evaluated at the .05 alpha level, and 95% confidence intervals were calculated for all obtained estimates. Data were analyzed using SPSS Statistical software (version 20, SPSS, INC, 2011) and R (version 3.6.1, R Foundation for Statistical Computing, 2019).
Results
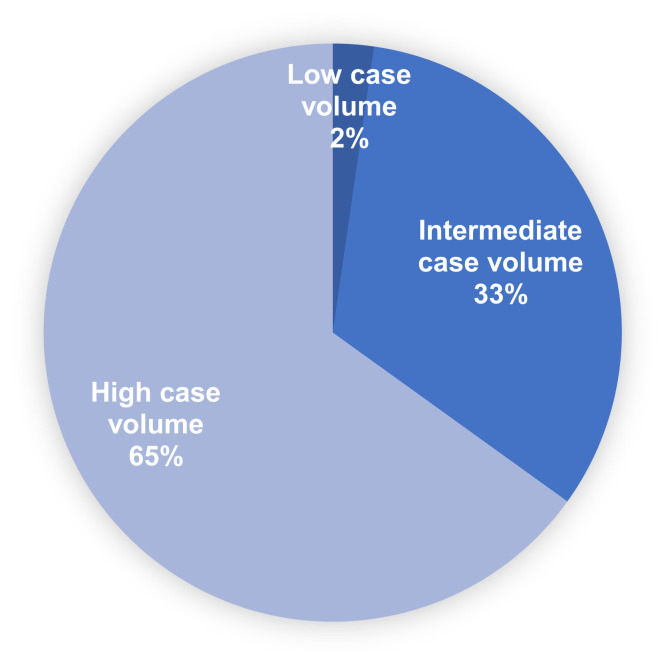
A total of 603 women visited the survey website between March 30, 2020, and April 13, 2020, and 555 (92%) completed the survey after providing electronic consent. The median age of participants was 58 years (range, 20–85). Most participants self-identified as white (469, 93.8%) (Table 1 ). There were participants from 41 states and 5 countries outside of the United States. A total of 313 participants (65.1%) came from states designated as high COVID-19 case volume by CDC (Figure 1 ).17 A total of 92 participants (16.6%) reported having a noncancer medical comorbidity, 45 participants (8.1%) reported having diabetes, 30 (5.4%) chronic lung disease, 21 (3.8%) chronic kidney disease, and 13 (2.3%) coronary artery disease.
Table 1.
Participants’ demographics and cancer history
| Characteristics | N | % |
|---|---|---|
| Age (n=511a) | ||
| <65 y | 376 | 73.58 |
| ≥65 y | 135 | 26.41 |
| Race (n=500a) | ||
| American Indian or Alaska Native | 3 | 0.60 |
| Asian or Asian American | 10 | 2.00 |
| Black or African American | 12 | 2.40 |
| White | 469 | 93.80 |
| Other | 6 | 1.20 |
| Ethnicity (n=495a) | ||
| Hispanic or Latino | 14 | 2.83 |
| Not Hispanic or Latino | 481 | 97.17 |
| Current relationship status (n=505a) | ||
| Married/civil union/domestic partnership | 340 | 67.33 |
| Divorced/widowed/separated | 87 | 17.23 |
| Single | 78 | 15.45 |
| Living alone (n=507a) | ||
| Yes | 101 | 19.92 |
| No | 406 | 80.08 |
| Highest level of education (n=506a) | ||
| Less than high school degree | 1 | 0.20 |
| High school degree or equivalent | 38 | 7.51 |
| Some college | 137 | 27.08 |
| Bachelor degree | 169 | 33.40 |
| Graduate degree | 161 | 31.82 |
| Ovarian cancer stage (n=505a) | ||
| I/II | 170 | 33.66 |
| III/IV | 321 | 63.56 |
| Uncertain | 14 | 2.77 |
| Completed first-line treatment (n=505a) | ||
| Yes | 476 | 94.26 |
| No | 20 | 3.96 |
| Uncertain | 9 | 1.78 |
| Currently receiving treatment (n=501a) | ||
| Yes | 217 | 43.31 |
| No | 284 | 56.69 |
Frey et al. Ovarian cancer during the COVID-19 pandemic. Am J Obstet Gynecol 2020.
