Abstract
Background
Using personal protective equipment (PPE) is one of several fundamental measures to prevent the transmission of infection and infectious diseases and is particularly pertinent in the current COVID-19 pandemic. Appropriate use of PPE by healthcare workers is, however, often suboptimal. Training and monitoring of PPE competency are essential components of an infection prevention and control program but there is a paucity of research and data on the content of such training programs across Australasia. This paper reports the results of a survey that characterised the nature of PPE training in Australian and New Zealand hospitals.
Methods
A population-based online survey was distributed to members of three major Australasian colleges representing infection prevention and control.
Results
Results indicate that, although training is frequently provided at orientation, many healthcare workers do not receive regular updates. Training programmes combine online and classroom sessions, but over a third do not include a practical component. The frequency of monitoring PPE competency is variable with one third of respondents indicating that no auditing occurs. PPE items used for high-level training are variable, with use of powered air purifying respirators (PAPRs) uncommon.
Conclusion
The results of this study suggest that HCWs’ confidence, competence and familiarity with PPE are a concern, which in the context of the current global COVID-19 pandemic is problematic. More research is needed into how PPE training programs could be better designed, to prepare HCWs for practice using PPE safely and confidently.
Keywords: Personal protective equipment, Infection prevention and control, Training, Education, COVID-19
Highlights
-
•
Most HCW orientation programmes include PPE training but fewer than half are updated annually.
-
•
One third of PPE programmes do not include a practical component.
-
•
Only two thirds of PPE training programmes monitor PPE compliance.
-
•
Future research should consider the design of PPE training programs to optimise HCW PPE practice.
Introduction
Healthcare associated infections (HAIs) cause significant morbidity and excess healthcare costs [1], and exacerbate the spread of antimicrobial resistance [2]. Personal protective equipment (PPE) is one of several important components of standard and transmission-based infection prevention and control (IPC) precautions [3] but sub-optimal use of PPE has been reported in varied hospital settings [[4], [5], [6]]. There are different configurations of PPE for different levels of standard and transmission-based precautions which include such items as protective masks, disposable gowns and gloves and face protection. Recurring outbreaks of viral haemorrhagic fever (VHF) [7] and the global pandemic of coronavirus disease (COVID-19), are contemporary reminders of the importance of PPE for the prevention and control of infectious diseases. Previous outbreaks of infectious disease have caused anxiety among healthcare workers (HCWs) and exposed deficiencies in their knowledge and understanding of IPC [8]. Failures of appropriate PPE use has contributed to hospital outbreaks [9].
In Australia the accreditation of hospitals via the Australian Council on Healthcare Standards (ACHS) [10] together with state and territory workplace health and safety legislation [11] mandates that all healthcare workers receive education and training in the workplace, including the use of PPE, and that the safety of work practices are actively monitored. While the National Health and Medical Research Council (NMRC) provides guidance about developing or implementing PPE training programs [3], this guidance does not translate to an defined accreditation standard. In New Zealand health and safety legislation requires organisations to provide information and training on PPE use and ensure it is worn in the workplace [12]. The New Zealand Ministry of Health IPC certification standards [13] require organisations to provide education and training in standard and transmission-based precautions which incorporates PPE but does not specify any monitoring requirements. The former Australian National Hand Hygiene Initiative led to improved hand hygiene compliance and was associated with reduction in the rate of healthcare associated Staphylococcus aureus bacteraemia [14], but hand hygiene is only one aspect of IPC practice. The formalisation of PPE training and auditing would improve the safety of staff and patients, and the prevention and control of infection and infectious disease.
Despite the importance of education, training and practice monitoring for optimal PPE use, there is a paucity of published information and research that documents PPE training programs in Australia and New Zealand. An understanding of them would assist in assuring more appropriate use of PPE to reduce the risk of occupational acquisition of and the spread of infectious diseases. This has been highlighted, particularly, during the current COVID-19 global pandemic where there are concerns from healthcare workers around PPE training [15,16] and the PPE supply chain is not guaranteed [17]. The purpose of this survey was to characterise and assess the extent and nature of PPE training in Australasian hospitals, and to inform future stakeholder consultations about, and gather respondents’ views on, other aspects of PPE training.
Methods
Study design, participants and recruitment
A cross-sectional, population-based, on-line survey of members of three Australasian IPC professional societies and colleges was undertaken over an eight-week period from August and October 2019. The three professional societies and colleges were the Australasian College of Infection Prevention and Control (ACIPC), the New Zealand Infection Prevention and Control Nurses College (IPCNC NZNO), and the Healthcare Infection Control Special Interest Group (HICSIG) of the Australasian Society for Infectious Diseases. The membership of the societies and colleges at the time of the survey was ACIPC 1143 members, IPCNC NZNO 630 members and HICSIG 250 members, with some individuals holding membership of at least two of these organisations.
