Abstract
Aims
Drug‐induced liver injury (DILI) is a heterogenous entity leading to liver damage. We have analysed the frequency, biochemical and histological patterns and clinical courses of DILI cases due to metamizole at our tertiary care centre in Hamburg, Germany.
Methods
Consecutive patients with DILI who presented to our clinic were analysed retrospectively. Causes of acute hepatitis other than DILI were excluded.
Results
In total, 154 DILI cases were admitted to our centre from 2008 to 2017. After phenprocoumon, metamizole was the second most frequent putative agent causing DILI (23 of all 154 DILI cases, 14,9%). The biochemical pattern on admission of metamizole‐induced DILI cases was hepatocellular with median levels of alanine transaminase (779 U/L, 64–3532 U/L) by far exceeding median alkaline phosphatase levels (131 U/L, 42–578 U/L). In 17 of the 23 cases (74%) liver biopsy was performed. Moderate to severe inflammatory histological activity and severe centrilobular necrosis (>30%) was present in 76.5 and 35.3%, respectively. Metamizole was involved in 2 DILI cases progressing to acute liver failure, then receiving liver transplantation and still alive at time of assessment. Our data were supported by re‐exposure in 4 patients. Furthermore, a database search for metamizole‐induced liver injury in the European Medicines Agency's database identified about 300 reports on suspected metamizole‐induced DILI in Europe.
Conclusion
Elevation of liver enzymes or acute liver failure are not mentioned in the German drug label of metamizole as potential side effects. Our study reveals that in Germany and Europe, metamizole is a frequent and underrated agent causing DILI.
Keywords: acute liver failure, drug‐induced liver injury, elevated liver enzymes, metamizole, side effects
What is already known about this subject
Metamizole is a popular analgesic frequently prescribed in countries such as Germany, Russia and South America.
In other countries such as the UK, the USA and Scandinavia, metamizole is off the market due to side effects, including agranulocytosis.
Liver‐related side effects are not in the German drug label of metamizole and have been published in very few case reports.
What this study adds
Metamizole frequently leads to severe acute liver injury at our tertiary care centre in Germany, which is supported by several reports of liver‐related side effects throughout Europe.
Liver injury due to metamizole is characterized by a hepatocellular biochemical pattern and infiltration of immune cells and necrosis on liver histology.
Awareness for liver injury due to metamizole must be raised worldwide.
1. INTRODUCTION
Drug‐induced liver injury (DILI) is a heterogenous entity with a broad spectrum of pathogenetic mechanisms and clinical appearances.1 A huge variety of drugs and herbal and dietary supplements can lead to liver injury.2 With regard to drug groups and single agents, antimicrobials including amoxicillin–clavulanate are the most frequent agents causing DILI due to large registry or prospective population‐based studies.3, 4, 5 Since no single test system reliably confirms that a certain drug or its metabolites were causing liver injury, diagnosis of DILI relies on causality assessment scores, such as the RUCAM score (Roussel Uclaf Causality Assessment Method).6, 7 Diagnostic scores for DILI lack sensitivity since drugs that the treating physician does not associate with DILI will not be considered and tested with causality assessment score. Therefore, establishing the diagnosis of DILI is still based on experience and expert opinion. As a consequence, misbeliefs on DILI prevail in the broad medical community, such as the overestimation of the hepatotoxicity of statins or the underrated toxicity of herbal and dietary supplements, which have been recently recognized more and more in western countries as a major group of agents causing DILI.8, 9, 10, 11, 12
Metamizole is synonymous with dipyrone.13 It is mainly prescribed for its analgesic effects. Due to side effects such as agranulocytosis, metamizole has been banished from the market in several countries, such as the UK, Scandinavia, the USA or Canada.13 A prospective German case–control study reported an incidence of metamizole‐induced agranulocytosis of 0.96 (95% confidence interval 0.95–0.97) cases per million per year.14 In other regions and countries, such as Spain, Poland, Russia or Latin America, metamizole still is popular on the market. In some countries such as Poland and Russia, metamizole is even distributed as an over‐the‐counter agent. In Germany, metamizole is a popular analgesic with large defined daily doses (DDD), only available on prescription.15 The DDD of metamizole increased in Germany from 32 million DDD in 2000 to 225 million DDD in 2018.14, 16 Due to the German drug label, metamizole is indicated for severe pain after trauma or surgery and for pain due to cancer, but only if pain does not respond to other analgesics. Elevation of liver enzymes, liver injury and acute liver failure are not mentioned in the German drug label of metamizole as side effects. Here we report on metamizole as a frequent drug causing severe DILI at our tertiary care centre in Germany.
2. METHODS
Consecutive idiosyncratic, non‐paracetamol DILI cases presenting to the I. Department of Medicine at the University Medical Centre Hamburg‐Eppendorf, Germany, were analysed retrospectively. Patients were referred to our centre from general practitioners or primary, secondary or tertiary care centres or presented themselves initially at our emergency department because of jaundice. DILI was defined as the following: peak alanine transaminase (ALT) levels >5 times upper limit of the normal (5×ULN) or peak ALT levels >3×ULN and total bilirubin levels >2×ULN or alkaline phosphatase (ALP) levels >2×ULN.17 The calculation of the biochemical pattern was based on the ratio (R) between ALT and ALP, each in relation to their respective ULN. The biochemical pattern was defined as hepatocellular when R > 5, as mixed when R was 2–5 and as cholestatic when R < 2. For the calculation of R, the first available blood tests at the time of initial presentation were used.