Number of respondents answering survey question.
Figure 1.
Distribution of COVID-19 case volume of participants’ states by the Centers for Disease Control and Prevention designation as high, intermediate, and low
COVID-19, coronavirus disease 2019.
Frey et al. Ovarian cancer during the COVID-19 pandemic. Am J Obstet Gynecol 2020.
At the time of survey completion, 217 (43.3%) participants were receiving cancer-directed therapy. Treatments included intravenous chemotherapy (61, 28.1%), oral chemotherapy (12, 5.5%), poly (ADP-ribose) polymerase (PARP) inhibitor therapy (70, 32.3%), immunotherapy (10, 4.6%), PARP inhibitor combined with immunotherapy (3, 1.4%), hormonal therapy (25, 11.5%), antiangiogenic therapy (29, 13.4%), other targeted therapy (3, 1.4%), and radiation (4, 1.8%). Overall, 175 participants (33.3%) reported a delay in some component of their cancer care. Among the 217 participants scheduled for nonsurgical cancer-directed therapy, 18 (8.3%) reported that their treatment was postponed and 199 (91.7%) underwent treatment without delay. Planned treatments that were postponed included intravenous chemotherapy (7), oral chemotherapy (1), PARP inhibitor therapy (4), immunotherapy (1), hormonal therapy (3), antiangiogenic therapy (1), and other targeted therapy (1). A total of 38 participants (6.9%) reported that they were scheduled for surgical treatment of ovarian cancer. Among these women, 10 (26.3%) reported that the surgery was delayed because of COVID-19. A total of 133 participants (24.0%) reported that an appointment with their gynecologic oncologist was postponed, 84 participants (15.1%) reported that a cancer-related laboratory test was postponed, and 53 participants (9.6%) reported that cancer-related imaging was postponed.
Of 503 participants, 252 (50.1%) responded that they self-described as immunocompromised, with 129 (25.6%) not self-describing as immunocompromised and 122 (24.3%) uncertain. The correlation between self-described as immunocompromised and receiving active cancer treatment was modest (kappa=0.39). Of 516 responders, 129 (25.0%) reported they had used telemedicine for gynecologic oncology care, with 224 (43.4%) reporting they had not and 163 (31.6%) uncertain. When asked whether or not they had access to online or in-person counseling, 287 (55.8%) of the 514 responders said yes, 111 (21.6%) said no, and 116 (22.6%) were uncertain. Of 517 respondents, 415 (80.3%) participated in networks for ovarian cancer survivors, 89 (17.2%) did not, and 13 (2.5%) were uncertain. Of note, when participants were asked about participation in telemedicine, counseling, and survivor networks, the services were not further defined.
QOL was measured with 2 survey instruments that have been validated in the oncology literature, CWS and HADS. Among the 552 participants completing CWS, the median CWS score was 14 (range, 6–24). A total of 489 participants (88.6%) reported significant cancer worry. Among the 555 participants completing HADS, the median anxiety score was 8 (range, 0–21) and the median depression score was 5 (range, 0–21). A total of 285 participants (51.4%) reported borderline or abnormal anxiety (borderline: 155, 27.9%; abnormal: 130, 23.4%) and 147 (26.5%) borderline or abnormal depression (borderline: 105, 18.9%; abnormal: 42, 7.6%).
The demographics, cancer, and treatment history of participants were evaluated for associations with cancer worry, anxiety, and depression. On univariate analysis, age less than 65 years, being scheduled for cancer treatment or cancer surgery, delay in oncology care, self-described as immunocompromised, and use of telemedicine were all associated with higher levels of cancer worry. Higher anxiety scores were associated with age less than 65 years and self-described as immunocompromised. Higher depression scores were associated with age less than 65 years, being scheduled for cancer surgery, delay in oncology care, self-described as immunocompromised, and use of telemedicine. Depression scores were significantly lower in participants reporting access to counseling (Table 2 ).
Table 2.
