Abstract
Vaccines are one of the most powerful technologies supporting public health. The adaptive immune response induced by immunization arises following appropriate activation and differentiation of T and B cells in lymph nodes. Among many parameters impacting the resulting immune response, the presence of antigen and inflammatory cues for an appropriate temporal duration within the lymph nodes, and further within appropriate subcompartments of the lymph nodes– the right timing and location– play a critical role in shaping cellular and humoral immunity. Here we review recent advances in our understanding of how vaccine kinetics and biodistribution impact adaptive immunity, and the underlying immunological mechanisms that govern these responses. We discuss emerging approaches to engineer these properties for future vaccines, with a focus on subunit vaccines.
Keywords: Immunization, vaccine kinetics, infectious disease, alum, adjuvants, nanoparticles
Graphical abstract
1. Introduction
Vaccines play a critical role in public health. Prophylactic vaccines against infectious disease have nearly eliminated once common debilitating diseases such as poliomyeltitis, measles, and smallpox [1], and currently 96 vaccines are licensed by the US Food and Drug Administration. Vaccines can also be used to curb emerging epidemics. During the 2014 ebola outbreak in west Africa, “ring” vaccination was employed, where immediate contacts of a suspected new infected individual were immunized in an effort to limit the spread of the disease. This trial was implemented using a new Zaire ebolavirus vaccine, and early results suggested the vaccine was 100% effective in preventing new infections within the immunized cohorts [2]. The development of new vaccines remains a global health priority. A universal vaccine against influenza to replace seasonal immunization and vaccines for longstanding global epidemics of malaria, tuberculosis, and HIV remain urgent unmet needs [[3], [4], [5], [6]]. Epidemics of new pathogens that induce significant morbidity and mortality continue to emerge, such as the Zika virus outbreak in the Americas in 2015-2016. As this review is being written, the global COVID-19 pandemic caused by the emergence of the SARS-CoV-2 coronavirus in 2019 continues unabated, with greater than three million cases of confirmed disease globally. Rapid development of a protective vaccine is an urgent world health priority [7,8]. Therapeutic vaccines used to treat existing disease are also in preclinical and clinical development for cancer, HIV, other infectious diseases. Thus, the potential public health impact of vaccine technologies continues to expand.
Vaccines are formulations that expose the immune system to a target antigen (or antigens), aiming to induce long-lived protective immunity in the form of antibody-producing plasma cells, memory B cells, and memory T cells. The majority of licensed vaccines are thought to protect through the induction of antibodies [9], some of which are capable of neutralizing pathogens prior to their entry into the body at mucosal surfaces. Vaccines can be created from genetically attenuated forms of a live pathogen, a chemically-inactivated form of a pathogen, from recombinant proteins or polysaccharides derived from the pathogen, from repurposed viruses or bacteria that express antigens from the pathogen of interest, or from synthetic nucleic acids that encode for the target antigen [1]. Therapeutic vaccines in cancer have even been generated by modifying a patient’s own immune cells, loading them with antigen ex vivo and then injecting back into the patient [10]. Much of modern prophylactic vaccine development is focused on the development of purified recombinant protein/polysaccharide antigens (subunit vaccines), which typically must be paired with adjuvants, compounds or formulations that provide inflammatory cues that stimulate the immune response against the co-administered antigen [11,12].
Despite this breadth in composition, the efficacy of all vaccines is influenced by a common set of factors that control the safe generation of a desired immune response: First, antigens must be identified that present a molecular structure to the immune system capable of eliciting protective antibody or T cell responses. In modern vaccine design for infectious disease, this is often approached through “reverse vaccinology”, whereby protective antibody or T cell responses generated naturally in infected humans are used to guide the selection of appropriate antigens to re-elicit this response through vaccination [[13], [14], [15]]. However, antigen selection/design is only part of the equation. Antigen and inflammatory cues must reach inductive sites– lymphoid tissues (most typically, lymph nodes), accessing subcompartments of these organs that govern T cell and B cell activation, and the timing and concentration of antigen and inflammatory cues at these sites must be appropriate to optimally trigger immune priming.
Controlling timing and location in vaccines is a challenging drug delivery problem, and the focus of this review. To limit the scope, we focus our discussion of vaccine localization on the trafficking of antigens/adjuvant compounds from injection sites, and do not discuss the additional challenges facing the development of vaccines designed to cross tissue barriers, such as oral, transcutaneous, or mucosal vaccines; we refer the reader to other recent reviews on these topics [[16], [17], [18], [19]]. The discussion focuses primarily on subunit vaccines, motivated by the general move of the vaccine industry toward subunit or vectored vaccine approaches rather than live attenuated vaccines in the interest of manufacturing advantages and increased safety in future vaccines. We also will not discuss important issues about the engagement of vaccine antigens/adjuvants with immune cells at the single cell level (e.g., designing multivalent antigens to crosslink B cell receptors, formulations that promote dendritic cell activation and cross presentation of antigen, etc.), though this is also an important issue in vaccine design [20]. Vaccine technologies aiming to promote tolerance for tissue transplantation or treatment of autoimmune disease are also in development, but these topics are also beyond the scope of the present discussion and we refer the interested reader to other excellent recent reviews [[21], [22], [23], [24]].
2. Influences of vaccine localization on the safety and efficacy of immunization
2.1. Role of antigen and adjuvant dose in lymphoid tissues in programming the immune response
Prophylactic vaccines function by activating antigen-specific B cells and T cells, leading to the production of 3 major cellular products– memory B cells, plasma cells, and memory T cells. Memory B cells and T cells provide a rapid response to subsequent encounter with a pathogen, and can further localize in peripheral tissues to provide a near-immediate antigen-specific first line of defense, bolstering the protection provided by innate immunity [25]. Plasma cells are B cells that have differentiated into long-lived antibody factories, which home to peripheral tissues and the bone marrow [26]. Antibodies are produced constitutively by bone marrow plasma cells, and these cells sustain levels of circulating pathogen-specific antibody in the blood (IgG) and mucosal surfaces (IgA) that enable sterilizing immunity that blocks microbes before they can establish infection.
Lymph nodes (LNs) are the critical command center of the immune response, housing T cells, B cells, and antigen presenting cells (APCs) that orchestrate adaptive immunity. Activation of B cells is triggered by binding of antigen to the B cell receptor, which can occur through binding to soluble antigen or via B cell recognition of antigens captured on cell membranes in the lymph node. T cells by contrast are activated through interactions with professional antigen presenting cells, dendritic cells (DCs). DCs capture antigen, proteolyze it into short peptide fragments, and load these peptides onto class I and class II major histocompatibility complex molecules, which are then physically presented on the cell surface to CD8+ and CD4+ T cells, respectively. These antigen recognition events must occur in the context inflammatory cues that direct the type of immune response the immune system should mount to the antigen [27]- such inflammatory cues come in the form of molecules that activate innate immune system pattern recognition receptors (e.g., Toll-like receptors, TLRs) or cytokines and chemokines induced in the lymph node in response to immunization. For subunit vaccines, these inflammatory cues are provided by the adjuvant. The role of these inflammatory cues is most completely understood for T cell activation: inflammation triggers activation of dendritic cells, which in response upregulate the expression of costimulatory receptors and secrete key cytokines such as IL-12 that are critical for T cells to proliferate and become fully functional effector and memory cells [28]. Studies of lymph node-targeted TLR agonists have shown a direct relationship between the magnitude of CD8+ T cell responses and the amount of TLR agonist accumulated in draining lymph nodes, pointing to the importance of providing sufficient inflammatory signals during immunization [29]. Thus, both antigen and inflammatory cues must be effectively localized in lymph nodes for vaccines to achieve their effect.
Both B and T cell activation exhibit antigen dose-dependent behavior in vivo, meaning that the ability of vaccines to accumulate in lymph nodes plays a critical role in the subsequent immune response. For both CD8+ and CD4+ T cells, lymphocyte expansion is limited by the availability of activated APCs bearing antigen [30,31]. Immunization studies in preclinical models suggest that large amounts of antigen are required to drive CD8+ T cell responses, which may be linked to the inefficiency of antigen processing and the process of cross presentation, where extracellular antigens are captured by dendritic cells and presented on class I MHC [32]. Differentiation of T cells is also influenced by the level of antigen, with higher levels of available antigen associated with greater production of follicular helper T cells that govern germinal center responses [33]. Above a minimal threshold, B cell responses appear to be less sensitive to antigen dose [[34], [35], [36]], and B cells are capable of responding to very small doses of antigen if efficiently delivered to lymph nodes [37]. However, booster vaccinations administered to subjects who already have high antibody titers benefit from higher antigen dose, likely as a consequence of “dosing through” the clearance of antigen by the pre-existing antibody [38]. Hence, the antigen dose accumulated in lymphoid tissues impacts diverse aspects of the adaptive immune response.
2.2. Anatomy of vaccination
Humans have approximately 400-600 lymph nodes distributed throughout the body, which are organized in clusters and chains connected by lymphatic vessels that collect fluid from all tissues and portals of entry into the body. The majority of licensed vaccines are administered as a bolus syringe injection into the muscle, skin, or subcutaneous space, but on entry into the tissue, vaccines must be transported to the draining lymph nodes where activation of T and B lymphocytes is coordinated. Material is transported out of tissues either by entering the blood vasculature or convecting into lymphatic vessels [39], and these potential fates have distinct implications for the outcome of immunization: Inflammatory cues provided by adjuvants must act to activate antigen presenting cells in these same lymph nodes, to avoid tolerance (Fig. 1 ). Adjuvant compounds that enter the systemic circulation not only fail to act at the draining LNs where they are needed, they introduce the risk of systemic toxicity. Thus, strategies to enhance delivery of both antigen and adjuvant compounds to lymph nodes can increase both the safety and efficacy of vaccines.
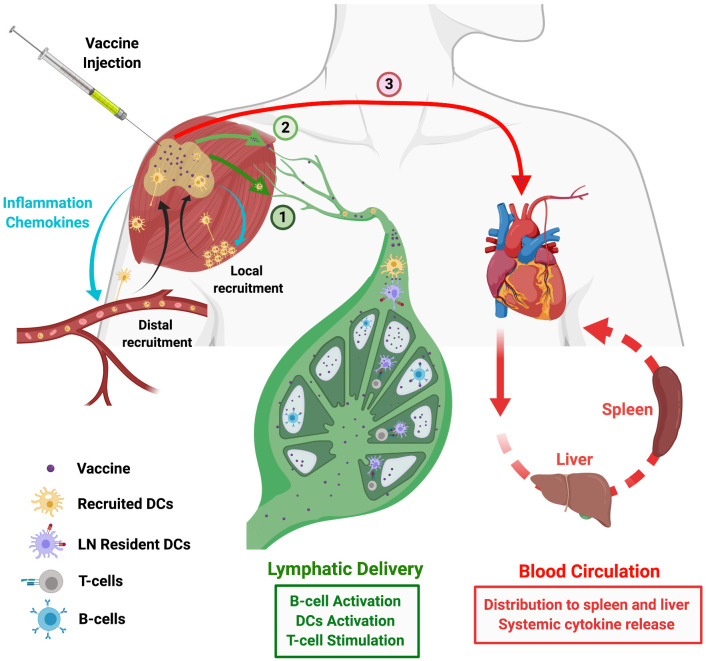
Fig. 1.
Vaccine biodistribution after injection. Schematic view of potential fates of vaccine antigen/adjuvant molecules following injection. Vaccines are typically administered parenterally, such through intramuscular injection. Shown are 3 distinct fates for vaccine component: (1) Vaccine trafficking via cell-mediated transport, where antigen presenting cells recruited from the blood or from the surrounding tissue migrate to the injection site, take up vaccine, and then migrate to the draining lymph node. (2) Vaccine molecules/particles directly convect with lymph into lymphatic vessels and thereby into the draining LN. (3) Vaccine components diffuse into the blood vasculature and enter the systemic circulation, reaching distal organs such as liver, spleen, and kidneys. Drainage of soluble antigen into the lymphatics (route 2) or systemic circulation (route 3) is heavily influenced by the physicochemical properties of the vaccine such as particle size and presence of molecular chaperones. Lymphatic drainage shown in route 2 directly delivers antigens to B-cells or LN-resident DCs to initiate an immune response. However, drainage into blood circulation (route 3) can lead to toxicity from activation of immune cells in the blood or at distal sites such as the liver or spleen.
What lymph nodes are involved in the response to vaccine injection? In large animals and humans, lymph nodes are distributed as clusters at key tissue-draining sites, with the exact number located at a given location being variable site to site and individual to individual. Injections of lymphatic tracers or fluorescently-labeled antigens has revealed that in large animal models, lymph transports material to the immediate draining LNs closest to the immunization site, and to a lesser extent may accumulate in the next 1-2 sets of lymph nodes in the lymphatic chain [[40], [41], [42], [43], [44], [45]]. In rhesus macaques, the large-animal vaccine model thought to be closest in anatomy and genetics to humans, distal LNs do not show evidence of antigen accumulation or reactive lymphocytes for at least 10 days following protein/adjuvant immunization [44]. Thus, responses to typical immunizations are focused in the nearest lymph nodes draining the immunization site.
APCs such as monocytes and dendritic cells can internalize antigens/adjuvant compounds at the injection site and physically carry the vaccine to lymph nodes, but this process is relatively inefficient compared to direct lymphatic transport of the vaccine. In addition, lymph node-resident DCs possess important functions such as cross presentation for priming CD8+ T-cells which are absent in some tissue-resident DCs [46]. Migratory DCs can transfer antigen to lymph node-resident DCs [47,48], but a comparison of the proportion of APCs that acquire antigen (and the amount of antigen acquired) in the setting of migratory DC-mediated antigen transport vs. efficient lymph node-targeted vaccines suggests the latter approach can load many more dendritic cells with antigen, and to higher levels (Table 1 ). Thus, vaccine formulation strategies that promote direct vaccine uptake into lymphatic vessels and accumulation in lymph nodes are of great interest.
Table 1.
Antigen uptake by lymph node dendritic cells following immunization with depot-forming and LN-targeted vaccines.
| Vaccine category | Material | Time | % DC Uptake* | Particle Size | Injection Site | Ref |
|---|---|---|---|---|---|---|
| Depot forming vaccines (Cell mediated antigen trafficking) | ||||||
|
Alum |
Alum Alum |
48 hrs 48 hrs |
0.01% 0.0065% |
-- -- |
i.m. i.m. |
[44] [102] |
|
Injected scaffolds |
Fibrous MR scaffold Macroporous PLGA scaffold |
168 hrs 168 hrs |
2% 0.64% |
-- -- |
s.c. (flank) s.c. |
[272] [80] |
|
Large Particulates (200 to 2000 nm) |
PRINT particles |
48 hrs |
2% |
1000 nm |
s.c. (flank) |
[333] |
| LN Targeting vaccines | ||||||
|
Liposomes |
Liposomes Liposomes |
24 hrs 48 hrs |
6% 3% |
150 nm 200 nm |
s.c. (tail base) s.c. (tail base) |
[161] [112] |
|
Small Nanoparticles |
alhydrogel NPs PRINT particles PPS NPs PPS NPs polymer nanogels poly-γ glutamic acid NPs |
24 hrs 48 hrs 24 hrs 24 hrs 24 hrs 24 hrs |
2% 20% 5% 30% 45% 40% (CD205+ DCs) |
85 nm 180x80 nm 100 nm 45 nm 40 nm 38 nm |
s.c. (footpad) s.c. (flank) s.c. s.c. s.c. (footpad) s.c. (tail base) |
[169] [333] [116] [116] [167] [334] |
|
Molecular Chaperones |
Protein G albumin binding domain recombinant antigen albumin-binding lipid vaccine albumin-binding small molecule vaccine anti-DEC205 Ab chaperone |
24 hrs 24 hrs 24 hrs 24 hrs |
12% 65% 71% 38% |
-- 6 nm 6 nm -- |
s.c. (tail base) s.c. (tail base) s.c. (tail base) s.c. (footpad) |
[127] [29] [128] [133] |
*Percentage of lymph node CD11c+ cells found to be positive for antigen acquisition by flow cytometry at 24-48 hr post immunization.
i.m.: intramuscular, s.c.: subcutaneous
Within lymph nodes, T cells and B cells are compartmentalized into specific sites, with T cells residing primarily in the paracortex deeper in the lymph nodes while B cells reside in follicles (Fig. 1). Vaccine particles carried to the lymph node by lymph or in lymph-migrating APCs arrive at the subcapsular sinus of the draining lymph node. The sinus is lined by a layer of macrophages and epithelial cells that form a barrier limiting access to the LN parenchyma. Material in the sinus can either drain to the medullary sinuses and subsequently exit the node through efferent lymphatics, which travel to the next lymph node in the chain, be passed into the LN parenchyma via gated, narrow collagen conduits [[49], [50], [51], [52]], or be physically captured and transferred across the sinus barrier by sinus-lining macrophages [53,54].
Transport within the lymph node impacts access of antigen/adjuvant compounds to key cells moderating lymphocyte activation: dendritic cells that specialize in priming CD4+ helper vs. CD8+ cytolytic T cells are localized in distinct sites, with CD8-priming DCs located deeper in the paracortex [55,56]. B cells that receive initial signaling by binding to cognate antigens enter specialized subregions of the follicles termed germinal centers (GCs), which are split into a light zone and a dark zone. Follicular dendritic cells in the light zone capture and retain antigen via Fc and complement receptors, which they can present for prolonged periods to local B cells [57,58]. Within the GC, B cells undergo the cyclic process of affinity maturation: In the dark zone, B cells proliferate and mutate their antigen receptors; as a round of proliferation ends, GC B cells migrate to the light zone, where they can acquire antigen from FDCs that is processed and presented on class II MHC to follicular helper T cells (Tfh). Tfh cells play a crucial role in providing survival and costimulatory cues to B cells that determine the amount of proliferation and antigen receptor mutation they carry out on returning to the dark zone, and competition among B cell clones for Tfh help determines the outcome of the GC response [[59], [60], [61]]. B cells which fail to receive sufficient signals from Tfh undergo apoptosis [62]. B cells can exit the GC to become short-lived antibody producing cells termed plasmablasts, long-lived antibody-producing plasma cells, or memory cells; the cues governing these fate decisions remain poorly understood. B cell-Tfh interactions are ultimately governed by the antigen acquired by the GC B cell [63], and the amount of antigen accumulated in lymph nodes directly correlates with the number of Tfh and GC B cells that develop in immunized lymph nodes [44]. Thus, the efficiency of antigen delivery to FDCs will also affect responses to immunization.
3. Vaccine delivery to lymph nodes
3.1. Technologies promoting cell-mediated trafficking of antigen to lymphoid tissues
A classic fundamental function of vaccine adjuvants has been thought to be the promotion of cell-mediated trafficking of antigen to draining lymph nodes. In general, this mode of vaccine delivery is promoted by attracting migratory immune cells to the injection site from the local tissue or the blood, which can internalize antigen and subsequently migrate to the draining lymphatic vessels to carry vaccine components to the draining LN (Fig. 1, pathway 1). Two licensed adjuvants, alum (Fig. 2A) and MF59 (Fig. 2B, an oil-in-water nanoemulsion), are thought to promote vaccine responses in large part through such mechanisms [64]. Both alum and MF59 induce the local production of chemokines recruiting neutrophils, monocytes, DCs, and macrophages to the injection site [44,65,66]. These recruited cells subsequently take up antigen and transport it to draining lymph nodes, increasing the amount of antigen+ cells compared to antigen alone and triggering activation of APCs in the dLNs [44,65]. These mechanisms can promote both T cell and B cell responses due to the ability of dendritic cells to preserve antigen intact in intracellular compartments and recycle it to the cell surface for access by B cells [67]; this function in fact appears to be promoted by MF59 [68]. These processes related to cell-mediated antigen trafficking are also induced by particulate adjuvants such as GSK’s liposomal adjuvant AS01b, which is comprised of liposomes with a size suitable for direct transport via lymphatics to the lymph node [69]. Thus, even lymph node-targeted adjuvants (discussed below) likely act in part through effects on immune cell recruitment at the injection site.
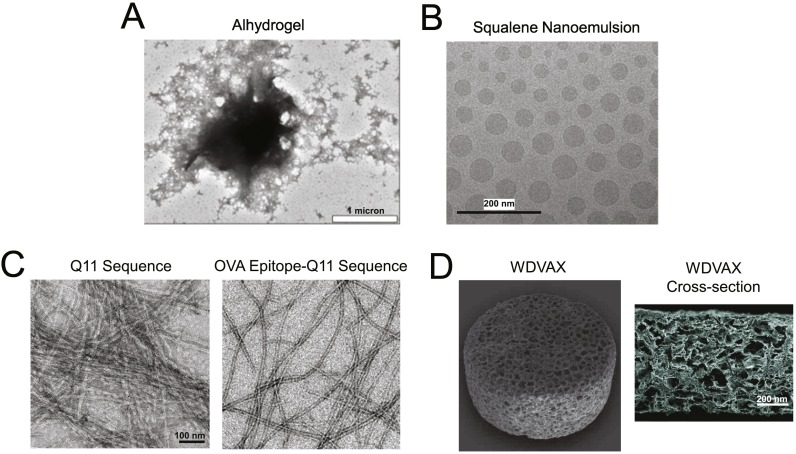
Fig. 2.
Technologies promoting cell-mediated antigen trafficking to lymph nodes. (A) TEM image of Alhydrogel adjuvant (Adapted from [64]). (B) Cryo-TEM images of an oil-in-water emulsion adjuvant similar to the licensed adjuvant MD59 (Adapted from [144]). (C) Peptide sequence Q11 forming beta-sheet-enriched nanofibers in vitro with and without N-terminal OVA peptide (Adapted from [74]). (D) PLGA macroporous implantable scaffold used as a vaccine platform that can be loaded with cancer cell lysates or peptides, CpG adjuvant, and GM-CSF to promote DC recruitment, antigen loading, and activation (Adapted from [83]). Scale bar: 200 microns.
In addition to these classic adjuvants, a number of novel vaccine strategies aim to promote cell-mediated trafficking of antigen. Cationic liposomes have been developed as adjuvants that form antigen depots via ionic interactions with both antigen and the surrounding extracellular matrix at the site of injection. CAF01, a two-component liposomal adjuvant system comprised of the cationic lipid dimethyl dioctadecylammonium stabilized by a glycolipid adjuvant trehalose dibehenate, was shown to remain at the injection site longer than larger liposomes with neutral charge [70]. In murine tuberculosis models, CAF01 added to the live attenuated bacille Calmette-Guérin vaccine promoted significantly higher CD4 central memory T cell expansion needed for improved immunity [71]. Motivated by this preclinical data, a CAF01-adjuvanted vaccine incorporating the protein antigens ESAT-6 and Ag85B was translated into human trials and was shown to be safe, although only a small proportion of the patients showed long lasting memory T-cell formation after 3 years [72].
A second class of promising new vaccine delivery technology is based on peptides that self-assemble into fibers with diameters of only a few nm but lengths of microns, which form an injectable hydrogel under physiologic conditions (Fig. 2C) [73,74]. By tethering peptides and/or whole proteins to the subunits, these nanofibers can elicit both T cell and antibody responses in the absence of additional adjuvants [[75], [76], [77], [78]]. Although the mechanism by which these fibers promote immune response is still under study, it seems likely that the nanofibers act as a depot for antigen acquisition by infiltrating immune cells over some time period, although they might also “shed” smaller assemblies over time that could traffic to lymph nodes or be phagocytosed for transport by APCs, as recently reported for self-assembling block copolymer nanofibers [79]. Such a dynamic process could also have implications for vaccine kinetics discussed later.