Causality for DILI was assessed according to the RUCAM score.6, 7 Alternative causes of acute liver injury were excluded by the following18: ultrasound examination revealed no signs of biliary obstruction or vascular liver disease in all cases; acute viral hepatitis was excluded by serology; viral hepatitis E infection was excluded in all cases by polymerase chain reaction. The majority of DILI cases at our centre were treated with steroids. Autoimmune hepatitis (AIH) was widely excluded by clinical follow‐up assuring that liver enzymes did not rise again after complete weaning of steroids. Acute Wilson's disease was excluded by serum ceruloplasmin levels >20 mg/dL and a ratio of ALP to total bilirubin >4 and a ratio of aspartate transaminase (AST) to ALT <2.19 Metamizole was prescribed for its analgesic effects in all cases. Four of the metamizole‐induced DILI cases were accidently re‐exposed to metamizole causing several episodes of liver injury. In these patients, the latest episode of acute hepatitis was included in the analysis. Data are summarized as counts and percentages or median and range, respectively.
Our analysis was supported by a database search for metamizole‐induced liver injury in the European Medicines Agency's (EMA) database (EudraVigilance data analysis system, EVDAS). The database search was performed by the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM). This database search was performed for Germany and for the European Economic Area (EEA) on 11 September 2018. The search criterion was ‘hepatobiliary disorders’. Results of the database search included spontaneous reports and reports from clinical studies since the year 1995 when the database was founded. The results for the following terms of potential side effects of metamizole are not shown in the results because they were considered to be unlikely a consequence of metamizole intake and/or did not represent a proper metamizole‐induced DILI case: autoimmune hepatitis (n = 1), bile duct stone (n = 1), biliary colic (n = 1), cholangitis (n = 4), cholecystitis (n = 7), cholelithiasis (n = 1), chronic hepatitis B (n = 1), granulomatous liver disease (n = 1), haemochromatosis (n = 2), hepatic cyst (n = 1), hepatic haematoma (n = 1), hepatitis viral (n = 1), metastases to the liver (n = 1), oesophageal varices haemorrhage (n = 1), porphyria acute (n = 10), primary biliary cholangitis (n = 1) and Reyes syndrome (n = 1). All cases of metamizole‐induced liver injury identified at our centre were reported to the BfArM, but after the EVDAS database search was performed. This study was approved by the local ethics committee (WF‐064/12).
3. RESULTS
3.1. Clinical characteristics and outcome of patients with metamizole‐induced liver injury
In total, 154 idiosyncratic, non‐paracetamol DILI cases were identified presenting at our centre in the time from 2008 to 2017. After phenprocoumon, metamizole was the second most frequent putative agent causing DILI (23 of all 154 DILI cases, 14.9%). The 10 most frequent causative agents for DILI at our centre are presented in Supplementary Table S1. Clinical characteristics of patients with metamizole‐induced DILI are displayed in Table 1. Median patient age at time of diagnosis was 40 years (26–79 years) and about 65% of patients were female. Median body mass index was 27 kg/m2 (20.8–33.2 kg/m2) and median RUCAM score was 7 (probable) including 4 cases that were caused by metamizole with high probability. Six of 23 of patients (about 26%) received concurrent causative medication (ibuprofen and amoxicillin–clavulanate were coadministered with metamizole in 1 case, the other 5 cases received either duloxetine, levetiracetam, atorvastatin, diclofenac or ibuprofen in combination with metamizole). The prevailing biochemical pattern of metamizole‐induced liver injury was hepatocellular (14 of 23 cases, 61%). Mixed and cholestatic patterns were less frequent (22% and 17%, respectively; Table 1). A representative time course of liver enzymes of 1 patient is shown in Supplementary Figure S1. At first presentation, 9 patients (39%) were jaundiced with initial bilirubin levels >3 mg/dL and 11 patients (48%) fulfilled Hy's law (ALT >3×ULN and total bilirubin >2×ULN). Antinuclear antibodies (ANA) were present in about 50% of metamizole‐DILI cases with median titres of 1:160 and with peak titres of 1:5120 (Table 1). Two patients were positive for anti‐smooth muscle antibodies (both with titres of 1:160) and 3 were positive for anti‐mitochondrial antibodies (AMA; 2 with titres of 1:160 and 1 with a titre of 1:1280). In those patients who tested positive for AMA, neither pre‐existing elevation of cholestatic liver enzymes nor persistent elevated cholestatic liver enzymes were known at last follow‐up. Therefore, the presence of AMA was considered as being unspecific due to severe acute hepatitis and not as diagnostic for primary biliary cholangitis.20 All patients were negative for anti‐liver kidney microsomal antibodies and anti‐soluble liver antigen/liver‐pancreas antigen antibodies. Median IgG level on admission was 12.2 g/L (5.6–16.8 g/L; normal range 7–16 g/L). Median follow‐up of patients was about 5 months, ensuring that liver enzymes did not rise again after resolution of acute liver injury and after total weaning of steroids. Steroids were discontinued in all patients. Median latency from first administration of metamizole until detection of elevated liver enzymes or onset of jaundice was about 4 weeks. In 4 of the 23 metamizole‐induced DILI cases (17.4%), patients were accidently re‐exposed to metamizole, resulting in another episode of acute icteric hepatitis. In 1 case, inadvertent administration of metamizole led to 3 episodes of acute icteric hepatitis until metamizole was identified as the cause of liver damage. Initial and peak liver enzymes did not differ significantly between first and second episodes of liver injury due to metamizole (Supplementary Table S2).
