Abstract
As of May 14, 2020, the World Health Organization has reported approximately 4.3 million cases of the novel Coronavirus Disease (COVID-19) with approximately 294,046 deaths worldwide [1]. Solid organ transplant recipients who are on chronic immunosuppressants fall within a special population of COVID-19 patients since they are more susceptible to complications secondary to COVID-19. Currently, we do not have data on treating COVID-19 patients with solid organ transplants with tocilizumab, an interleukin-6 (IL-6) inhibitor. We report a case of COVID-19 in a patient with a kidney and liver transplant and discuss the early use of tocilizumab to prevent the cytokine storm and attempt to reduce the likelihood of progression to Acute Respiratory Distress Syndrome (ARDS). In addition, we present other COVID-19 related transplant cases reported in the literature outlining the presenting clinical signs and outcomes.
Keywords: COVID-19, Renal transplant, Liver transplant
Introduction
As of May 14, 2020, the World Health Organization has reported 4,248,386 cases of COVID-19 with 294,046 reported deaths secondary to complications related to the novel Coronavirus [1]. In the United States, the Center for Disease Control (CDC) reported 1,364,061 total cases and 82,246 COVID-19 related deaths [2]. A common complication reported secondary to COVID-19 is Acute Respiratory Distress Syndrome (ARDS) requiring endotracheal intubation and ventilator management. Experts currently believe that ARDS possibly results from a state of hyper-inflammation mediated by a cytokine storm in COVID-19 patients [3]. Recipients of solid organ transplants are included in a special population of patients who may face increased risks of COVID-19 related complications given their use of chronic immunosuppressants. Immunosuppression in this population can further reduce cell-mediated immunity, which may prolong viral shedding and increase the risk of COVID related complications [3]. As reported by McGonagle et al., interleukin-6 (IL-6) plays an important role in lung repair following viral insults and the administration of IL-6 inhibitor drugs may be time-sensitive [3]. We report a case of COVID-19 in a patient with kidney and liver transplant and discuss the use of IL-6 inhibitor to prevent a cytokine storm in this setting.
Case report
The patient is a 63-year-old male kidney and liver transplant recipient who presented to the Emergency Department (ED) after developing symptoms of mild fever, shortness of breath, and cough. His vitals and physical exam in the Emergency Department were within normal limits, except for a temperature of 38 degrees Celsius. His initial chest x-ray on the day of admission (Day 1) was unremarkable. He tested negative for influenza and COVID-19 via polymerase chain reaction (PCR) and was admitted to the hospital. At the time of admission, he had a normal white blood cell count (6.94 × 109/liter), decreased absolute lymphocyte count (2.9 %), and normal liver function tests. Cytomegalovirus and Bordetella PCR serology were also negative. His home immunosuppressant regimen consisted of mycophenolic acid 500 milligrams daily, prednisone 5 milligrams daily, tacrolimus 2 milligrams twice a day.
On day 2 of the admission, the patient was switched from oral prednisone to methylprednisolone 40 milligrams administered every eight hours intravenously. On day 3, he received cefepime 1 g over six hours. He also received five doses of hydroxychloroquine 400 milligrams on days 3–7.