Technologies promoting cell-mediated antigen trafficking to dLNs are also being developed to enhance CD8+ T cell-based immunity. One recently developed approach employs biodegradable polymer scaffolds implanted under the skin as a matrix to recruit DC precursors from the surrounding tissue and blood, differentiate them in situ, load them with antigen, and activate them to migrate to draining LNs: Poly(lactide-co-glycolide) foam discs containing GM-CSF were shown to be highly efficient at recruiting DCs to the scaffold implantation site (Fig. 2D) [[80], [81], [82], [83]]. By further incorporating adjuvants such as CpG and antigens such as tumor lysates or peptide antigens, it was shown that DCs can be recruited to the scaffold, activated to produce inflammatory cytokines, and prime CD8+ T cells, promoting tumor rejection in mouse models of melanoma. Success in these preclinical studies has led to evaluation of this approach in a phase I trial in melanoma patients [84]. The approach taken here has also been implemented using collapsible hydrogels to achieve similar DC recruitment/programming in an injectable material [81,82].
3.2. Nanoparticles for selective LN trafficking of vaccine immunogens
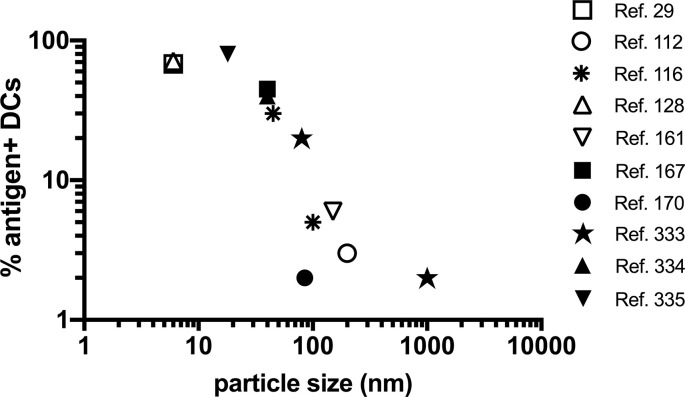
An alternative to vaccines promoting cell-mediated trafficking of antigen to LNs is to engineer efficient entry of the vaccine into lymphatic vessels for direct transport in lymph fluid to the draining LN (Fig. 1, pathway 2). Amongst many possible approaches, designing vaccines in a particulate form is one well established strategy to promote such direct lymph node targeting. Particles smaller than ~5 nm efficiently diffuse through the basement membrane of the blood endothelium and enter the circulation, while particles larger than ~100 nm are often trapped in the extracellular matrix at the injection site only to be displaced by antigen presenting cells or macrophages that phagocytose the particles [85,86]. Thus, only nanoparticles (NPs) with sizes roughly between 5 and 100 nm enter the lymphatics and efficiently reach lymph nodes through direct transport in lymph. (Note however that when considering literature studies, many variables such as the volume of vaccine solution injected, animal model used, and site of injection impact the uptake of particles into lymphatics and their transport, and transient edema induced by the injection itself can enable larger particles to enter lymph vessels). For small protein antigens, these transport requirements can be met by linking antigen to a particulate carrier. A broad range of technologies have been developed for this purpose including virus-like particles (VLPs), protein based self-assembling NPs, liposomes, and synthetic particles [85,87,88]. Variables such as the surface chemistry, shape, and stiffness of particles undoubtedly impact the efficiency of lymphatic trafficking [86,89]. However, it is striking that quantifying antigen delivery to lymph node dendritic cells (as a crude measure of LN delivery efficiency) as a function of particle size alone reveals a strong influence of particle size, with particles < 50 nm in diameter showing an ability to deliver antigen to a majority of LN DCs within 24-48 hr irrespective of particle composition (Table 1, Fig. 3 ). This analysis also shows that relative to technologies promoting cell-mediated antigen trafficking, directly lymphatic targeting with particulate vaccines leads to much greater antigen levels in draining lymph nodes (Table 1).
Fig. 3.
Relationship between vaccine particle size and antigen uptake by lymph node dendritic cells. Shown are compiled data of parenteral immunization with a broad range of subunit vaccines based on antigen linked to or encapsulated within particles of varying composition and size (references as indicated in the legend). Data is shown from studies where the frequency of antigen+ CD11c+ dendritic cells were measured by flow cytometry at 24-48 hr post immunization.
VLPs are formed from virus-derived proteins that assemble into a viral capsid and can either display its endogenous viral antigen or are chemically or genetically modified to display a heterologous antigen of interest [90]. These natural nanoparticles have already been proven an effective basis for vaccines; the licensed human papilloma virus and hepatitis B vaccines are based on VLPs and many additional VLP-based vaccines are in clinical development [88]. The origin of the viruses for these particles include bacteriophages, animal viruses, and plant viruses all of which can display dense array of antigens to strongly crosslink B cell receptors [[91], [92], [93]]. Moreover, they can be generated within eukaryotic or prokaryotic cells with tunable sizes ranging from 20 to 100 nm and display both whole proteins or peptides.
Protein-based nanoparticles created from chemically crosslinked or self-assembled subunits of non-viral origin have also been used to create particulate forms of immunogens. Genetic fusion of antigens a variety of self-assembling proteins including ferritin, E2 (Geobacillus stearothermophilus), and lumazine synthase (hyperthermophile Aquifex Aeolicus), has been used to create particles ranging from ~15-30 nm in diameter displaying up to 60 copies of selected immunogens [[94], [95], [96], [97], [98]]. Sixty-mer nanoparticles formed by the fusion of a gp120 antigen with lumazine synthase [[98], [99], [100], [101]] recently entered clinical trials (Fig. 4A, clinicaltrials.gov: NCT03547245). Vaccine nanoparticles formed by alcohol-mediated desolvation of protein antigens followed by chemical crosslinking has been used to formulate multiple influenza antigens into the same particle, promoting broad influenza strain protection in mouse models [102]. Advances in protein engineering have now also allowed de novo self-assembling protein designs to be implemented to create well-defined, monodisperse antigen-particles [103,104]. For example, using a pair of synthetic protein subunits designed to self-assemble together into three-dimensional particles, Sanders and colleagues created a system that forms icosahedral NPs presenting stabilized HIV Env trimer immunogens [104]. This two-component particle assembly carried out using purified individual subunits reduced misfolding of the trimers and improved trimer homogeneity on the particle surfaces, overcoming drawbacks of NPs assembled intracellularly during cellular production.
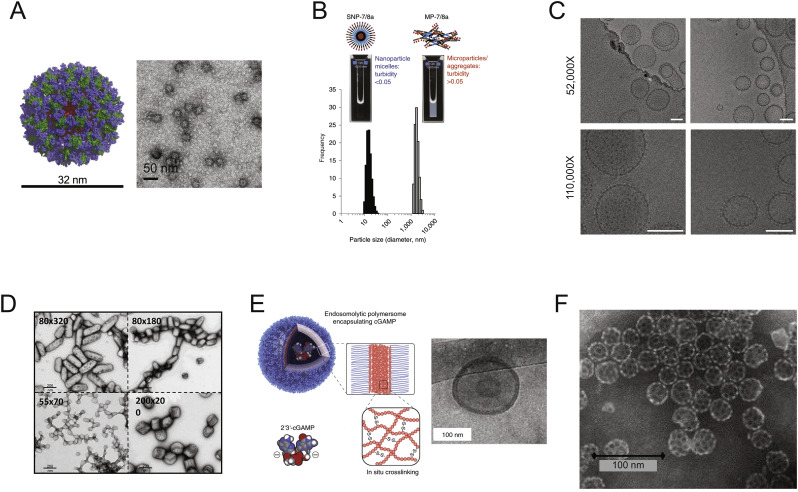
Fig. 4.
Nanoparticle vaccine formulations for LN delivery. (A) Molecular model and TEM images of eOD-60mer nanoparticles used as a priming immunogen to elicit CD4 binding site-directed antibody responses against HIV (Adapted from [110]). (B) DLS measurements of two classes of peptide-TLR7/8 conjugate vaccines. Left represents a self-assembling nanoparticle micelle and right represents a microparticle/aggregate format. (Adapted from [106]). (C) TEM images of HIV envelope trimers attached to liposomes via metal affinity coordination bonds, scale bar: 100 nm. (Adapted from [109]). (D) TEM images of PLGA-based asymmetric PRINT Dengue Virus vaccine nanoparticles. (Adapted from [118]). (E) Schematic of endosomolytic polymersomes assembled from disulfide-linked diblock copolymers encapsulating the STING agonist cGAMP (left) and TEM of polymersomes (right). (Adapted from [331]). (F) TEM of saponin-based immune stimulating complexes (ISCOMs) [332].
In addition to engineered larger proteins, peptide sequences that form NPs have been developed. By incorporating flagellin (TLR5 agonist) and avian or human influenza epitope sequences into a self-assembling peptide framework, self-adjuvanted NPs were generated that elicited neutralizing antibody responses and protected mice from challenge with a lethal dose of influenza virus [105]. To achieve a generalizable technology for formulating peptide antigens of interest for cancer vaccines into a uniform nanoparticle state, Lynn et al. recently described a platform based on the linkage of antigen epitopes with charge-modifying peptide blocks that promote hydrophobic self-assembly and also carry chemically-linked small molecule Toll-like receptor 7/8 agonists as built-in adjuvants [106]. These peptide nanomaterials are designed to self-assemble in water into 20 nm nanoparticles irrespective of the peptide antigen sequence, facilitating application to personalized neoantigen-based vaccines for cancer (Fig. 4B). This platform provides the added benefit of codelivery of small molecule adjuvants ensuring potent immune activation [106].
Liposomes have also been extensively used for targeted vaccine delivery to LNs. Liposomes can be formed in sizes suitable for lymphatic trafficking, and display antigens attached to the exterior membrane surface, or carry antigen or adjuvant compounds within the vesicle lumen [107]. By selecting lipids with functional groups such as maleimides or chelatable metal ions such as cobalt and nickel, proteins ranging from targeting antibodies to histidine-tagged immunogens can be densely tethered with control over antigen orientation on the surface of liposomes (Fig. 4C) [78,102,[108], [109], [110], [111]]. Lipid vesicles can be engineered as multilamellar structures with crosslinks between bilayers to increase their stability in vivo, thereby increasing the proportion of dendritic cells that acquire antigen in lymph nodes [112]. Lipids can also be self-assembled with peptides derived from lipoproteins to form vaccine-carrying nanodiscs that exhibit efficient LN accumulation following parental injection [113].
In addition to using biological constituents, synthetic materials such as biodegradable polymers or inorganic materials can be used to create vaccine NPs. Solid polymer nanoparticles less than 50 nm in diameter show efficient LN targeting following parenteral injection [[114], [115], [116]]. New manufacturing technologies such as PRINT (Particle Replication in Nonwetting Templates) enable the shape of polymeric particles to be controlled in addition to size (Fig. 4D) [117]. Using this technology, poly (lactic-co-glycolic acid) particles with tunable size and aspect ratios were used to absorb DENV-E protein from Dengue virus and demonstrated to be potent vaccine delivery vehicles compared to soluble antigen. Interestingly, asymmetric and smaller particles elicited higher overall antigen binding titers although comparable results were observed for neutralizing titers and retention at the injection site for all particles [118]. These PRINT particles were also shown to be scalable and have been translated into Phase I clinical trials for influenza vaccines (clinicaltrials.gov: NCT01224262). In addition to synthetic moieties, NPs can also be generated by complexation of soluble polymers through ionic interactions. For example, a combination of positively charged chitosan and negatively charged dextran sulfate forms NPs that have been shown to interact with and co-deliver an HIV peptide antigen and the TLR3 agonist Poly(I:C). The targeted delivery of both antigen and adjuvant resulted in high B and T cell responses in vivo [119]. Unique to synthetic polymers, recent NP technologies have exploited the capacity of certain polymers to induce rupture of antigen-containing endosomes in APCs prior to fusion with lysosomes. Such approaches maximize delivery of cargo into the cytosol thus increasing antigen presentation on class I MHC to CD8+ T cells. To this end, poly(propyl acrylic acid)-based NPs that that induce endosomal escape were found to effectively deliver tumor specific peptides to DCs and induce anti-tumor immunity (Fig. 4E) [120]. In a similar vein, ultra-pH-sensitive NPs that act as proton sponges to buffer the pH of endocytic organelles were shown to significantly improve antigen uptake and display by DCs. These particles were also found to activate the STING pathway thereby served as an effective stimulatory vehicle without the addition of adjuvants [121]. Lastly, nanoparticles composed of inorganic materials are also being explored as vaccine delivery vehicles. For example, gold NPs (GNPs) have been explored as antigen carriers, motivated by their ease of synthesis and tunable size [85]. Recent findings have suggested that these materials may have intrinsic adjuvant-like properties, as GNPs used as peptide vaccine carriers did not require an added adjuvant to induce anti-tumor T cell responses in mice [122]. In addition, GNPs can present a wide array of antigens including whole proteins or be coated with membranes of bacteria or red blood cells [123,124].
3.3. Molecular chaperones for LN targeting
In addition to formulating vaccines as nanoparticles, molecular approaches to target vaccine components to lymph nodes have garnered considerable attention. These strategies include approaches to associate vaccine antigens/adjuvants with larger protein carriers, which passively target the vaccine to LNs though molecular size, and molecular targeting approaches, where vaccines are linked to antibodies or other engineered binding proteins that both have a size suitable to promote lymphatic uptake and enable specific binding to target cell populations in the LN, such as dendritic cells.
Molecules with a hydrodynamic diameter of ~5 nm or larger are of sufficient size to exhibit preferential trafficking into lymph rather than clearance into the blood following parenteral injection [86,89]. This enables any globular protein of ~40 kDa or larger to serve as an effective chaperone to direct small antigens to the lymph nodes [125,126]. Albumin, the most prevalent protein in blood and tissue interstitial fluid, serves as an ideal chaperone as it constitutively traffics from blood to lymph. One of albumin’s main functions is to serve as a fatty acid transporter, and hence one strategy to promote lymph node targeting of vaccines is to link antigens or molecular adjuvants to phospholipid tails selected for efficient albumin binding [29,127]. An important element in this strategy is the inclusion of a solubilizing polymer spacer (e.g., poly(ethylene glycol)) between the cargo and the albumin binding lipid moiety, to ensure the conjugate is soluble and able to interact with albumin. Vaccines can also achieve lymph node targeting by covalent linkage to albumin or through peptides, protein subunits, or small molecules that exhibit high-affinity binding to albumin [[127], [128], [129]]. Albumin is not unique in serving as an efficient chaperone for lymph node uptake of vaccines, and similar lymph node targeting can be achieved by fusing antigens to other large proteins [129]. However, a potential advantage of using lipid-based non-covalent association with albumin to mediate LN trafficking is the subsequent transfer of the vaccine conjugates to cell membranes in the lymph node [130]–which may promote enhanced capture and prolonged antigen presentation in LNs [127]. For antigens covalently linked to large protein chaperones, the carrier should be designed to have a short circulatory half life, because antigen not captured in the draining LN will eventually pass through the thoracic duct into the systemic circulation. If the carrier has a long half life on reaching the blood, substantial accumulation in distal lymphoid organs (the spleen, distal lymph nodes) can occur– and this antigen taken up in lymphoid tissues far from the injection site can lead to antigen presentation in the absence of adjuvant cues, leading to tolerogenic priming that may blunt the overall magnitude of resulting immune responses [129]. Using a protein chaperone with large size for LN trafficking and short blood half-life can minimize this effect and increases T cell priming.
Antibodies are also effective chaperones that can specifically target antigens to key immune cells such as DCs to initiate adaptive immune responses. In fact, the targeting of receptors CD40, CD11c, DEC-205, and Clec9A has been shown to direct vaccines towards DCs, promote higher antigen uptake, and improve subsequent T cell activation through increased peptide-MHC display [131,132]. Early work by the lab of Nobel laureate Ralph Steinman demonstrated that fusion of antigens with antibodies against the DC-expressed lectin molecule DEC-205 could lead to robust cellular and humoral immunity, with CD8+ T cell responses induced by immunization with nanograms of antigen [133,134]. These promising preclinical findings have led to clinical trials of DC-targeted antigens. In phase I studies, CDX-1401, an anti-DEC-205 monoclonal antibody conjugated to full length tumor antigen NY-ESO-1, was not found to show dose-limiting toxicities although only a portion of the participants across different treatment regimen and doses showed CD8+ T cell responses towards NY-ESO-1 derived peptides post-treatment [135]. However, the efficacy of CDX1401 was substantially improved by pretreatment of patients with CDX-301, a recombinant FMS-like tyrosine kinase-3 ligand (Flt3L) that increases DC numbers within peripheral blood, resulting in higher T-cell responses across all patients (clinicaltrials.gov: NCT02129075) [136]. Note that these dendritic cell-targeted antigens must still be combined with adjuvants to stimulate upregulation of DC co-stimulatory molecules required for effective T-cell priming. Recent strategies have attempted to combine targeted antigen and adjuvant delivery to DCs. Examples include antibody-conjugated polymer nanoparticles loaded with both antigen and adjuvants, or Clec9A-targeting oil-in-water nanoemulsions that were found to possess adjuvant-like properties [131,132].
Besides using antibodies directed at immune cell receptors, antibodies bound to antigens to form immune complexes (ICs) have been pursued as vaccines that are targeted to Fc and/or complement receptors on immune cells. The targeting of specific Fc receptors, which can be modulated by the isotype of the Ab and glycosylation patterns on the Fc domain, is crucial since the choice of Fc receptor can stimulate or inhibit target immune cells [[137], [138], [139]]. Interestingly, a recently developed IC vaccine for influenza was designed to trigger inhibitory Fc receptor, FcgRIIB, on B-cells to limit the proliferation of low affinity BCRs, leading to enrichment and production of high affinity antibodies [140]. In addition to Fc receptor interactions, IC vaccines often elicit complement activation. Deposition of complement on ICs is crucial for FDCs to acquire and maintain antigens throughout an immune response as well as promote B-cell survival through co-engagement of BCR and CD21 [57]. As such, recombinant IC vaccines comprised of antibody-antigen fusions have been developed and reported to successfully elicit immunity [141,142].
3.4. LN targeting of adjuvant compounds
Modern subunit vaccines, comprised of purified recombinant antigens from pathogens, enable the immune system to respond to pathogens without the potential dangers of classical live-attenuated vaccines. Such antigens however, are often unable to elicit efficient immunity on their own and typically require the co-administration of adjuvants. Adjuvants are an integral component of modern vaccines and are crucial in initiating the immune response [12,16,143]. Classical adjuvants such as alum and oil-in-water emulsions non-specifically trigger mild inflammation at the injection site and in draining lymph nodes [11,144]. However, more potent adjuvants are being pursued in clinical and preclinical studies that act through defined immunological pathways. These molecular adjuvants are often ligands for pattern recognition receptors (such as Toll-like receptors (TLRs), RIG-I-like receptors, or NOD-like receptors) in APCs and other cells of the innate immune system, which trigger an inflammatory cascade that promotes efficient activation of the adaptive arm of the immune system [27]. Many of these newer-generation adjuvants are low molecular weight materials, meaning that their systemic dissemination following administration as neat compounds poses a significant risk of systemic toxicity. A canonical example of this class of candidate adjuvant compounds are TLR7/8 agonist imidazoquinolines, small molecules with potent innate immune stimulatory activity in vitro, but which are poorly tolerated when administered as free compounds in vivo ([[145], [146], [147]]). Such adjuvants thus benefit from many of the lymph node targeting strategies described above for antigen targeting to lymph nodes.
Novartis demonstrated one effective strategy to concentrate small molecule adjuvant action in the injection site and draining lymph nodes using the most common clinical vaccine adjuvant, alum [148]. In an effort to address pharmacokinetic (PK) and pharmacodynamic (PD) limitations, derivatives of Imidazoquinolines were synthesized containing a phosphate moiety, enabling ligand exchange with the hydroxyl or phosphate groups at the surface of the aluminum salts used in clinical aluminum adjuvant formulations. Through this engineered alum binding, systemic exposure of the TLRa was reduced and resulted in improved humoral responses following anthrax protein vaccination. These effects were shown to be functionally significant as mice that received phosphate-modified TLR7/8 agonist showed significantly improved survival in a Bacillus anthracis challenge model [148]. This approach should be generalizable to a broad range of candidate molecular adjuvants. Recent data demonstrating trafficking of alum particles to lymph nodes following immunization [149] further suggests this approach may also promote lymph node targeting of the TLR ligands.
Saponins are glycosylated triterpenes originally isolated from the Chilean soap bark tree Quillaja saponaria [150]. Saponins activate the NLRP3 inflammasome and have membrane-disruptive activity in vitro, which promote inflammatory cytokine and chemokine production in vivo and make them effective vaccine adjuvants [151,152]. However, these membrane-interactive small molecules elicited substantial injection site reactions and side effects in humans [153,154], motivating the need for formulations to control their biodistribution and membrane-interaction activity. One fascinating approach to this problem is to complex saponins with cholesterol and phospholipids, which leads to the self-assembly of striking ~40 nm diam. cage-like structures termed ISCOMs (Fig. 4F) [155]. These nanoparticle adjuvants have significantly improved biodistribution properties and efficiently traffic to draining lymph nodes [156]. This class of adjuvant is highly promising at inducing a balanced Th1/Th2 immune response and is being used in clinical trials for cancer vaccines as well as infectious diseases [157].
Liposomes have also been widely utilized to improve LN trafficking properties of small molecule adjuvants to the lymph node [158]. An excellent example of liposomal adjuvants is the clinically approved AS01b adjuvant developed by GSK. AS01b is licensed for use in the Herpes Zoster vaccine Shingrix as well as the malaria vaccine Mosquirix in the European Union, and has demonstrated excellent clinical efficacy [159,160]. AS01b is comprised of 50 - 100nm diam. dioleoyl phosphatidylcholine (DOPC)-based liposomes incorporating the TLR4 agonist monophosphoryl Lipid A (MPLA) as well as the purified saponin fraction QS-21 into the vesicle bilayer. The AS01b-adjuvanted zoster vaccine demonstrated an efficacy of over 90% in adults 70 years and older. Although the Shingrix vaccine caused increased injection site reactogenicity over placebo (79% vs 30%), these adverse events were generally mild and subsided within 3 days of injection [159]. Liposomes have also been used to target small molecule adjuvant compounds such as cyclic di-nucleotide STING agonists to lymph nodes, increasing both the safety and efficacy of these vaccine adjuvants [161]. Immunostimulatory spherical nucleic acids (IS-SNAs) are another form of liposomal-based systems that can promote targeted delivery of nucleic acid-based TLR ligands. Preclinical studies have shown that delivery of the TLR9a CpG 1826 in IS-SNA form exhibits 700-fold higher antibody titers and 400-fold higher cellular responses compared to injection of free adjuvant [162]. Phase 1b/2 clinical studies evaluating the safety and efficacy of this technology for treatment of Merkel Cell Carcinoma and Metastatic Melanoma are underway (clinicaltrials.gov: NCT03684785). Co-formulation of adjuvant compounds with antigens in one delivery vehicle has also been demonstrated using IS-SNAs, liposomes or lipoprotein-mimicking nanodiscs [111,112,161,[163], [164], [165]].