Study instrument
The survey tool was developed using expert opinion and reference to PPE training in relevant IPC guidelines [3] and comprised four sections. Questions from sections 1–3 are shown in Table 1 (Appendix). Section 1 gathered data about respondents’ roles in, and demographics of the facility/organisation in which they worked. Section 2 explored details of training programmes for routine PPE, including content and delivery modes, responsibility for providing the training and auditing PPE practice. Section 3 asked questions about training for high-level PPE including types of PPE used and who delivered and received this level of training. Section 4 collected mainly qualitative data about PPE, analysis of which will be reported separately.
For the purposes of this research ‘routine PPE’ was defined as those items of PPE which are used by HCWs for standard and transmission-based precautions for commonly encountered infectious diseases and pathogens, while ‘high-level PPE’ referred to an ensemble of alternative PPE items that are used with rare infectious diseases of high consequence such as a powered air purifying respirator (PAPR).
Data collection and analysis
Study data were collected via a survey and managed using REDCap™ electronic data capture tools hosted at University of Sydney [18]. Participants accessed an anonymous, online survey via an electronic link generated in REDCap™. Invitations to participate were distributed by each society or college to its own members by organisational websites, members' discussion forums, social media pages and Presidents'/Chairpersons’ e-newsletters. Only those respondents who indicated they were employed in an active IPC role in a healthcare facility were invited to participate and the survey requested that, where possible, only one respondent per facility participate.
Frequencies and percentages were calculated for all relevant variables using Microsoft Excel (2019).
Ethics
The research project was approved by the University of Sydney Human Research Ethics Committee (2019/614). Informed consent was implied in completing and submitting the survey. The survey was subsequently accepted for dissemination by the three professional organisations.
Results
Section 1. respondents and facilities
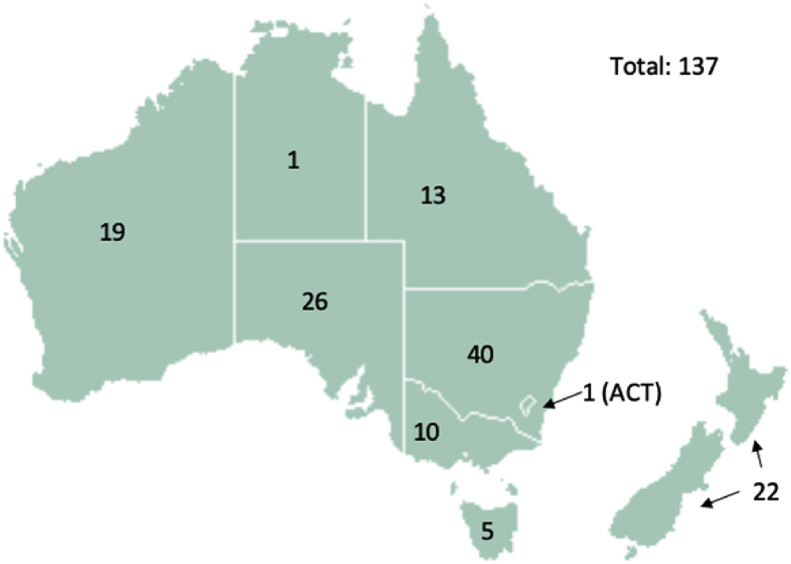
The geographic distribution of 137 survey respondents and the facilities they identified with are shown in Fig. 1 .
Fig. 1.
Geographical distribution of 137 survey respondents in Australia and New Zealand.
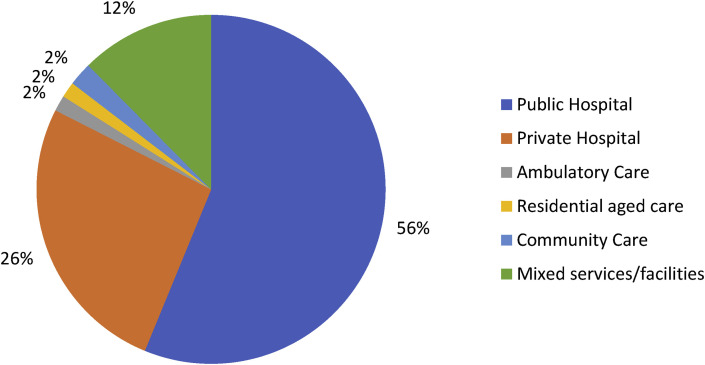
Respondents had worked in IPC for an average of 8.5 (range 0.5–43) years. Seventy-seven (56%) reported working in a public hospital, 36 (26%) in a private hospital and 24 (18%) mixed public/private or non-inpatient facilities/services representing approximately 10% and 5% of public and private hospitals respectively as shown in Fig. 2 .
Fig. 2.
Reported frequencies of healthcare facility types represented by respondents.