Table 1.
Clinical characteristics of patients with metamizole‐induced liver injury
| Group of metamizole‐induced DILI cases | n | Age | Sex | Time of follow‐up | RUCAM score | Re‐challenge | Concurrent causative medication | Presence of ANA | ANA titre | Biochemical pattern (R) | Latency | HE | Steroid treatment | Infectious complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| y | Female/male | mo | Hepatocellular/mixed/cholestatic | Weeks | ||||||||||
| All | 23 | 40 (26–79) | 15/23 (65.2%) | 4.5 | 7 (4–11) | 4/23 (17.4%) | 6/23 (26.1%) | 12/23 (52.2%) | 1:160 (1:80–1:5120) | 14/4/5 | 4 (1–135) | 2/23 (8.7%) | 16/23 (69.6%) | 0/23 (0%) |
| Recovery | 21 | 40 (26–79) | 14/2 (54.5) | 4.5 | 7 (4–11) | 4/21 (19.0%) | 4/21 (19%) | 11/21 (52.4%) | 1:160 (1:160–1:5120) | 13/4/4 | 4.5 (1–135) | 0/21 (0%) | 14/21 (66.7%) | 0/21 (0%) |
| ALF and LTX | 2 | 50 (33–67) | 1/2 (50%) | 1 (until LTX‡) | 8 (7–8) | 0/2 (0%) | 2/2 (100%) | 1/2 (50%) | 1:80 | 1/0/1 | 6 (4–8) | 2/2 (100%) | 2/2 (100%) | 0/2 (0%) |
ALF, acute liver failure; ANA, anti‐nuclear antibodies; HE, hepatic encephalopathy; DILI, drug‐induced liver injury; LTX, liver transplantation; RUCAM, Roussel UCLAF Causality Assessment Method (possible 4–5; probable 6–8; highly probable >8).
In 70% of patients, prednisolone (median dosage 1 mg/kg bodyweight) was administered for resolution of acute hepatitis. No infectious complications occurred as a consequence of steroid treatment.
In 2 of the 23 metamizole‐DILI cases (8.7%), acute liver failure (ALF) developed with hepatic encephalopathy and an international normalized ratio > 1.5 being present. Both patients progressing to ALF received other potentially hepatotoxic drugs apart from metamizole: In 1 patient with ALF, amoxicillin–clavulanate was coadministered and in the other, ibuprofen and duloxetine were applied in addition to metamizole. Both patients with ALF received steroids, underwent liver transplantation and were still alive at the time of analysis. No mortality occurred in the follow‐up of all metamizole‐DILI cases. The 2 patients progressing to ALF had similar levels of total bilirubin (0.3 and 8.4 mg/dL) and similar levels of model of end stage liver disease (MELD) scores (6 and 23) at initial presentation compared to the other metamizole‐DILI cases not developing ALF (median total bilirubin level 1.9 mg/dL, 0.3–21.1 mg/dL; median MELD score 12, 6–25; Table 2). The 2 patients developing ALF had higher peak MELD scores (23 and 33), peak total bilirubin levels (24.5 and 30.5 mg/dL) and peak ALT (3502 and 3574 U/L) and AST levels (1998 and 4172 U/L) than those patients who did not develop ALF (median peak MELD score 13, 6–27; median peak total bilirubin 3.7 mg/dL, 0.7–40.7 mg/dL; median peak ALT level 1374 U/L, 683–3636 U/L; median peak AST level 864 U/L, 335‐2774 U/L).
Table 2.
Biochemical patterns of patients with metamizole‐induced liver injury: at initial presentation and peak values
| Group of metamizole‐induced DILI cases | n | AST (initial) | ALT (initial) | ALP (initial) | GGT (initial) | Total bilirubin (initial) | AST (peak) | ALT (peak) | ALP (peak) | GGT (peak) | Total bilirubin (peak) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 50 U/L (male) <35 U/L (female) | < 50 U/L (male) <35 U/L (female) | < 129 U/L (male) < 104 U/L (female) | < 65 U/L (male) < 38 U/L (female) | <1.2 mg/dL | < 50 U/L (male) <35 U/L (female | < 50 U/L (male) <35 U/L (female) | < 129 U/L (male) <104 U/L (female) | < 65 U/L (male) <38 U/L (female) | <1,2 mg/dL | ||
| All | 23 | 574 (39–4172) | 779 (64–3532) | 131 (42–578) | 196 (10–1345) | 1.9 (0.3–21.1) | 1033 (514–4172) | 1391 (683–3636) | 176 (85–854) | 300 (61–2165) | 3,8 (0.7–40.7) |
| Recovery | 21 | 574 (39–2774) | 784 (64–3532) | 131 (66–578) | 205 (38–1345) | 1.9 (0.3–21.1) | 864 (335–2774) | 1374 (683–3636) | 153 (85–854) | 306 (61–2165) | 3.7 (0.7–40.7) |
| ALF and LTX | 2 | 2115 (57–4172) | 1822 (69–3574) | 104 (42–166) | 69 (10–128) | 4.3 (0.3–8.4) | 3085 (1998–4172) | 3538 (3502–3574) | 235 (227–243) | 270 (239–300) | 27.5 (24.5–30.5) |
ALF, acute liver failure; ALT, alanine transaminase; ALP, alkaline phosphatase; AST; aspartate transaminase; DILI, drug‐induced liver injury; GGT, γ‐glutamyltransferase; LTX, liver transplantation.