Formulation of adjuvant compounds in biocompatible polymer or inorganic particles is another promising engineering solution to improve both the safety and efficacy profile of vaccine adjuvants. For example, conjugation of small molecule TLR7/8 agonists to a water-soluble polymer backbone with different types of hydrophobic linker led to self-assembly of the resulting conjugates into particles of varying size in aqueous solution [166]. Conjugates forming particles of ~700 nm size achieved substantially better LN homing, LN cytokine production, and T cell immunity compared to unconjugated TLR7/8 agonists. Given the near micron size of these particles, it is expected that the main mode of transport to LNs is through active APC transport [115]. Consistent with these properties, these large polymer adjuvants were found primarily within DCs, monocytes, and macrophages in the LN. Biodegradable polymer nanogels have also been developed to promote LN targeting of TLR7/8 agonists: Reversible addition-fragmentation chain transfer (RAFT) polymerization was used to generate well-defined amphiphilic block-polymers conjugated with TLR7/8 agonists. These amphiphiles self-assembled into particles ~50 nm in diameter and exhibited efficient LN targeting. Compared to soluble TLR7/8a that was widely distributed to several organs following injection, TLRa-nanogels were localized to the injection site and the dLN [167]. Synthetic particles also enable the capacity to co-target multiple molecular adjuvants and/or antigens together to lymph nodes. For example, Kasturi et al. encapsulated influenza HA, the TLR4 agonist MPLA, and the TLR7/8 agonist R848 together into ~300 nm diam. poly(lactide-co-glycolide) nanoparticles. Immunization with particle-formulated TLRa led to substantially greater humoral immune responses and higher titers of virus-neutralizing antibodies compared to injection of “free” adjuvant compounds [168]. Alum adjuvant formed into 80 nm nanoparticles through mechanical dispersal of aluminum hydroxide nanocrystals followed by stabilization with polyanions was able to co-deliver CpG and antigen more efficiently to LN APCs and represents another example of how co-delivery of multiple adjuvants combined with antigen can improve humoral responses [169].
Finally, molecular chaperones are also of interest for targeting adjuvant compounds to lymphatics. Albumin-binding lipid conjugates of TLR9 agonist CpG DNA concentrated the adjuvant in lymph nodes and blocked systemic dissemination, both lowering toxicity and increasing the adjuvant’s potency [29,127]. This molecular adjuvant will enter first-in-human clinical testing with a cancer vaccine in late 2020/early 2021. Similar approaches have been applied to target TLR7/8 agonist compounds to lymph nodes for enhanced vaccine activity [170].
3.5. Biodistribution of nucleic acid and viral/bacterial-vectored vaccines
Nucleic acids encoding antigens and/or adjuvant molecules can also serve as vaccines, when delivered into host cells through synthetic agents (e.g., formulation in lipid nanoparticles, LNPs) or as recombinant viral or bacterial vectors. These technologies have gained steady momentum over the past 20 years, and preclinical studies have shown that nucleic acid vaccines are effective against a broad range of pathogens including influenza, Zika, tuberculosis, and HIV, creating significant interest for the translational prospect of this technology [[171], [172], [173]]. Ervebo, a recombinant vesicular stomatitis virus encoding ebola virus antigens, became the first viral-vectored vaccine licensed by the US FDA in 2019. In general, these approaches utilize the host’s cellular machinery to express foreign proteins of interest to induce anti-pathogen immunity. In this case, antigen localization is determined by what cells in what locations are transfected by the delivery system, be it syringe injection of naked nucleic acids, a synthetic nanoparticle, or a viral particle.
DNA-based vaccines are most often administered as naked plasmid molecules directly into the muscle, with or without transfection-promoting treatments such as in vivo electroporation, and thereby primarily transfect muscle cells and produce antigen at the injection site, which is either secreted or acquired from dying muscle cells by migratory antigen presenting cells [[174], [175], [176]]. Recently, an approach termed in vivo electroporation has made headway as a clinically-relevant approach to promote DNA delivery into the muscle for vaccines against precancerous lesions associated with HPV infection [177,178]. Similar to naked DNA injection, this approach appears to also primarily confine transfection to the muscle [179]. However, gene gun technology, which ballistically implants gold particles coated with DNA into the skin, has been reported to directly transfect dendritic cells in the skin [180,181].
An alternative to plasmid DNA is mRNA encoding vaccine antigens and/or adjuvant molecules. mRNA vaccine molecules consist of 5 major components: a 5’ cap structure, a 5’ untranslated region (5’ UTR), an open reading frame that encodes the antigen of interest, a 3’ UTR, and a polyA tail. These vaccine mRNAs are typically less than 3kb in length and are generally delivered through the use of cationic lipid nanoparticle (LNP) formulations, functionally similar in design to the LNP formulation in the approved siRNA drug Patisiran [182]. In studies evaluating a library of PEGylated LNP compositions for mRNA vaccine delivery, Oberli et al. reported that LNPs with slightly negative zeta potentials administered subcutaneously in mice transfected cells at the injection site and also in immediate and next-draining lymph nodes [183]. By flow cytometry, 3-5% of myeloid and lymphoid DCs were transfected by the particles in draining lymph nodes. By contrast, LNPs of a similar general composition but employing a different (undefined) ionizable lipid in the composition administered intramuscularly were reported to transfect not only muscle cells but also to exhibit transient gene expression in the liver, indicating systemic dissemination of the LNPs from the injection site [184]. Subcutaneous or intradermal administration of these particles were found to elicit gene expression only at the injection site. Most LNP formulations under study for mRNA delivery are formulated with a net positive charge, deriving from the ionizable lipid or cationic helper lipids in the formulation. Kranz et al. demonstrated that for i.v. delivery, near-neutral or negatively-charged LNPs localized almost exclusively to the spleen, and led to mRNA expression primarily in CD11c+ cells [185]. Based on these promising preclinical findings, this approach was translated into a first-in-humans clinical trial for therapeutic cancer vaccination, and in a small number of patients, induction of de novo antigen-specific T cell responses against a set of tumor-associated antigens was reported [185]. Thus, antigen localization with such LNP-formulated vaccines depends on both the chemistry of the lipid carrier as well as the route of injection.
Recombinant viral or bacterial vectors are a third class of nucleic acid vaccine. Many viruses are particles < 100 nm in diameter that are well suited for direct lymphatic trafficking from a parenteral injection site. For example, after a single dose of intra-dermal injection, adenovirus (Ad) serotype 2 vector was found primarily at the injection site and the skin draining LNs [186]. However, multiple doses of (6 injections over a span of 40 days) did result in detection of vector DNA within the spleen. Intramuscular (i.m.) injection of adenovectors has been shown to give gene expression primarily in the muscle itself [187,188]. By contrast, recombinant vesicular stomatitis virus vectors (rVSV) administered intramuscularly in macaques showed short term (24 hr) systemic distribution with vector RNA detected in the spleen, lymph nodes, and ileum at day 28 [189]. I.m. injection of an rVSV-vectored ebola vaccine in a clinical trial also showed signs of adverse systemic distribution in small percentages of the patients (2% for low dose and 15% for high dose), although most patients did not show serious systemic reactions. Interestingly, this study reported the presence of vasculitis only in rVSV containing the ebola envelope protein and not wild type VSV, suggesting that endothelial cells are potential targets for this vaccine vector [190]. Highly sensitive PCR analysis of recombinant modified vaccinia virus Ankara (rMVA) administered intradermally into SCID mice was only found at the injection site after 49 days and not in reproductive tissues, brain, spleen, or liver [191]. Intravenous, intranasal, and intraperitoneal administration of viral vectors has been shown to result in systemic distribution of the virus with the liver being the most prevalent site of gene expression followed by lungs, spleen, and heart [187,[192], [193], [194]]. Thus, antigen localization can vary widely for viral-vectored vaccines depending on the physical characteristics of the virus and its tropism for host cells.
4. Transport of antigens within lymph nodes
4.1. Entry of soluble and cell-carried antigens into the lymph node parenchyma
Antigens arrive at lymph nodes either via the draining lymph fluid as free particles or carried there by migrating neutrophils, monocytes, or dendritic cells. Migrating APCs can enter the lymph node parenchyma to present antigens to T cells or B cells [[195], [196], [197]]. Lymph-borne antigen accesses the LN parenchyma through several different pathways: it can enter collagen conduits that start in the subcapsular sinus (SCS) and lead into B cell follicles or the deeper paracortex [51,52], be captured by macrophages lining the subcapsular or medullary sinuses [53], or be captured by dendritic cells probing the sinuses with dendrites that permit antigen capture and subsequent migration into the LN [198,199]. Recent studies have also revealed that lymphatic endothelial cells can capture, store, and present antigen [200,201].
Small lymph-borne antigens can enter lymph nodes by passing into the collagen conduits emanating from the SCS. These conduits are gated by a protein filter formed by the plasmalemma vesicle-associated protein, PLVAP, which caps transendothelial channels in the lymphatic endothelial cells lining the sinus [49]. Proteins larger than ~70 kDa are excluded from conduits [202], and this size-mediated exclusion is governed by PLVAP as larger macromolecules are capable of permeating the conduits in PLVAP-deficient mice [49]. Dendritic cells line the collagen conduits and are thought to have preferential access to antigens small enough to enter these passages [52]. However, soluble antigen appears to quickly clear from conduits deeper in the T cell paracortex within several hours, while conduits closer to the sinuses continue to permeate antigen across the subcapsular sinus over more prolonged periods [55]. Interestingly, a recent study has presented evidence that during infection, large viruses can also pass through the conduits [50]; this may be relevant for immune responses triggered by potent adjuvants.
4.2. Complement and the transport of large/particulate antigens into the lymph node
Excluded from conduits, large macromolecular and particulate antigens must instead enter the LN by transfer across the sinuses by macrophages or dendritic cells. SCS macrophages can transfer antigen to antigen-specific B cells that migrate near the sinus [203], and also to antigen-non-specific B cells [204,205]. They appear to have evolved for this role in transferring antigen into the lymph node, as they express a variety of scavenger receptors and integrins that aid in capturing opsonized antigens, and internalize captured material into non-degradative endocytic compartments [54,206]. Transfer to non-specific B cells is mediated by complement [110,204,205]. Binding of complement receptors (CRs) on migrating B cells leads to antigen acquisition from the macrophage, and these non-specific B cells then transfer the captured antigen to follicular dendritic cells, a hand-off thought to be mediated by the higher level of CRs on FDCs [54]. Concentration of antigen in the follicles through these pathways has been shown to amplify GC and Tfh responses and increase both output antibody titers and the production of long-lived plasma cells following immunization [110]. The removal of this antigen relay system decreases the amount of antigen in germinal centers and hinders affinity maturation, suggesting that passive antigen transport may be more limited [207]. The relay system is also responsible for shuttling immune complexes (ICs) that are formed from antibodies generated during an ongoing GC. Shuttled ICs are thought to limit access to B-cell receptors (BCRs) with lower affinities than that of the Abs decorating the ICs, while providing GC B-cells with higher affinities antigen required during the germinal center selection process. This competition may explain why genetic removal of the relay system in mice lacking CR on non-cognate B cells inhibits affinity maturation but not the initial GC reaction [208].
Based on these findings, designing vaccines that activate complement may be an effective approach to enhance humoral immunity and particularly promote germinal center responses that are important for controlling the breadth and affinity of the antibody response. Strategies to promote complement activation include the creation of antigen-complement fusions [209], immunization with pre-formed immune complexes [137,204], or creation of virus-like particles that are recognized by natural IgM [210]. Complement activation can also be engineered into synthetic vaccine particles: polypropylene sulfide nanoparticles functionalized with hydroxylated poly(ethylene glycol) were shown to induce C3b deposition through the alternative pathway of complement activation, promoting antibody responses in mice in the absence of additional adjuvants [114]. Vaccine nanoparticles bearing dense glycosylation can trigger complement activation and FDC targeting through binding of mannose binding lectin, a conserved innate immunity pathway evolved for recognition of bacteria and other heavily glycosylated microbes (Fig. 5A) [110]. Similar trafficking behavior to the follicles has also been recently observed for immunization of non-human primates with liposomes surface-conjugated with glycosylated HIV envelope trimers [45]. As revealed by these diverse examples, nanoparticle formulations appear particularly efficient at eliciting complement-mediated trafficking to follicles. Recently, a first study using gold nanoparticles surface-functionalized with antigen explored the role of particle size in this process. Here, 10-100 nm diameter GNPs were deposited on FDCs, yet only particles larger than 50 nm were retained on FDC dendrites for several weeks, while smaller particles were rapidly cleared (Fig. 5B) [211]. FDC trapping of antigen can also be promoted through “extended dosing” immunization regimens, where antigen is continuously supplied over a week or more (e.g., through repeated injections, slow release devices, or nucleic acid vaccines, as discussed in detail below) (Fig. 5 C). If antigen is still arriving at the LN at time points ~7 days after initial exposure when the first affinity-matured antigen-specific antibodies are being produced in the responding lymph node, these newly-produced antibodies can opsonize the incoming antigen, forming immune complexes that activate complement and triggering the same complement-mediated pathway for FDC deposition. Correlating with this enhanced antigen capture, such extended-dosing strategies have been shown to increase the magnitude of GC and Tfh responses, increase the number of B cell clones participating in the GC response, and increase production of neutralizing antibody responses in response to HIV Env antigen immunizations [212,213]. Thus, a variety of strategies may be exploited to enhance antigen transport to FDCs/germinal centers, and this may provide an important approach for enhancing responses to vaccination.
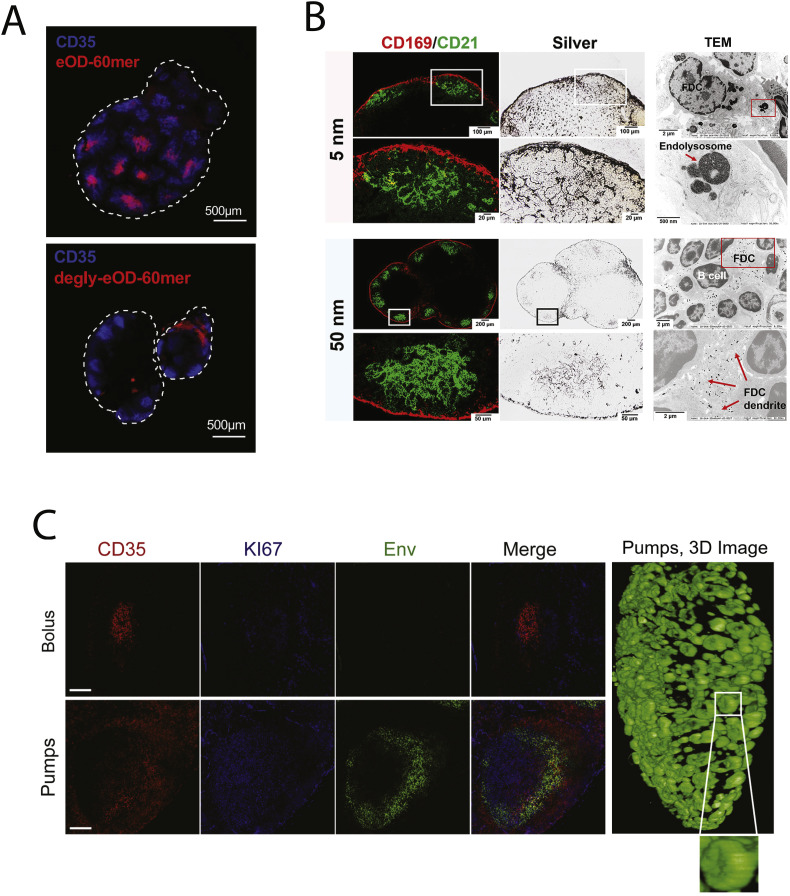
Fig. 5.
Selective antigen trafficking to FDCs within lymph nodes. (A) Heavily glycosylated HIV Env antigens arrayed on protein nanoparticles are trafficked to CD35+ FDCs following immunization, but this targeting is lost following deglycosylation of the particles (Adapted from [110]). (B) Preferential capture of 50 but not 5 nm antigen-conjugated gold nanoparticles following parenteral immunization, observed through silver staining, on FDC networks in the lymph node denoted by CD169/CD21 antibody staining. 50 nm NPs were found on FDC dendrites while 5 nm particles reside in endolysosomes prior to being rapidly cleared (Adapted from [211]). (C) Extended dosing of an HIV Env trimer vaccine using osmotic pumps leads to capture and retention of antigen in the follicles of non-human primate lymph nodes, in constract to bolus immunization with the same antigen and adjuvant (Adapted from [212]).
5. Immunological impact of vaccine kinetics
Vaccine kinetics refers to the temporal changes in the concentration of antigens and/or adjuvants the immune system is exposed to over time, parameters that significantly affect responses to immunization. A variety of basic and vaccine development-focused studies have identified multifaceted effects of antigen and inflammatory cue kinetics on the magnitude and quality of the immune response, with extended exposure over at least a few weeks (reminiscent of antigen persistence following acute infection) being associated with enhancements in both cellular and humoral immune responses. Note that these durations are quite distinct from chronic antigen/inflammation exposure associated with immune dysfunction, e.g., during chronic infection– see Fig. 6 . However, conventional bolus vaccination with soluble antigens is often followed by rapid clearance of antigen from the lymph node following a primary immunization [111,214,215]. Antigen presentation by DCs following bolus injection of protein immunogens typically wanes within ~7 days [174], and can decay even faster following peptide immunization [127].
Fig. 6.
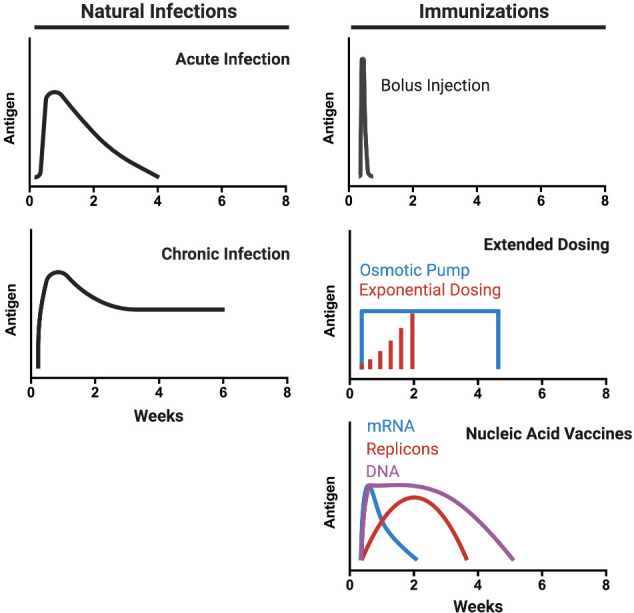
Kinetics of natural infections vs. immunization. Schematics of antigen concentration in the LN as a function of time for natural infections (acute or chronic) and different vaccination technologies. Natural infections are accompanied by a rapid escalation of antigen levels over 1-2 weeks followed by a gradual decay in antigen levels over ~4 weeks (acute) or sustained levels of antigen (chronic). Bolus injections provide a rapid burst and decline in antigen levels due to rapid clearance. This transient exposure can be adjusted by using repeated injection dosing or osmotic pump-driven vaccination. Recently developed nucleic acid vaccines can sustain antigen expression over prolonged periods.
For T-cells, vaccine kinetics modulate lymphocyte proliferation and persistence throughout an immune response. Detailed studies with repetitive adoptive transfer of antigen-pulsed DCs or DCs transduced with inducible expression of peptide-MHC complexes have demonstrated greater T cell proliferation in response to extended durations of antigen presentation [31,[216], [217], [218]]. Computational modeling of T cell expansion based on experimental data has predicted that despite the lack of affinity-based selection for T-cells, increased antigen availability over time will result in larger populations of T-cells with high affinity TCRs [30]. A practical challenge in achieving extended antigen exposure to promote T cell responses is that antigen depots formed at the injection site can become a “sink” for activated lymphocytes, where activated effector cells accumulate in response to the residual antigen and can undergo apoptosis if insufficient inflammatory cues are present [219].
Vaccine kinetics also have a major impact on B cell responses, which stems at least in part from the function of germinal centers (GC) to affinity mature B cell receptors (BCRs). Availability of antigen to B cells in the GC determines their ability to receive help from Tfh cells, a process which initiates within several days following immunization but can continue for months [59,212,220]. In parallel, antigens arriving in the subcapsular sinus can quickly begin to be proteolyzed [221]. It has been hypothesized that GCs might be a location where antigen is protected from proteolysis [54], but this remains largely conjecture. An analysis of the BCR sequences of B cells in GCs after a single injection of soluble influenza hemagglutinin showed broad diversification of the BCR repertoire to the extent that a sizable portion of GC cells did not bind to the original (intact) antigen [222]. From such findings, an alternate emerging hypothesis is that antigens are susceptible to degradation in the LN and can expose immunodominant epitopes on these breakdown products that are irrelevant for protection [[222], [223], [224]]. Thus, strategies to ensure that sufficient amounts of antigen in the correct structural state are present for GC B cells over time for effective affinity maturation may be important for optimal affinity maturation and clonal selection.
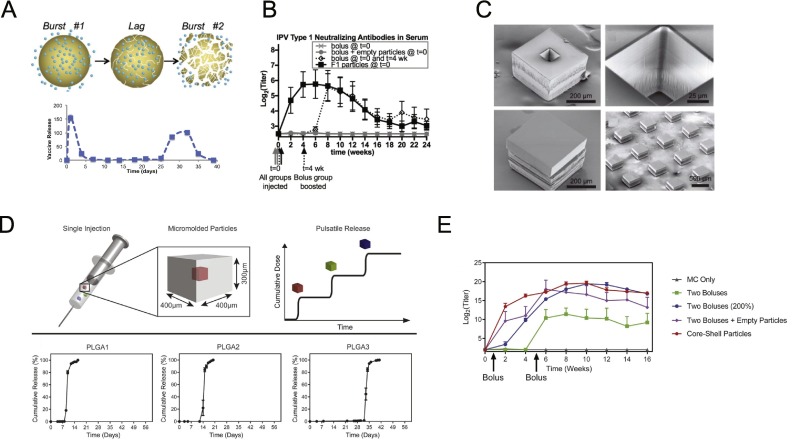
Recent studies have illustrated that alternative vaccine dosing regimens (collectively termed “extended dosing”, Fig. 6) achieve prolonged antigen availability and thereby improve humoral responses compared to bolus injections. In mice, “extended dosing” immunization through repeated injections or through the implantation of osmotic pumps releasing vaccine at a constant rate over several weeks led to 3-5 fold increases in GC B cells and antigen-specific Tfh cells compared to bolus immunization, and antibody titers were increased more than 10-fold for any given total dose of vaccine [[225], [226], [227]]. Similar substantial enhancements in antigen-specific IgG responses were reported for extended dosing regimens of inactivated poliovirus vaccines administered as 4 or 8 daily doses [228]. Mechanistically, antigen arriving at the LN at late times when the first affinity-matured antibodies were being produced (~7-10 days after initial antigen injection) led to enhanced capture and retention of antigen on FDCs within follicles. Computational modeling suggested that this increased antigen availability drives the GC response more effectively [213]. Interestingly, in line with experimental results in mice, the computational model showed that for repeated injection immunization, a “dose escalation” regimen administering vaccine in an exponentially-increasing dosage over time was superior to constant or decaying dose patterns, by maximizing antigen available for capture in immune complexes at the end of the dosing scheme [213]. These results were extended in a non-human primate (NHP) model of HIV vaccination, where osmotic pump or “extended dosing” (ED) vaccination with HIV envelope trimers outperformed traditional bolus immunizations [42,212]. Longitudinal tracking of the immune response in NHPs through fine needle aspirates revealed that bolus immunization triggered a GC response that only expanded over 2 weeks and then plateaued, while pump and ED immunizations led to GC responses that expanded continuously over ~5 weeks. Similar to the findings in mice, binding antibody titers were much higher for pump/ED immunization compared to bolus vaccination, and importantly, tier 2 neutralizing antibody responses against the Env trimer were greatly enhanced by the extended dosing strategies. Sequencing of antigen-binding B cells from GCs in these animals revealed that extended dosing immunizations did not increase the amount of mutations in the BCRs of responding B cells, but did increase the number of clones entering GCs [212]. This striking finding correlated with electron microscopy analysis of the specificity of the antibody response, which revealed that bolus immunization only elicited antibodies binding the base of the Env trimer, while extended dosing elicited additional responses towards non-base regions of the trimer, including putative neutralizing epitopes [212]. An interesting contrast was obtained in a study testing extended dosing approaching with a glycan immunogen meant to elicit antibodies against the high mannose patch of HIV. In this study, extended dosing increased overall binding titers, but antibodies against the intact, structurally faithful glycan target were highest with traditional bolus immunization [226]. This may reflect the need for high instantaneous concentrations of glycan immunogens in the lymph node to overwhelm tissue glycosidase activity, or other factors that are not yet fully understood for glycan antigens. Overall, these studies highlight the potential for extended dosing of vaccines to promote potent and directed immunity against neutralizing epitopes on pathogens.