Univariate analysis of predictors of cancer worry, anxiety, and depression
| Predictor | Cancer Worry Scale |
HADS-Anxiety |
HADS-Depression |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Min | Max | P value | N | Median | Min | Max | P value | N | Median | Min | Max | P value | |
| Age | <.001 | .003 | .041 | ||||||||||||
| ≥65 y | 135 | 13.0 | 6 | 23 | 135 | 7.0 | 0 | 18 | 135 | 4.0 | 0 | 21 | |||
| <65 y | 373 | 15.0 | 6 | 24 | 376 | 8.0 | 0 | 21 | 376 | 5.0 | 0 | 19 | |||
| Living alone | .599 | .619 | .129 | ||||||||||||
| No | 404 | 14.0 | 6 | 24 | 406 | 8.0 | 0 | 20 | 406 | 5.0 | 0 | 21 | |||
| Yes | 100 | 14.0 | 6 | 23 | 101 | 8.0 | 0 | 21 | 101 | 5.0 | 0 | 19 | |||
| State | .756 | .84 | .568 | ||||||||||||
| High case volume | 312 | 14.0 | 6 | 24 | 313 | 8.0 | 0 | 20 | 313 | 5.0 | 0 | 17 | |||
| Moderate case volume | 156 | 14.5 | 6 | 24 | 157 | 8.0 | 0 | 21 | 157 | 5.0 | 0 | 21 | |||
| Low case volume | 11 | 14.0 | 9 | 21 | 11 | 7.0 | 1 | 13 | 11 | 4.0 | 1 | 11 | |||
| Cancer stage | .106 | .121 | .672 | ||||||||||||
| 1 | 109 | 13.0 | 6 | 22 | 109 | 109 | |||||||||
| 2 | 61 | 15.0 | 6 | 24 | 61 | 9.0 | 0 | 21 | 61 | 5.0 | 0 | 21 | |||
| 3 | 241 | 14.0 | 6 | 24 | 244 | 7.0 | 0 | 20 | 244 | 4.0 | 0 | 19 | |||
| 4 | 77 | 15.0 | 6 | 23 | 77 | 8.0 | 0 | 18 | 77 | 5.0 | 0 | 15 | |||
| Unknown | 14 | 15.5 | 7 | 24 | 14 | 7.5 | 1 | 15 | 14 | 6.0 | 0 | 12 | |||
| Scheduled for cancer treatment | <.001 | .645 | .062 | ||||||||||||
| No | 284 | 13.0 | 6 | 24 | 284 | 8.0 | 0 | 20 | 284 | 4.0 | 0 | 19 | |||
| Yes | 214 | 15.5 | 6 | 24 | 217 | 8.0 | 0 | 21 | 217 | 5.0 | 0 | 19 | |||
| Scheduled for cancer surgery | .03 | .304 | .022 | ||||||||||||
| No | 485 | 14.0 | 6 | 24 | 488 | 8.0 | 0 | 21 | 488 | 5.0 | 0 | 19 | |||
| Yes | 38 | 17.0 | 6 | 23 | 38 | 9.0 | 1 | 18 | 38 | 7.0 | 0 | 21 | |||
| Delay in oncology care | .002 | .068 | .032 | ||||||||||||
| No | 350 | 14.0 | 6 | 24 | 351 | 8.0 | 0 | 20 | 351 | 5.0 | 0 | 21 | |||
| Yes | 173 | 15.0 | 7 | 24 | 175 | 8.0 | 0 | 21 | 175 | 5.0 | 0 | 19 | |||
| Medical Comorbidity (noncancer) | .443 | .233 | .071 | ||||||||||||
| No | 431 | 14.0 | 6 | 24 | 434 | 8.0 | 0 | 21 | 434 | 5.0 | 0 | 21 | |||
| Yes | 92 | 15.0 | 8 | 24 | 92 | 8.0 | 0 | 20 | 92 | 5.5 | 0 | 18 | |||
| Access to counseling | .142 | .136 | .026 | ||||||||||||
| No | 110 | 15.5 | 6 | 24 | 111 | 9.0 | 0 | 20 | 111 | 6.0 | 0 | 21 | |||
| Yes | 285 | 14.0 | 6 | 24 | 287 | 8.0 | 0 | 21 | 287 | 5.0 | 0 | 19 | |||
| Uncertain | 116 | 15.0 | 6 | 24 | 116 | 8.0 | 0 | 18 | 116 | 5.0 | 0 | 19 | |||
| Self-described as immunocompromised | <.001 | .05 | <.001 | ||||||||||||
| No | 128 | 12.5 | 6 | 23 | 129 | 7.0 | 0 | 18 | 129 | 4.0 | 0 | 17 | |||
| Yes | 250 | 16.0 | 6 | 24 | 252 | 8.0 | 0 | 21 | 252 | 5.0 | 0 | 21 | |||
| Uncertain | 122 | 14.0 | 6 | 23 | 122 | 8.0 | 0 | 20 | 122 | 4.0 | 0 | 19 | |||
| Use of telemedicine | <.001 | .139 | .025 | ||||||||||||
| No | 224 | 13.0 | 6 | 24 | 224 | 7.0 | 0 | 20 | 224 | 4.0 | 0 | 21 | |||