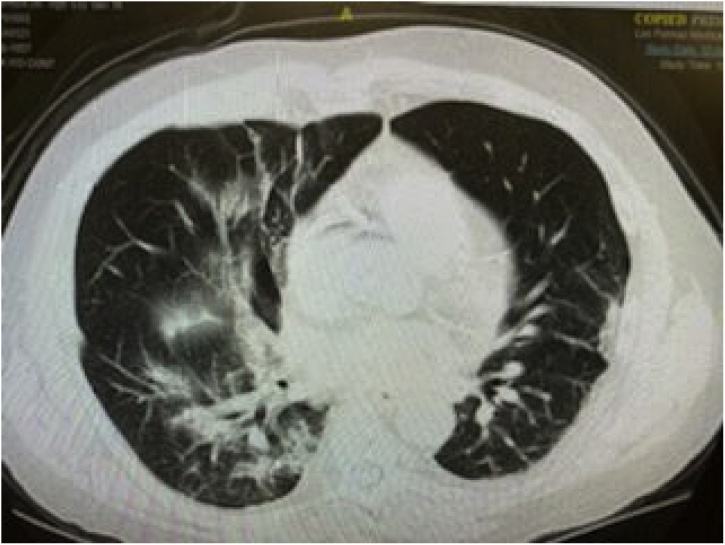
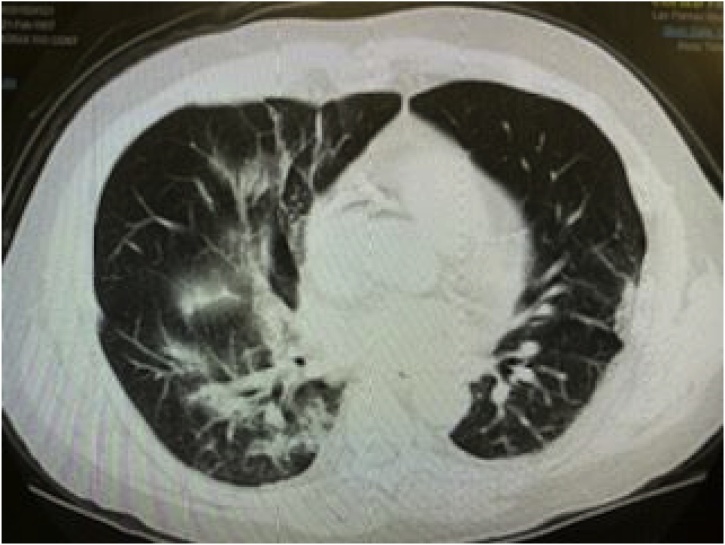
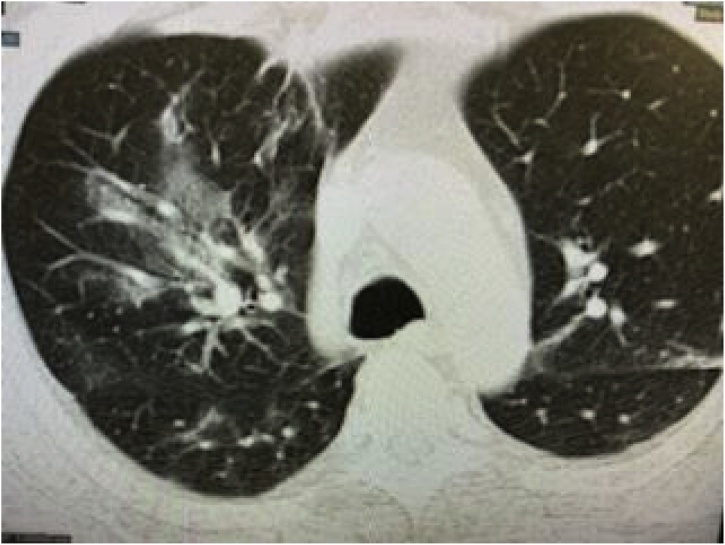
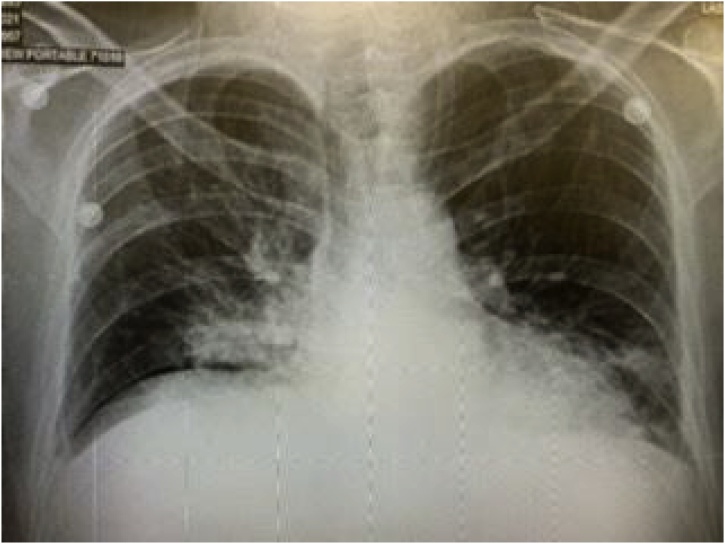
On day 4, the fever subsided and vitals remained within normal limits. However, he developed increasing shortness of breath with new diffuse expiratory wheezes on physical exam. Repeat chest x-ray showed right lower lobe infiltrates and CT Thorax without contrast showed right upper, middle, and lower lobe infiltrates with ground glass appearance consistent with a viral pneumonitis. This later progressed to bilateral ground glass opacities as pictured in Fig. 1, Fig. 2, Fig. 3 and warranted transfer to the Intensive Care Unit (ICU). Test for COVID-19 serum antibodies performed at this time was positive for COVID-19 IgG antibodies. On Day 4, he received one dose of tocilizumab 4 mL/kilogram. Within 24 h of receiving this medication, his shortness of breath started to improve. His oxygen requirement dropped to 2–3 liters via nasal cannula with exercise while maintaining an oxygen saturation of 86–94 % over the next two days. His chest x-ray also showed improvement, as shown in Fig. 4. Overall, he began to show clinical signs of improvement. Mycophenolic acid and tacrolimus were discontinued on day 5. Table 1 shows the lab results for the patient through the first seven days of his hospital stay.
Fig. 1.
CT Thorax without contrast: bilateral ground glass opacities.
Fig. 2.
CT Thorax without contrast: bilateral ground glass opacities.
Fig. 3.