Taken together, modulation of vaccine kinetics to increase availability of “fresh” antigen for extended periods of time have the potential to (i) sustain and improve affinity maturation for GC B-cells by enabling the recruitment of Tfh help and minimize BCR diversification and (ii) promote greater expansion of CD4+ and CD8+ T-cells of potentially high mean affinity to aid protective immunity.
6. Modulating kinetics of subunit vaccines
6.1. Vaccine kinetics with the most common clinical adjuvant, alum
Osmotic pumps and repeated injections are not convenient for prophylactic immunization in healthy subjects, but a variety of other technologies are in development to address this need. Aluminum hydroxide or aluminum phosphate (collectively referred to by the chemical misnomer “alum”) are the most common adjuvants used in approved vaccines. Aluminum hydroxide is physically comprised of noncovalent aggregates of tiny needle-like aluminum hydroxide nanocrystals (Fig. 2 A). Alum was originally conceived of as acting as a depot, which could adsorb antigen and slowly release it over time to promote the immune response in vivo [64,229]. These features have been well documented since the 1930s when injection sites of alum and antigen from immunized animals were extracted, homogenized, and transplanted into non-immunized animals and shown to elicit an antigen-specific humoral response [230]. By using sheep as a model system, Meeusen and colleagues tracked and characterized lymphatic flow into draining LNs from the injection site and found that alum did not affect the velocity of lymph flow, although a substantial decrease in antigen concentration was observed in the lymph when immunizing with alum compared to injection of antigen alone. After 72 hrs, antigen was no longer detected within the lymph irrespective of the presence of alum, but alum-associated antigens at the injection site were found to be taken up by migratory DCs that continually migrated to the draining LN [214]. Contrasting these findings, Gupta et al. observed that alum-adsorbed diphtheria toxoid antigen was not retained at the injection site beyond ~3 days [231]. Other studies have shown that aluminum phosphate also failed to act as a depot for antigen, although antibody titers were enhanced by the adjuvant [232]. More recent studies have shown similar immune responses following traditional alum immunization or vaccinations where the alum injection site was surgically removed 2 hr after immunization [233]. Such findings are consistent with experiments showing that non-adsorbed antigens co-injected with alum elicit effective humoral immune responses [234]. Fundamental studies have demonstrated diverse mechanisms by which alum can potentiate the immune response independent of any depot activity, including induction of cell death at the injection site, production of chemokines and cytokines recruiting immune cells to the injection site and draining lymph nodes (LNs) [[235], [236], [237]], activation of innate immunity signaling pathways [[238], [239], [240]] and complement [241,242], activation of APCs through interactions with the plasma membrane [243], and altering the duration of antigen presentation by APCs [244].
As highlighted by the discussion above, antigens often fail to remain bound to alum following administration. However, fundamental studies by Hem and colleagues demonstrated that phosphorylated proteins bind very tightly to alum, by undergoing ligand exchange reactions with surface hydroxyls of aluminum hydroxide particles [245,246]. This reaction can be exploited to achieve highly stable binding of immunogens to alum particles, by introducing short phosphopeptide tags in a site-specific manner to an antigen of interest [247]. Introduction of phosphoserine (pSer) tags to HIV Env immunogens dramatically altered the kinetics of antigen clearance from the injection site with alum, from a few days to ~3 weeks. This slow release behavior correlated with the slow dispersal of alum particles from the injection site, as revealed by matching kinetics of antigen and alum particle appearance in draining lymph nodes [247]. In addition, by remaining stably bound, the immunogen becomes multimerized on the alum particles, enhancing B cell triggering and allowing irrelevant surfaces of the immunogen (such as the base of the Env trimer) to be masked by apposition against the alum particle surface. Together, these features led to major enhancements in antigen-specific GC B cell responses, induction of long-lived plasma cells, and enhancements in induction of neutralizing antibody responses [247]. As discussed earlier, ligand-exchange reactions have also been exploited to create alum-binding molecular adjuvants [148].
6.2. Novel technologies for modulating vaccine kinetics
Beyond alum, biomaterials are also being explored as drug depots capable of altering the kinetics of drug delivery. Vaccines can be encapsulated within biodegradable polymers that release material over time through hydrolysis, or entrapped within hydrogels for slow release. For hydrogels, the rate of release for encapsulated cargo is dependent on the relative difference between the size of the entrapped molecule and mesh size of the polymer network, assuming a lack of significant molecular interactions between the two [248]. For example, hydrogel particles formed from dehydrated collagen have been shown to released entrapped antigens over a period of 7 days in vivo [249]. “Smart” hydrogels can also be employed, which remain in a liquid state prior to injection but form a gel in response to temperature or pH in the body. Phosphorylated chitosan, capable of gelling at a pH greater than 7, was effectively used to form an injectable gel releasing ovalbumin (OVA) within muscle tissue over several days, leading to marked improvement in OVA-specific antibody titers compared to bolus immunization [250]. Temperature-responsive polymers that gel in response to body temperature have also been used to deliver antigens to the nasal cavity [251]. Natural polymers have also been employed as antigen-releasing hydrogels. Skin-implantable microneedles prepared from silk fibroin protein were shown to slow-release antigens and adjuvant compounds over two weeks in vivo, enhancing humoral responses to entrapped protein antigen [252].
Solid polymer matrices can also be used to extend vaccine kinetics, and have been extensively explored over the last 30 years as candidate vaccine carriers, particularly as microparticles or nanoparticles encapsulating vaccines for slow release. Poly(lactic-co-glycolic acid) based polymers have a long track record of safe use in humans as resorbable sutures, and have also been explored as matrices to encapsulate vaccine antigens for tunable release kinetics [[253], [254], [255]]. Months-long release of vaccine antigens from PLGA particles has been demonstrated, leading to antibody responses equivalent to or superior to alum for a variety of vaccine antigens [255]. PLGA microparticles releasing antigen over more than a month have also been found to promote stronger T cell responses in mice compared to faster-clearing vaccine formulations [256]. Despite the appeal of PLGA’s well established safety, challenges in scalable manufacturing, and issues of antigen degradation and/or aggregation during encapsulation have limited successful commercial translation of this approach for vaccines [257,258]. However, progress in overcoming these issues applicable to PLGA and other resorbable polymers continues [[259], [260], [261]]. To avoid issues in encapsulation, an alternative is to adsorb or covalently attach antigens to the surface of PLGA particles, an approach that has been commercialized by Liquidia Technologies and moved into first-in-humans testing [262], though the ability of this approach to augment immune responses by changes in vaccine kinetics has not been studied in detail.
Polyanhydrides (PAs) are a second important class of biocompatible polymers with regions of hydrophobic hydrocarbons concatenated by short hydrophilic anhydride bonds and are susceptible to hydrolysis [263]. Hydrophobic PAs are resistant to water penetration into their bulk, and therefore undergo surface erosion. These materials have been used as nanoparticles or bulk implants containing antigens and adjuvants and have been shown to facilitate slow release antigens and other cargos for up to one month [264,265], and promising vaccine protection has been demonstrated with PA nanoparticles in animal models of influenza, bovine brucelliosis, and plague [264,[266], [267], [268]]. Natural polymers have also been used as slow-release matrices. Vaccination with peptide antigens encapsulated in L-tyrosine-based microparticles led to prolonged antigen presentation in draining lymph nodes lasting ~2 weeks, providing greatly enhanced T cell expansion in response to the vaccine [269]. This study notably ruled out direct effects of the microparticle immunization on DC activation or the inflammasome pathway (known to be triggered in response to phagocytosis of particles in innate immune cells [239,270]), strengthening the conclusion that alterations in antigen availability kinetics was the key driver of these effects.
New materials systems are opening up additional ways to modulate vaccine kinetics. It is possible that antigen-displaying peptide nanofiber vaccines as discussed in section 3.1 above slowly clear from injection sites, prolonging antigen availability as part of their mechanisms of action [77,271]. Mooney and colleagues recently described the use of mesoporous silica rods as a matrix for co-delivery of antigen and molecular adjuvants [272]. Here, vaccine molecules were loaded and released from nanoscale pores of an injectable suspension of silica rods over 10 days in vivo. Karabin et al. recently described an injectable hydrogel material based on oxidation-sensitive self-assembling resorbable block copolymers that spontaneously form nanoscale filaments in water. This unique system demonstrated a one month sustained release of material from the injection site as slow oxidation of the filaments led to continuous release of micelles from the matrix, which drained into local LNs [79]. This elegant system can readily be envisioned as a platform for extended antigen/adjuvant release in vaccines.
6.3. Modulating the kinetics of adjuvant/inflammatory cues
“Extended dosing” immunization regimens can be used to prolong exposure to adjuvant as well as antigen. In studies of an escalating-dosing vaccination where peptide antigen mixed with the TLR9 agonist CpG as an adjuvant was administered as 8 injections spread over 8 days instead of as a bolus at one time, enhanced T cell responses obtained with escalating-dosing were only obtained when both the antigen and adjuvant were administered together in the increasing-dosing pattern [31]. Bolus administration of CpG at the beginning or ending of extended dosing led to substantially reduced T cell immunity [31]. In a similar vein, a study examining protein immunization combined with the TLR3 agonist polyI:C showed that repeated daily immunization antigen and adjuvant over 4 days could trigger a dramatic enhancement in antigen-specific CD8+ T cell responses in mice [273]. Encapsulation of molecular adjuvants in materials to facilitate controlled release may be an effective strategy to achieve such effects without the need for repeated injections. For example, intranodal injections of protein antigens mixed with biodegradable polymer microparticles releasing polyI:C over > 5 days elicited profound CD8+ T cell responses, which could not be matched by a 10-fold higher dose of soluble adjuvant injected with antigen in the same manner [274].
The use of highly hydrophobic molecular adjuvants is another strategy that prolongs the pharmacokinetics of small-molecule adjuvants [148]. To localize the action of small-molecule TLR7/8 agonists, Novartis developed lipophilic derivatives of imidazoquinolines and showed that these modifications significantly limited the compounds’ biodistribution and subsequent systemic inflammation. PK/PD analysis showed that these modified compounds were nearly undetectable in serum and remained in the injection site (muscle) for greater than 2 weeks. Functionally, these lipophilic TLRa outperformed the hydrophilic parent compound R848 in terms of antigen-specific antibody titers while reducing systemic inflammatory cytokine production by 10-fold, even though R848 is a more potent immune stimulatory compound in vitro. Taking a different approach to enhance the safety and efficacy of TLR7/8 agonists, Lynn et al. developed polymer conjugates of imidazoquinolines, which aggregated through hydrophobic self-associations into submicron-sized particles [166]. Particulate forms of the TLRa trafficked to lymph nodes and were persistent in the tissue for ~20 days, giving rise to 400-fold increases in total area-under-the-curve of lymph node exposure compared to the parent TLRa compound. This increased accumulation and persistence led to prolonged localized IL-12 expression in draining lymph nodes lasting >2 weeks, correlating with greatly enhanced CD4+/CD8+ T cell responses and enhancements in antibody titers.
A contrasting example comes from a study focused on humoral immunity induced by microneedle vaccines. Here, microneedle patches designed to implant vaccine-releasing resorbable silk microdepots in the skin were tested for their effects on humoral immune responses to HIV Env antigens [252]. In these experiments, immunizations were carried out comparing silk depots that encapsulated both antigen and TLR3/TLR2 agonist molecular adjuvants, just the antigen (with adjuvant bolus-injected at the same site), or the adjuvant only (with antigen bolus injected). Intriguingly, antibody responses were weak and comparable to bolus immunization if the adjuvant was slow-released from silk in tandem with bolus antigen injection, but slow release of antigen+adjuvant was comparable to slow release of antigen combined with bolus adjuvant injection [252]. These distinct outcomes might reflect differences in the behavior of the distinct innate immune pathways being targeted.
7. Vaccine kinetics of nucleic acid and viral-vectored vaccines
7.1. Expression patterns of nucleic acid vaccines
Nucleic acid vaccines based on mRNA, RNA replicons, or DNA access a range of different kinetic patterns for antigen/adjuvant expression in vivo (Fig. 6). DNA-based vaccines for example, can induce sustained foreign protein expression for greater than two months post injection [275]. However, potent immune response elicited by naked DNA administered to the muscle or skin in small animal models have not translated well into large animal models or human subjects in the absence of boosting using another vaccine modality [276,277]. This poor translation is thought to be due at least in part to hydrodynamic effects of the injection promoting transfection in small animals, which are absent in the much greater tissue volumes of large animals and humans. Improvements in delivery such as in vivo electroporation can increase the level of transfection by a 1000-fold and significantly increases expression longevity and the overall immunogenicity of DNA vaccines [278]. The company Inovio has developed clinical stage in vivo electroporation devices for delivery of a DNA-based therapeutic vaccine against cervical intraepithelial neoplasia (CIN), a precancerous condition caused by human papilloma virus. In a phase 2b clinical trial, this vaccine encoding HVP E6 and E7 proteins was the first to show therapeutic efficacy against CIN [279]. The use of RNA interference (RNAi) to modulate expression of apoptotic genes is another strategy that can be used to enhance the expression duration of DNA vaccines. As transfected cell apoptosis inherently limits the duration of antigen expression, targeting death pathways with RNAi is an effective method to improve the durability of nucleic acid vaccines [280]. Codelivery of DNA vaccines and siRNA targeting the pro-apoptotic genes Bax and Bak was recently shown to significantly increase the survival of antigen-expressing DCs and led to improved anti-tumor immunity [281]. Combining DNA vaccination with RNAi knocking down caspase 12 and Fas ligand has also been shown to improve immune responses in animal models, presumably due to prolonged antigen expression kinetics [282,283]. Additionally, the co-delivery of DNA-encoded molecular adjuvants such as IL-2 improves adaptive immunity and augments antibody secretion and cytotoxic T cell activation [284]. In vivo cytokine production following immunization with DNA encoding IL-2 has been shown to persist for 12 days post injection [285] and induced sustained activation of peripheral T cells in a non-human primate HIV vaccination model. Monkeys that received DNA-encoded IL-2 produced 30-fold higher antigen-specific titers and 5-fold higher frequencies of cytotoxic T cells compared to DNA vaccines encoding antigen alone. Additionally, DNA-encoded IL-2 was shown to outperform IL-2 protein injection, presumably due to the sustained availability of cytokine [284]. Timing of DNA-encoded adjuvant administration can also have a pronounced effect on the phenotype of the ensuing immune response. Administration of DNA encoding GM-CSF 3 days prior to immunization was shown to increase IL-4 secretion and promote a Th2-type immune response whereas administration 3 days post immunization increased IFN-γ production and skewed the response toward Th1 [286]. Although the use of genetically encoded adjuvants has been shown to be generally well tolerated in phase 1 clinical trials, there exist concerns over chronic inflammation from persistent expression of proinflammatory cytokines (clinicaltrials.gov: NCT00111605, NCT02960594, NCT00069030).
mRNA vaccines are a second key nucleic acid vaccine platform. mRNA is inherently unstable in vivo and antigen expression levels are significantly more transient than observed with DNA vaccination, typically only persisting for several days [183,275]. This is at least in part due to viral RNA sensing molecules such as TLRs 3, 7, and 8 as well as RIG-I and MDA-5. Activation of these innate immune sensors triggers a signaling cascade that enhances RNA clearance via 2’-5’-oligoadenylate synthetase (OAS) and RNase L and also leads to transfected cell apoptosis, limiting antigen production [287,288]. Although these events are proinflammatory, it may reduce a potential adaptive immune response due to suppression of antigen expression. The use of modified nucleotides such as 5-methyl cytosine or pseudouridine can limit recognition by RIG-I and OAS and enables transfected cells to express antigen for significantly longer timeframes [[288], [289], [290]]. In a luciferase expression model, mice that received pseudouridine-modified RNA had higher protein translation capacity that extended for longer than 2 weeks after injection, contrasting to the abbreviated expression profile of non-modified RNA which was nearly undetectable within 7 days [290]. Pseudouridine-modified RNA induced significantly lower acute IFN-α secretion from transfected cells compared to controls, possibly explaining the expression improvements over non-modified RNA controls [289] Additionally, incorporation of RNA capping analogues have been reported to reduce innate sensing and increase the duration of antigen production in vivo, leading to enhanced immune responses to the encoded antigen [291]. Aside from nucleoside modifications and capping analogs, the purity of RNA appears to be another important factor affecting expression kinetics [292]. Synthesis contaminants such as double stranded RNA can trigger innate RNA sensor molecules such as TLR3, RNA-dependent protein kinase (PKR), and OAS and can directly decrease translation upon activation. HPLC purification of RNA prior to transfection was reported to increase expression levels by over 1000-fold [293]. The temporal pattern of mRNA expression is also significantly affected by delivery routes in vivo [184]: Although intravenous delivery of LNP-formulated mRNA vaccines has been reported to provide the highest levels of peak gene expression, antigen production dissipates within 5 days of injection. Intradermal and intramuscular injections on the other hand provided local antigen production for up to 10 days, albeit at lower peak levels. Recent clinical trials from Moderna using intramuscular delivery of nucleoside modified RNA show that mRNA vaccines are safe and capable of inducing effective humoral responses against H10N8 and H7N9 influenza strains in humans [294]. In this trial, virus-neutralizing antibody responses were sustained for over 6 months after immunization. Additional clinical trials are underway against SARS-Cov-2, Zika, RSV, CMV, and cancer antigens (clinicaltrials.gov: NCT03382405, NCT04064905, NCT03897881, NCT02316457, NCT04368728).
A third category of nucleic acid vaccines are based on self-amplifying RNA derived from viral replicons, most commonly alphaviruses. Like mRNA vaccines, replicon vaccines are typically delivered using lipid nanoparticles and utilize the host’s cellular machinery to generate proteins of interest in the cytosol of transfected cells [295,296]. Replicon vaccines are considerably larger than nonreplicating mRNA vaccines, typically over 10kb in length. In addition to the basic elements of mRNA vaccines, self-replicating RNA vaccines encode a set of non-structural proteins that form an RNA-dependent RNA polymerase that copies the replicon in the cytoplasm of transfected cells, providing self-amplification. Compared to non-replicating mRNA, replicon vaccines induce substantially longer durations of antigen expression. Luciferase reporter systems show that protein expression can be sustained at high levels for 2 to 3 weeks with very low doses of RNA [275,297]. A potential trade-off in this enhanced antigen expression duration is the possibility of immune responses against the replicon non-structural proteins, which might theoretically limit responses following repeated administrations. Interestingly, low doses of LNP-replicon vaccine exhibit a pattern of escalating expression over a period of 10-14 days as the RNA undergoes replication in transfected host cells [297], mimicking “escalating dose” immunizations that help drive humoral and T cell responses as discussed in Section 5 [31,212,213]. A recent study showed that although replicon-induced total germinal center responses were comparable to protein-based immunization, replicon vaccinated animals produced ~2-fold greater antigen-specific GC responses in mice for up to one-month post immunization. This difference may be due to the sustained production of “fresh” antigen from transfected muscle cells in vivo compared to acute antigen exposure achieved by bolus protein immunization [297,298]. Other studies have shown replicon vaccines encoding H1N1 hemagglutinin induced equivalent antigen-specific humoral responses and protection as mRNA vaccines, at a 50-fold lower dose of RNA [299]. These results exemplify the impact of replicon self-amplification and could be a significant advantage when considering the large-scale clinical production i.e. emerging epidemic and pandemic pathogens. In a recent pre-clinical SAR-Cov-2 study, McKay et al. showed that replicon vaccines can induce potent neutralizing antibody titers that were significantly greater than equivalent doses of DNA vaccine [300]. At the time of this review GSK has an ongoing study to evaluate the safety and immunogenicity of a replicon-based rabies vaccine in healthy adults (clinicaltrials.gov: NCT04062669).
7.2. Expression patterns of viral- and bacteria-vectored vaccines
The kinetics of antigen expression achieved by non-replicating recombinant viral- and bacteria-vectored vaccines can vary widely. Similar to nucleic acid vaccines, expression profiles are highly influenced by innate immune recognition of the vector. Recent studies by Quinn et al. show that there is a strong inverse correlation between the intensity of innate immunity induced by viral vectors and the magnitude and kinetics of antigen expressed in vivo [301]. Viral vectors typically induce rapid peak antigen expression that slowly dissipates over time [301,302]. Transcriptomic analysis of persistent viral vectors such as recombinant adenovirus serotype 5 (rAd5) or chimpanzee adenovirus serotype 3 can induce antigen expression for at least two weeks post injection [301]. These durable vectors have relatively attenuated type 1 IFN and STING pathway activation, suggesting that these signaling programs are key to suppressing expression levels. Administration of the type 1 IFN activator Poly I:C in conjunction with rAd5 vector significantly reduces antigen expression, indicating a direct inhibitory effect of triggering strong innate immunity on expression from these vectors. Similar results were seen when a viral vector that induces strong innate signaling activation, such as chimpanzee adenovirus serotype 63, was co-delivered with rAd5. Studies in mice with these vectors showed that the extent of antigen production directly correlated with the level of antigen-specific CD8+ T cell activation and protection from L. monocytogenes bacterial challenge. Other studies using temporally-regulated adenoviral vectors also underscore the importance of sustained antigen expression to the ensuing immune response. Using a SIINFEKL-luciferase-expressing vector under control of a tetracyclin promoter, studies were conducted where antigen expression could be turned off via the administration of doxycycline at various time points after immunization [303]. Mice that did not receive doxycycline retained antigen expression for up to 60 days post injection. Terminating antigen expression at timepoints earlier than 30 days post-immunization reduced the magnitude of the primary response as well as protection from vaccinia virus challenge.
Altering the promotor used for viral-vectored vaccines is a simple way to affect expression kinetics. For example, adenoviral vectors under the control of a strong promoter such as CMV achieve peak antigen expression levels within one day after immunization, but this expression quickly dissipates and returns to baseline over the course of 2-3 weeks [304]. Weaker promoters such as promoters derived from RSV generate 100-fold lower initial antigen expression levels, but expression increases exponentially over the course of 2 weeks. RSV-controlled antigen expression levels thus overtake those of CMV-controlled vectors and remains persistent for greater than 3 weeks post immunization
Vaccine administration routes are also an important factor controlling antigen expression kinetics from microbial vectors. Intramuscular and intraperitoneal injections of rAd5 promote high initial expression levels that slowly taper off over the course of three weeks [302]. Interestingly, although intranasal administration led to low initial expression, antigen level increased exponentially over time, peaking at 9 days post injection, and waned in a similar manner to parenteral injections. These kinetic profiles were directly dependent on anti-vector immunity as the expression profile in athymic mice persisted for over 100 days post injection in immunized animals. Although viral-vectored vaccines have the intrinsic benefits of potent cellular uptake, anti-vector immunity has the potential to reduce the effectiveness of repeated boosting and represents a potential detriment to this vaccine approach [305].