| Yes | 129 | 16.0 | 6 | 24 | 129 | 8.0 | 0 | 21 | 129 | 5.0 | 0 | 19 | |||
| Uncertain | 163 | 15.0 | 8 | 24 | 163 | 8.0 | 0 | 20 | 163 | 4.0 | 0 | 15 | |||
HADS, Hospital Anxiety and Depression Scale; Max, maximum; Min, minimum.
Frey et al. Ovarian cancer during the COVID-19 pandemic. Am J Obstet Gynecol 2020.
We performed a multivariable linear regression model incorporating age less than 65 years, living alone, COVID-19 case volume by state, cancer stage, scheduled for cancer treatment or surgery, delay in oncology care, medical comorbidities, access to counseling, self-described as immunocompromised, and use of telemedicine for oncology care. On multivariable analysis, cancer worry was independently associated with age less than 65 years, being scheduled for cancer treatment, and self-described as immunocompromised. Anxiety was independently associated with age less than 65 years, delay in oncology care, and self-described as immunocompromised. Depression was independently associated with age less than 65 years, living alone, delay in oncology care, lack of access to counseling, and self-described as immunocompromised (Table 3 ).
Table 3.
Multivariable linear regression predicting cancer worry, anxiety, and depression
| Characteristic Term |
Cancer Worry Scale |
HADS-Anxiety |
HADS-Depression |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β coefficient | 95% CI: low | 95% CI: high | P value | β coefficient | 95% CI: low | 95% CI: high | P value | β coefficient | 95% CI: low | 95% CI: high | P value | |
| Intercept | 11.835 | 9.115 | 14.555 | <.001 | 7.217 | 4.273 | 10.161 | <.001 | 4.353 | 1.797 | 6.909 | <.001 |
| Age ≥65 y | −1.236 | −2.052 | −0.42 | .003 | −1.016 | −1.899 | −0.133 | .024 | −0.821 | −1.588 | −0.054 | .036 |
| Living alone | −0.306 | −1.235 | 0.622 | .517 | 0.006 | −0.999 | 1.011 | .99 | 1.118 | 0.246 | 1.991 | .012 |
| Scheduled for nonsurgical cancer treatment | 1.124 | 0.258 | 1.99 | .011 | −0.203 | −1.138 | 0.733 | .671 | 0.409 | −0.403 | 1.222 | .322 |
| Scheduled for cancer surgery | 0.764 | −1.583 | 3.11 | .523 | −0.183 | −2.724 | 2.357 | .887 | 0.712 | −1.493 | 2.918 | .526 |
| Delay in oncology care | 0.604 | −0.184 | 1.392 | .133 | 0.947 | 0.097 | 1.797 | .029 | 0.813 | 0.075 | 1.552 | .031 |
| Medical comorbidity (noncancer) | 0.504 | −0.416 | 1.425 | .282 | 0.876 | −0.12 | 1.872 | .085 | 0.799 | −0.066 | 1.663 | .07 |
| Access to counseling (Yes vs no [referent]) | −0.786 | −1.733 | 0.162 | .104 | −0.592 | −1.612 | 0.428 | .255 | −1.148 | −2.033 | −0.262 | .011 |
| Self-described as immunocompromised (Yes vs no [referent]) | 1.705 | 0.761 | 2.649 | <.001 | 1.295 | 0.278 | 2.311 | .013 | 1.324 | 0.442 | 2.206 | .003 |
| Use of telemedicine (Yes vs no [referent]) | 0.754 | −0.226 | 1.734 | .131 | −0.324 | −1.379 | 0.731 | .546 | 0.203 | −0.713 | 1.119 | .663 |
CI, confidence interval; HADS, Hospital Anxiety and Depression Scale.
