Abstract
Aims
This study aimed to establish the psychometric properties of a questionnaire measure of patients' adherence to medications to elicit patients' report of medication use in a variety of clinical samples. The reliability and validity were assessed in patients with hypertension. Additional analyses were performed on other patient groups.
Methods
Using a cross‐sectional study design, a 10‐item version of the Medication Adherence Report Scale (©Professor Rob Horne) was piloted in two samples of patients receiving treatment for hypertension (n = 50 + 178), asthma (n = 100) or diabetes (n = 100) at hospital outpatient or community clinics in London and the south‐east of England. Following principal components analysis, five items were retained to form MARS‐5 (©Professor Rob Horne). Evaluation comprised internal reliability, test‐retest reliability, criterion‐related validity (relationship with blood pressure control) and construct validity (relationship with patients' beliefs about medicines).
Results
The MARS‐5 demonstrated acceptable reliability (internal and test‐retest) and validity (criterion‐related and construct validity) in these patient groups. Internal reliability (Cronbach's α) ranged from 0.67 to 0.89 across all patient groups; test‐retest reliability (Pearson's r) was 0.97 in hypertension. Criterion‐related validity was established with more adherent hypertension patients showing better blood‐pressure control (χ2 = 4.24, df = 1, P < .05). Construct validity with beliefs about medicines was demonstrated with higher adherence associated with stronger beliefs in treatment necessity and lower treatment concerns.
Conclusions
The MARS‐5 performed well on several psychometric indicators in this study. It shows promise as an effective self‐report tool for measuring patients' reports of their medication use across a range of health conditions.
Keywords: adherence, MARS‐5, Medication Adherence Report Scale, self‐report, validation
What is already known about this subject
Medication nonadherence is a significant problem that prevents the achievement of optimum outcomes in patients with long‐term conditions.
While electronic adherence monitoring is considered the gold‐standard method to assess adherence, its high costs and lack of information regarding the types of nonadherence (intentional or unintentional) means that there is a still a need for valid and reliable methods for capturing patient self‐reports.
What this study adds
This study demonstrated the reliability and validity of a questionnaire designed to elicit self‐report use of medications.
The five‐item Medication Adherence Report Scale (MARS‐5) shows promise as an effective tool for assessing adherence, identifying patients reporting low adherence and the specific types of nonadherence behaviours (eg, forgetting, deliberately missing doses).
1. INTRODUCTION
Nonadherence to medication is thought to be a major impediment to achieving optimum outcomes in chronic illness.1, 2 The measurement of patients' adherence to treatment is, however, fraught with difficulties.3 The “gold standard” of adherence measurement is currently thought to be the use of direct observation therapy or electronic adherence monitoring.4 However, direct observation of medication taking is not practical on a long‐term basis because it is intrusive and resource‐intensive.3 Whilst electronic adherence monitoring is able to provide a detailed profile of usage over time,4 the opening of the container does not guarantee ingestion of the medication: the dose might simply be discarded.3 An additional issue is that ethically patients should be told in advance that their adherence is being monitored. This awareness of monitoring may cause a temporary change in behaviour, as the patient modifies their behaviour in the short‐term to match the expectations of the observer, also known as the Hawthorne effect.5 Importantly, electronic monitors cannot be fitted to many of the conventional dosage forms and packaging used in practice, limiting its widespread implementation. Electronic monitoring is also expensive and does not provide information about the reasons for nonadherence.3, 5
Nonadherence may be a complex set of behaviours with both intentional and unintentional causes. Unintentional nonadherence occurs when the patient wants to adhere but is unable to because they lack capability or resources (eg, lack of understanding of instruction or forgetting to remember the treatment). Intentional nonadherence occurs when the patient decides not to follow the recommendations. It is best understood in terms of the perceptual factors (eg, beliefs and preferences) influencing motivation to start and continue with treatment.6 There is evidence demonstrating the utility of this approach in explaining nonadherence across several conditions and types of nonadherence behaviour.7 Although some measures, such as the Morisky Medication Adherence Scale (MMAS), capture data on intentional and unintentional nonadherence, most current methods for assessing adherence are typically not able to capture the specific types of nonadherent behaviours, for example differences between unintentional forgetting versus intentionally missing or reducing doses.4, 5