Inpatient bed numbers as of 1 July 2019 in these facilities, as reported by the participants, ranged from 14 to 1300 (mean 300, median 200). More than half of the respondents reported that their facility had the following specialist services: an emergency department, 89 (65%); critical care, 87 (63.5%); and paediatrics, 71 (52%). Respondents also stated that 46 (33.5%) facilities had a respiratory ward and 31 (23%) housed dedicated infectious disease beds. The majority (75%–97%) of the respondents who identified the different specialist services worked in a public hospital.
In Australia there are thirteen formally designated VHF biocontainment units – between one and three in each State and Territory - and four such units in New Zealand. Ten of the respondents reported working in a facility that was designated as a State/Territory VHF biocontainment unit. One of these respondents was from New Zealand and nine were from four Australian states; New South Wales, Western Australia, South Australia, and Victoria. At least four VHF biocontainment units were not represented. Completed the survey.
Section 2: training programmes for routine PPE
Two out of the 137 respondents reported that no PPE training was provided at their facility. Respondents indicated a combination of training frequencies; 96 (70%) included PPE training at new staff orientation, 55 (40%) provided annual updates and 84 (61%) provided training on an ad hoc/on request basis, at intervals ranging from monthly to every 5 years. Annual refresher sessions were delivered more frequently in private facilities (21/36; 58%) than public hospitals (29/37; 38%). Three quarters of respondents (n = 105:77%) indicated that some training was provided for all staff who work in clinical areas, while 21 (15%) and 11 (8%) responses stated that training was limited to professional clinical staff and specific occupational groups respectively. In the ‘other comments’ field about which staff receive training, 26 respondents identified other non-clinical occupational groups including cleaners, orderlies, maintenance, security, catering and other support staff e.g. “Kitchen staff, housekeeping and admin staff who go to wards” (respondent [R]40) “we train our maintenance staff’ (R99).
Most respondents (n = 104, 76%) stated that the IPC team delivered the PPE training, supported, in 26 (19%) cases, by IPC link representatives (i.e. nurses and other clinical staff nominated in wards and departments given additional training to promote and advise fellow staff on good IPC practice). Other personnel reported as providing PPE training, in 34 (25%) of responses, included clinical nurse educators or train-the-trainer programme leaders. Six respondents listed other staff including managers, training officers and staff development officers. Two respondents commented that medical staff, other than students and interns, were difficult to capture for training.
A variety of training and delivery methods were reported, including one or more of the following: classroom settings (n = 112, 83%), online training modules (n = 81, 60%), and simulation (n = 13, 10%). Virtual reality and video-reflexive methods were reported by three and eight respondents respectively. Most, but not all, respondents (n = 106, 78.5%) stated that training focused on standard and transmission-based precautions including hand hygiene associated with glove use. More than half (n = 83, 61%) of the respondents reported that training included demonstrations by facilitators followed by participant practice, and 42 (31%) used videos to demonstrate donning and doffing of PPE. In the additional comments field, two respondents referred to the benefits of ultraviolet marker technology; for example, this statement from Respondent 4; “We use fluorescent glow gel to demonstrate bad technique of removal of PPE”.
Most respondents (n = 116, 85%) used one or more common PPE items - non-sterile disposable gloves, long-sleeved gown, surgical mask and eye protection–in training. Respondents from public hospitals reported the inclusion of N95/P2 respirators more frequently than those from private facilities (76% vs 62%).
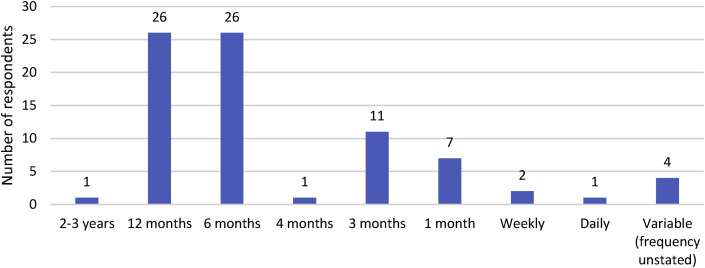
The survey also collected data on auditing processes for routine PPE. Auditing PPE compliance commonly comprises observational methods to collect data relating to the correct choice, and methods of donning and doffing, of PPE in clinical situations. There were 124 responses to this survey section, of which 80 (64%) indicated that compliance audits for routine PPE use were undertaken in their organisation. A higher percentage of private hospitals undertook audits than public hospitals (78% vs 54%). Of these 80 respondents, 79 provided details of who undertook audits and how frequently they occurred. The responsibility for undertaking audits was reported to be the IPC team and IPC link representatives by 54 (68%) and 18 (22%) respondents respectively. A number of responses (n = 22, 27%) specified other staff who undertook audits, including nurse managers and other ward staff. 31 (39%) respondents indicated that the audit tool used had been developed in-house. Of the remaining responses (n = 48, 61%) that identified a standardised tool was used, 14 were state or district instruments. The reported frequency of audits ranged from daily to every two to three years. The most common frequencies of auditing were annually and 6-monthly (Fig. 3 ).