Frey et al. Ovarian cancer during the COVID-19 pandemic. Am J Obstet Gynecol 2020.
Of 526 responders, 57 (10.8%) reported having a symptom of COVID-19 and 12 (2.3%) underwent COVID-19 testing. Among those tested, 3 had a positive test and 9 had a negative test. Reporting COVID-19 symptoms was not predictive of the CWS score (15 [6–24] vs 14 [6–24], P=.70), HADS-A score (9 [0–18] vs 8 [0–21], P=.102), or HADS-D score (7 [0–21] vs 5 [0–19], P=.074). Participants who had undergone COVID-19 testing reported significantly higher anxiety scores (10.5 [1–15] vs 8 [0–21], P=.012) and depression scores (5.5 [1–16] vs 5 [0–21], P=.05).
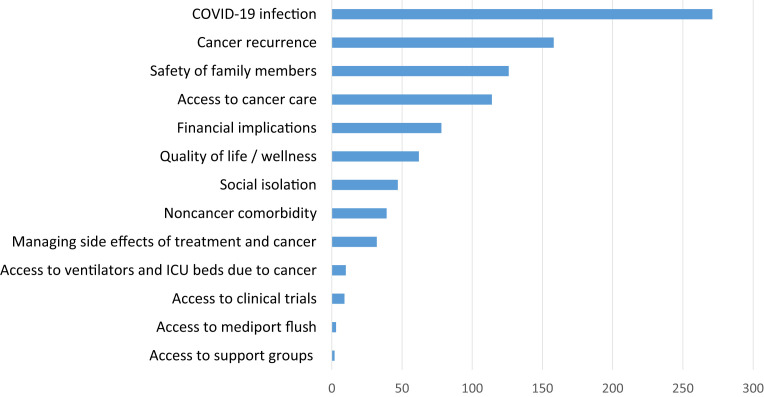
Participants were asked to provide, via free text, their top concerns. The most commonly listed concerns among the 469 responding participants included COVID-19 infection (271, 57.8%), cancer recurrence (158, 33.7%), safety of family members (126, 26.9%), access to cancer care (114, 24.3%), financial implications of COVID-19 (78, 16.6%), overall QOL and wellness (62, 13.2%), and social isolation (47, 10.0%) (Figure 2 ).
Figure 2.
Participants’ top concerns during the COVID-19 crisis
COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Frey et al. Ovarian cancer during the COVID-19 pandemic. Am J Obstet Gynecol 2020.
Comment
People living with cancer and providers face unprecedented challenges during the COVID-19 crisis. Our survey of 555 ovarian cancer survivors demonstrates that many have experienced interruptions in their cancer-directed care. Of the participants, 33% reported a delay in at least one component of their cancer care including surgery, nonsurgical cancer-directed therapy, physician appointment, laboratory tests, and imaging. Among the 225 participants scheduled for surgery or chemotherapy, 11% (28) experienced a delay in their cancer treatment. Surgical delays were more common than delays of nonsurgical cancer treatments (26% vs 8%); however, the cohort of women scheduled for surgery was small. These significant interruptions in cancer care are not surprising given that medical staff and resources have been redeployed to manage COVID-19, hospital policies call for cancellation of nonemergent surgical procedures, and general recommendations are to avoid hospital visits because of infection risk and the current strategy of restrictive social distancing.