The simplest and easiest way to obtain information is patient self‐report of adherence, which is an inexpensive and convenient method of adherence assessment in naturalistic studies and clinical practice.3 However, whilst self‐report adherence questionnaires can be an inexpensive and practical alternative to electronic adherence monitoring, there are several limitations with existing questionnaires which may affect the accuracy of adherence reporting from patients. Patients may exaggerate their adherence if they believe that reports of nonadherence will disappoint their health provider.8 Importantly, the wording of questionnaire items may discourage accurate reports of adherence behaviours. For example, if nonadherence is described as “careless” or “sloppy” behaviour, this could be misinterpreted by the respondent as judgmental and increase their reluctance to truthfully report nonadherent behaviour. Lastly, errors in reporting can occur if questionnaire items combine reports of nonadherence with reasons for nonadherence. For example, a statement such as “I take less medication if I am feeling better” may be difficult for the patient to interpret: how should the patient respond if they take less medication, but not because they feel better? This use of “double‐barrel” questioning can reduce the accuracy of self‐report9 yet it is often used in self‐report adherence measures.8, 10 Stirratt et al noted that within hypertension alone, at least five questionnaires include at least some items which evaluate reasons for nonadherence as well as measures of medication‐taking.8 Most current measures have also been primarily developed for the research setting, thus often have not considered the time required for completion in everyday clinical practice.11
The National Institutes of Health (NIH) Adherence Network recently released a paper on the use of self‐report medication adherence measures.8 The report acknowledges the advantages of using self‐report in clinical practice – such as speed and efficiency – and produced a set of recommendations on how to increase the quality of self‐report, which included using validated scales, developing the questionnaire based on proper constructs and wording the questionnaire to reduce social desirability bias. There is a need for validated self‐report measures that are easy to administer but meet quality requirements to ensure accurate self‐reported adherence.
The Medication Adherence Report Scale‐10 (MARS‐10, ©Professor Rob Horne)12, 13 is a 10‐item self‐report adherence scale which assesses both intentional (“I avoid using it if I can”) and nonintentional nonadherence (“I forget to use it”). It is designed to address some of the limitations of self‐report measures minimising social desirability bias and setting a tone where nonadherence is considered normal.8 While the questionnaire has been shown to be reliable in predicting nonadherence across several conditions,12, 14 the current study assesses the validity and reliability of a shorter number of items for clinical utility. Whilst there have been studies utilising a shorter form of MARS, based on an initial validation study in asthma patients by Horne and Weinman12 these have shown variability in its reported accuracy in various conditions,15, 16 or have focused on validation in other languages or countries.17, 18 The Medication Adherence Report Scale (MARS‐5, ©Professor Rob Horne) – a shorter form of MARS‐10 – comprises items which describe a range of nonadherent behaviours, with items phrased in a nonthreatening and nonjudgmental way to normalise nonadherence, and a response scale that allows the categorisation of patients in terms of their position along the “adherence dimension” rather than on the basis of a “yes/no” or “high/low” dichotomous response, thus providing more detail and differentiation between individuals.
The aim of the present study was to establish the psychometric properties of the MARS‐5 in a variety of clinical settings by testing reliability, criterion‐related validity and construct validity.
2. METHOD
The validity and reliability testing of this questionnaire was conducted using a historical dataset collected using a cross‐sectional study design as described below.
2.1. Participant recruitment
2.1.1. Hypertension
Participants with hypertension were recruited and comprised two groups: Hypertension A and Hypertension B. These two groups were approached to increase the variation in the sample as it was hypothesised that patient characteristics may differ between the two clinics. Patients were included if they were 18 years and over and had hypertension as a primary diagnosis. The Hypertension A group consisted of consecutive hypertensive patients attending the thrice‐weekly cardiology outpatient clinics of two consultant cardiologists at two district general hospitals in south‐west London. The target number to recruit for this sample was 50. For the Hypertension B group, consecutive attendees at a weekly hypertension clinic in a London teaching hospital were approached by a trained researcher and invited to take part in a study of “patients' views about high blood pressure and its treatment”.
2.1.2. Asthma
Participants with asthma were recruited to ensure the properties of MARS‐5 could be tested across other conditions. The Asthma group comprised 100 participants recruited consecutively from attendees at asthma clinics in two general practitioner surgeries in Sussex. Patients were eligible to take part if they were given a clear diagnosis of asthma (obtained from the medical notes) and had been on regular preventer medication (inhaled corticosteroids) for a period of at least 1 month prior to attendance at the clinic.