Fig. 3.
Reported frequency of compliance audits for PPE use.
Section 3: training programmes for high-level PPE
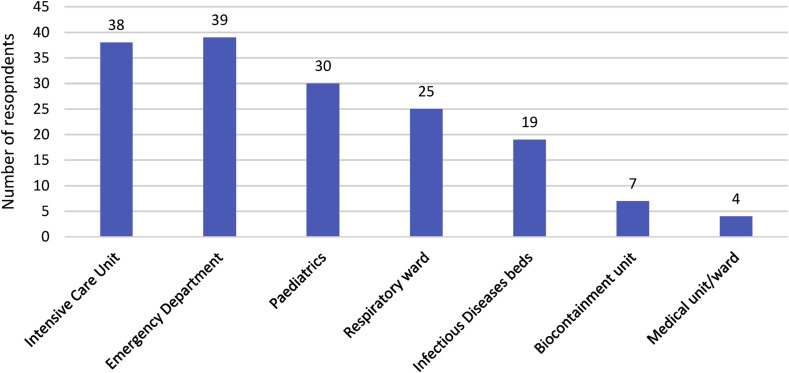
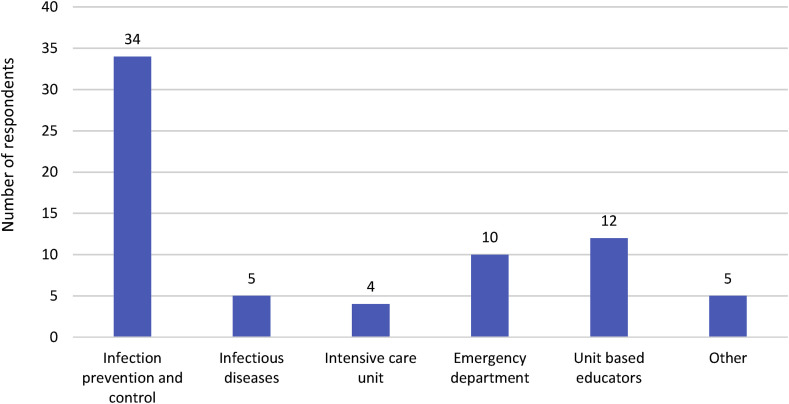
Just over one-third (n = 46, 37%) out of 125 respondents across Australia [39] and New Zealand [7], indicated that their organisation provided training in the use of high-level PPE. The majority of these respondents were from public hospitals (n = 38, 83%) and seven worked in a facility with a biocontainment unit. Just under half of these responses (n = 22, 48%) described a dedicated team of clinicians responsible for this training. Emergency department and intensive care units were reported to be most frequently users of the training as seen in Fig. 4 .
Fig. 4.
Reported distribution among 162 specific departments that received training in high-level PPE, in 125 facilities.
Three quarters of respondents (n = 35, 76%) stated that training in high-level PPE was provided ‘just-in-time’ at the time of an infectious diseases threat, with five of these also providing additional regular training sessions. Few (n = 15) respondents indicated that regular training was delivered, annually [11], 6-monthly [1] and 3-monthly [3], while one respondent did not provide any details of training frequency. One organisation also provided high level training to all new ED clinical staff.
Of the 45 respondents who provided details about which staff received high-level PPE training, 45 (100%) stated nurses and midwives, 35 (78%) doctors, 19 (42%) support workers and 12 (27%) trained allied healthcare workers. Responses for the methods of training in high-level PPE included face-to-face classroom (n = 42, 93%), online module (n = 16, 35%), and simulation (n = 8, 18%). Infection prevention and control staff were the craft group most frequently reported (n = 34, 74%) to deliver face-to face training for high-level PPE. Other departments also delivered this training as shown in Fig. 5 .
Fig. 5.
Reported frequencies of departments responsible for delivering training in high-level PPE.
Training programmes for high-level PPE contained one or more teaching modalities as reported by respondents: facilitator demonstration of PPE donning and doffing (n = 34, 78%), participant practice in PPE donning and doffing (n = 39, 87%), video(s) (n = 21, 47%), practice in actual isolation rooms (n = 14, 31%), simulation of procedures while in PPE (n = 9, 20%).
The frequency of different PPE items as used for high-level training is shown in Fig. 6 . In addition to items commonly used for routine PPE, e.g. gowns, N95 respirators, and eye/face protection, other high-level PPE frequently used included long-cuff gloves (n = 35, 88%), bootees (n = 37, 81%) and a hood (n = 28, 65%). Only 11 (23%) of respondents stated staff are trained in the use of a PAPR, 10 from public hospitals, including three with a biocontainment unit. Two respondents reported that PAPRs were purchased in preparation for SARS patients (in 2003) but were not currently used.
Fig. 6.
Reported frequency of Personal Protective Equipment (PPE) items used in training for high-level PPE in Australasia.