Intracellular bacterial strains such as Listeria monocytogenes and Mycobacterium bovis have also been used as vectors for prophylactic and therapeutic vaccines. Due to rapid uptake by macrophages and dendritic cells, bacterial vectors are highly effective at stimulating innate system activation and promoting robust antigen processing and presentation through MHC-I and MHC-II molecules. Preclinical models have shown that Listeria replication is sustained for up to one week after immunization and can induce potent CD8 T cell responses [306,307]. Introduced mutations to virulent factors such as ActA and Internalin B significantly reduce undesirable hepatocyte infection but maintain functional tropism towards antigen presenting cells. These modifications reduce the duration of bacterial gene expression to ~48hrs post immunization and dramatically reduce liver toxicity by 1000-fold. Interestingly, despite this reduction in virulence factors and duration of infection, the mutated Listeria strain maintains potent and durable effector and memory T cell responses. This is in accordance with previous studies that show that the magnitude and kinetics of antigen-specific T cells responses in this system are determined within the first 24 hrs of bacterial infection and are dependent on initial infection intensity but not on the duration of infection [308,309]. In a CT26 lung metastasis model, Brockstedt and colleagues showed that a high dose vaccination with Listeria ΔActA/ΔInternalin B expressing tumor-associated antigens was an effective anti-tumor therapy [306]. Clinical trials are currently being conducted with live-attenuated Listeria targeting cervical carcinoma [310] (clinicaltrials.gov: NCT01266460). Along similar lines, Tullilus and colleagues have shown that recombinant Mycobacterium bovis BCG engineered to undergo limited replication in vivo are safer and at least as effective as the parental BCG vaccine and may be especially useful for immunocompromised individuals [311].
Overall Nucleic acid, viral-vectored and bacterial-vectored vaccines provide a powerful platform to vaccine development. Not only do these vaccines provide a balanced cellular and humoral immune response, their genetically tunable expression profile will likely provide key advantages to future vaccine development.
8. Technologies toward single-shot vaccines
Most approved vaccines require multiple immunizations to provide long-lasting protection from pathogens [312]. In order to provide better compliance and promote global vaccine coverage in the developing world, it would be highly beneficial to develop vaccines that are efficacious from a single shot [313]. Although single bolus immunizations typically lead to seroconversion, these beneficial responses often lack durability and are typically only suitable for seasonal vaccines. By contrast, traditional booster doses significantly expand memory B cells and long-lived plasma cells and lead to sustained protection spanning years [314]. Recent studies have suggested that the optimal time for boosting is after the effector phase of the immune response has subsided, typically 1 to 3 months after initial immunization [[315], [316], [317]]; this immunology poses a major technical challenge to creating single-shot vaccines.
Classic early studies demonstrated that implanted biomaterials releasing vaccine antigens over 1 or more months could prime humoral immune responses comparable to a prime and boost with bolus protein immunization [318]. More recent examples of this strategy include a two-component injectable system containing chitosan and chondroitin sulphate along with Montanide and antigen, which created depots at the injection site for up to 20 weeks and elicited strong antibody response for at least a year in mice [319]. However, it is unclear if these principles will be sustained in large animals and/or with optimized adjuvants, as sustained vaccine release in non-human primates over 1 month still benefits significantly from the administration of a boost dose [42,212]. One strategy to achieve intermittent immune stimulation required for potent and durable immune response from a single injection is to use biomaterials with programmable, pulsatile vaccine release. PLGA polymers can be designed to release antigen in a pulsatile manner that is analogous to traditional bolus dosing (Fig 7 A ). Tseng and colleagues developed a pulsatile-release PLGA-based microsphere formulation that is capable of releasing bursts of polio vaccine roughly one month apart [320,321]. Preclinical studies have shown that these formulations yielded similar or better humoral immune response compared to a typical prime-boost regimen (Fig 7 B). A novel microfabrication technology termed StampEd Assembly of polymer Layers (SEAL) is capable of generating PLGA-based microstructures with a fillable core that can be loaded with vaccine formulations [322] (Fig 7 C). These microstructures can be engineered to release its cargo at various timepoints based on chemical modifications made to the PLGA polymer. A mixture of microstructures with different release kinetics could be used in a vaccine formulation to enable a pulsatile burst release of antigen at several different timepoints (Fig 7 D). Using ovalbumin as a model antigen, McHugh and colleagues showed that mice that received ovalbumin-filled microstructures with release profiles of ~7 and ~40 days post injection induced antigen-specific antibody titers that were equivalent or better than bolus prime-boost injections of corresponding levels of soluble OVA protein (Fig 7 E).
Fig. 7.
Technologies toward single-shot vaccines. (A) Schematic of pulsatile release PLGA microspheres. Initial release burst results from release of antigen from on or near the surface of the microspheres. This phase is followed by a lag phase as the PLGA polymer starts to degrade. The polymer then fully degreades leading to a second burst of antigen. (B) Humoral immune response against a polio vaccine where the pulsatile release platform (labeled F1) was tested against bolus protein injections. (C) Representative TEM of 3D microstructures that contain a fillable reservoir for loading vaccine formulations.(A-C adapted from [320]). (D) Schematic of potential multi-burst vaccine strategy elicited from a single shot. Microstructures composed of varying PLGA modifications degrade at different rates in vivo leading to burst releases of vaccines at different timepoints. (E) Humoral immune response following immunization with ovalbumin. The immune response induced by core-shell microstructures were compared to that of bolus injection (Adapted from [322]).
In another recently-developed approach, Garcea et. al used atomic layer deposition (ALD) to coat microparticles of alum and HPV capsomere antigen with trimethyaluminum vapor [323]. This approach aims to simultaneously thermostabilize the vaccine and provide a slowly-dissolving alum coating that can unmask and release the vaccine over tunable time periods. The extent of aluminum deposition could be tuned to optimize the level of antigen/adjuvant retention in the injection site. One hundred layers of ALD applied to alum-adsorbed HPV immunogen led to antigen retention of around 4 weeks at the injection site following administration, whereas 500 ALD layers led to retention for over 10 weeks post injection. Animals immunized with HPV vaccines using the ALD formulation strategy had improved humoral responses compared to a typical prime/boost vaccination regimen that persisted for greater than 20 weeks.
Despite the promise of single-shot vaccines, several challenges will need to be overcome before this strategy can be implemented clinically. Like any implanted foreign material, these extended delivery platforms will need to show exceedingly safe properties to warrant replacement of current standards. Care must be taken to ensure these platforms do not induce injection site reactogenicity and chronic inflammation, particularly if they incorporate molecular adjuvants to enhance immunogenicity. Importantly, it will be critical that antigens are preserved in their native conformation to ensure the induced immune response recognizes the intended pathological target, and not merely degraded antigen. Although this prospect can be achieved for antigens such as inactivated poliovirus, complex antigens such as HIV trimer or influenza HA trimer may be challenging to engineer for prolonged stability in situ at 37°C [222,298,320].
As discussed in section 7, the prolonged expression profile of nucleic acid vaccines may overcome some of these challenges. Preclinical studies have shown that a single injection of RNA replicon vaccines is capable of generating protective immunity against a broad spectrum of pathogens including Ebola, H1N1 Influenza, Toxoplasma gondii and Zika [324,325]. Nucleic acid vaccines can also be combined with traditional protein vaccines to provide a heterologous antigen delivery modality where bolus protein injection provides acute immune stimulation and the co-administered nucleic acid vaccine provides prolonged antigen availability. This combinatorial strategy promotes both humoral and cellular immune responses [212,326,327]. In a simian immunodificiency virus (SIV) infection model, DNA and viral protein co-immunization was able to induce significant protection against SIV infection from serial rechallenge [328]. Monkeys that received the heterologous vaccine developed more potent and durable humoral immune responses compared to DNA alone or a DNA prime and protein boost regimen.
Another potential approach for achieving single-dose vaccination through nucleic acid vaccines is through the use of molecular switches controlled by orally-available small molecule drugs [329]. Genetically engineered molecular circuits such as the Tet repressor, destabilizing domains, or synthetic riboswitches could potentially be employed to develop single-shot vaccines where antigens are expressed in a pulsatile manner similar to traditional vaccination, regulated by doses of a small molecule drug taken as an oral pill. Such a strategy might also be utilized to induce a sequential expression profile of heterologous antigens that could be beneficial to control the activation and mutational pathway of B cells and could prove important to generating effective responses against challenging pathogens such as HIV [330].
Overall significant progress has been made towards the deployment of safe and effective single-shot vaccines. The benefits that such technologies could provide are immense from a global health standpoint and continued research on both materials science- and nucleic acid vaccine-based approaches are warranted.
9. Conclusions and outlook
Vaccines are one of the oldest tools for promoting public health, but their importance has only grown as we face newly-arising pathogens. We expect that technologies addressing the issues of timing and localization discussed here will be critical for achieving the goals of such vaccine development programs. Further, to overcome longstanding challenges posed by difficult pathogens that establish chronic infections and/or have evolved effective means of evading immunity, optimizing vaccine targeting and timing may be crucial in multiple respects: As antigen availability plays a deterministic role in the strength and type of B cell and T cell responses induced by immunization, optimizing targeting of vaccines to lymph nodes and prolonging antigen availability over an optimal temporal window become key parameters shaping the immune response. As illustrated by recent studies, these are not just “nice to have” features, but can determine the difference between a vaccine inducing neutralizing antibodies or not [35,212,247]. In addition, as we seek vaccines capable of driving more diverse and robust humoral immune responses or more effectively establish protective T cell responses, adjuvants play an increasingly important role, and balancing adjuvant safety vs. efficacy will be strongly influenced by our ability to focus adjuvant activity into draining lymphoid tissues and away from the systemic circulation. Of importance in the development of new strategies to control vaccine kinetics and targeting, the need for high levels of safety at low cost remain critical issues that will ultimately govern what approaches can move forward in the clinic vs. remain preclinical proofs of concept.
Acknowledgments
This was work was supported in part by the Ragon Institute of MGH, MIT, and Harvard, the NIH (awards UM1AI144462, AI048240, AI125068, CA247632, EB025854), the Marble Center for Nanomedicine, and the Dept. of Defense (contract W911NF-18-2-0048). DJI is an investigator of the Howard Hughes Medical Institute. MS is an NRSA Fellow (T32 AI07386).
References
- 1.Nabel G.J. Designing tomorrow's vaccines. N. Engl. J. Med. 2013;368:551–560. doi: 10.1056/NEJMra1204186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henao-Restrepo A.M., Longini I.M., Egger M., Dean N.E., Edmunds W.J., Camacho A., Carroll M.W., Doumbia M., Draguez B., Duraffour S., Enwere G., Grais R., Gunther S., Hossmann S., Konde M.K., Kone S., Kuisma E., Levine M.M., Mandal S., Norheim G., Riveros X., Soumah A., Trelle S., Vicari A.S., Watson C.H., Keita S., Kieny M.P., Rottingen J.A. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–866. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 3.Draper S.J., Sack B.K., King C.R., Nielsen C.M., Rayner J.C., Higgins M.K., Long C.A., Seder R.A. Malaria vaccines: recent advances and new horizons. Cell Host Microbe. 2018;24:43–56. doi: 10.1016/j.chom.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei C.J., Crank M.C., Shiver J., Graham B.S., Mascola J.R., Nabel G.J. Next-generation influenza vaccines: opportunities and challenges. Nat. Rev. Drug Discov. 2020;19:239–252. doi: 10.1038/s41573-019-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes B.F., Burton D.R. Developing an HIV vaccine. Science. 2017;355:1129–1130. doi: 10.1126/science.aan0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrager L.K., Vekemens J., Drager N., Lewinsohn D.M., Olesen O.F. The status of tuberculosis vaccine development. Lancet Infect. Dis. 2020;20:e28–e37. doi: 10.1016/S1473-3099(19)30625-5. [DOI] [PubMed] [Google Scholar]
- 7.Amanat F., Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 9.Plotkin S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F., Redfern C.H., Ferrari A.C., Dreicer R., Sims R.B., Xu Y., Frohlich M.W., Schellhammer P.F., Investigators I.S. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 11.Di Pasquale A., Preiss S., Da Silva F.T., Garcon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 13.Graham B.S., Gilman M.S.A., McLellan J.S. Structure-based vaccine antigen design. Annu. Rev. Med. 2019;70:91–104. doi: 10.1146/annurev-med-121217-094234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker L.M., Burton D.R. Rational antibody-based HIV-1 vaccine design: current approaches and future directions. Curr. Opin. Immunol. 2010;22:358–366. doi: 10.1016/j.coi.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rappuoli R., Bottomley M.J., D'Oro U., Finco O., De Gregorio E. Reverse vaccinology 2.0: human immunology instructs vaccine antigen design. J. Exp. Med. 2016;213:469–481. doi: 10.1084/jem.20151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moyer T.J., Zmolek A.C., Irvine D.J. Beyond antigens and adjuvants: formulating future vaccines. J. Clin. Invest. 2016;126:799–808. doi: 10.1172/JCI81083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neutra M.R., Kozlowski P.A. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowski P.A., Aldovini A. Mucosal vaccine approaches for prevention of HIV and SIV transmission. Curr. Immunol. Rev. 2019;15:102–122. doi: 10.2174/1573395514666180605092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyaka P.N. Inducing mucosal IgA: A challenge for vaccine adjuvants and delivery systems. J. Immunol. 2017;199:9–16. doi: 10.4049/jimmunol.1601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z., Kulp D.W. Protein engineering and particulate display of B-cell epitopes to facilitate development of novel vaccines. Curr. Opin. Immunol. 2019;59:49–56. doi: 10.1016/j.coi.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Serra P., Santamaria P. Antigen-specific therapeutic approaches for autoimmunity. Nat. Biotechnol. 2019;37:238–251. doi: 10.1038/s41587-019-0015-4. [DOI] [PubMed] [Google Scholar]
- 22.Luo X., Miller S.D., Shea L.D. Immune tolerance for autoimmune disease and cell transplantation. Annu. Rev. Biomed. Eng. 2016;18:181–205. doi: 10.1146/annurev-bioeng-110315-020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozsgay J., Szekanecz Z., Sarmay G. Antigen-specific immunotherapies in rheumatic diseases. Nat. Rev. Rheumatol. 2017;13:525–537. doi: 10.1038/nrrheum.2017.107. [DOI] [PubMed] [Google Scholar]
- 24.Baekkeskov S., Hubbell J.A., Phelps E.A. Bioengineering strategies for inducing tolerance in autoimmune diabetes. Adv. Drug Deliv. Rev. 2017;114:256–265. doi: 10.1016/j.addr.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Schenkel J.M., Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nutt S.L., Hodgkin P.D., Tarlinton D.M., Corcoran L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Panhuys N., Klauschen F., Germain R.N. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Moynihan K.D., Zheng Y., Szeto G.L., Li A.V., Huang B., Van Egeren D.S., Park C., Irvine D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer A., Zhang Y.J., Perelson A.S., Wingreen N.S. Regulation of T cell expansion by antigen presentation dynamics. Proc. Natl. Acad. Sci. U. S. A. 2019;116:5914–5919. doi: 10.1073/pnas.1812800116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansen P., Storni T., Rettig L., Qiu Z.Y., Der-Sarkissian A., Smith K.A., Manolova V., Lang K.S., Senti G., Mullhaupt B., Gerlach T., Speck R.F., Bot A., Kundig T.M. Antigen kinetics determines immune reactivity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5189–5194. doi: 10.1073/pnas.0706296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burchill M.A., Tamburini B.A., Pennock N.D., White J.T., Kurche J.S., Kedl R.M. T cell vaccinology: exploring the known unknowns. Vaccine. 2013;31:297–305. doi: 10.1016/j.vaccine.2012.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumjohann D., Preite S., Reboldi A., Ronchi F., Ansel K.M., Lanzavecchia A., Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Frey S.E., Harrison C., Pass R.F., Yang E., Boken D., Sekulovich R.E., Percell S., Izu A.E., Hirabayashi S., Burke R.L., Duliege A.M. Effects of antigen dose and immunization regimens on antibody responses to a cytomegalovirus glycoprotein B subunit vaccine. J. Infect. Dis. 1999;180:1700–1703. doi: 10.1086/315060. [DOI] [PubMed] [Google Scholar]
- 35.Pauthner M.G., Nkolola J.P., Havenar-Daughton C., Murrell B., Reiss S.M., Bastidas R., Prevost J., Nedellec R., von Bredow B., Abbink P., Cottrell C.A., Kulp D.W., Tokatlian T., Nogal B., Bianchi M., Li H., Lee J.H., Butera S.T., Evans D.T., Hangartner L., Finzi A., Wilson I.A., Wyatt R.T., Irvine D.J., Schief W.R., Ward A.B., Sanders R.W., Crotty S., Shaw G.M., Barouch D.H., Burton D.R. Vaccine-induced protection from homologous tier 2 shiv challenge in nonhuman primates depends on serum-neutralizing antibody titers. Immunity. 2018;50(1):241–252. doi: 10.1016/j.immuni.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang J., Zhang N., Tao X., Zhao G., Guo Y., Tseng C.T., Jiang S., Du L., Zhou Y. Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus. Hum. Vaccin. Immunother. 2015;11:1244–1250. doi: 10.1080/21645515.2015.1021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bershteyn A., Hanson M.C., Crespo M.P., Moon J.J., Li A.V., Suh H., Irvine D.J. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J. Control. Release. 2012;157:354–365. doi: 10.1016/j.jconrel.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrikx L.H., Berbers G.A., Veenhoven R.H., Sanders E.A., Buisman A.M. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: effect of vaccine antigen content. Vaccine. 2009;27:6530–6536. doi: 10.1016/j.vaccine.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 39.Trevaskis N.L., Kaminskas L.M., Porter C.J. From sewer to saviour - targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 40.Smedley J., Turkbey B., Bernardo M.L., Del Prete G.Q., Estes J.D., Griffiths G.L., Kobayashi H., Choyke P.L., Lifson J.D., Keele B.F. Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Havenar-Daughton C., Carnathan D.G., Boopathy A.V., Upadhyay A.A., Murrell B., Reiss S.M., Enemuo C.A., Gebru E.H., Choe Y., Dhadvai P., Viviano F., Kaushik K., Bhiman J.N., Briney B., Burton D.R., Bosinger S.E., Schief W.R., Irvine D.J., Silvestri G., Crotty S. Rapid germinal center and antibody responses in non-human primates after a single nanoparticle vaccine immunization. Cell Rep. 2019;29:1756–1766. doi: 10.1016/j.celrep.2019.10.008. e1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pauthner M., Havenar-Daughton C., Sok D., Nkolola J.P., Bastidas R., Boopathy A.V., Carnathan D.G., Chandrashekar A., Cirelli K.M., Cottrell C.A., Eroshkin A.M., Guenaga J., Kaushik K., Kulp D.W., Liu J., McCoy L.E., Oom A.L., Ozorowski G., Post K.W., Sharma S.K., Steichen J.M., de Taeye S.W., Tokatlian T., de la Pena A. Torrents, Butera S.T., LaBranche C.C., Montefiori D.C., Silvestri G., Wilson I.A., Irvine D.J., Sanders R.W., Schief W.R., Ward A.B., Wyatt R.T., Barouch D.H., Crotty S., Burton D.R. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity. 2017;46:1073–1088. doi: 10.1016/j.immuni.2017.05.007. e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin J.T., Hartwell B.L., Melo M.B., Carnathan D., Cossette B.J., Adams J., Tokatlian T., Silvestri G., Kumarapperuma S.C., Schief W.R., Ruprecht R.M., Irvine D.J. 2020. Imaging the Fate of HIV Immunogens in Rhesus Macaques with Whole Organ and whole Animal Imaging Techniques. submitted. [Google Scholar]
- 44.F. Liang, G. Lindgren, K.J. Sandgren, E.A. Thompson, J.R. Francica, A. Seubert, De Gregorio, E., S. Barnett, D.T. O'Hagan, N.J. Sullivan, R.A. Koup, R.A. Seder, K. Lore, Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake, Sci. Transl. Med., 9 (2017). [DOI] [PubMed]
- 45.Ols S., Yang L., Thompson E.A., Pushparaj P., Tran K., Liang F., Lin A., Eriksson B., Karlsson Hedestam G.B., Wyatt R.T., Lore K. Route of vaccine administration alters antigen trafficking but not innate or adaptive immunity. Cell Rep. 2020;30:3964–3971. doi: 10.1016/j.celrep.2020.02.111. e3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allan R.S., Smith C.M., Belz G.T., van Lint A.L., Wakim L.M., Heath W.R., Carbone F.R. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 47.Gurevich I., Feferman T., Milo I., Tal O., Golani O., Drexler I., Shakhar G. Active dissemination of cellular antigens by DCs facilitates CD8(+) T-cell priming in lymph nodes. Eur. J. Immunol. 2017;47:1802–1818. doi: 10.1002/eji.201747042. [DOI] [PubMed] [Google Scholar]
- 48.Allan R.S., Waithman J., Bedoui S., Jones C.M., Villadangos J.A., Zhan Y., Lew A.M., Shortman K., Heath W.R., Carbone F.R. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Rantakari P., Auvinen K., Jappinen N., Kapraali M., Valtonen J., Karikoski M., Gerke H., Iftakhar E.K.I., Keuschnigg J., Umemoto E., Tohya K., Miyasaka M., Elima K., Jalkanen S., Salmi M. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 2015;16:386–396. doi: 10.1038/ni.3101. [DOI] [PubMed] [Google Scholar]