2.1.3. Diabetes
The Diabetes group (n = 100) was recruited as part of a wider study at three teaching hospitals and nine nonteaching hospitals in the south‐east of England. The sample consisted of patients treated with oral antihypoglycaemic agents.
2.2. Sample size
The sample size recommendations vary for validation studies, but is usually based on the item:subject ratio.19 A “rule of thumb” of at least 10 participants per scale item has been previously been suggested20 therefore for this study we aimed to recruit at least 50 participants for each condition to validate the five‐item MARS‐5.
3. DATA COLLECTION
3.1. Instruments
Participants were invited to complete a brief questionnaire on baseline demographics and the MARS‐5. In the Asthma and Diabetes groups, participants completed an additional questionnaire, the Beliefs about Medicines Questionnaire (BMQ)21 (see Psychometric Evaluation of MARS‐5 section below).
3.2. Procedures
All participants were invited to self‐complete the questionnaires and return them to the researchers at the time of recruitment as described below.
Hypertension A: Participants who agreed to take part were seen by the researcher after their appointment with their consultant. The researcher completed demographic data and details of the patient's current medication, after which the patient completed the MARS‐5 questionnaire. This group was invited to complete the questionnaire a second time at home 2 weeks after the initial approach at the outpatient clinic to evaluate test‐retest reliability (see Reliability section below).
Hypertension B: Patients were invited to participate by a trained researcher or member of the hospital/clinic staff. Questionnaires were completed by patients in the hospital outpatient or community‐based clinic.
Asthma: A trained researcher invited patients to participate in a study of “patients' views about asthma and its treatment”. Participants completed the questionnaire while waiting to see the practice nurse or doctor.
Diabetes: Data from a convenience sample were collected by 24 pharmacy graduates undertaking their pre‐registration training within the hospitals. Each was asked to collect data from at least five consecutive patients attending a diabetes outpatient clinic. Patients were invited to take part and complete the questionnaires while waiting for their clinic appointment.
3.2.1. Item pool and scoring: Development of MARS‐5
The MARS‐10 questionnaire consists of 10 items (see Table 1) designed to elicit patients' reports of nonadherence, an approach that has been validated in previous studies.8, 22 In order to diminish the effects of self‐presentational bias on reports of adherence, the following statement prefaced the list of items: “Many people find a way of using their medicines which suits them. This may differ from the instructions on the label or from what their doctor had said. Here are some ways in which people have said they use their medicines. For each statement, please tick the box which best applies to you” (©Professor Rob Horne).
Table 1.
Structure matrix for the principal components factor analysis of the MARS‐5 item pool
| Item number | Description of adherence‐related behaviour | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|---|
| 10 | I take less than instructed | 0.81 | 0.44 | 0.28 |
| 6 | I stop taking it for a while | 0.81 | 0.14 | 0.30 |
| 8 | I miss out a dose | 0.77 | 0.28 | 0.28 |
| 5 | I alter the dose | 0.74 | 0.33 | 0.19 |
| 4 | I forget to take it | 0.73 | 0.03 | 0.28 |
| 3 | I avoid taking it if I can | 0.69 | 0.32 | 0.61 |
| 7 | I take it exactly as prescribed | 0.12 | 0.84 | –0.12 |
| 2 | I take it regularly every day | 0.38 | 0.75 | 0.32 |
| 1 | I take it only when I need it | 0.25 | 0.00 | 0.90 |
| 9 | I take more than instructed | 0.45 | 0.56 | 0.58 |
Bold numbers indicate the highest loading for that factor.
Adherence was measured using a continuous scale rather than as a dichotomous division into adherent/nonadherent categories. Participants were asked to rate the frequency with which they engaged in each of the adherence‐related behaviours on a five‐point scale, where 5 = never, 4 = rarely, 3 = sometimes, 2 = often and 1 = always. Scores for each item were summed to give a total score, with higher scores indicating higher levels of reported adherence.