A total of 89% of participants demonstrated significant cancer worry on CWS. This was a vastly greater proportion than has previously been reported among women with ovarian cancer (88.6% vs 57.9% or 19.3%). However, previous studies of CWS evaluated women after the completion of treatment and our cohort included women during and after treatment.18 , 19 In contrast, HADS-A scores indicated that 23.4% of participants completing our survey had abnormal levels of anxiety, which correlates with previously reported levels of clinically abnormal anxiety outside of the COVID-19 time frame (which spanned 13.9%–48.1%). HADS-D scores indicated that 7.6% of participants met the criteria for abnormal depression. This is comparable with the rates of previously published posttreatment HADS-D scores among women both undergoing treatment and having completed treatment, ranging from 5% to 33.7%.20, 21, 22, 23, 24, 25 The pandemic did not seem to negatively affect anxiety and depression; however, these results may be due to our patient population being super users of counseling (56%) and survivor networking (80%) and possibly, as indicated by their willingness to complete a 65-item survey during these times, especially generous, a character trait associated with happiness.26
On multivariable regression analysis, age less than 65 years and self-describing as immunocompromised were associated with higher levels of cancer worry, anxiety, and depression. It was surprising to find younger age being associated with increased worry, anxiety, and depression during the COVID-19 crisis because preliminary reports suggest that older age is associated with increased COVID-19 mortality.27, 28, 29 However, pre–COVID-19 literature on QOL and age for women with ovarian cancer is inconsistent. Several studies confirm our findings that younger age is associated with higher cancer worry, depression, and anxiety, often suggesting that the threat of aggressive illness and its consequences is greater for younger women.19 , 21 Other studies have found no difference based on age or even greater depression with older age.22 , 25 , 30 The association between self-describing as immunocompromised and heightened worry, anxiety, and depression was less surprising because the CDC includes individuals who are immunocompromised owing to cancer treatment among those at highest risk for severe COVID-19 illness.31 Delay in oncology care was predictive of both anxiety and depression. It is important that gynecologic oncologists consider this because, although the intent behind postponing oncology-related appointments may be to minimize COVID-19 infection in a vulnerable patient population, this decision may negatively affect QOL. Interestingly, living in a higher burden COVID-19 state did not negatively affect QOL on any of the measures used, although this may be due to our cohort being composed of a small number of women from low-volume COVID-19 states. Education, cancer stage, and medical comorbidities were not significantly associated with QOL scores.
At the conclusion of the survey, participants were asked to share their top concerns during the COVID-19 pandemic. The most commonly cited concerns were about COVID-19, cancer recurrence, and the safety of family members. The fifth most commonly shared concern, reported by 17% of participants, was the financial implications of the crisis. Limited data on cost of care for ovarian cancer suggest substantial overall costs as well as survivor out-of-pocket expenditure.32 , 33 A recent study has reported that individuals with a cancer diagnosis were 2.5 times more likely to file for bankruptcy than individuals without cancer.34 The country is only beginning to experience the financial implications of COVID-19, and it is critical that the oncology community is attentive to the potential financial toxicity experienced by ovarian cancer survivors.
There are an estimated 233,000 women living with ovarian cancer in the United States, and therefore, our cohort of 555 women represents a small proportion of ovarian cancer survivors.35 Furthermore, our cohort may not be representative of the greater population of women with ovarian cancer. Women with ovarian cancer who participate in online surveys via survivor networks and Twitter and Facebook groups may be more reflective and informed than those who do not. Those well enough to respond to online survey invitations may represent a limited spectrum of women with current or prior ovarian cancer with computer literacy and access to computers. In addition, most participants identified as non-Hispanic white with extremely limited diversity in race and ethnicity. Emerging data suggest that the COVID-19 pandemic has disproportionately affected many minority and marginalized populations in the United States.36, 37, 38 All these selection biases could have skewed our findings. Furthermore, as demonstrated in Figure 1, our cohort tended to live in areas of higher COVID-19 case density, which could certainly affect the generalizability of these results. The survey did not capture information on insurance status, employment, or income, which certainly may affect the impact of COVID-19 on QOL. However, our study did include a large number of women with a current or prior diagnosis of ovarian cancer with diversity in geographic location, age, education, and spectrum on the treatment continuum, and the lessons learned are likely relevant to other people with cancer.