CT Thorax without contrast: bilateral ground glass opacities.
Fig. 4.
Chest x-ray improved after six days of treatment.
Table 1.
Patient labs from Day 1–10.
| Labratory Test Results | 4/11/20 Day 1 |
4/13/20 day 3 |
4/14/20 day 4 |
4/15/20 day 5 |
4/16/20 day 6 |
4/17/20 day 7 |
4/18/20 day 8 |
4/20/20 day 10 |
|---|---|---|---|---|---|---|---|---|
| WBC (4.5–11 × 109/L) |
6.94 | 6.73 | 12.13 | 8.27 | 8.03 | 8.89 | ||
| Lymphocytes (30–45 %) |
2.90 % | 3.70 % | 3.30 % | 6.50 % | ||||
| Creatinine (Male: 0.70−1.30 mg/dL) |
1.20 | 1.30 | 1.50 | 1.10 | 1.00 | 0.90 | 0.90 | |
| AST (10−40 units/L) |
21 | 25 | 29 | 34 | 37 | 25 | 22 | |
| ALT (10−40 units/L) |
29 | 26 | 28 | 45 | 64 | 53 | 51 | |
| Alkaline phosphatase (30−120 units/L) |
81 | 75 | 72 | 68 | 63 | 63 | 72 | |
| LDH (80–225 units/L) |
226.00 | 344.00 | ||||||
| C-reactive protein (<0.8 mg/dL) | 3.70 | 1.20 | 0.60 | |||||
| IL-6 (5−15 pg/mL) |
18.2 | 670.8 | 392.8 | 280.7 | ||||
| Prograf/ Tacrolimus levels (5.0−15.0 ng/mL) |
17.30 | 14.80 | 7.90 | |||||
| Ferritin (male: 54–755 pmol/L) |
336.50 | 225.90 | ||||||
| VBG | pH 7.47 pO2 81 pCO2 32 HCO3 23.20 |
|||||||
| ABG | pH 7.42 pO2 72 pCO2 33 HCO3 21.4 |
|||||||
| Serology | ||||||||
| Influenza | Negative | |||||||
| Coronavirus | Negative | Positive |
However, on Day 7, he decompensated requiring intubation and mechanical ventilation. The critical care team also placed him on a rotator bed. On day 8, his oxygen requirements substantially increased requiring an increase of oxygen to 1 L. At this time, he received a second dose of tocilizumab.