Principal components analysis (PCA) was performed on data pooled from all three patient samples to establish a coherent set of items measuring a single construct and to allow a subset of the original item pool to be selected to form a parsimonious measure. The PCA indicated that the data were adequately modelled with three factors and that the first factor was unambiguously associated with five items (see Table 1). Item 3 (“I avoid taking it when I can”) was found to load on all three factors and was therefore not considered to be unambiguously associated with the first factor. Items 10, 6, 8, 5 and 4 were selected to form a short form consisting of five items. This shortened form of MARS‐10, ie, the MARS‐5 scale, was used for subsequent psychometric evaluation. MARS‐5 follows the same structure as MARS‐10 and asks respondents to rate the frequency with which the five different medication‐taking behaviours occur, scoring each item on a five‐point scale (5 = never, 4 = rarely, 3 = sometimes, 2 = often, 1 = very often), with higher scores indicating higher reported adherence.12
4. DATA ANALYSIS: PSYCHOMETRIC EVALUATION OF MARS‐5
4.1. Reliability and validity testing
The MARS‐5 questionnaire was evaluated in terms of its reliability (internal consistency and test‐retest reliability) and validity (criterion‐related and construct validity) using data from the Hypertension A and B groups. Additional analyses were also performed on the other patient groups.
4.2. Reliability
Reliability refers to whether a questionnaire is measuring what is intended in a reproducible manner and can be assessed in several ways.23 Internal consistency estimates the extent to which items within a scale are assessing a single construct and is assessed by computing a Cronbach's α coefficient. The internal consistency of the MARS‐5 was calculated for all of the diagnostic groups comprising the study sample.
Test‐retest reliability refers to the likelihood that a given measure will yield the same description of a given phenomenon if that measurement is repeated at a later date. This was assessed using the Hypertension A group, as described in the Procedures section above.
4.3. Validity
Validity refers to whether the questionnaire measures what it intends to measure.23 Two types of validity were examined in this study: criterion and construct. Criterion‐related validity refers to the degree of agreement between scores on a questionnaire and some independent, nontest criterion. The criterion for the hypertension sample was a measure of blood pressure control, involving patients from the Hypertension B group. Patients were classified as (a) “high adherers” or “low adherers”, using the median value of the MARS‐5 data as a cut‐off point, and (b) within or outside the target range of blood pressure as specified by their consultant according to the mean of their three most recent measurements of blood pressure. A chi‐square analysis was conducted to examine the association between self‐reported adherence and blood pressure control. It was predicted that patients classified as “high adherers” would be significantly more likely to be within range in terms of their blood pressure than those classified as “low adherers”.
For the Asthma and Diabetes samples, criterion validity was more difficult to evaluate as obtaining measures of asthma and diabetes control, respectively, were deemed more intrusive and practically more difficult. As such, construct validity was used to assess validity in these samples. Construct validity is demonstrated by linking the attribute that is being measured in a questionnaire with another attribute via a particular hypothesis or construct.23 This was assessed in terms of relationships between scores on MARS‐5 and the beliefs that patients held about their medication in the Asthma and Diabetes groups. Previous research has shown that levels of adherence are related to beliefs about the personal necessity of prescribed medication and concerns about taking it. Specifically, stronger necessity beliefs are associated with higher adherence and greater concerns are linked to lower adherence.7, 14 These beliefs were examined in the present study using Beliefs about Medicines Questionnaire (BMQ‐Specific).12, 21 The BMQ Specific comprises two scales: a Necessity scale assessing beliefs about personal need for prescribed medicines and a Concerns assessing medication concerns. Each scale comprises five items that are scored on a Likert‐type scale, where 1 = strongly disagree, 2 = disagree, 3 = uncertain, 4 = agree and 5 = strongly agree. Higher scores indicate stronger beliefs in the necessity of medication and greater concerns about taking it. The BMQ has shown good reliability and validity in a range of chronic illnesses.21 It was predicted that higher MARS‐5 scores would be associated with stronger necessity beliefs and lower MARS‐5 scores would be associated with stronger concerns.
4.4. Ethical considerations
As the validation study was conducted using a historical dataset which was collected as part of routine health service delivery at the time, no additional ethics approval was deemed to be required.
5. RESULTS
5.1. Population characteristics
The MARS‐5 was evaluated in four groups of patients (see Table 2 for sample characteristics). Overall, a high completion rate was achieved with the questionnaires as described below.
Table 2.