- 50.Reynoso G.V., Weisberg A.S., Shannon J.P., McManus D.T., Shores L., Americo J.L., Stan R.V., Yewdell J.W., Hickman H.D. Lymph node conduits transport virions for rapid T cell activation. Nat. Immunol. 2019;20:602–612. doi: 10.1038/s41590-019-0342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roozendaal R., Mempel T.R., Pitcher L.A., Gonzalez S.F., Verschoor A., Mebius R.E., von Andrian U.H., Carroll M.C. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sixt M., Kanazawa N., Selg M., Samson T., Roos G., Reinhardt D.P., Pabst R., Lutz M.B., Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Junt T., Moseman E.A., Iannacone M., Massberg S., Lang P.A., Boes M., Fink K., Henrickson S.E., Shayakhmetov D.M., Di Paolo. N.C. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 54.Cyster J.G. B cell follicles and antigen encounters of the third kind. Nat. Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 55.Gerner M.Y., Casey K.A., Kastenmuller W., Germain R.N. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J. Exp. Med. 2017;214:3105–3122. doi: 10.1084/jem.20170335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerner M.Y., Torabi-Parizi P., Germain R.N. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42:172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 57.Heesters B.A., Chatterjee P., Kim Y.A., Gonzalez S.F., Kuligowski M.P., Kirchhausen T., Carroll M.C. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38:1164–1175. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kranich J., Krautler N.J. How Follicular Dendritic Cells Shape the B-Cell Antigenome. Front. Immunol. 2016;7:225. doi: 10.3389/fimmu.2016.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mesin L., Ersching J., Victora G.D. Germinal Center B Cell Dynamics. Immunity. 2016;45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gitlin A.D., Mayer C.T., Oliveira T.Y., Shulman Z., Jones M.J., Koren A., Nussenzweig M.C. HUMORAL IMMUNITY. T cell help controls the speed of the cell cycle in germinal center B cells. Science. 2015;349:643–646. doi: 10.1126/science.aac4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shulman Z., Gitlin A.D., Weinstein J.S., Lainez B., Esplugues E., Flavell R.A., Craft J.E., Nussenzweig M.C. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayer C.T., Gazumyan A., Kara E.E., Gitlin A.D., Golijanin J., Viant C., Pai J., Oliveira T.Y., Wang Q., Escolano A., Medina-Ramirez M., Sanders R.W., Nussenzweig M.C. The microanatomic segregation of selection by apoptosis in the germinal center. Science. 2017;358 doi: 10.1126/science.aao2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gitlin A.D., Shulman Z., Nussenzweig M.C. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.M. Mold, E. Shardlow, C. Exley, Insight into the cellular fate and toxicity of aluminium adjuvants used in clinically approved human vaccinations, Sci. Rep., 6 (2016). [DOI] [PMC free article] [PubMed]
- 65.Calabro S., Tortoli M., Baudner B.C., Pacitto A., Cortese M., O'Hagan D.T., De Gregorio E., Seubert A., Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29:1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 66.Lu F., Hogenesch H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine. 2013;31:3979–3986. doi: 10.1016/j.vaccine.2013.05.107. [DOI] [PubMed] [Google Scholar]
- 67.Bergtold A., Desai D.D., Gavhane A., Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 68.Cantisani R., Pezzicoli A., Cioncada R., Malzone C., De Gregorio E., D'Oro U., Piccioli D. Vaccine adjuvant MF59 promotes retention of unprocessed antigen in lymph node macrophage compartments and follicular dendritic cells. J. Immunol. 2015;194:1717–1725. doi: 10.4049/jimmunol.1400623. [DOI] [PubMed] [Google Scholar]
- 69.Didierlaurent A.M., Collignon C., Bourguignon P., Wouters S., Fierens K., Fochesato M., Dendouga N., Langlet C., Malissen B., Lambrecht B.N., Garcon N., Van Mechelen M., Morel S. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 2014;193:1920–1930. doi: 10.4049/jimmunol.1400948. [DOI] [PubMed] [Google Scholar]
- 70.Henriksen-Lacey M., Christensen D., Bramwell V.W., Lindenstrom T., Agger E.M., Andersen P., Perrie Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J. Control. Release. 2010;145:102–108. doi: 10.1016/j.jconrel.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 71.Lindenstrom T., Knudsen N.P., Agger E.M., Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J. Immunol. 2013;190:6311–6319. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 72.van Dissel J.T., Joosten S.A., Hoff S.T., Soonawala D., Prins C., Hokey D.A., O'Dee D.M., Graves A., Thierry-Carstensen B., Andreasen L.V., Ruhwald M., de Visser A.W., Agger E.M., Ottenhoff T.H.M., Kromann I., Andersen P. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32:7098–7107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 73.Rudra J.S., Mishra S., Chong A.S., Mitchell R.A., Nardin E.H., Nussenzweig V., Collier J.H. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33:6476–6484. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudra J.S., Tian Y.F., Jung J.P., Collier J.H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hudalla G.A., Modica J.A., Tian Y.F., Rudra J.S., Chong A.S., Sun T., Mrksich M., Collier J.H. A Self-adjuvanting supramolecular vaccine carrying a folded protein Antigen. Adv. Healthcare Mater. 2013;2:1114–1119. doi: 10.1002/adhm.201200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hudalla G.A., Sun T., Gasiorowski J.Z., Han H.F., Tian Y.F., Chong A.S., Collier J.H. Gradated assembly of multiple proteins into supramolecular nanomaterials. Nat. Mater. 2014;13:829–836. doi: 10.1038/nmat3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pompano R.R., Chen J.J., Verbus E.A., Han H.F., Fridman A., McNeely T., Collier J.H., Chong A.S. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+Helper T cell and antibody outputs. Adv. Healthcare Mater. 2014;3:1898–1908. doi: 10.1002/adhm.201400137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tokatlian T., Kulp D.W., Mutafyan A.A., Jones C.A., Menis S., Georgeson E., Kubitz M., Zhang M.H., Melo M.B., Silva M., Yun D.S., Schief W.R., Irvine D.J. Enhancing humoral responses against HIV Envelope TRIMx`x`ERS via nanoparticle delivery WITH stabilized synthetic liposomes. Sci. Rep. 2018;8:16527. doi: 10.1038/s41598-018-34853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karabin N.B., Allen S., Kwon H.K., Bobbala S., Firlar E., Shokuhfar T., Shull K.R., Scott E.A. Sustained micellar delivery via inducible transitions in nanostructure morphology. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali O.A., Emerich D., Dranoff G., Mooney D.J. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci. Transl. Med. 2009;1 doi: 10.1126/scitranslmed.3000359. 8ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bencherif S.A., Warren Sands R., Ali O.A., Li W.A., Lewin S.A., Braschler T.M., Shih T.Y., Verbeke C.S., Bhatta D., Dranoff G., Mooney D.J. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015;6:7556. doi: 10.1038/ncomms8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shah N.J., Najibi A.J., Shih T.Y., Mao A.S., Sharda A., Scadden D.T., Mooney D.J. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat. Biomed. Eng. 2020;4:40–51. doi: 10.1038/s41551-019-0503-3. [DOI] [PubMed] [Google Scholar]
- 83.Ali O.A., Doherty E., Mooney D.J., Emerich D. Relationship of vaccine efficacy to the kinetics of DC and T-cell responses induced by PLG-based cancer vaccines. Biomatter. 2011;1:66–75. doi: 10.4161/biom.1.1.16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang H., Mooney D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018;17:761–772. doi: 10.1038/s41563-018-0147-9. [DOI] [PubMed] [Google Scholar]
- 85.Jiang H., Wang Q., Sun X. Lymph node targeting strategies to improve vaccination efficacy. J. Control. Release. 2017;267:47–56. doi: 10.1016/j.jconrel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 86.Schudel A., Francis D.M., Thomas S.N. Material design for lymph node drug delivery. Nat. Rev. Mater. 2019;4:415–428. doi: 10.1038/s41578-019-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Irvine D.J., Hanson M.C., Rakhra K., Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015;115(15):11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donaldson B., Lateef Z., Walker G.F., Young S.L., Ward V.K. Virus-like particle vaccines: immunology and formulation for clinical translation. Expert Rev. Vaccines. 2018;17:833–849. doi: 10.1080/14760584.2018.1516552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rohner N.A., Thomas S.N. Flexible macromolecule versus rigid particle retention in the injected skin and accumulation in draining lymph nodes are differentially influenced by hydrodynamic size. ACS Biomater. Sci. Eng. 2017;3:153–159. doi: 10.1021/acsbiomaterials.6b00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rohovie M.J., Nagasawa M., Swartz J.R. Virus-like particles: next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med. 2017;2:43–57. doi: 10.1002/btm2.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peabody D.S., Manifold-Wheeler B., Medford A., Jordan S.K., Caldeira J.D., Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J. Mol. Biol. 2008;380:252–263. doi: 10.1016/j.jmb.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Billaud J.N., Peterson D., Barr M., Chen A., Sallberg M., Garduno F., Goldstein P., McDowell W., Hughes J., Jones J., Milich D. Combinatorial approach to hepadnavirus-like particle vaccine design. J. Virol. 2005;79:13656–13666. doi: 10.1128/JVI.79.21.13656-13666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shukla S., Myers J.T., Woods S.E., Gong X.J., Czapar A.E., Commandeur U., Huang A.Y., Levine A.D., Steinmetz N.F. Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials. 2017;121:15–27. doi: 10.1016/j.biomaterials.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 94.Kanekiyo M., Wei C.J., Yassine H.M., McTamney P.M., Boyington J.C., Whittle J.R.R., Rao S.S., Kong W.P., Wang L.S., Nabel G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013;499 doi: 10.1038/nature12202. 102-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sliepen K., Ozorowski G., Burger J.A., van Montfort T., Stunnenberg M., LaBranche C., Montefiori D.C., Moore J.P., Ward A.B., Sanders R.W. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology. 2015;12 doi: 10.1186/s12977-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He L.L., de Val N., Morris C.D., Vora N., Thinnes T.C., Kong L., Azadnia P., Sok D., Zhou B., Burton D.R., Wilson I.A., Nemazee D., Ward A.B., Zhu J. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 2016;7 doi: 10.1038/ncomms12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sliepen K., Han B.W., Bontjer I., Mooij P., Garces F., Behrens A.J., Rantalainen K., Kumar S., Sarkar A., Brouwer P.J.M., Hua Y.Z., Tolazzi M., Schermer E., Torres J.L., Ozorowski G., van der Woude P., de la Pena A.T., van Breemen M.J., Camacho-Sanchez J.M., Burger J.A., Medina-Ramirez M., Gonzalez N., Alcami J., LaBranche C., Scarlatti G., van Gils M.J., Crispin M., Montefiori D.C., Ward A.B., Koopman G., Moore J.P., Shattock R.J., Bogers W.M., Wilson I.A., Sanders R.W. Structure and immunogenicity of a stabilized HIV-1 envelope trimer based on a group-M consensus sequence. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-10262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jardine J., Julien J.P., Menis S., Ota T., Kalyuzhniy O., McGuire A., Sok D., Huang P.S., MacPherson S., Jones M., Nieusma T., Mathison J., Baker D., Ward A.B., Burton D.R., Stamatatos L., Nemazee D., Wilson I.A., Schief W.R. Rational HIV Immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sok D., Briney B., Jardine J.G., Kulp D.W., Menis S., Pauthner M., Wood A., Lee E.C., Le K.M., Jones M., Ramos A., Kalyuzhniy O., Adachi Y., Kubitz M., MacPherson S., Bradley A., Friedrich G.A., Schief W.R., Burton D.R. Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice. Science. 2016;353:1557–1560. doi: 10.1126/science.aah3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jardine J.G., Kulp D.W., Havenar-Daughton C., Sarkar A., Briney B., Sok D., Sesterhenn F., Ereno-Orbea J., Kalyuzhniy O., Deresa I., Hu X., Spencer S., Jones M., Georgeson E., Adachi Y., Kubitz M., de Camp A.C., Julien J.P., Wilson I.A., Burton D.R., Crotty S., Schief W.R. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science. 2016;351:1458–1463. doi: 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jardine J.G., Ota T., Sok D., Pauthner M., Kulp D.W., Kalyuzhniy O., Skog P.D., Thinnes T.C., Bhullar D., Briney B., Menis S., Jones M., Kubitz M., Spencer S., Adachi Y., Burton D.R., Schief W.R., Nemazee D. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science. 2015;349:156–161. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang W.C., Deng B.B., Lin C.Y., Carter K.A., Geng J.M., Razi A., He X.D., Chitgupi U., Federizon J., Sun B.Y., Long C.A., Ortega J., Dutta S., King C.R., Miura K., Lee S.M., Lovell J.F. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat. Nanotechnol. 2018;13 doi: 10.1038/s41565-018-0271-3. 1174-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marcandalli J., Fiala B., Ols S., Perotti M., de van der Schueren W.D., Snijder J., Hodge E., Benhaim M., Ravichandran R., Carter L., Sheffler W., Brunner L., Lawrenz M., Dubois P., Lanzavecchia A., Sallusto F., Lee K.K., Veesler D., Correnti C.E., Stewart L.J., Baker D., Lore K., Perez L., King N.P. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell. 2019;176 doi: 10.1016/j.cell.2019.01.046. 1420-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brouwer P.J.M., Antanasijevic A., Berndsen Z., Yasmeen A., Fiala B., Bijl T.P., Bontjer I., Bale J.B., Sheffler W., Allen J.D., Schorcht A., Burgers J.A., Camacho M., Ellis D., Cottrell C.A., Behrens A.J., Catalano M., Del Moral-Sanchez I., Ketas T.J., LaBranche C., van Gils M.J., Sliepen K., Stewart L.J., Crispin M., Montefiori D.C., Baker D., Moore J.P., Klasse P.J., Ward A.B., King N.P., Sanders R.W. Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-12080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karch C.P., Li J.P., Kulangara C., Paulillo S.M., Raman S.K., Emadi S., Tan A.M., Helal Z.H., Fan Q., Khan M.I., Burkhard P. Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge. Nanomed. Nanotechnol. Biol. Med. 2017;13:241–251. doi: 10.1016/j.nano.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 106.Lynn G.M., Sedlik C., Baharom F., Zhu Y., Ramirez-Valdez R.A., Coble V.L., Tobin K., Nichols S.R., Itzkowitz Y., Zaidi N., Gammon J.M., Blobel N.J., Denizeau J., de la Rochere P., Francica B.J., Decker B., Maciejewski M., Cheung J., Yamane H., Smelkinson M.G., Francica J.R., Laga R., Bernstock J.D., Seymour L.W., Drake C.G., Jewell C.M., Lantz O., Piaggio E., Ishizuka A.S., Seder R.A. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol. 2020;38:320–332. doi: 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henriksen-Lacey M., Korsholm K.S., Andersen P., Perrie Y., Christensen D. Liposomal vaccine delivery systems. Expert Opin. Drug Deliv. 2011;8:505–519. doi: 10.1517/17425247.2011.558081. [DOI] [PubMed] [Google Scholar]
- 108.Dubrovskaya V., Tran K., Ozorowski G., Guenaga J., Wilson R., Bale S., Cottrell C.A., Turner H.L., Seabright G., O'Dell S., Torres J.L., Yang L.F., Feng Y., Leaman D.P., Bernat N.V., Liban T., Louder M., McKee K., Bailer R.T., Movsesyan A., Doria-Rose N.A., Pancera M., Hedestam G.B.K., Zwick M.B., Crispin M., Mascola J.R., Ward A.B., Wyatt R.T. Vaccination with glycan-modified HIV NFL envelope trimer-liposomes elicits broadly neutralizing antibodies to multiple sites of vulnerability. Immunity. 2019;51 doi: 10.1016/j.immuni.2019.10.008. 915-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ingale J., Stano A., Guenaga J., Sharma S.K., Nemazee D., Zwick M.B., Wyatt R.T. High-density array of well-ordered HIV-1 spikes on synthetic liposomal nanoparticles efficiently activate B Cells. Cell Rep. 2016;15:1986–1999. doi: 10.1016/j.celrep.2016.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tokatlian T., Read B.J., Jones C.A., Kulp D.W., Menis S., Chang J.Y.H., Steichen J.M., Kumari S., Allen J.D., Dane E.L., Liguori A., Sangesland M., Lingwood D., Crispin M., Schief W.R., Irvine D.J. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. 2019;363:649–654. doi: 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moon J.J., Suh H., Li A.V., Ockenhouse C.F., Yadava A., Irvine D.J. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moon J.J., Suh H., Bershteyn A., Stephan M.T., Liu H., Huang B., Sohail M., Luo S., Um S.H., Khant H., Goodwin J.T., Ramos J., Chiu W., Irvine D.J. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017;16 doi: 10.1038/nmat4822. 489-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reddy S.T., van der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'Neil C.P., Lee L.K., Swartz M.A., Hubbell J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 115.Manolova V., Flace A., Bauer M., Schwarz K., Saudan P., Bachmann M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 116.Reddy S.T., Rehor A., Schmoekel H.G., Hubbell J.A., Swartz M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 117.Perry J.L., Herlihy K.P., Napier M.E., DeSimone J.M. PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Acc. Chem. Res. 2011;44:990–998. doi: 10.1021/ar2000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Metz S.W., Tian S., Hoekstra G., Yi X., Stone M., Horvath K., Miley M.J., DeSimone J., Luft C.J., de Silva A.M. Precisely molded nanoparticle displaying DENV-E proteins induces robust serotype-specific neutralizing antibody responses. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dacoba T.G., Omange R.W., Li H.Z., Crecente-Campo J., Luo M., Alonso M.J. Polysaccharide nanoparticles can efficiently modulate the immune response against an HIV peptide antigen. ACS Nano. 2019;13:4947–4959. doi: 10.1021/acsnano.8b07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qiu F., Becker K.W., Knight F.C., Baljon J.J., Sevimli S., Shae D., Gilchuk P., Joyce S., Wilson J.T. Poly(propylacrylic acid)-peptide nanoplexes as a platform for enhancing the immunogenicity of neoantigen cancer vaccines. Biomaterials. 2018;182:82–91. doi: 10.1016/j.biomaterials.2018.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luo M., Wang H., Wang Z.H., Cai H.C., Lu Z.G., Li Y., Du M.J., Huang G., Wang C.S., Chen X., Porembka M.R., Lea J., Frankel A.E., Fu Y.X., Chen Z.J.J., Gao J.M. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017;12 doi: 10.1038/nnano.2017.52. 648-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Almeida J.P.M., Lin A.Y., Figueroa E.R., Foster A.E., Drezek R.A. In vivo Gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small. 2015;11:1453–1459. doi: 10.1002/smll.201402179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao W.W., Fang R.H., Thamphiwatana S., Luk B.T., Li J.M., Angsantikul P., Zhang Q.Z., Hu C.M.J., Zhang L.F. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403–1409. doi: 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao W.W., Hu C.M.J., Fang R.H., Luk B.T., Su J., Zhang L.F. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv. Mater. 2013;25:3549–3553. doi: 10.1002/adma.201300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McLennan D.N., Porter C.J., Charman S.A. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov. Today Technol. 2005;2:89–96. doi: 10.1016/j.ddtec.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 126.Supersaxo A., Hein W.R., Steffen H. Effect of molecular-weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 1990;7:167–169. doi: 10.1023/a:1015880819328. [DOI] [PubMed] [Google Scholar]
- 127.Moynihan K.D., Holden R.L., Mehta N.K., Wang C., Karver M.R., Dinter J., Liang S., Abraham W., Melo M.B., Zhang A.Q., Li N., Le Gall S., Pentelute B., Irvine D.J. Enhancement of peptide vaccine immunogenicity by increasing lymphatic drainage and boosting serum stability. Cancer Immunol. Res. 2018;6(9):1025–1038. doi: 10.1158/2326-6066.CIR-17-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu G., Lynn G.M., Jacobson O., Chen K., Liu Y., Zhang H., Ma Y., Zhang F., Tian R., Ni Q., Cheng S., Wang Z., Lu N., Yung B.C., Wang Z., Lang L., Fu X., Jin A., Weiss I.D., Vishwasrao H., Niu G., Shroff H., Klinman D.M., Seder R.A., Chen X. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat. Commun. 2017;8:1954. doi: 10.1038/s41467-017-02191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.N.K. Mehta; R.V. Pradhan; Soleimany, A.P.; Moynihan, K.K.; Rothschild, A.M.; Momin, N.; Rakhra, K.; Mata-Fink, J.; Bhatia, S.N.; Wittrup, K.D.; Irvine, D.J., 2020 Pharmacokinetic tuning of protein-antigen fusions to enhance the potency of therapeutic T cell vaccines, Nat. Biomed. Eng., in press., (2020). [DOI] [PMC free article] [PubMed]
- 130.Ma L., Dichwalkar T., Chang J.Y.H., Cossette B., Garafola D., Zhang A.Q., Fichter M., Wang C., Liang S., Silva M., Kumari S., Mehta N.K., Abraham W., Thai N., Li N., Wittrup K.D., Irvine D.J. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365:162–168. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeng B., Middelberg A.P., Gemiarto A., MacDonald K., Baxter A.G., Talekar M., Moi D., Tullett K.M., Caminschi I., Lahoud M.H., Mazzieri R., Dolcetti R., Thomas R. Self-adjuvanting nanoemulsion targeting dendritic cell receptor Clec9A enables antigen-specific immunotherapy. J. Clin. Invest. 2018;128:1971–1984. doi: 10.1172/JCI96791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cruz L.J., Rosalia R.A., Kleinovink J.W., Rueda F., Lowik C.W., Ossendorp F. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8(+) T cell response: a comparative study. J. Control. Release. 2014;192:209–218. doi: 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 133.Bonifaz L.C., Bonnyay D.P., Charalambous A., Darguste D.I., Fujii S.I., Soares H., Brimnes M.K., Moltedo B., Moran T.M., Steinman R.M. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boscardin S.B., Hafalla J.C., Masilamani R.F., Kamphorst A.O., Zebroski H.A., Rai U., Morrot A., Zavala F., Steinman R.M., Nussenzweig R.S., Nussenzweig M.C. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J. Exp. Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dhodapkar M.V., Sznol M., Zhao B., Wang D., Carvajal R.D., Keohan M.L., Chuang E., Sanborn R.E., Lutzky J., Powderly J., Kluger H., Tejwani S., Green J., Ramakrishna V., Crocker A., Vitale L., Yellin M., Davis T., Keler T. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008068. 232ra251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhardwaj N.F., Pavlick A.C., Ernstoff M.S., Gastman B., Hanks B.A., Albertini M.R., Luke J.J., Yellin M.J., Keler T., Davis T.A., Crocker A., Vitale L., Morishima C., Cheever M.A., Fling S.P. A phase II, open-label, multicenter, randomized study of CDX-1401, a dendritic cell targeting NY-ESO-1 vaccine, in patients with malignant melanoma pre-treated with CDX-301, a dRecombinant human Flt3 ligand. J. Clin. Oncol. 2016;34 [Google Scholar]
- 137.Wang X.Y., Wang B., Wen Y.M. From therapeutic antibodies to immune complex vaccines. NPJ Vaccines. 2019;4:2. doi: 10.1038/s41541-018-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ben Mkaddem S., Benhamou M., Monteiro R.C. Understanding Fc receptor involvement in inflammatory diseases: from mechanisms to new therapeutic tools. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Caaveiro J.M.M., Kiyoshi M., Tsumoto K. Structural analysis of Fc/FcR complexes: a blueprint for antibody design. Immunol. Rev. 2015;268:201–221. doi: 10.1111/imr.12365. [DOI] [PubMed] [Google Scholar]
- 140.Maamary J., Wang T.T., Tan G.S., Palese P., Ravetch J.V. Increasing the breadth and potency of response to the seasonal influenza virus vaccine by immune complex immunization. Proc. Natl. Acad. Sci. U. S. A. 2017;114:10172–10177. doi: 10.1073/pnas.1707950114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pepponi I., Diogo G.R., Stylianou E., van Dolleweerd C.J., Drake P.M.W., Paul M.J., Sibley L., Ma J.K.C., Reljic R. Plant-derived recombinant immune complexes as self-adjuvanting TB immunogens for mucosal boosting of BCG. Plant Biotechnol. J. 2014;12:840–850. doi: 10.1111/pbi.12185. [DOI] [PubMed] [Google Scholar]