3.2. Histopathological patters of metamizole‐induced liver injury
In 17 of the 23 cases of metamizole‐induced liver injury (74%) mini‐laparoscopically‐guided liver biopsy was performed. Histological characteristics of these patients are shown in Table 3. Seventy‐six percent of patients who underwent liver biopsy showed moderate to severe liver inflammation with lymphocytic infiltrations on histology (Figure 1A‐C). Infiltration of eosinophils was rarely detected on liver histology (in 1 of 17 cases). Histological features associated with AIH such as emperipolesis or plasma cells were not present. Steatosis of more than 10% of hepatocytes and cholestatic changes were rare (both in 12%). Low‐grade fibrosis (stage 1 and 2) was detected in 29% of cases. High‐grade fibrosis and liver cirrhosis (stage 3 and 4) were absent. Extensive centrilobular necrosis (>30%) was prevalent in 35% of cases.
Table 3.
Histological patterns of patients with metamizole‐induced liver injury
| Group of metamizole‐induced DILI cases | n | Liver biopsy performed | Mild inflammatory activity | Moderate to severe inflammatory activity | Cholestasis | Steatosis | Low‐grade fibrosis | High‐grade fibrosis/cirrhosis | Eosino‐phil granulo‐cytes | Centri‐lobular damage | Necrosis | Necrosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >10% | Stage 1–2 | Stage 3–4 | <30% | >30% | ||||||||
| All | 23 | 17/23 (73.9%) | 3/17 (17.6%) | 13/17 (76.5%) | 2/17 (11.8%) | 2/17 (11.7%) | 5/17 (29.4%) | 0/17 (0%) | 1/17 (5.9%) | 7/17 (41.2%) | 3/17 (17.6%) | 6/17 (35.3%) |
| Recovery | 21 | 15/21 (71.4%) | 3/15 (20%) | 12/15 (80%) | 2/15 (13.3%) | 2/15 (13.3%) | 5/15 (33.3%) | 0/15 (0%) | 1/15 (6.7%) | 7/15 (46.7%) | 3/15 (20%) | 5/15 (33.3%) |
| ALF and LTX | 2 | 2/2 (100%) | 0/2 (0%) | 1/2 (50%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) | 1/2 (50%) |
ALF, acute liver failure; DILI, drug‐induced liver injury; LTX, liver transplantation.
Figure 1.
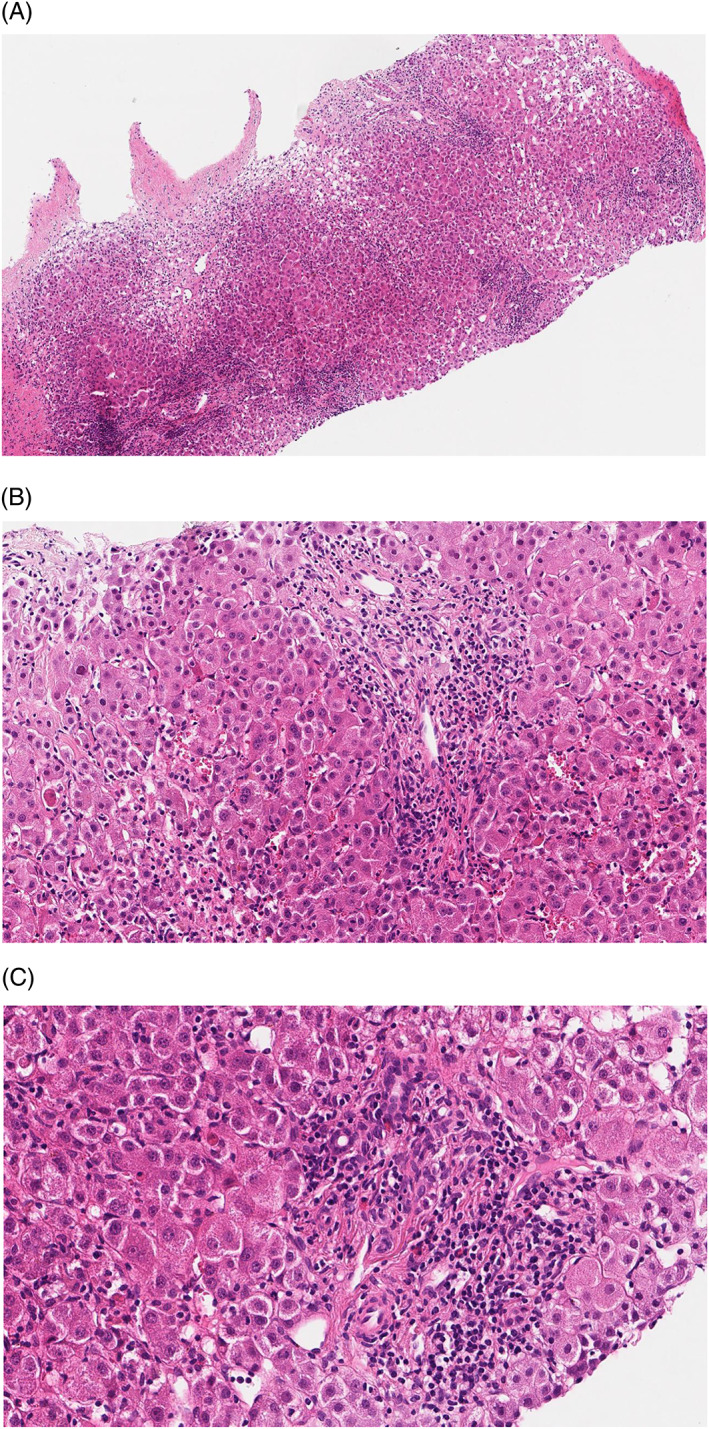
Liver histology of a representative patients with metamizole‐induced liver injury. Liver histology of a patient with metamizole‐induced liver injury shows infiltration of immune cells: 40× optic magnification and 4.5× digital zoom (A); 40× optic magnification and 14× digital zoom (B); and 40× optic magnification and 20× digital zoom (C)
3.3. German and European database search for metamizole‐induced liver injury
The EVDAS database search performed by the BfArM identified 143 reports on liver‐related side effects being associated with metamizole in Germany (Figure 2A) and 296 reports in the EEA (Figure 2B). In Germany, the 3 most frequent categories of liver‐related side effects being associated with metamizole were hepatic failure, drug‐induced liver injury and jaundice, together representing 69 of the 143 reports (48%). Eighty‐five reports (59%) were entitled with categories of liver‐related side effects that hint at hepatic malfunction and/or impaired synthesis (such as acute hepatic failure or hypoalbuminaemia). In the EEA, the 3 most frequent categories were hepatitis, hepatic failure and jaundice, representing 121 of the 296 reports (41%). There were 131 reports in the EEA database (44%) entitled with liver‐related side effects implying hepatic malfunction and/or impaired synthesis. The most relevant difference between the database search in Germany and in the EEA was the category hepatitis cholestatic: in the EEA, this category was chosen to describe liver injury due to metamizole in 32 of 296 reports (11%), but was less frequently mentioned in Germany (3%).