Discussion
The aim of this study was to characterise the PPE training programmes in Australian and New Zealand healthcare facilities. There were no obvious differences in training programmes private or public facilities. The results indicate that training in routine PPE for healthcare workers is a routine occurrence in most Australasian hospitals, particularly for new employees, but overall fewer than half provide annual or other regular refresher education. This is sub-optimal, as it risks staff being unable to maintain confidence and competence in their donning and doffing skills. In an observational study of 30 clinical staff, removing PPE used for routine care, only around 50% doffed PPE in the recommended manner and few followed the correct order [19]. Poor PPE donning and doffing techniques are unsafe for healthcare workers and patients, contribute to transmission of infection, and are symptomatic of the lack of experience and expertise in PPE use that is due to a just-in-time approach to PPE training during infectious disease emergency responses [20].
The current COVID-19 pandemic has resulted in widespread anxiety, lack of confidence and concern for their personal safety amongst HCWs, particularly related to PPE use [21,22], as was noted during previous infectious disease emergencies [23,24].
PPE training should be a blend of both theory and practice, but our results indicate that over a third (39%) of respondents do not include a practical component in their training programme for routine PPE. The World Health Organization emphasises the importance of practical training for ensuring competency in IPC practices [25] and research has shown the benefits of practical PPE demonstration with feedback [26]. Theoretical training content can be provided through on-line modules. However one of the barriers to incorporating practice is the high burden of human resources required. In spite of theoretical content in PPE training, COVID-19 has also exposed limited understanding of the reasons for recommended choices, and methods of use, of PPE, among healthcare workers [21]. Eight respondents used video-reflexive methods [27] for training, an approach which the authors have used extensively in both routine and high level PPE training, and in which participants report greater enjoyment and satisfaction, than conventional training (manuscript in preparation).
In this study the IPC team was responsible for the majority of PPE training although several respondents reported the use of non-IPC personnel incorporating the train-the-trainer model, a technique which has been used successfully to improve hand hygiene compliance in Australia and overseas [14,28]. Three respondents have used virtual reality in their training programmes, a technology which can provide individual practical training while eliminating PPE use for training when resources are scarce, as seen in the current COVID-19 pandemic [29]. This technology has been gaining momentum in IPC training [30].
PPE use is both a quality and safety and a work safety IPC measure which should be evaluated. However, in this study one third (36%) of respondents did not undertake auditing to monitor the correct use of PPE. Although the Australian Guidelines for the Prevention and Control of Infection in Healthcare [3] include statements about PPE training, they are limited in that they do not prescribe the content and frequency of this training, nor include detailed guidance for monitoring PPE use in practice. Improving national hand hygiene compliance through a centralised auditing and reporting programme has been associated with reduction in the incidence of health-care-associated S. aureus bacteraemia (HA-SAB) [14]. There is evidence that the Australian National Hand Hygiene Initiative (NHHI) and the inclusion of hand hygiene monitoring in hospital accreditation standards has ensured its standardisation and place in organisations’ patient safety programmes [14]. In comparison, the implementation of a national policy for aseptic technique in Australia has been associated with multiple challenges [31]. Hand hygiene monitoring has also drawn criticism and scepticism that direct observation scores do not accurately reflect real hand hygiene practice [32], which could equally apply to PPE compliance audits. The substantial increase in HCWs undergoing PPE training and the importance of PPE as an IPC measure, mainly for the protection of HCWs, during the current COVID-19 pandemic, presents additional challenges for ensuring PPE is used correctly. An examination of research into PPE compliance within the industrial sector may identify innovative ways to monitor compliance such as non-observational sensor methods [33].
The limited number of responses for training in high-level PPE identified variability in training frequency, who delivers the training, and the equipment used. Most respondents indicated that regular high-level training is not currently provided, and is only provided at time of threat, limiting local, regional, state/territory and national bio-preparedness. In the wake of the 2014 EVD outbreak, research and expert commentary [[34], [35], [36]] recommended regular skills training to ensure safety in donning and doffing high-level PPE, but in practice it has been very difficult for IPC teams to implement without additional educational resources. Another notable finding from the survey was that front-line staff are often involved in training which assists in bridging the gap between IPC principles and hands-on practice [[37], [38], [39]]. Conversely, in this study 11 out of the 45 respondents stated that IPC professionals were not involved in the training of high-level PPE. All PPE training is a fundamental component of an infection control programme, and therefore IPC should be involved in and have oversight of all PPE training.