The patient remained critically ill following intubation. Seven days after intubation he developed a right sided pneumothorax and required a chest tube. The liver function tests, blood urea nitrogen (BUN), and creatinine remained stable. During his ICU stay, he was fully therapeutically heparinized as well. He developed no evidence of secondary bacterial infection. We obtained consent and administered 200 mL of convalescent plasma. He remained stable without significant change in his condition over the next several days.
On Day 14, the pneumothorax resolved and he began weaning off the ventilator.
Discussion
Severe Acute Respiratory Disease 2 (SARS-CoV-2) is a highly infectious, novel coronavirus that emerged in Wuhan, China in December of 2019 [4,5]. As of May 14, 2020, the World Health Organization has reported 4,248,386 cases of COVID-19 with 294,046 reported deaths secondary to complications related to the novel Coronavirus [1]. While this lethal disease has not spared immunocompetent patients, populations who have the highest mortality risk require special attention. At this time, there are limited case reports on solid organ transplant (SOT) patients [6]. There is insufficient data on the clinical presentation and management of immunosuppressant regimens in SOT recipients [4]. These patients may differ from the immunocompetent population in regards to presentation, diagnosis, and clinical course of Covid-19 [4]. Notably, transplant patients may present with mild or atypical symptoms and without fever. Thus, physicians must maintain broad differential diagnoses and high clinical suspicions [7]. One case series of five patients reported the most common symptoms on admission were fever, cough, myalgia/fatigue, and sputum production [5]. Other case reports have identified patients as having vague abdominal discomfort [4]. Table 2 outlines the reported cases on COVID-19 in SOT recipients. We did not find any reports of patients with double solid organ transplants reported in the literature.
Table 2.
Reported cases of COVID 19 in transplant patients. MMF = Mycophenolate Mofetil. WBC = White blood cell count. CRP = C-reactive protein. LDH = lactate dehydrogenase.
| Age/ Sex | Solid Organ Transplant | Chronic Immunomodulator |
Medications given during admission while receiving COVID treatment |
IL-6 levels | WBC (4.5–11 × 109/L) |
Lymphocytes (30–45 %) | CRP (<0.8 mg/dL; <76.2 nmol/L) |
LDH (80–225 units/L) |
Outcome | Total Days of Illness | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 37M | Liver | Tacrolimus | Oseltamivir, phosphate capsules, cefoperazone sulbactam sodium, methylprednisolone Tacrolimus discontinued |
Not Reported | 2.46 | 48% | Not reported | Not Reported | Acute transplant rejection, but recovered after tacrolimus was resumed Full recovery |
15 days | [4] |
| 48M | Kidney, 2003 | Tacrolimus MMF | Oseltamivir, abidol moxifloxacin, recombinant human interferon alpha, methylprednisolone, human IVIG Tacrolimus MMF discontinued |
Not Reported | 2.49 | 64% | 31.25 | Not Reported | Bone marrow suppression due to MMF Full recovery |
42 days | [4] |
| 38M | Kidney, 2019 | Glucocorticoid MMF Tacrolimus | Oseltamivir, arbidol Glucocorticoids Tacrolimus MMF discontinued |
Not Reported | 4.73 | 63% | 0.37 | 193 | Full recovery | 26 days | [5] |
| 64M | Kidney, 2016 | Glucocorticoid MMF rapamycin | Oseltamivir or arbidol cefepime and IVIG Glucocorticoids Tacrolimus MMF discontinued |
Not Reported | 17.67 | 55% | 1.26 | 180 | Remained hospitalized | Not Reported | [5] |
| 37F | Kidney, 2019 | Glucocorticoid MMF Tacrolimus | Oseltamivir or arbidol cefepime IVIG Glucocorticoids MMF discontinued Tacrolimus discontinued, then restarted at half the dose |