Figure 2.
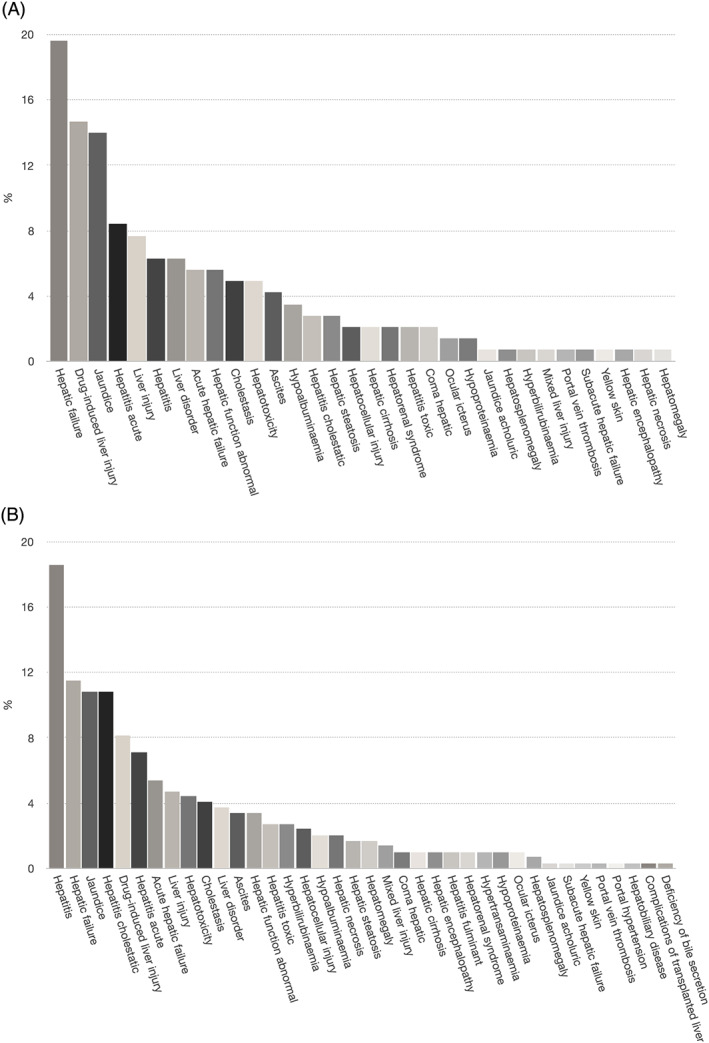
German‐ and European‐wide database search for metamizole‐induced liver injury. The EudraVigilance data analysis system database search performed by the Bundesinstitut für Arzneimittel und Medizinprodukte identified 143 reports on liver‐related side effects being associated with metamizole in Germany (A) and 296 reports in the European Economic Area (B). The categories of liver‐related side effects are shown, ordered by frequency. Categories that were considered to be unlikely a consequence of metamizole intake and/or did not represent a proper metamizole‐induced liver injury case are not shown
4. DISCUSSION
According to a report by the EMA from 2018, metamizole is prescribed frequently for its analgesic effects in several European countries such as Spain, Portugal, Italy, Austria, Luxemburg, Belgium, Poland, Slovakia and others.21 Outside of Europe, it is sold in Latin America, Africa and Asia. In other countries, such as the UK, Scandinavia, the USA and Canada, metamizole has been taken off the market mainly due to its risk of agranulocytosis. However, cases of metamizole‐induced side effects may still occur in these countries as a result of increasing migration, working abroad and foreign travelling.22 As an example, 1 study reported about metamizole exposure in regions of Texas, USA, close to the Mexican border.23 Besides, metamizole can be purchased worldwide via the internet without prescription.24
In Germany, metamizole is a very popular analgesic. The German drug label of metamizole does not mention elevation of liver enzymes or acute icteric liver injury. In this study we report on metamizole as the second most frequent cause of severe DILI, including ALF, at a tertiary care centre in Germany. Very few case reports have been published on liver injury due to metamizole. Herdeg et al. reported on a 66‐year old man suffering from exanthema and elevation of liver enzymes after the intake of metamizole.25 Liver histology of this case showed lymphocytic infiltration and perivenular confluent necrosis. Another case report was published on a 50‐year‐old patient being accidentally re‐exposed to metamizole which led to severe hyperbilirubinaemia.26 Very recently, a case report of metamizole‐induced acute liver failure was published showing necrosis and infiltration of immune cells on liver histology.24 In each of these 3 case reports, lymphocyte transformation tests confirmed immunological sensitization to metamizole metabolites.25, 26, 27 Next to these case reports, a case–control study for the quantification of the risk of DILI, performed in several departments and hospitals in Berlin, Germany, has identified 13 outpatient cases