Some items of PPE, in particular PAPRs, were reportedly seldom used for training which may reflect the infrequency of HCID events requiring PAPRs and the lack of actual cases occurring in Australasia from HCID events including SARS-CoV-1, MERS-CoV and EVD outbreaks. Global interest in PAPRs began at the time of the 2003 SARS-CoV-1 global outbreak and increased significantly during the West African EVD outbreak 2013–2015 [40]. Several respondents stated they still had older PAPRs from the SARS-CoV-1 and one reported that PPE preparedness for the 2003 SARS-CoV-1 global outbreak identified difficulties in safe doffing of a PAPR. PAPR hoods have been associated with an increased risk of contamination both during patient care and when doffing [41]. However other research suggests that PAPRs and assisted doffing protocols may reduce high-level PPE doffing errors [42]. Maintaining a skilled workforce in the use of high-level PPE is an important part of pandemic preparedness, particularly for hospitals with biocontainment capacity. Research from the USA showed that HCWs expect PAPRs to be available for them to use with EVD patients [43]. However, PAPR components are relatively expensive and the cost of maintaining an in-date stock of PAPR components between HCID events or outbreaks may be a deterrent to using them for training, especially if the risk of an actual case is very low. The current COVID-19 pandemic has resulted in misconceptions among some specialist clinical groups about the utility of a PAPR, with guidelines demanding that PAPRs be approved for use with COVID-19 patients [44]. While there may be limited indications for PAPR in the current pandemic [45], a PAPR and hood does not provide a greater level of protection for aerosolised SARS-CoV-2 than a correctly fitted N95/P2 respirator and eye protection. It is important that training programmes for HCID are based on the modes of transmission of disease and the protection required based on current epidemiological evidence.
The findings of this research have implications for training programmes outside of Australasia, particularly in countries with fewer IPC resources. Further research may be beneficial in identifying novel ways of providing PPE training in these locations.
This study, like all studies, have several limitations. The survey design did not prevent more than one response from the same facility, and the number of facilities from which there were more than one is unknown. However, a detailed examination of the demographic data indicates that this occurrence was uncommon. The data are based on self-report and it is not possible to verify the responses provided. The survey targeted members of three professional organisations; data relating to training programmes delivered by non-member IPC professionals was not captured. As the respondents were from the Because of overlapping memberships of the three organisations surveyed, the exact number of potential respondents (and hence the response rate) cannot be accurately estimated.
Conclusions
This study identifies variability in the extent and nature of training within Australian and New Zealand hospitals in the use of both routine and high-level PPE. The current COVID-19 pandemic has challenged the confidence and competence of many HCWs in using PPE, indicating inadequate preparedness for HCID. Regular audits of PPE skills may help to identify occupational groups or clinical areas that would benefit from refresher training. The study findings can help to inform further discussion about any proposed national standards for training and auditing PPE within healthcare facilities in Australia and New Zealand.
Ethics
Ethical approval was granted by the University of Sydney Human Research Ethics Committee (2019/614).
Authorship statement
RB Conceptualization, Methodology, Formal Analysis, Investigation, Writing - Original Draft, Review and Editing, Visualization. Project Management GLG: Conceptualization, Writing - Review & Editing, Supervision, Funding acquisition RS: Writing - Review & Editing, Supervision. All authors approved the final version of the manuscript.
Declaration of Competing Interest
RS is a Senior Editor of Infection, Disease and Health and LG is a member of the International Editorial Board of Infection, Disease and Health but neither had any role in peer review or editorial decision-making of the manuscript whatsoever. The authors declare no other conflict of interest.
Funding
This work was supported by The Australian Partnership for Preparedness Research on Infectious Diseases Emergencies (APPRISE) of which author GLG is a chief investigator and author RB is recipient of a doctoral scholarship. The research presented in this article is solely the responsibility of the authors and does not reflect the views of APPRISE.