Not Reported | 5.67 | 31% | 2.03 | 160 | Remained hospitalized | 22 days | [5] |
| 47M | Kidney, 2019 | Glucocorticoid MMF Tacrolimus | Oseltamivir or arbidol Glucocorticoids MMF discontinued Tacrolimus stopped and restarted |
Not Reported | 3.99 | 51% | 0.45 | 235 | Remained hospitalized | Not Reported | [5] |
| 38M | Kidney, 2017 | Glucocorticoid MMF Tacrolimus | Oseltamivir or arbidol Glucocorticoids MMF Tacrolimus | Not Reported | 6.44 | 91% | 0.39 | 248 | Full recovery | 23 days | [5] |
| 50 M | Liver | Tacrolimus | Methylprednisolone. Umifenovir, Lopinavir/ritonavir, IVIG, Cefoperazone, alpha interferon Tacrolimus discontinued for 4 weeks due to lymphopenia |
Not Reported | 5.9 | 72% | 32.1 | Not Reported | Full recovery | 28 days | [6] |
| 49M | Kidney | Cyclosporine MMF Prednisone | Lopinavir, ritonavir, ribavirin, interferon alpha-2b, methylprednisolone, MMF, prednisone Cyclosporine discontinued |
Not Reported | 7.18 | 59% | 22.73 | Not Reported | Full recovery | 14 days | [19] |
| 50M | Kidney, 1992, 2016 | MMF Tacrolimus | MMF, Tacrolimus, ceftriaxone |
26.22 | 3.2 | 60% | Not Reported | Not Reported | Full recovery | 13 days | [17] |
| 58M | Kidney, 2017 | Belatacept MMF Prednisone | Belatacept Cyclosporine Prednisone MMF discontinued during treatment |
29 | 5.04 | 16% | 14 | Not Reported | Full recovery | 18 days | [20] |
| 36F | Kidney, 1993, 1995 | Tacrolimus Prednisone | Hydroxychloroquine, opinavir/ritonavir, ceftriaxone, tacrolimus, methlyprednisolone | Within normal limits (reported elevated IL-8) |
High neutrophil | normal | 67 | Not Reported | Full recovery | 9 days | [21] |
| 58M | Kidney | MMF Prednisone | Methylprednisolone MMF discontinued during treatment |
Not Reported | Not Reported | Not Reported | Not Reported | Not Reported | Mechanical ventilation; death due to multi-organ failure on Day 40 | 40 days | [22] |
| 52 M | Kidney | Tacrolimus MMF Prednisone | Imifovir, Moxifloxacin Methylprednisolone, Biapenem, interferon alpha All immunosuppressive agents discontinued during treatment |
Day 2: 19.53 | Day 2: 5.54; Day 11 11.68 | Day 2: 17.9 % Day 11: 12 % | Day 2: 54; Day 11: 1.4 | Not Reported | Full recovery | 18 days | [23] |
Recent proposals regarding the pathophysiology of COVID-19 suggest a hyper-inflammatory state resulting in COVID-19 related acute respiratory distress syndrome (ARDS) [3]. Once infected, there is loss of primary antiviral defense because of virus-induced interferon suppression with lymphopenia [8]. Subsequently, the body activates a second defense mechanism, known as the “second wave,” which results in a cytokine storm and severe tissue damage [3]. Increases in many inflammatory markers, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum ferritin, interleukins 6, 8, and 10, procalcitonin, and interleukin-receptor have been found in COVID-19 patients [9]. Interleukin-6 (IL-6) is a marker of recent interest because of its role in the “second wave” and resulting cytokine storm. [10]
IL-6 signal transduction occurs via three main pathways: classical signal transduction, trans signal transduction, and trans presentation [11]. The classical signal transduction involves IL-6 binding to the IL-6R and forming a complex; the IL6-IL6R complex then binds to membrane gp130 and subsequently initiates intracellular transduction [11]. The trans signal transduction pathway occurs with IL-6 binding to sIL-6R and forming a complex; the IL6-sIL6R complex then binds to membrane gp130, which then initiates intracellular signal transduction [11]. The trans presentation signal involves sgp130 forming a complex with sIL-6R to prevent sIL-6R from binding to membrane-bound gp130; the JAK-STATA, RAS-RAF, and ATK-PI3K pathways are then activated [11].