of metamizole‐DILI and an odds ratio of 5.2 (95% CI 2.0–13.4) for liver injury after the intake of metamizole. To our knowledge, these are the only published reports on metamizole‐induced liver injury.28 In contrast, a systematic review and meta‐analysis of metamizole‐associated adverse events did not detect liver injuries.29 Treatment duration of metamizole in most of the studies included in this meta‐analysis was shorter than 1–2 weeks. Besides, the case–control study mentioned above did not identify liver injuries due to metamizole for inpatients. Since median latency from first administration of metamizole until detection of elevated liver enzymes was about 4 weeks in our analysis, these discrepancies could hint a cumulative dosage effect for the hepatotoxicity of metamizole. Prospective studies with a sufficient follow‐up time are needed for metamizole to clarify this aspect.
There are further reasons for metamizole being not recognized as an agent causing liver injury in the past. First, metamizole has been banned from the market in several countries such as the USA and large registry studies on DILI and its causative drugs derive from these countries. Second, metamizole is prescribed in clinical settings when other, potentially DILI‐causing drugs are coprescribed, such as antibiotics. Antibiotics are the most frequent drug group causing DILI, and there is much more awareness of antibiotics causing DILI than for metamizole. Furthermore, elevation of liver enzymes or liver damage is not mentioned in the drug label of metamizole in Germany. Third, since analgesics such as metamizole are taken on demand by the patient, they are underreported to the treating physician when taking the history for potential liver‐toxic agents.
In about 25% of our cohort, metamizole was coadministered with other potential DILI‐causing drugs. This was also the case in both patients progressing to ALF. We cannot assure that metamizole was either solely causative or potentiated the clinical course of DILI due to the other agents by drug–drug‐interactions. It might even have played no role at all in these patients. However, the cases of accidental re‐exposure in our study with reappearance of DILI strongly support that metamizole indeed was the causative agent. These cases of re‐exposure also stress the lack of awareness of metamizole potentially causing DILI.
Histological analyses of our cohort showed severe centrilobular necrosis in some of the metamizole‐induced DILI cases. This supports that metabolic and toxic mechanisms are involved in metamizole‐induced liver injury. One study could show that metamizole and its metabolites can induce the expression of the cytochrome P450 (CYP) enzymes CYP2B6 and CYP3A4.30 This could promote neoantigen formation and drug–drug‐interactions. Besides, since we also detected an infiltration of inflammatory cells and elevation of ANA in about 50% of patients, we assume an immune‐mediated pathogenesis of metamizole‐induced liver injury. This is supported by other well‐known side effects of metamizole such as allergic skin reactions or agranulocytosis which are also immune‐mediated.