Provenance and peer review
Not commissioned; externally peer reviewed.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.idh.2020.05.005.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.World Health Organisation . 2011. Report on the burden of endemic health care-associated infection worldwide.https://www.who.int/infection-prevention/publications/burden_hcai/en/ [Internet] Available from: [Google Scholar]
- 2.Weiner L.M., Webb A.K., Limbago B., Dudeck M.A., Patel J., Kallen A.J. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016 Nov;37(11):1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Australian guidelines for the prevention and control of infection in healthcare. National Health and Medical Research Council; Canberra: 2019. https://www.nhmrc.gov.au/about-us/publications [Internet] Available from: [Google Scholar]
- 4.Giard M., Laprugne-Garcia E., Caillat-Vallet E., Russell I., Verjat-Trannoy D., Ertzscheid M.-A. Compliance with standard precautions: results of a French national audit. Am J Infect Control. 2016 Jan;44(1):8–13. doi: 10.1016/j.ajic.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Gammon J., Morgan-Samuel H., Gould D. A review of the evidence for suboptimal compliance of healthcare practitioners to standard/universal infection control precautions. J Clin Nurs. 2008 Jan;17(2):157–167. doi: 10.1111/j.1365-2702.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 6.Williams V.R., Leis J.A., Trbovich P., Agnihotri T., Lee W., Joseph B. Improving healthcare worker adherence to the use of transmission-based precautions through application of human factors design: a prospective multi-centre study. J Hosp Infect. 2019 Sep;103(1):101–105. doi: 10.1016/j.jhin.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organisation . World Health Organization; Democratic Republic of the Congo: 2019. Ebola situation reports.https://www.who.int/ebola/situation-reports/drc-2018/en/ [Internet] Available from: [Google Scholar]
- 8.Gilbert G.L. Australia’s response to Ebola virus disease in West Africa, 2014–15. Public Heal Res Pract. 2016 Dec;26(5) doi: 10.17061/phrp2651661. [DOI] [PubMed] [Google Scholar]
- 9.Al-Tawfiq J.A., Abdrabalnabi R., Taher A., Mathew S., Rahman K.A. Infection control influence of Middle East respiratory syndrome coronavirus: a hospital-based analysis. Am J Infect Control. 2019 Apr;47(4):431–434. doi: 10.1016/j.ajic.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Australian Commission on Safety and Quality in Health Care . 2nd ed. ACSQHC; Sydney: 2017. National safety and quality health service standards.https://www.safetyandquality.gov.au/standards/nsqhs-standards/preventing-and-controlling-healthcare-associated-infection-standard Available from: [Google Scholar]
- 11.Safe Work Australia Personal protective equipment [Internet] https://www.safeworkaustralia.gov.au/ppe Available from:
- 12.WorkSafe New Zealand Government . 2018. Personal protective equipment – a guide for businesses.https://worksafe.govt.nz/topic-and-industry/personal-protective-equipment-ppe/personal-protective-equipment-a-guide-for-businesses/ [Internet] Available from: [Google Scholar]
- 13.Standards New Zealand. NZS 8134.3 . Standards New Zealand; Wellington: 2008. Health and disability services standards – health and disability services (infection prevention and control) standards. [Google Scholar]
- 14.Grayson M.L., Stewardson A.J., Russo P.L., Ryan K.E., Olsen K.L., Havers S.M. Effects of the Australian National Hand Hygiene Initiative after 8 years on infection control practices, health-care worker education, and clinical outcomes: a longitudinal study. Lancet Infect Dis. 2018 Nov;18(11):1269–1277. doi: 10.1016/S1473-3099(18)30491-2. [DOI] [PubMed] [Google Scholar]
- 15.Peng P.W.H., Ho P.-L., Hota S.S. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020 May;124(5):497. doi: 10.1016/j.bja.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamieson D.J., Steinberg J.P., Martinello R.A., Perl T.M., Rasmussen S.A. Obstetricians on the coronavirus disease 2019 (COVID-19) front lines and the confusing world of personal protective equipment. Obstet Gynecol. 2020 June;135(6):1257–1263. doi: 10.1097/AOG.0000000000003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahase E. Covid-19: retired doctors could be asked to return to work, says Hancock. BMJ. 2020 Mar 2;368:m831. doi: 10.1136/bmj.m831. [DOI] [PubMed] [Google Scholar]
- 18.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019 Jul;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zellmer C., Van Hoof S., Safdar N. Variation in health care worker removal of personal protective equipment. Am J Infect Control. 2015 Jul;43(7):750–751. doi: 10.1016/j.ajic.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barratt R., Gilbert G.L., Shaban R.Z., Wyer M., Hor S. Enablers of, and barriers to, optimal glove and mask use for routine care in the emergency department: an ethnographic study of Australian clinicians. Australas Emerg Care. 2019 June;23(2):105–113. doi: 10.1016/j.auec.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ault A. Medscape Medical News; 2020 Apr 06. COVID-19 Exposes Potential Gaps in PPE Training, Effectiveness.https://www.medscape.com/viewarticle/928163 [accessed 2020 Apr 08]; Available from: [Google Scholar]
- 22.Semple S., Cherrie J.W. COVID-19: Protecting worker health. Ann Work Expo Heal. 2020 Mar 23:1–4. doi: 10.1093/annweh/wxaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doll M., Feldman M., Hartigan S., Sanogo K., Stevens M., McReynolds M. Acceptability and necessity of training for optimal personal protective equipment use. Infect Control Hosp Epidemiol. 2017 Feb;38(2):226–229. doi: 10.1017/ice.2016.252. [DOI] [PubMed] [Google Scholar]