- 142.Kim M.Y., Reljic R., Kilbourne J., Ceballos-Olvera I., Yang M.S., Reyes-del Valle J., Mason H.S. Novel vaccination approach for dengue infection based on recombinant immune complex universal platform. Vaccine. 2015;33:1830–1838. doi: 10.1016/j.vaccine.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 143.Del Giudice G., Rappuoli R., Didierlaurent A.M. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin. Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 144.Fox C.B., Barnes L., Evers T., Chesko J.D., Vedvick T.S., Coler R.N., Reed S.G., Baldwin S.L. Adjuvanted pandemic influenza vaccine: variation of emulsion components affects stability, antigen structure, and vaccine efficacy. Influenza Other Respir. Viruses. 2013;7:815–826. doi: 10.1111/irv.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dummer R., Hauschild A., Becker J.C., Grob J.J., Schadendorf D., Tebbs V., Skalsky J., Kaehler K.C., Moosbauer S., Clark R., Meng T.C., Urosevic M. An exploratory study of systemic administration of the toll-like receptor-7 agonist 852A in patients with refractory metastatic melanoma. Clin. Cancer Res. 2008;14:856–864. doi: 10.1158/1078-0432.CCR-07-1938. [DOI] [PubMed] [Google Scholar]
- 146.Geller M.A., Cooley S., Argenta P.A., Downs L.S., Carson L.F., Judson P.L., Ghebre R., Weigel B., Panoskaltsis-Mortari A., Curtsinger J., Miller J.S. Toll-like receptor-7 agonist administered subcutaneously in a prolonged dosing schedule in heavily pretreated recurrent breast, ovarian, and cervix cancers. Cancer Immunol. Immunother. 2010;59:1877–1884. doi: 10.1007/s00262-010-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harrison L.I., Astry C., Kumar S., Yunis C. Pharmacokinetics of 852A, an imidazoquinoline Toll-like receptor 7-specific agonist, following intravenous, subcutaneous, and oral administrations in humans. J. Clin. Pharmacol. 2007;47:962–969. doi: 10.1177/0091270007303766. [DOI] [PubMed] [Google Scholar]
- 148.Wu T.Y., Singh M., Miller A.T., De Gregorio E., Doro F., D'Oro U., Skibinski D.A., Mbow M.L., Bufali S., Herman A.E., Cortez A., Li Y., Nayak B.P., Tritto E., Filippi C.M., Otten G.R., Brito L.A., Monaci E., Li C., Aprea S., Valentini S., Calabromicron S., Laera D., Brunelli B., Caproni E., Malyala P., Panchal R.G., Warren T.K., Bavari S., O'Hagan D.T., Cooke M.P., Valiante N.M. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3009980. 263ra160. [DOI] [PubMed] [Google Scholar]
- 149.Moyer T.J., Kato Y., Abraham W., Chang J.Y.H., Kulp D.W., Watson N., Turner H.L., Menis S., Abbott R.K., Bhiman J.N., Melo M.B., Simon H.A., Herrera-De la Mata S., Liang S., Seumois G., Agarwal Y., Li N., Burton D.R., Ward A.B., Schief W.R., Crotty S., Irvine D.J. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020;26:430–440. doi: 10.1038/s41591-020-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marciani D.J. Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018;39:573–585. doi: 10.1016/j.tips.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 151.Wilson N.S., Duewell P., Yang B., Li Y., Marsters S., Koernig S., Latz E., Maraskovsky E., Morelli A.B., Schnurr M., Ashkenazi A. Inflammasome-dependent and -independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant. J. Immunol. 2014;192:3259–3268. doi: 10.4049/jimmunol.1302011. [DOI] [PubMed] [Google Scholar]
- 152.Marty-Roix R., Vladimer G.I., Pouliot K., Weng D., Buglione-Corbett R., West K., MacMicking J.D., Chee J.D., Wang S., Lu S., Lien E. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J. Biol. Chem. 2016;291:1123–1136. doi: 10.1074/jbc.M115.683011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Evans T.G., McElrath M.J., Matthews T., Montefiori D., Weinhold K., Wolff M., Keefer M.C., Kallas E.G., Corey L., Gorse G.J., Belshe R., Graham B.S., Spearman P.W., Schwartz D., Mulligan M.J., Goepfert P., Fast P., Berman P., Powell M., Francis D., N.A.V.E. Group QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine. 2001;19:2080–2091. doi: 10.1016/s0264-410x(00)00415-1. [DOI] [PubMed] [Google Scholar]
- 154.Kashala O., Amador R., Valero M.V., Moreno A., Barbosa A., Nickel B., Daubenberger C.A., Guzman F., Pluschke G., Patarroyo M.E. Safety, tolerability and immunogenicity of new formulations of the Plasmodium falciparum malaria peptide vaccine SPf66 combined with the immunological adjuvant QS-21. Vaccine. 2002;20:2263–2277. doi: 10.1016/s0264-410x(02)00115-9. [DOI] [PubMed] [Google Scholar]
- 155.Morein B., Sundquist B., Hoglund S., Dalsgaard K., Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature. 1984;308:457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- 156.Scales H.E., Meehan G.R., Hayes A.J., Benson R.A., Watson E., Walters A., Tomura M., Maraskovsky E., Garside P., Baz Morelli A., Brewer J.M. A Novel Cellular Pathway of Antigen Presentation and CD4 T Cell Activation in vivo. Front. Immunol. 2018;9:2684. doi: 10.3389/fimmu.2018.02684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bigaeva E., van Doorn E., Liu H., Hak E. Meta-analysis on randomized controlled trials of vaccines with QS-21 or ISCOMATRIX adjuvant: safety and tolerability. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Detienne S., Welsby I., Collignon C., Wouters S., Coccia M., Delhaye S., Van Maele L., Thomas S., Swertvaegher M., Detavernier A. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep39475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cunningham A.L., Lal H., Kovac M., Chlibek R., Hwang S.-J., Díez-Domingo J., Godeaux O., Levin M.J., McElhaney J.E., Puig-Barberà J. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 160.Didierlaurent A., Berger A., Heineman T., Henderickx V., Da Silva F.T., Vekemans J., Voss G., Garçon N. Elsevier; 2017. The Development of the Adjuvant System AS01: A Combination of Two Immunostimulants MPL and QS-21 in Liposomes, Immunopotentiators in Modern Vaccines; pp. 265–285. [Google Scholar]
- 161.Hanson M.C., Crespo M.P., Abraham W., Moynihan K.D., Szeto G.L., Chen S.H., Melo M.B., Mueller S., Irvine D.J. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Invest. 2015;125:2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Radovic-Moreno A.F., Chernyak N., Mader C.C., Nallagatla S., Kang R.S., Hao L., Walker D.A., Halo T.L., Merkel T.J., Rische C.H. Immunomodulatory spherical nucleic acids. Proc. Natl. Acad. Sci. 2015;112:3892–3897. doi: 10.1073/pnas.1502850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fischer N.O., Rasley A., Corzett M., Hwang M.H., Hoeprich P.D., Blanchette C.D. Colocalized delivery of adjuvant and antigen using nanolipoprotein particles enhances the immune response to recombinant antigens. J. Am. Chem. Soc. 2013;135:2044–2047. doi: 10.1021/ja3063293. [DOI] [PubMed] [Google Scholar]
- 165.Guan C., Chernyak N., Dominguez D., Cole L., Zhang B., Mirkin C.A. RNA-based immunostimulatory liposomal spherical nucleic acids as potent TLR7/8 modulators. Small. 2018;14:1803284. doi: 10.1002/smll.201803284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lynn G.M., Laga R., Darrah P.A., Ishizuka A.S., Balaci A.J., Dulcey A.E., Pechar M., Pola R., Gerner M.Y., Yamamoto A., Buechler C.R., Quinn K.M., Smelkinson M.G., Vanek O., Cawood R., Hills T., Vasalatiy O., Kastenmuller K., Francica J.R., Stutts L., Tom J.K., Ryu K.A., Esser-Kahn A.P., Etrych T., Fisher K.D., Seymour L.W., Seder R.A. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015;33:1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nuhn L., Vanparijs N., De Beuckelaer A., Lybaert L., Verstraete G., Deswarte K., Lienenklaus S., Shukla N.M., Salyer A.C., Lambrecht B.N. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc. Natl. Acad. Sci. 2016;113:8098–8103. doi: 10.1073/pnas.1600816113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kasturi S.P., Skountzou I., Albrecht R.A., Koutsonanos D., Hua T., Nakaya H.I., Ravindran R., Stewart S., Alam M., Kwissa M., Villinger F., Murthy N., Steel J., Jacob J., Hogan R.J., García-Sastre A., Compans R., Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jiang H., Wang Q., Li L., Zeng Q., Li H.M., Gong T., Zhang Z.R., Sun X. Turning the old adjuvant from gel to nanoparticles to amplify CD8(+) T cell responses. Adv. Sci. 2018;5 doi: 10.1002/advs.201700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.De Vrieze J., Louage B., Deswarte K., Zhong Z., De Coen R., Van Herck S., Nuhn L., Kaas Frich C., Zelikin A.N., Lienenklaus S., Sanders N.N., Lambrecht B.N., David S.A., De Geest B.G. Potent lymphatic translocation and spatial control over innate immune activation by polymer-lipid amphiphile conjugates of small-molecule TLR7/8 agonists. Angew. Chem. Int. Ed. Eng. 2019;58:15390–15395. doi: 10.1002/anie.201905687. [DOI] [PubMed] [Google Scholar]
- 171.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li L., Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines. 2016;15:313–329. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines. 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rush C.M., Mitchell T.J., Garside P. A detailed characterisation of the distribution and presentation of DNA vaccine encoded antigen. Vaccine. 2010;28:1620–1634. doi: 10.1016/j.vaccine.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shirota H., Petrenko L., Hong C., Klinman D.M. Potential of transfected muscle cells to contribute to DNA vaccine immunogenicity. J. Immunol. 2007;179:329–336. doi: 10.4049/jimmunol.179.1.329. [DOI] [PubMed] [Google Scholar]
- 176.Dupuis M., Denis-Mize K., Woo C., Goldbeck C., Selby M.J., Chen M., Otten G.R., Ulmer J.B., Donnelly J.J., Ott G., McDonald D.M. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 2000;165:2850–2858. doi: 10.4049/jimmunol.165.5.2850. [DOI] [PubMed] [Google Scholar]
- 177.Bagarazzi M.L., Yan J., Morrow M.P., Shen X., Parker R.L., Lee J.C., Giffear M., Pankhong P., Khan A.S., Broderick K.E., Knott C., Lin F., Boyer J.D., Draghia-Akli R., White C.J., Kim J.J., Weiner D.B., Sardesai N.Y. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004414. 155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Trimble C.L., Morrow M.P., Kraynyak K.A., Shen X., Dallas M., Yan J., Edwards L., Parker R.L., Denny L., Giffear M., Brown A.S., Marcozzi-Pierce K., Shah D., Slager A.M., Sylvester A.J., Khan A., Broderick K.E., Juba R.J., Herring T.A., Boyer J., Lee J., Sardesai N.Y., Weiner D.B., Bagarazzi M.L. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dolter K.E., Evans C.F., Ellefsen B., Song J., Boente-Carrera M., Vittorino R., Rosenberg T.J., Hannaman D., Vasan S. Immunogenicity, safety, biodistribution and persistence of ADVAX, a prophylactic DNA vaccine for HIV-1, delivered by in vivo electroporation. Vaccine. 2011;29:795–803. doi: 10.1016/j.vaccine.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 180.Porgador A., Irvine K.R., Iwasaki A., Barber B.H., Restifo N.P., Germain R.N. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J. Exp. Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stoecklinger A., Eticha T.D., Mesdaghi M., Kissenpfennig A., Malissen B., Thalhamer J., Hammerl P. Langerin+ dermal dendritic cells are critical for CD8+ T cell activation and IgH gamma-1 class switching in response to gene gun vaccines. J. Immunol. 2011;186:1377–1383. doi: 10.4049/jimmunol.1002557. [DOI] [PubMed] [Google Scholar]
- 182.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., Anderson D.G., Langer R., Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H., Grunwitz C., Vormehr M., Husemann Y., Selmi A., Kuhn A.N., Buck J., Derhovanessian E., Rae R., Attig S., Diekmann J., Jabulowsky R.A., Heesch S., Hassel J., Langguth P., Grabbe S., Huber C., Tureci O., Sahin U. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 186.Plog M.S., Guyre C.A., Roberts B.L., Goldberg M., George J.A. St, Perricone M.A. Preclinical safety and biodistribution of adenovirus-based cancer vaccines after intradermal delivery. Hum. Gene Ther. 2006;17:705–716. doi: 10.1089/hum.2006.17.705. [DOI] [PubMed] [Google Scholar]
- 187.Gonin P., Gaillard C. Gene transfer vector biodistribution: pivotal safety studies in clinical gene therapy development. Gene Ther. 2004;11(Suppl. 1):S98–S108. doi: 10.1038/sj.gt.3302378. [DOI] [PubMed] [Google Scholar]
- 188.Maione D., Della Rocca C., Giannetti P., D'Arrigo R., Liberatoscioli L., Franlin L.L., Sandig V., Ciliberto G., La Monica N., Savino R. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Monath T.P., Fast P.E., Modjarrad K., Clarke D.K., Martin B.K., Fusco J., Nichols R., Heppner D.G., Simon J.K., Dubey S., Troth S.P., Wolf J., Singh V., Coller B.A., Robertson J.S. G. Brighton Collaboration Viral Vector Vaccines Safety Working, rVSVDeltaG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire Glycoprotein: Standardized template with key considerations for a risk/benefit assessment. Vaccine X. 2019;1 doi: 10.1016/j.jvacx.2019.100009. 100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huttner A., Dayer J.A., Yerly S., Combescure C., Auderset F., Desmeules J., Eickmann M., Finckh A., Goncalves A.R., Hooper J.W., Kaya G., Krahling V., Kwilas S., Lemaitre B., Matthey A., Silvera P., Becker S., Fast P.E., Moorthy V., Kieny M.P., Kaiser L., Siegrist C.A., Consortium V.S.-E. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2015;15:1156–1166. doi: 10.1016/S1473-3099(15)00154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hanke T., McMichael A.J., Dennis M.J., Sharpe S.A., Powell L.A.J., McLoughlin L., Crome S.J. Biodistribution and persistence of an MVA-vectored candidate HIV vaccine in SIV-infected rhesus macaques and SCID mice. Vaccine. 2005;23:1507–1514. doi: 10.1016/j.vaccine.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 192.Huyn S.T., Burton J.B., Sato M., Carey M., Gambhir S.S., Wu L. A potent, imaging adenoviral vector driven by the cancer-selective mucin-1 promoter that targets breast cancer metastasis. Clin. Cancer Res. 2009;15:3126–3134. doi: 10.1158/1078-0432.CCR-08-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Johnson M., Huyn S., Burton J., Sato M., Wu L. Differential biodistribution of adenoviral vector in vivo as monitored by bioluminescence imaging and quantitative polymerase chain reaction. Hum. Gene Ther. 2006;17:1262–1269. doi: 10.1089/hum.2006.17.1262. [DOI] [PubMed] [Google Scholar]
- 194.Sharma A., Bangari D.S., Tandon M., Pandey A., HogenEsch H., Mittal S.K. Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology. 2009;386:44–54. doi: 10.1016/j.virol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Itano A.A., McSorley S.J., Reinhardt R.L., Ehst B.D., Ingulli E., Rudensky A.Y., Jenkins M.K. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 196.Qi H., Egen J.G., Huang A.Y., Germain R.N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 197.Randolph G.J., Inaba K., Robbiani D.F., Steinman R.M., Muller W.A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 198.Woodruff M.C., Heesters B.A., Herndon C.N., Groom J.R., Thomas P.G., Luster A.D., Turley S.J., Carroll M.C. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J. Exp. Med. 2014;211:1611–1621. doi: 10.1084/jem.20132327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gonzalez S.F., Lukacs-Kornek V., Kuligowski M.P., Pitcher L.A., Degn S.E., Kim Y.A., Cloninger M.J., Martinez-Pomares L., Gordon S., Turley S.J., Carroll M.C. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat. Immunol. 2010;11:427–434. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kedl R.M., Lindsay R.S., Finlon J.M., Lucas E.D., Friedman R.S., Tamburini B.A.J. Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat. Commun. 2017;8:2034. doi: 10.1038/s41467-017-02247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vokali E., Yu S.S., Hirosue S., Rincon-Restrepo M., Duraes F.V., Scherer S., Corthesy-Henrioud P., Kilarski W.W., Mondino A., Zehn D., Hugues S., Swartz M.A. Lymphatic endothelial cells prime naive CD8(+) T cells into memory cells under steady-state conditions. Nat. Commun. 2020;11:538. doi: 10.1038/s41467-019-14127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gretz J.E., Norbury C.C., Anderson A.O., Proudfoot A.E., Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Carrasco Y.R., Batista F.D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 204.Phan T.G., Grigorova I., Okada T., Cyster J.G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 205.Junt T., Moseman E.A., Iannacone M., Massberg S., Lang P.A., Boes M., Fink K., Henrickson S.E., Shayakhmetov D.M., Di Paolo N.C., van Rooijen N., Mempel T.R., Whelan S.P., von Andrian U.H. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 206.C. Park, J.H. Kehrl, An integrin/MFG-E8 shuttle loads HIV-1 viral like particles onto follicular dendritic cells in mouse lymph node, Elife, 8 (2019). [DOI] [PMC free article] [PubMed]
- 207.Phan T.G., Green J.A., Gray E.E., Xu Y., Cyster J.G. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang Y., Meyer-Hermann M., George L.A., Figge M.T., Khan M., Goodall M., Young S.P., Reynolds A., Falciani F., Waisman A., Notley C.A., Ehrenstein M.R., Kosco-Vilbois M., Toellner K.M. Germinal center B cells govern their own fate via antibody feedback. J. Exp. Med. 2013;210:457–464. doi: 10.1084/jem.20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dempsey P.W., Allison M.E., Akkaraju S., Goodnow C.C., Fearon D.T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 210.Link A., Zabel F., Schnetzler Y., Titz A., Brombacher F., Bachmann M.F. Innate immunity mediates follicular transport of particulate but not soluble protein antigen. J. Immunol. 2012;188:3724–3733. doi: 10.4049/jimmunol.1103312. [DOI] [PubMed] [Google Scholar]
- 211.Zhang Y.N., Lazarovits J., Poon W., Ouyang B., Nguyen L.N.M., Kingston B.R., Chan W.C.W. Nanoparticle size influences antigen retention and presentation in lymph node follicles for humoral immunity. Nano Lett. 2019;19:7226–7235. doi: 10.1021/acs.nanolett.9b02834. [DOI] [PubMed] [Google Scholar]
- 212.Cirelli K.M., Carnathan D.G., Nogal B., Martin J.T., Rodriguez O.L., Upadhyay A.A., Enemuo C.A., Gebru E.H., Choe Y., Viviano F., Nakao C., Pauthner M.G., Reiss S., Cottrell C.A., Smith M.L., Bastidas R., Gibson W., Wolabaugh A.N., Melo M.B., Cossette B., Kumar V., Patel N.B., Tokatlian T., Menis S., Kulp D.W., Burton D.R., Murrell B., Schief W.R., Bosinger S.E., Ward A.B., Watson C.T., Silvestri G., Irvine D.J., Crotty S. Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell. 2019;177:1153–1171. doi: 10.1016/j.cell.2019.04.012. e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tam H.H., Melo M.B., Kang M., Pelet J.M., Ruda V.M., Foley M.H., Hu J.K., Kumari S., Crampton J., Baldeon A.D., Sanders R.W., Moore J.P., Crotty S., Langer R., Anderson D.G., Chakraborty A.K., Irvine D.J. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. U. S. A. 2016;113(43):E6639–E6648. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.de Veer M., Kemp J., Chatelier J., Elhay M.J., Meeusen E.N.T. The kinetics of soluble and particulate antigen trafficking in the afferent lymph, and its modulation by aluminum-based adjuvant. Vaccine. 2010;28:6597–6602. doi: 10.1016/j.vaccine.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 215.Liang F., Lindgren G., Sandgren K.J., Thompson E.A., Francica J.R., Seubert A., De Gregorio E., Barnett S., O'Hagan D.T., Sullivan N.J., Koup R.A., Seder R.A., Lore K. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal2094. [DOI] [PubMed] [Google Scholar]
- 216.Storni T., Ruedl C., Renner W.A., Bachmann M.F. Innate immunity together with duration of antigen persistence regulate effector T cell induction. J. Immunol. 2003;171:795–801. doi: 10.4049/jimmunol.171.2.795. [DOI] [PubMed] [Google Scholar]
- 217.He Y.K., Zhang J.Y., Mi Z.B., Robbins P., Falo L.D. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J. Immunol. 2005;174:3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 218.Obst R., van Santen H.M., Mathis D., Benoist I. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J. Exp. Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hailemichael Y., Dai Z., Jaffarzad N., Ye Y., Medina M.A., Huang X.-F., Dorta-Estremera S.M., Greeley N.R., Nitti G., Peng W. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pape K.A., Taylor J.J., Maul R.W., Gearhart P.J., Jenkins M.K. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Catron D.M., Pape K.A., Fife B.T., van Rooijen N., Jenkins M.K. A protease-dependent mechanism for initiating T-dependent B cell responses to large particulate antigens. J. Immunol. 2010;184:3609–3617. doi: 10.4049/jimmunol.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kuraoka M., Schmidt A.G., Nojima T., Feng F., Watanabe A., Kitamura D., Harrison S.C., Kepler T.B., Kelsoe G. Complex antigens drive permissive clonal selection in germinal centers. Immunity. 2016;44:542–552. doi: 10.1016/j.immuni.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Havenar-Daughton C., Lee J.H., Crotty S. Tfh cells and HIV bnAbs, an immunodominance model of the HIV neutralizing antibody generation problem. Immunol. Rev. 2017;275:49–61. doi: 10.1111/imr.12512. [DOI] [PubMed] [Google Scholar]
- 224.Cirelli K.M., Crotty S. Germinal center enhancement by extended antigen availability. Curr. Opin. Immunol. 2017;47:64–69. doi: 10.1016/j.coi.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tam H.H., Melo M.B., Kang M., Pelet J.M., Ruda V.M., Foley M.H., Hu J.K., Kumari S., Crampton J., Baldeon A.D., Sanders R.W., Moore J.P., Crotty S., Langer R., Anderson D.G., Chakraborty A.K., Irvine D.J. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E6639–E6648. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nguyen D.N., Redman R.L., Horiya S., Bailey J.K., Xu B., Stanfield R.L., Temme J.S., LaBranche C.C., Wang S., Rodal A.A., Montefiori D.C., Wilson I.A., Krauss I.J. The impact of sustained immunization regimens on the antibody response to oligomannose glycans. ACS Chem. Biol. 2020;15:789–798. doi: 10.1021/acschembio.0c00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hu J.K., Crampton J.C., Cupo A., Ketas T., van Gils M.J., Sliepen K., de Taeye S.W., Sok D., Ozorowski G., Deresa I., Stanfield R., Ward A.B., Burton D.R., Klasse P.J., Sanders R.W., Moore J.P., Crotty S. Murine antibody responses to cleaved soluble HIV-1 envelope trimers are highly restricted in specificity. J. Virol. 2015;89(20):10383–10398. doi: 10.1128/JVI.01653-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schipper P., van der Maaden K., Romeijn S., Oomens C., Kersten G., Jiskoot W., Bouwstra J. Repeated fractional intradermal dosing of an inactivated polio vaccine by a single hollow microneedle leads to superior immune responses. J. Control. Release. 2016;242:141–147. doi: 10.1016/j.jconrel.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 229.Glenny A.T., Buttle G.A.H., Stevens M.F. Rate of dissapearance of diptheria toxid injected into rabbits and guinea pigs: Toxoid precipitated with alum. J. Pathol. Bacteriol. 1931;34:267–275. [Google Scholar]
- 230.Harrison W.T. Some observations on the use of alum precipitated diphtheria toxoid. Am. J. Public Health Nations Health. 1935;25:298–300. doi: 10.2105/ajph.25.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gupta R.K., Chang A.C., Griffin P., Rivera R., Siber G.R. In vivo distribution of radioactivity in mice after injection of biodegradable polymer microspheres containing C-14-labeled tetanus toxoid (vol 14, pg 1412, 1996) Vaccine. 1996;14(15):1412–1416. doi: 10.1016/s0264-410x(96)00073-4. [DOI] [PubMed] [Google Scholar]
- 232.Noe S.M., Green M.A., HogenEsch H., Hem S.L. Mechanism of immunopotentiation by aluminum-containing adjuvants elucidated by the relationship between antigen retention at the inoculation site and the immune response. Vaccine. 2010;28:3588–3594. doi: 10.1016/j.vaccine.2010.02.085. [DOI] [PubMed] [Google Scholar]