Although it is assumed that the immune system contributes to liver injury in idiosyncratic forms of DILI, steroids as a treatment for DILI are controversial.31, 32, 33, 34, 35, 36 Due to clinical guidelines, steroid treatment is not generally recommended for DILI.37, 38 At our centre, we made the experience that in the setting of acute icteric hepatitis it is not easy to differentiate between DILI and AIH. Therefore, we start steroids in uncertain cases and the course of liver enzymes after weaning of steroids will help to differentiate both entities: in AIH, liver enzymes will increase again and then, long‐term immunosuppression must be started. In DILI, liver enzymes will not rise again after the weaning of steroids. We, as other European centres, prefer in this clinical setting a dosage of 1 mg/kg bodyweight prednisolone.36 Others would prefer lower dosages.35 Since the majority of our cohort was treated with prednisolone, we were not able to analyse whether such treatment prevents progression to ALF in metamizole‐induced liver injury. The 2 severe cases in our cohort who progressed to ALF were also treated with steroids. Therefore, treatment of DILI with steroids should not be regarded as standard. As long it is unknown whether immune‐mediated DILI should be treated with corticosteroids, correct identification and prompt stopping of the causative agent is essential to prevent progression of DILI. Therefore, awareness for metamizole‐induced liver injury must be raised both in those countries where it is on the market as well as in those where it is banished. To overcome underreporting, actively asking for the intake of metamizole should be in the mind of every physician taking the history of a patient with acute hepatitis.
In summary, we here show that metamizole is among the drugs with high potential for liver injury. Awareness for metamizole as a cause of DILI needs to be raised. Thereby, unintended re‐exposition or severe courses leading to ALF could potentially be avoided.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
M. Sebode analysed the data and wrote the manuscript. M. Reike‐Kunze provided and analysed the primary data. S. Weidemann analysed the liver histologies. R. Zenouzi, J. Hartl, M. Peiseler, T. Liwinski, L. Schulz, C. Weiler‐Normann and M. Sterneck were essential for the inclusion of patients and provided primary data. A.W. Lohse and C. Schramm contributed to the design of the study, reviewed and edited the manuscript. All authors approved the final version of the article, including the authorship list.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting information
TABLE S1 The 10 most frequent causative drugs of drug‐induced liver injury at the University Medical Centre Hamburg‐Eppendorf, Hamburg, Germany.
TABLE S2 Clinical characteristics of 4 drug‐induced liver injury patients accidently re‐exposed to metamizole
FIGURE S1 Supporting Information
ACKNOWLEDGEMENTS
We thank the German Federal Institute for Drugs and Medical Devices (BfArM) for performing the database search for metamizole‐induced liver injuries in the EMA database (EVDAS).
This study was supported by the YAEL Foundation and the Helmut and Hannelore Greve Foundation. M.S., A.WL. and C.S. were supported by the ‘Deutsche Forschungsgemeinschaft’ (DFG award numbers SE 2665/1–1, SFB841 and KFO306).
Sebode M, Reike‐Kunze M, Weidemann S, et al. Metamizole: An underrated agent causing severe idiosyncratic drug‐induced liver injury. Br J Clin Pharmacol. 2020;86:1406–1415. 10.1111/bcp.14254
The authors confirm that the Principal Investigator for this paper is Marcial Sebode and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Fontana RJ. Pathogenesis of idiosyncratic drug‐induced liver injury and clinical perspectives. Gastroenterology. 2014;146(4):914‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology. 2016;63(2):590‐603. [DOI] [PubMed] [Google Scholar]
- 3. Andrade RJ, Lucena MI, Fernandez MC, et al. Drug‐induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10‐year period. Gastroenterology. 2005;129(2):512‐521. [DOI] [PubMed] [Google Scholar]
- 4. Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug‐induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419‐1425. [DOI] [PubMed] [Google Scholar]
- 5. Chalasani N, Bonkovsky HL, Fontana R, et al. Features and outcomes of 899 patients with drug‐induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danan G, Benichou C. Causality assessment of adverse reactions to drugs‐‐I. a novel method based on the conclusions of international consensus meetings: application to drug‐induced liver injuries. J Clin Epidemiol. 1993;46(11):1323‐1330. [DOI] [PubMed] [Google Scholar]
- 7. Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs‐‐II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46(11):1331‐1336. [DOI] [PubMed] [Google Scholar]
- 8. Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long‐term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek atorvastatin and coronary heart disease evaluation (GREACE) study: a post‐hoc analysis. Lancet. 2010;376(9756):1916‐1922. [DOI] [PubMed] [Google Scholar]
- 9. Bader T. Liver tests are irrelevant when prescribing statins. Lancet. 2010;376(9756):1882‐1883. [DOI] [PubMed] [Google Scholar]
- 10. Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post‐marketing. J Hepatol. 2012;56(2):374‐380. [DOI] [PubMed] [Google Scholar]
- 11. Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65(1):363‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medina‐Caliz I, Garcia‐Cortes M, Gonzalez‐Jimenez A, et al. Herbal and dietary supplement‐induced liver injuries in the Spanish DILI registry. Clin Gastroenterol Hepatol. 2018;16:1495‐1502. [DOI] [PubMed] [Google Scholar]
- 13. Levy M, Zylber‐Katz E, Rosenkranz B. Clinical pharmacokinetics of dipyrone and its metabolites. Clin Pharmacokinet. 1995;28(3):216‐234. [DOI] [PubMed] [Google Scholar]
- 14. Huber M, Andersohn F, Sarganas G, et al. Metamizole‐induced agranulocytosis revisited: results from the prospective Berlin case‐control surveillance study. Eur J Clin Pharmacol. 2015;71:219‐227. [DOI] [PubMed] [Google Scholar]
- 15. Böger R, Schmidt G. In: Schwabe U, Paffrath D, eds. Arzneiverordnungsreport. Heidelberg: Springer Medizin Verlag Heidelberg; 2005:277‐290. [Google Scholar]
- 16. WIdO Research Institute (‘Wissenschaftliches Institut der AOK’) , Berlin, Germany (2019) Arzneimittel. https://arzneimittel.wido.de/PharMaAnalyst. Accessed 02 Jan 2020.