- 24.Barratt R. Infection prevention and control lessons learned from the management of the first suspected Ebola virus disease case admitted to a New Zealand hospital. Healthc Infect. 2015 Jun;20(2):78–80. [Google Scholar]
- 25.World Health Organization . World Health Organization; Geneva: 2019. Minimum requirements for infection prevention and control programmes. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 26.Poller B., Hall S., Bailey C., Gregory S., Clark R., Roberts P. ‘VIOLET’: a fluorescence-based simulation exercise for training healthcare workers in the use of personal protective equipment. J Hosp Infect. 2018 Jun;99(2):229–235. doi: 10.1016/j.jhin.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iedema R., Carroll K., Collier A., Hor S.Y., Mesman J., Wyer M. CRC Press; 2018 Dec 21. Video-reflexive ethnography in health research and healthcare improvement: theory and application. [Google Scholar]
- 28.Tartari E., Fankhauser C., Masson-Roy S., Márquez-Villarreal H., Fernández Moreno I., Rodriguez Navas M.L. Train-the-Trainers in hand hygiene: a standardized approach to guide education in infection prevention and control. Antimicrob Resist Infect Control. 2019 Dec;8(1):206. doi: 10.1186/s13756-019-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance. https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov- IPCPPE_use-2020.1-eng.pdf [Internet]
- 30.Eubanks J.C., Somareddy V., McMahan R.P., Lopez A.A. Springer; Cham: 2016 Jul 17. Full-body portable virtual reality for personal protective equipment training. International conference on virtual, augmented and mixed reality; pp. 490–501. [Google Scholar]
- 31.Havers S.M., Russo P.L., Page K., Wilson A., Hall L. Clinician perspectives of policy implementation: A qualitative study of the implementation of a national infection prevention policy in Australian hospitals. Am J Infect Control. 2019 Apr;47(4):366–370. doi: 10.1016/j.ajic.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Gould D.J., Creedon S., Jeanes A., Drey N.S., Chudleigh J., Moralejo D. Impact of observing hand hygiene in practice and research: a methodological reconsideration. J Hosp Infect. 2017 Feb;95(2):169–174. doi: 10.1016/j.jhin.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H., Yan X., Li H., Jin R., Fu H. Real-time alarming, monitoring, and locating for non-hard-hat use in construction. J Constr Eng Manag. 2019 Mar;145(3) [Google Scholar]
- 34.Verbeek J.H., Ijaz S., Tikka C., Ruotsalainen J.H., Mäkelä E., Neuvonen K. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Occup Environ Med. 2018;75:A177. [Google Scholar]
- 35.Mulvey D., Mayer J., Visnovsky L., Samore M., Drews F. Frequent and unexpected deviations from personal protective equipment guidelines increase contamination risks. Am J Infect Control. 2019 Sep;47(9):1146–1147. doi: 10.1016/j.ajic.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Sykes A. 2018. An international review of high level isolation units.https://www.wcmt.org.uk/sites/default/files/report-documents/Sykes%20A%202018%20Final.pdf [Internet] Available from. [Google Scholar]
- 37.Corley A., Hammond N.E., Fraser J.F. The experiences of health care workers employed in an Australian intensive care unit during the H1N1 Influenza pandemic of 2009: A phenomenological study. Int J Nurs Stud. 2010 May;47(5):577–585. doi: 10.1016/j.ijnurstu.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Den Boon S., Vallenas C., Ferri M., Norris S.L. Incorporating health workers’ perspectives into a WHO guideline on personal protective equipment developed during an Ebola virus disease outbreak. F1000Research. 2018;7 doi: 10.12688/f1000research.12922.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chughtai A.A., Seale H., Rawlinson W.D., Kunasekaran M., Macintyre C.R. Selection and use of respiratory protection by healthcare workers to protect from infectious diseases in hospital settings. Ann Work Expo Heal. 2020 Apr 30;64(4):368–377. doi: 10.1093/annweh/wxaa020. [DOI] [PubMed] [Google Scholar]
- 40.Wizner K., Stradtman L., Novak D., Shaffer R. Prevalence of respiratory protective devices in U.S. Health Care Facilities. Workplace Health Saf. 2016 Aug;64(8):359–368. doi: 10.1177/2165079916657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herlihey T.A., Gelmi S., Flewwelling C.J., Hall T.N.T., Bañez C., Morita P.P. Personal protective equipment for infectious disease preparedness: a human factors evaluation. Infect Control Hosp Epidemiol. 2016 Sep;37(9):1022–1028. doi: 10.1017/ice.2016.124. [DOI] [PubMed] [Google Scholar]
- 42.Chughtai A.A., Chen X., Macintyre C.R. Risk of self-contamination during doffing of personal protective equipment. Am J Infect Control. 2018 Dec 1;46(12):1329–1334. doi: 10.1016/j.ajic.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Bleasdale S.C., Sikka M.K., Moritz D.C., Fritzen-Pedicini C., Stiehl E., Brosseau L.M. Experience of Chicagoland acute care hospitals in preparing for Ebola virus disease, 2014–2015. J Occup Environ Hyg. 2019 Aug 3;16(8):582–591. doi: 10.1080/15459624.2019.1628966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020 Feb;12:1–9. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Australian Government Department of Health Guidance on the use of personal protective equipment (PPE) in hospitals during the COVID-19 outbreak. https://www.health.gov.au/sites/default/files/documents/2020/05/guidance-on-the-use-of-personal-protective-equipment-ppe-in-hospitals-during-the-covid-19-outbreak.pdf Available from.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.