- 233.Hutchison S., Benson R.A., Gibson V.B., Pollock A.H., Garside P., Brewer J.M. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012;26:1272–1279. doi: 10.1096/fj.11-184556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Romero Mendez I.Z., Shi Y., HogenEsch H., Hem S.L. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine. 2007;25:825–833. doi: 10.1016/j.vaccine.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 235.Kool M., Fierens K., Lambrecht B.N. Alum adjuvant: some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012;61:927–934. doi: 10.1099/jmm.0.038943-0. [DOI] [PubMed] [Google Scholar]
- 236.McKee A.S., Marrack P. Old and new adjuvants. Curr. Opin. Immunol. 2017;47:44–51. doi: 10.1016/j.coi.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.HogenEsch H., O’Hagan D.T., Fox C.B. 2018. Optimizing the Utilization of Aluminum Adjuvants in vaccines: You Might Just Get What you want. NPJ Vaccines, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kool M., Petrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I.M., Castillo R., Lambrecht B.N., Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 239.Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L., Fitzgerald K.A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Eisenbarth S.C., Colegio O.R., O’connor W., Sutterwala F.S., Flavell R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ramanathan V.D., Badenoch-Jones P., Turk J.L. Complement activation by aluminium and zirconium compounds. Immunology. 1979;37:881–888. [PMC free article] [PubMed] [Google Scholar]
- 242.Guven E., Duus K., Laursen I., Hojrup P., Houen G. Aluminum hydroxide adjuvant differentially activates the three complement pathways with major involvement of the alternative pathway. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Flach T.L., Ng G., Hari A., Desrosiers M.D., Zhang P., Ward S.M., Seamone M.E., Vilaysane A., Mucsi A.D., Fong Y., Prenner E., Ling C.C., Tschopp J., Muruve D.A., Amrein M.W., Shi Y. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 244.Ghimire T.R., Benson R.A., Garside P., Brewer J.M. Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunol. Lett. 2012;147:55–62. doi: 10.1016/j.imlet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Iyer S., HogenEsch H., Hem S.L. Relationship between the degree of antigen adsorption to aluminum hydroxide adjuvant in interstitial fluid and antibody production. Vaccine. 2003;21:1219–1223. doi: 10.1016/s0264-410x(02)00556-x. [DOI] [PubMed] [Google Scholar]
- 246.Morefield G.L., Jiang D., Romero-Mendez I.Z., Geahlen R.L., Hogenesch H., Hem S.L. Effect of phosphorylation of ovalbumin on adsorption by aluminum-containing adjuvants and elution upon exposure to interstitial fluid. Vaccine. 2005;23:1502–1506. doi: 10.1016/j.vaccine.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 247.Moyer T.J., Kato Y., Abraham W., Chang J.Y.H., Kulp D.W., Watson N., Turner H.L., Menis S., Abbott R.K., Bhiman J.N., Melo M.B., Simon H.A., Herrera-De la Mata S., Liang S., Seumois G., Agarwal Y., Li N., Burton D.R., Ward A.B., Schief W.R., Crotty S., Irvine D.J. Engineered immunogen binding to alum adjuvant enhances humoral immunity. Nat. Med. 2020;26:430–440. doi: 10.1038/s41591-020-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1 doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Higaki M., Azechi Y., Takase T., Igarashi R., Nagahara S., Sano A., Fujioka K., Nakagawa N., Aizawa C., Mizushima Y. Collagen minipellet as a controlled release delivery system for tetanus and diphtheria toxoid. Vaccine. 2001;19:3091–3096. doi: 10.1016/s0264-410x(01)00039-1. [DOI] [PubMed] [Google Scholar]
- 250.Wei J., Xue W., Yu X., Qiu X., Liu Z. pH Sensitive phosphorylated chitosan hydrogel as vaccine delivery system for intramuscular immunization. J. Biomater. Appl. 2017;31:1358–1369. doi: 10.1177/0885328217704139. [DOI] [PubMed] [Google Scholar]
- 251.Wu Y., Wei W., Zhou M., Wang Y., Wu J., Ma G., Su Z. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials. 2012;33:2351–2360. doi: 10.1016/j.biomaterials.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 252.Boopathy A.V., Mandal A., Kulp D.W., Menis S., Bennett N.R., Watkins H.C., Wang W., Martin J.T., Thai N.T., He Y.P., Schief W.R., Hammond P.T., Irvine D.J. Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proc. Natl. Acad. Sci. U. S. A. 2019;116:16473–16478. doi: 10.1073/pnas.1902179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yue H., Ma G. Polymeric micro/nanoparticles: particle design and potential vaccine delivery applications. Vaccine. 2015;33:5927–5936. doi: 10.1016/j.vaccine.2015.07.100. [DOI] [PubMed] [Google Scholar]
- 254.O’Hagan D., Malyala P. In: Immunopotentiators in Modern Vaccines. Schijns V.E.J.C., O'Hagan D., editors. Academic Press; 2017. Polymeric particles as vaccine delivery systems; pp. 231–248. [Google Scholar]
- 255.Gupta R.K., Singh M., O'Hagan D.T. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv. Drug Deliv. Rev. 1998;32:225–246. [PubMed] [Google Scholar]
- 256.Demento S.L., Cui W.G., Criscione J.M., Stern E., Tulipan J., Kaech S.M., Fahmy T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957–4964. doi: 10.1016/j.biomaterials.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cleland J.L. Protein delivery from biodegradable microspheres. Pharm. Biotechnol. 1997;10:1–43. doi: 10.1007/0-306-46803-4_1. [DOI] [PubMed] [Google Scholar]
- 258.Jiang W., Gupta R.K., Deshpande M.C., Schwendeman S.P. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Deliv. Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 259.Zhu G., Mallery S., Schwendeman S. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat. Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 260.Desai K.G., Schwendeman S.P. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J. Control. Release. 2013;165:62–74. doi: 10.1016/j.jconrel.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Reinhold S.E., Desai K.G., Zhang L., Olsen K.F., Schwendeman S.P. Self-healing microencapsulation of biomacromolecules without organic solvents. Angew. Chem. Int. Ed. Eng. 2012;51:10800–10803. doi: 10.1002/anie.201206387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Galloway A.L., Murphy A., DeSimone J.M., Di J., Herrmann J.P., Hunter M.E., Kindig J.P., Malinoski F.J., Rumley M.A., Stoltz D.M., Templeman T.S., Hubby B. Development of a nanoparticle-based influenza vaccine using the PRINT technology. Nanomedicine. 2013;9:523–531. doi: 10.1016/j.nano.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 263.Tamada J., Langer R. The development of polyanhydrides for drug delivery applications. J. Biomater. Sci. Polymer Edition. 1992;3:315–353. doi: 10.1163/156856292x00402. [DOI] [PubMed] [Google Scholar]
- 264.Boggiatto P.M., Schaut R.G., Kanipe C., Kelly S.M., Narasimhan B., Jones D.E., Olsen S.C. Sustained antigen release polyanhydride-based vaccine platform for immunization against bovine brucellosis. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kipper M.J., Shen E., Determan A., Narasimhan B. Design of an injectable system based on bioerodible polyanhydride microspheres for sustained drug delivery. Biomaterials. 2002;23:4405–4412. doi: 10.1016/s0142-9612(02)00181-3. [DOI] [PubMed] [Google Scholar]
- 266.Wagner D.A., Kelly S.M., Petersen A.C., Peroutka-Bigus N., Darling R.J., Bellaire B.H., Wannemuehler M.J., Narasimhan B. Single-dose combination nanovaccine induces both rapid and long-lived protection against pneumonic plague. Acta Biomater. 2019;100:326–337. doi: 10.1016/j.actbio.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ross K., Senapati S., Alley J., Darling R., Goodman J., Jefferson M., Uz M., Guo B., Yoon K.J., Verhoeven D., Kohut M., Mallapragada S., Wannemuehler M., Narasimhan B. Single dose combination nanovaccine provides protection against influenza A virus in young and aged mice. Biomater. Sci. 2019;7:809–821. doi: 10.1039/c8bm01443d. [DOI] [PubMed] [Google Scholar]
- 268.Ulery B.D., Kumar D., Ramer-Tait A.E., Metzger D.W., Wannemuehler M.J., Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Khong H., Volmari A., Sharma M., Dai Z.M., Imo C.S., Hailemichael Y., Singh M., Moore D.T., Xiao Z.L., Huang X.F., Horvath T.D., Hawke D.H., Overwijk W.W. Peptide vaccine formulation controls the duration of antigen presentation and magnitude of tumor-specific CD8(+) T cell response. J. Immunol. 2018;200:3464–3474. doi: 10.4049/jimmunol.1700467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sharp F.A., Ruane D., Claass B., Creagh E., Harris J., Malyala P., Singh M., O'Hagan D.T., Petrilli V., Tschopp J., O'Neill L.A., Lavelle E.C. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chesson C.B., Huante M., Nusbaum R.J., Walker A.G., Clover T.M., Chinnaswamy J., Endsley J.J., Rudra J.S. Nanoscale peptide self-assemblies boost bcg-primed cellular immunity against mycobacterium tuberculosis. Sci. Rep. 2018;8:12519. doi: 10.1038/s41598-018-31089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kim J., Li W.A., Choi Y., Lewin S.A., Verbeke C.S., Dranoff G., Mooney D.J. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 2015;33:64–U241. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wick D.A., Martin S.D., Nelson B.H., Webb J.R. Profound CD8+ T cell immunity elicited by sequential daily immunization with exogenous antigen plus the TLR3 agonist poly(I:C) Vaccine. 2011;29:984–993. doi: 10.1016/j.vaccine.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 274.Jewell C.M., Lopez S.C., Irvine D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Geall A.J., Verma A., Otten G.R., Shaw C.A., Hekele A., Banerjee K., Cu Y., Beard C.W., Brito L.A., Krucker T. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. 2012;109:14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Liu M.A. Immunologic basis of vaccine vectors. Immunity. 2010;33:504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 277.Kalams S.A., Parker S., Jin X., Elizaga M., Metch B., Wang M., Hural J., Lubeck M., Eldridge J., Cardinali M., Blattner W.A., Sobieszczyk M., Suriyanon V., Kalichman A., Weiner D.B., Baden L.R., Network N.H.V.T. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sardesai N.Y., Weiner D.B. Electroporation delivery of DNA vaccines: prospects for success. Curr. Opin. Immunol. 2011;23:421–429. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Trimble C.L., Morrow M.P., Kraynyak K.A., Shen X., Dallas M., Yan J., Edwards L., Parker R.L., Denny L., Giffear M. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Li L., Saade F., Petrovsky N. The future of human DNA vaccines. J. Biotechnol. 2012;162:171–182. doi: 10.1016/j.jbiotec.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Geiben-Lynn R., Frimpong-Boateng K., Letvin N.L. Modulation of plasmid DNA vaccine antigen clearance by caspase 12 RNA interference potentiates vaccination. Clin. Vaccine Immunol. 2011;18:533–538. doi: 10.1128/CVI.00390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kim T.W., Lee J.-H., He L., Boyd D.A., Hardwick J.M., Hung C.-F., Wu T.C. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–316. [PubMed] [Google Scholar]
- 283.Huang B., Mao C.-P., Peng S., Hung C.-F., Wu T.-C. RNA interference-mediated in vivo silencing of fas ligand as a strategy for the enhancement of DNA vaccine potency. Hum. Gene Ther. 2008;19:763–773. doi: 10.1089/hum.2007.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Barouch D.H., Craiu A., Kuroda M.J., Schmitz J.E., Zheng X.X., Santra S., Frost J.D., Krivulka G.R., Lifton M.A., Crabbs C.L., Heidecker G., Perry H.C., Davies M.-E., Xie H., Nickerson C.E., Steenbeke T.D., Lord C.I., Montefiori D.C., Strom T.B., Shiver J.W., Lewis M.G., Letvin N.L. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. 2000;97:4192–4197. doi: 10.1073/pnas.050417697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Barouch D.H., Truitt D.M., Letvin N.L. Expression kinetics of the interleukin-2/immunoglobulin (IL-2/Ig) plasmid cytokine adjuvant. Vaccine. 2004;22:3092–3097. doi: 10.1016/j.vaccine.2004.01.065. [DOI] [PubMed] [Google Scholar]
- 286.Kusakabe K.-i., Xin K.-Q., Katoh H., Sumino K., Hagiwara E., Kawamoto S., Okuda K., Miyagi Y., Aoki I., Nishioka K., Klinman D., Okuda K. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. J. Immunol. 2000;164:3102–3111. doi: 10.4049/jimmunol.164.6.3102. [DOI] [PubMed] [Google Scholar]
- 287.Besch R., Poeck H., Hohenauer T., Senft D., Häcker G., Berking C., Hornung V., Endres S., Ruzicka T., Rothenfusser S. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon–independent apoptosis in human melanoma cells. J. Clin. Invest. 2009;119:2399–2411. doi: 10.1172/JCI37155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Anderson B.R., Muramatsu H., Jha B.K., Silverman R.H., Weissman D., Karikó K. Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011;39:9329–9338. doi: 10.1093/nar/gkr586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Andries O., Mc Cafferty S., De Smedt S.C., Weiss R., Sanders N.N., Kitada T. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release. 2015;217:337–344. doi: 10.1016/j.jconrel.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 291.Kuhn A., Diken M., Kreiter S., Selmi A., Kowalska J., Jemielity J., Darzynkiewicz E., Huber C., Türeci Ö., Sahin U. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 2010;17:961–971. doi: 10.1038/gt.2010.52. [DOI] [PubMed] [Google Scholar]
- 292.Weissman D., Pardi N., Muramatsu H., Karikó K. Springer; 2013. HPLC purification of in vitro transcribed long RNA, Synthetic Messenger RNA and Cell Metabolism Modulation; pp. 43–54. [DOI] [PubMed] [Google Scholar]
- 293.Kariko K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Feldman R.A., Fuhr R., Smolenov I., Ribeiro A.M., Panther L., Watson M., Senn J.J., Smith M., Almarsson Ӧ., Pujar H.S. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 295.Schott J.W., Morgan M., Galla M., Schambach A. Viral and synthetic RNA vector technologies and applications. Mol. Ther. 2016;24:1513–1527. doi: 10.1038/mt.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lundstrom K. Alphaviruses in gene therapy. Viruses. 2015;7:2321–2333. doi: 10.3390/v7052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Melo M., Porter E., Zhang Y., Silva M., Li N., Dobosh B., Liguori A., Skog P., Landais E., Menis S., Sok D., Nemazee D., Schief W.R., Weiss R., Irvine D.J. Immunogenicity of RNA replicons encoding HIV Env immunogens designed for self-assembly into nanoparticles. Mol. Ther. 2019;27:2080–2090. doi: 10.1016/j.ymthe.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Finney J., Yeh C.H., Kelsoe G., Kuraoka M. Germinal center responses to complex antigens. Immunol. Rev. 2018;284:42–50. doi: 10.1111/imr.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Vogel A.B., Lambert L., Kinnear E., Busse D., Erbar S., Reuter K.C., Wicke L., Perkovic M., Beissert T., Haas H. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 2018;26:446–455. doi: 10.1016/j.ymthe.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McKay P.F., Hu K., Blakney A.K., Samnuan K., Bouton C.R., Rogers P., Polra K., Lin P.J., Barbosa C., Tam Y. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine induces equivalent preclinical antibody titers and viral neutralization to recovered COVID-19 patients. bioRxiv. 2020 doi: 10.1101/2020.04.22.055608. https://www.biorxiv.org/about/FAQ [DOI] [Google Scholar]
- 301.Quinn K.M., Zak D.E., Costa A., Yamamoto A., Kastenmuller K., Hill B.J., Lynn G.M., Darrah P.A., Lindsay R.W., Wang L. Antigen expression determines adenoviral vaccine potency independent of IFN and STING signaling. J. Clin. Invest. 2015;125:1129–1146. doi: 10.1172/JCI78280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Geiben-Lynn R., Greenland J.R., Frimpong-Boateng K., Letvin N.L. Kinetics of recombinant adenovirus type 5, vaccinia virus, modified vaccinia ankara virus, and DNA antigen expression in vivo and the induction of memory T-lymphocyte responses. Clin. Vaccine Immunol. 2008;15:691–696. doi: 10.1128/CVI.00418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Finn J.D., Bassett J., Millar J.B., Grinshtein N., Yang T.C., Parsons R., Evelegh C., Wan Y., Parks R.J., Bramson J.L. Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J. Virol. 2009;83:12027–12036. doi: 10.1128/JVI.00593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Chen P., Tian J., Kovesdi I., Bruder J.T. Promoters influence the kinetics of transgene expression following adenovector gene delivery. J. Gene Med. 2008;10:123–131. doi: 10.1002/jgm.1127. [DOI] [PubMed] [Google Scholar]
- 305.Sharpe S., Polyanskaya N., Dennis M., Sutter G., Hanke T., Erfle V., Hirsch V., Cranage M. Induction of simian immunodeficiency virus (SIV)-specific CTL in rhesus macaques by vaccination with modified vaccinia virus Ankara expressing SIV transgenes: influence of pre-existing anti-vector immunity. J. Gen. Virol. 2001;82:2215–2223. doi: 10.1099/0022-1317-82-9-2215. [DOI] [PubMed] [Google Scholar]
- 306.Brockstedt D.G., Giedlin M.A., Leong M.L., Bahjat K.S., Gao Y., Luckett W., Liu W., Cook D.N., Portnoy D.A., Dubensky T.W. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl. Acad. Sci. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Starks H., Bruhn K.W., Shen H., Barry R.A., Dubensky T.W., Brockstedt D., Hinrichs D.J., Higgins D.E., Miller J.F., Giedlin M. Listeria monocytogenes as a vaccine vector: virulence attenuation or existing antivector immunity does not diminish therapeutic efficacy. J. Immunol. 2004;173:420–427. doi: 10.4049/jimmunol.173.1.420. [DOI] [PubMed] [Google Scholar]
- 308.Mercado R., Vijh S., Allen S.E., Kerksiek K., Pilip I.M., Pamer E.G. Early programming of T cell populations responding to bacterial infection. J. Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 309.Badovinac V.P., Porter B.B., Harty J.T. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 310.Maciag P.C., Radulovic S., Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 311.Tullius M.V., Harth G., Masleša-Galić S., Dillon B.J., Horwitz M.A. A replication-limited recombinant Mycobacterium bovis BCG vaccine against tuberculosis designed for human immunodeficiency virus-positive persons is safer and more efficacious than BCG. Infect. Immun. 2008;76:5200–5214. doi: 10.1128/IAI.00434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Bergmann-Leitner E.S., Leitner W.W. Adjuvants in the driver’s seat: how magnitude, type, fine specificity and longevity of immune responses are driven by distinct classes of immune potentiators. Vaccines. 2014;2:252–296. doi: 10.3390/vaccines2020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bloom B.R. 1990. Vaccines for the Third World. [Google Scholar]
- 314.Kardani K., Bolhassani A., Shahbazi S. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 2016;34:413–423. doi: 10.1016/j.vaccine.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 315.Masopust D., Ha S.-J., Vezys V., Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 316.Thompson E.A., Beura L.K., Nelson C.E., Anderson K.G., Vezys V. Shortened intervals during heterologous boosting preserve memory CD8 T cell function but compromise longevity. J. Immunol. 2016;196:3054–3063. doi: 10.4049/jimmunol.1501797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Griffin J., Mackintosh C., Rodgers C. Factors influencing the protective efficacy of a BCG homologous prime-boost vaccination regime against tuberculosis. Vaccine. 2006;24:835–845. doi: 10.1016/j.vaccine.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 318.Preis I., Langer R.S. A single-step immunization by sustained antigen release. J. Immunol. Methods. 1979;28:193–197. doi: 10.1016/0022-1759(79)90341-7. [DOI] [PubMed] [Google Scholar]
- 319.Chua B.Y., Sekiya T., Al Kobaisi M., Short K.R., Mainwaring D.E., Jackson D.C. A single dose biodegradable vaccine depot that induces persistently high levels of antibody over a year. Biomaterials. 2015;53:50–57. doi: 10.1016/j.biomaterials.2015.02.066. [DOI] [PubMed] [Google Scholar]
- 320.Tzeng S.Y., McHugh K.J., Behrens A.M., Rose S., Sugarman J.L., Ferber S., Langer R., Jaklenec A. Stabilized single-injection inactivated polio vaccine elicits a strong neutralizing immune response. Proc. Natl. Acad. Sci. 2018;115:E5269–E5278. doi: 10.1073/pnas.1720970115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Guarecuco R., Lu J., McHugh K.J., Norman J.J., Thapa L.S., Lydon E., Langer R., Jaklenec A. Immunogenicity of pulsatile-release PLGA microspheres for single-injection vaccination. Vaccine. 2018;36:3161–3168. doi: 10.1016/j.vaccine.2017.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.McHugh K.J., Nguyen T.D., Linehan A.R., Yang D., Behrens A.M., Rose S., Tochka Z.L., Tzeng S.Y., Norman J.J., Anselmo A.C. Fabrication of fillable microparticles and other complex 3D microstructures. Science. 2017;357:1138–1142. doi: 10.1126/science.aaf7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Garcea R.L., Meinerz N.M., Dong M., Funke H., Ghazvini S., Randolph T.W. Single-administration, thermostable human papillomavirus vaccines prepared with atomic layer deposition technology. npj Vaccines. 2020;5:1–8. doi: 10.1038/s41541-020-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Erasmus J.H., Khandhar A.P., Guderian J., Granger B., Archer J., Archer M., Gage E., Fuerte-Stone J., Larson E., Lin S. A nanostructured lipid carrier for delivery of a replicating viral RNA provides single, low-dose protection against Zika. Mol. Ther. 2018;26:2507–2522. doi: 10.1016/j.ymthe.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chahal J.S., Khan O.F., Cooper C.L., McPartlan J.S., Tsosie J.K., Tilley L.D., Sidik S.M., Lourido S., Langer R., Bavari S. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. 2016;113:E4133–E4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Jalah R., Kulkarni V., Patel V., Rosati M., Alicea C., Bear J., Yu L., Guan Y., Shen X., Tomaras G.D. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in macaques. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Li J., Valentin A., Kulkarni V., Rosati M., Beach R.K., Alicea C., Hannaman D., Reed S.G., Felber B.K., Pavlakis G.N. HIV/SIV DNA vaccine combined with protein in a co-immunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine. 2013;31:3747–3755. doi: 10.1016/j.vaccine.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Patel V., Jalah R., Kulkarni V., Valentin A., Rosati M., Alicea C., von Gegerfelt A., Huang W., Guan Y., Keele B.F. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc. Natl. Acad. Sci. 2013;110:2975–2980. doi: 10.1073/pnas.1215393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Andries O., Kitada T., Bodner K., Sanders N.N., Weiss R. Synthetic biology devices and circuits for RNA-based ‘smart vaccines’: a propositional review. Expert Rev. Vaccines. 2015;14:313–331. doi: 10.1586/14760584.2015.997714. [DOI] [PubMed] [Google Scholar]
- 330.Haynes B.F., Kelsoe G., Harrison S.C., Kepler T.B. B-cell–lineage immunogen design in vaccine development with HIV-1 as a case study. Nat. Biotechnol. 2012;30:423. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Shae D., Becker K.W., Christov P., Yun D.S., Lytton-Jean A.K., Sevimli S., Ascano M., Kelley M., Johnson D.B., Balko J.M. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019;14:269–278. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Demana P.H., Davies N.M., Vosgerau U., Rades T. Pseudo-ternary phase diagrams of aqueous mixtures of Quil A, cholesterol and phospholipid prepared by the lipid-film hydration method. Int. J. Pharm. 2004;270:229–239. doi: 10.1016/j.ijpharm.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 333.Mueller S.N., Tian S.M., DeSimone J.M. Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Mol. Pharm. 2015;12:1356–1365. doi: 10.1021/mp500589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kim S.Y., Noh Y.W., Kang T.H., Kim J.E., Kim S., Um S.H., Oh D.B., Park Y.M., Lim Y.T. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials. 2017;130:56–66. doi: 10.1016/j.biomaterials.2017.03.034. [DOI] [PubMed] [Google Scholar]