- 17. Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug‐induced liver injury. Clin Pharmacol Ther. 2011;89(6):806‐815. [DOI] [PubMed] [Google Scholar]
- 18. Teschke R, Danan G. Drug induced liver injury with analysis of alternative causes as confounding variables. Br J Clin Pharmacol. 2018;84(7):1467‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Korman JD, Volenberg I, Balko J, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008;48(4):1167‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leung PS, Rossaro L, Davis PA, et al. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology. 2007;46(5):1436‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. https://www.ema.europa.eu/documents/psusa/metamizole-list-nationally-authorised-medicinal-products-psusa/00001997/201804_en.pdf.
- 22. Bonkowsky JL, Frazer JK, Buchi KF, Byington CL. Metamizole use by Latino immigrants: a common and potentially harmful home remedy. Pediatrics. 2002;109:e98. [DOI] [PubMed] [Google Scholar]
- 23. Forrester MB. Pattern of dipyrone exposure in Texas, 1998 to 2004. J Med Toxicol. 2006;2:101‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raine C, Webb DJ, Maxwell SR. The availability of prescription‐only analgesics purchased from the internet in the UK. Br J Clin Pharmacol. 2009;67:250‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herdeg C, Hilt F, Büchtemann A, Bianchi L, Klein R. Allergic cholestatic hepatitis and exanthema induced by metamizole: verification by lymphocyte transformation test. Liver. 2002;22(6):507‐513. [DOI] [PubMed] [Google Scholar]
- 26. Federmann G, Becker EW, Tautorat H, Penschuck C, Berg PA. Demonstration by lymphocyte transformation test of the allergic genesis in a case of acute hepatitis [article in German]. Dtsch Med Wochenschr. 1988;113(43):1676‐1679. [DOI] [PubMed] [Google Scholar]
- 27. Krisai P, Rudin D, Grünig D, et al. Acute liver failure in a patient treated with Metamizole. Front Pharmacol. 2019;10:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Douros A, Bronder E, Andersohn F, et al. Drug‐induced liver injury: results from the hospital‐based Berlin case‐control surveillance study. Br J Clin Pharmacol. 2015;79:988‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kötter T, da Costa BR, Fässler M, et al. Metamizole‐associated adverse events: a systematic review and meta‐analysis. PLoS One. 2015;10:e0122918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saussele T, Burk O, Blievernicht JK, et al. Selective induction of human hepatic cytochromes P450 2B6 and 3A4 by metamizole. Clin Pharmacol Ther. 2007;82(3):265‐274. [DOI] [PubMed] [Google Scholar]
- 31. Rakela J, Mosley JW, Edwards VM, Govindarajan S, Alpert E. A double‐blinded, randomized trial of hydrocortisone in acute hepatic failure. The acute hepatic failure study group. Dig Dis Sci. 1991;36(9):1223‐1228. [DOI] [PubMed] [Google Scholar]
- 32. EASL . Randomised trial of steroid therapy in acute liver failure. Report from the European Association for the Study of the liver (EASL). Gut. 1979;20:620‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karkhanis J, Verna EC, Chang MS, Stravitz RT, Schilsky M, Lee WM. Brown RS Jr; acute liver failure study group. Steroid use in acute liver failure. Hepatology. 2014;59:612‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wree A, Dechêne A, Herzer K, et al. Steroid and ursodesoxycholic acid combination therapy in severe drug‐induced liver injury. Digestion. 2011;84:54‐59. [DOI] [PubMed] [Google Scholar]
- 35. Björnsson ES, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S. Drug‐induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol. 2017;15:1635‐1636. [DOI] [PubMed] [Google Scholar]
- 36. Borlak J, van Bömmel F, Berg T. N‐acetylcysteine and prednisolone treatment improved serum biochemistries in suspected flupirtine cases of severe idiosyncratic liver injury. Liver Int. 2018;38(2):365‐376. [DOI] [PubMed] [Google Scholar]
- 37. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG clinical guideline: the diagnosis and management of idiosyncratic drug‐induced liver injury. Am J Gastroenterol. 2014;109:950‐966. [DOI] [PubMed] [Google Scholar]
- 38. European Association for the Study of the Liver . EASL clinical practice guidelines: drug‐induced liver injury. J Hepatol. 2019;70:1222‐1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 The 10 most frequent causative drugs of drug‐induced liver injury at the University Medical Centre Hamburg‐Eppendorf, Hamburg, Germany.
TABLE S2 Clinical characteristics of 4 drug‐induced liver injury patients accidently re‐exposed to metamizole
FIGURE S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