Our findings suggest that ovarian cancer survivors are experiencing delays in cancer-directed treatment during the COVID-19 crisis. Furthermore, women with ovarian cancer participating in this study reported high levels of cancer worry, anxiety, and depression. These components of QOL were negatively affected by younger age, self-described as immunocompromised, and delays in oncology care. Access to counseling services may mitigate some of the depression experienced by these women. People with cancer value communication with their treating oncologist and shared decision making throughout the disease spectrum, especially at times of treatment modifications.7 , 8 , 16 , 39 Oncologists must therefore remain well informed on the emerging data addressing COVID-19 and cancer, dynamically incorporating the COVID-19 climate into patient care to achieve an acceptable balance between risk of infection and risk of untreated cancer. Furthermore, oncologists must screen patients for their psychosocial well-being and establish a robust framework of patient support, recognizing that communication is a critical clinical tool to improve QOL in these times.
Footnotes
K.H. serves as a consultant for Johnson and Johnson and receives research support from Fujirebio Diagnostics. The remaining authors report no conflict of interest.
C.T. and P.J.C. were both partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
This communication has been published in the middle of the COVID-19 pandemic and is available via expedited publication to assist patients and healthcare providers.
Cite this article as: Frey MK, Ellis AE, Zeligs K, et al. Impact of the coronavirus disease 2019 pandemic on the quality of life for women with ovarian cancer. Am J Obstet Gynecol 2020;223:725.e1-9.
References
- 1.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannistra S.A., Haffty B.G., Ballman K. Challenges faced by medical journals during the COVID-19 pandemic. J Clin Oncol. 2020 doi: 10.1200/JCO.20.00858. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning D.L., Dickens C. Cancer information and support centres: fixing parts cancer drugs cannot reach. Eur J Cancer Care (Engl) 2007;16:33–38. doi: 10.1111/j.1365-2354.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 6.Fitch M.I., McAndrew A., Harth T. Perspectives from older adults receiving cancer treatment about the cancer-related information they receive. Asia Pac J Oncol Nurs. 2015;2:160–168. doi: 10.4103/2347-5625.160971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey M.K., Philips S.R., Jeffries J. A qualitative study of ovarian cancer survivors’ perceptions of endpoints and goals of care. Gynecol Oncol. 2014;135:261–265. doi: 10.1016/j.ygyno.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Frey M.K., Ellis A., Shyne S., Kahn R., Chapman-Davis E., Blank S.V. Bridging the gap: a priorities assessment tool to support shared decision making, maximize appointment time, and increase patient satisfaction in women with ovarian cancer. J Oncol Pract. 2020;16:e148–e154. doi: 10.1200/JOP.19.00455. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y., Jin R., Zhao J., Li W., Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H., Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutikov A., Weinberg D.S., Edelman M.J., Horwitz E.M., Uzzo R.G., Fisher R.I. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172:756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey M.K., Blank S.V. Coronavirus concerns: what do women with gynecologic cancer need to know during the COVID-19 crisis? Gynecol Oncol. 2020;158:32–33. doi: 10.1016/j.ygyno.2020.04.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 14.Custers J.A.E., Kwakkenbos L., van de Wal M., Prins J.B., Thewes B. Re-validation and screening capacity of the 6-item version of the Cancer Worry Scale. Psychooncology. 2018;27:2609–2615. doi: 10.1002/pon.4782. [DOI] [PubMed] [Google Scholar]
- 15.Lerman C., Daly M., Masny A., Balshem A. Attitudes about genetic testing for breast-ovarian cancer susceptibility. J Clin Oncol. 1994;12:843–850. doi: 10.1200/JCO.1994.12.4.843. [DOI] [PubMed] [Google Scholar]
- 16.Frey M.K., Ellis A.E., Koontz L.M. Ovarian cancer survivors’ acceptance of treatment side effects evolves as goals of care change over the cancer continuum. Gynecol Oncol. 2017;146:386–391. doi: 10.1016/j.ygyno.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19): cases in the U.S. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Available at: Accessed April 17, 2020.