Sample characteristics by patient group
| Asthma (n = 100) | Diabetes (n = 100) | Hypertension A (n = 50) | Hypertension B (n = 178) | ||
|---|---|---|---|---|---|
| Gender (%) | Male | 39 | 68 | 62 | 52 |
| Female | 61 | 32 | 38 | 48 | |
| Age, years (SD) | 49.1 (18.1) | 58.2 (15.9) | 62.3 (13.4) | 53.6 (14.6) | |
5.1.1. Hypertension
In the Hypertension A sample, a total of 50 consecutive patients were recruited, of which 86% (43/50) completed the questionnaire. In the Hypertension B group, 224 patients were approached and 178 gave consent to participate in the study. Of these, 126 returned the questionnaires; a completion rate of 71%. In the Hypertension B sample, there were some missed items on the questionnaires. In order to overcome the problem of these occasionally missed items, every scale was scored on a pro rata basis when the patient had completed 70% or more of the items. Of the 126 questionnaires returned, 17 had less than 70% of items completed on each scale, leaving 109 for inclusion in the statistical analyses (49% of the original sample).
5.1.2. Asthma
In the Asthma group, a total of 100 participants were included. These 100 participants were recruited from 119 approaches, representing an acceptance rate of 84%. The number of questionnaires with fully completed MARS‐5 scales in the Asthma group was 98/100 (98%).
5.1.3. Diabetes
In the Diabetes group, 100 participants were included. The sample characteristics of each patient group are shown in Table 1. All participants in this group completed the questionnaires fully.
5.2. Psychometric evaluation
5.2.1. MARS‐5 reliability
The MARS‐5 showed good internal consistency in all of the patient groups, with Cronbach's α coefficients of 0.68 (Hypertension A), 0.67 (Hypertension B), 0.84 (Asthma) and 0.89 (Diabetes).
For test‐retest reliability testing in the Hypertension A group, a total of 43 questionnaires were completed and returned. There was a significant correlation between the MARS‐5 scores of the Hypertension A group recorded at the two time points (r =0.97, P < .001), indicating excellent test‐retest reliability.
5.2.2. MARS‐5 validity
Blood pressure measurements were able to be obtained for 63 patients in the Hypertension B sample. The frequency distribution of patients classified as “high adherers” and “low adherers” as a function of blood pressure control (within range or out of range) is shown in Figure 1. As predicted, there was a significant association between adherence and blood pressure control (χ2 [1, N = 63] = 4.24, P < .05). Patients in the Hypertension B group who were classified as “high adherers” were significantly more likely to be within range in terms of their blood pressure than those classified as “low adherers”.
Figure 1.
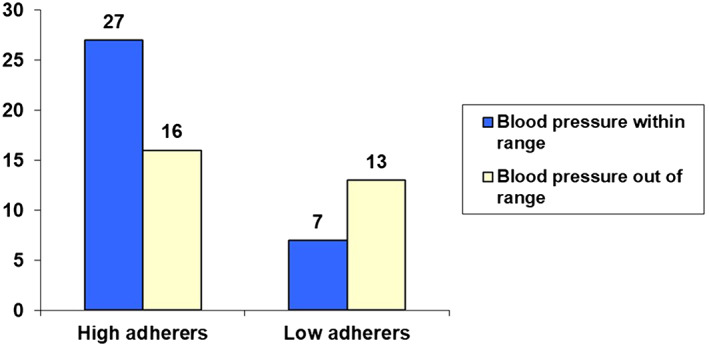
Bar chart of “high adherers” vs “low adherers” as a function of clinical outcome (blood pressure within or out of range) in the Hypertension B group
Correlations between scores on the MARS‐5 and those on the BMQ Necessity and Concerns subscales are shown in Table 3. There is a significant positive correlation between MARS‐5 and BMQ Necessity scores in the Asthma group, with higher levels of self‐reported adherence associated with stronger beliefs in the necessity of taking prescribed medication, though correlation for the Diabetes group was not significant for Necessity scores. There were significant negative correlations between MARS‐5 and BMQ Concerns scores in the Asthma and Diabetes groups, with lower adherence associated with stronger concerns about taking the medication.
Table 3.
Correlations of MARS‐5 scores with BMQ necessity and concerns scores
Abbreviations: BMQ, Beliefs about Medicines Questionnaire; MARS‐5, Medication Adherence Report Scale.