Chen et al. found in a sample of 48 patients that increased levels of IL-6 significantly correlated to disease severity [12]. Another study reported a significant association between lymphopenia and increased IL-6 levels in COVID-19 non-survivors compared with survivors. [12,13] These findings suggest monitoring IL-6 levels to evaluate cytokine storm as a prognostic tool [12]. However, it is likely that physicians do not routinely follow IL-6 levels in COVID-19 patients with solid organ transplants, as it was not a commonly reported lab value in the studies we evaluated (Table 2).
Treatment with tocilizumab, a monoclonal antibody that binds to the IL-6 Receptor, has been recently published [11]. By binding to the IL-6R, tocilizumab can inhibit both the classical and trans signaling pathways leading to a reduction in the “second wave” response and preventing cytokine storm [11].
The standard treatment dosing according to the Diagnosis and Treatment Protocol for COVID-19 (7th Edition) is a first dose of 4−8 mg/kg/day, with 400 mg diluted to 100 mL with 0.9 % normal saline, infused over a 1 h period [14]. The maximal dose is 800 mg, and the maximal number of administrations is two [14]. This dosing is based on a small trial of 21 patients that received tocilizumab, in addition to the standard care recommended by the Diagnosis and Treatment Protocol for Covid-19 (6th Edition) including lopinavir, methylprednisolone, other symptom relievers, and oxygen therapy [15]. Given the small sample size, we need more research on appropriate dosing. At this time, there is a multicenter randomized controlled trial currently ongoing in China [16]. Another potential medication that decreases IL-6 levels is metronidazole [13]. In vitro and in vivo studies have shown that metronidazole decreases serum IL-6 levels [13].
Close monitoring of immunosuppressive therapy in SOT recipients infected with COVID-19 is necessary. There is a balance of allowing adequate immune response to suppress viral load and preventing transplant rejection. Previous case reports have hypothesized that immunosuppressive therapy protects SOT recipients by dampening the cytokine storm [17]. Other case reports have noted that viral RNA levels remain positive for a longer period than in immunocompetent patients [4]. This is notable because previous studies have demonstrated that ARDS occurs in SARS patients despite a decreased viral load [18]. This is important because it indicates antiviral therapy alone is inadequate treatment and supports the hypothesis that the ARDS results from the cytokine storm [18]. One case report of a liver transplant recipient that tested positive for COVID-19 suffered from acute transplant rejection despite maintaining adequate immunosuppression based on lymphocyte subtests. 4 This suggests that early IL-6 intervention to prevent the cytokine storm could improve outcomes in COVID-19 positive, SOT recipients.
One of the main concerns with IL-6 blockade in the treatment of COVID-19, however, is the appropriate timing of when to start treatment. It is possible that blocking IL-6 signal transduction can lead to a reduction in viral clearance [3]. In patients that are already immunosuppressed, this could lead to a much higher viral load as compared with immunocompetent patients. Additionally, we do not know whether IL-6 alters the levels of circulating tacrolimus. This may suggest cessation of immunosuppressive therapy until the initial cytokine storm as resolved. Temporary removal of immunosuppressive regiment is likely beneficial for transplant recipients because clinical outcomes of COVID-19 seems to mostly dependent on the virus-host interaction.