- 18.Daniele A., Olivero F., Menato G., Biglia N. Fear of cancer recurrence in ovarian cancer survivors. Eur J Obstet Gynecol Reprod Biol. 2016;206:E30. [Google Scholar]
- 19.Krok-Schoen J.L., Naughton M.J., Bernardo B.M., Young G.S., Paskett E.D. Fear of recurrence among older breast, ovarian, endometrial, and colorectal cancer survivors: findings from the WHI LILAC study. Psychooncology. 2018;27:1810–1815. doi: 10.1002/pon.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisseling K.C., Kondalsamy-Chennakesavan S., Bekkers R.L., Janda M., Obermair A. Depression, anxiety and body image after treatment for invasive stage one epithelial ovarian cancer. Aust N Z J Obstet Gynaecol. 2009;49:660–666. doi: 10.1111/j.1479-828X.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 21.Hipkins J., Whitworth M., Tarrier N., Jayson G. Social support, anxiety and depression after chemotherapy for ovarian cancer: a prospective study. Br J Health Psychol. 2004;9:569–581. doi: 10.1348/1359107042304542. [DOI] [PubMed] [Google Scholar]
- 22.Wen Q., Shao Z., Zhang P., Zhu T., Li D., Wang S. Mental distress, quality of life and social support in recurrent ovarian cancer patients during active chemotherapy. Eur J Obstet Gynecol Reprod Biol. 2017;216:85–91. doi: 10.1016/j.ejogrb.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Shand L.K., Brooker J.E., Burney S., Fletcher J., Ricciardelli L.A. Symptoms of posttraumatic stress in Australian women with ovarian cancer. Psychooncology. 2015;24:190–196. doi: 10.1002/pon.3627. [DOI] [PubMed] [Google Scholar]
- 24.Hodgkinson K., Butow P., Fuchs A. Long-term survival from gynecologic cancer: psychosocial outcomes, supportive care needs and positive outcomes. Gynecol Oncol. 2007;104:381–389. doi: 10.1016/j.ygyno.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 25.Mielcarek P., Nowicka-Sauer K., Kozaka J. Anxiety and depression in patients with advanced ovarian cancer: a prospective study. J Psychosom Obstet Gynaecol. 2016;37:57–67. doi: 10.3109/0167482X.2016.1141891. [DOI] [PubMed] [Google Scholar]
- 26.Park S.Q., Kahnt T., Dogan A., Strang S., Fehr E., Tobler P.N. A neural link between generosity and happiness. Nat Commun. 2017;8:15964. doi: 10.1038/ncomms15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price M.A., Butow P.N., Costa D.S. Prevalence and predictors of anxiety and depression in women with invasive ovarian cancer and their caregivers. Med J Aust. 2010;193:S52–S57. doi: 10.5694/j.1326-5377.2010.tb03929.x. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention People who are at increased risk for severe illness. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-increased-risk.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fpeople-at-higher-risk.html Available at: Accessed April 17, 2020.
- 32.Bercow A.S., Chen L., Chatterjee S. Cost of care for the initial management of ovarian cancer. Obstet Gynecol. 2017;130:1269–1275. doi: 10.1097/AOG.0000000000002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urban R.R., He H., Alfonso-Cristancho R., Hardesty M.M., Goff B.A. The cost of initial care for Medicare patients with advanced ovarian cancer. J Natl Compr Canc Netw. 2016;14:429–437. doi: 10.6004/jnccn.2016.0049. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey S., Blough D., Kirchhoff A. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Cancer Institute Surveillance, Epidemiology, and End Results. Cancer stat facts: uterine cancer. http://seer.cancer.gov/statfacts/html/corp.html Available at: Accessed April 17, 2020.
- 36.Wang Z., Tang K. Combating COVID-19: health equity matters. Nat Med. 2020;26:458. doi: 10.1038/s41591-020-0823-6. [DOI] [PubMed] [Google Scholar]
- 37.Laurencin C.T., McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farley J.H., Hines J., Lee N.K. Promoting health equity in the era of COVID-19. Gynecol Oncol. 2020;158:25–31. doi: 10.1016/j.ygyno.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winckworth-Prejsnar K., Lentz L.K., Nardi E.A., Pruthi S., Fitzgerald C.L., Carlson R.W. Value tools for patients in cancer care. J Natl Compr Canc Netw. 2017;15:872–877. doi: 10.6004/jnccn.2017.0118. [DOI] [PubMed] [Google Scholar]