Another interesting finding in many patients with COVID-19 is the potential for a second cytokine storm. A second cytokine storm may have happened to the patient in this case report as he clinically improved and then developed a recurrence of respiratory distress that required the intubation. The IL-6 levels were also markedly elevated at this time suggesting an ongoing cytokine response. This may be the reason why several of these patients become severely hypoxic after a few days of stabilization. This may also suggest that we administer the tocilizumab in an interval fashion, approximately 4–6 days after the first administration.
In conclusion, there is limited data on the clinical presentation, management, and appropriate treatment of COVID-19 patients who are recipients of solid organ transplants. The use of IL-6 inhibitor in our patient resulted in clinical improvement initially, but it is difficult to determine if the tocilizumab played a role in the management of this patient, specifically in preventing respiratory failure. The cytokine storm appears to play a major role in these patients, and it is possible that several of them experience a second storm that results in severe respiratory distress and intubation. Thromboembolic phenomena also appear to play a role in respiratory failure. A single case does not represent the complex strategy needed when considering the treatment of COVID-19 patients with solid organ transplants. Further studies are necessary to investigate treatment modalities for COVID-19 in special populations.
References
- 1.The World Health Organization . 2020. Coronavirus disease 2019 (COVID-19) situation report – 87. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200416-sitrep-87-covid-19.pdf?sfvrsn=9523115a_2 [Accessed 16 April 2020] [Google Scholar]
- 2.Centers for Disease Control and Prevention . 2020. Coronavirus disease 2019 (COVID-19) in the U.S. [online] Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html [Accessed 16 April 2020] [Google Scholar]
- 3.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong Z., Zhang Q., Xia H. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020 doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Chen Y., Yuan Q. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020 doi: 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bin L., Yangzhong W., Yuanyuan Z., Huibo S., Fanjun Z., Zhishui C. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020 doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manuel O., Estabrook M. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9) doi: 10.1111/ctr.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G., Wu D., Guo W. Clinical and immunologic features in severe and moderate forms of coronavirus disease 2019. J Clin Invest. 2020;(April) doi: 10.1101/2020.02.16.20023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry B.M., Oliveira M.H.S.D., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 10.Russell B., Moss C., George G. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortaliy. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X., Zhao B., Qu Y. 2020. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharebaghi R., Heidary F., Moradi M., Parvizi M. Metronidazole a potential novel addition to the COVID-19 treatment regimen. SSRN Electron J. 2020 doi: 10.2139/ssrn.3559020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18(1) doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X., Han M., Li T., Sun W., Wang D., Fu B. 2020. Effective treatment of severe COVID-19 patients with tocilizumab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q., Wang Y., Qi C., Shen L., Li J. Clinical trial analysis of 2019‐nCoV therapy registered in China. J Med Virol. 2020;92(6):540–545. doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seminari E., Colaneri M., Sambo M. SARS Cov2 infection in a renal transplanted patients. A case report. Am J Transplant. 2020 doi: 10.1111/ajt.15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Li X., Cao G., Wu X., Wang Z., Yan T. COVID-19 in a kidney transplant patient. Eur Assoc Urol. 2020 doi: 10.1016/j.eururo.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marx D., Moulin B., Fafi-Kremer S. First case of COVID-19 in a kidney transplant recipient treated with belatacept. Am J Transplant. 2020 doi: 10.1111/ajt.15919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartiromo M., Borchi B., Botta A. Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID-19) Transpl Infect Dis. 2020 doi: 10.1111/tid.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J., Lin H., Wu Y. COVID-19 in post-transplantation patients- report of two cases. Am J Transplant. 2020 doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L., Xu X., Ma K. Successful recovery of COVID‐19 pneumonia in a renal transplant recipient with long‐term immunosuppression. Am J Transplant. 2020 doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]