P < .05
P < .01
6. DISCUSSION
This paper reports on the development and psychometric evaluation of MARS‐5, a practical adherence measure that can be used to identify patients at risk of nonadherence in a clinical setting. The measure was shown to have good reliability and validity across three different long‐term conditions: hypertension, asthma and diabetes. MARS‐5 performed well on several psychometric indicators, showing good internal consistency across the range of illness groups and excellent test‐retest reliability among a sample of patients with hypertension. This is similar to the psychometric measures for the 10‐item MARS‐10 questionnaire, which had similar internal reliability values in asthma (α = 0.85) as seen in our MARS‐5 sample (α = 0.84), with higher test‐retest reliability (r = 0.65, P < .001 for MARS‐10 vs r = 0.97, P < .001 in this sample).13
Validity was demonstrated in a number of ways. Patients diagnosed with hypertension and classified as “high adherers” on the basis of their MARS‐5 scores were more likely to achieve blood pressure control than those classified as “low adherers” (criterion‐related validity). This is in line with adherence studies in patients with hypertension which show that individuals with high adherence achieve lower blood pressure compared to individuals with poor adherence.24 Construct validity was demonstrated in terms of relationships between MARS‐5 scores and the treatment beliefs held by patients with asthma or diabetes. Consistent with previous findings7, 21 lower adherence was related to stronger concerns about the potential adverse effects of medication. Higher adherence was associated with stronger beliefs in the necessity of taking medication for patients with asthma – a finding in line with the construct validity testing conducted for the MARS‐1013 and also other studies which have looked at the association of treatment beliefs and adherence in asthma.7, 25 Necessity beliefs, however, were not associated with adherence in those diagnosed with diabetes and treated with oral antihypoglycaemic agents. This finding is consistent with results from other studies showing that in individuals with diabetes, concerns may be stronger determinants of adhernece than necessity beliefs.7, 26, 27 The mechanism behind this is unknown but it could be postulated that asthma is a more symptom‐driven condition compared to diabetes, which may reinforce the necessity of medication in asthma to greater extent than in diabetes. However, further work is needed in this area to understand the associations observed and how the strength of the relationship between treatment beliefs and adherence varies across conditions. Higher concerns were associated with lower adherence in both samples, which is similar to previous reported findings.7
The MARS‐5 may address some of the limitations of existing self‐report measures of adherence to medication.8, 11 One of its advantages is that the instructions that precede its items are worded in a way designed to normalise the reporting of nonadherence, thus reducing the likelihood of self‐presentational bias. To improve the quality of information provided, the response scale allows patients to be graded along an adherence dimension in terms of the frequency with which they engage in nonadherent behaviours, rather than a dichotomous ‘yes/no’ classification. The current MARS‐5 also has advantages over the existing longer MARS‐10 questionnaire as the shorter length is more practical for use in a clinical setting, without compromising the reliability and validity of the questionnaire, and still enabling the user to identify key reasons for nonadherence, which can serve as a starting point to guide adherence discussions.
6.1. Practical implications
The testing of MARS‐5 in the current study leads to several crucial implications. For patients scoring low on MARS‐5, the pattern of responses to individual items can indicate whether their poor adherence is largely unintentional (“I forget to take it”) or intentional (eg, “I take less than instructed” or “I miss out a dose”). This, in turn, can serve as a guide as to which interventions would be most suitable for improving their level of adherence. Unintentional nonadherence can be addressed using a variety of strategies that are designed to act as a reminder or cue for medicine taking. Examples include electronic reminders,28 specialised pill packaging29 and tailoring the drug regimen to daily routines.30 With regards to intentional adherence, the correlations between MARS‐5 and BMQ scores in the present study suggest that intentional nonadherence may stem in part from the patient's beliefs about their medication. For example, the endorsement of the item “I stop taking it for a while” may reflect concerns about the long‐term effects of the medication and the risk of dependence. Counselling‐based interventions would seem to be a more appropriate intervention in this case by offering the patient an opportunity to discuss any doubts about the effects and effectiveness of their treatment.6
6.2. Limitations
Whilst MARS‐5 showed good reliability and validity on psychometric evaluation, there are a few limitations to consider. The evaluation of MARS‐5 was hampered by the lack of an opportunity to examine both test‐retest reliability and the two indicators of validity in all of the illness groups sampled. Additional research is required to assess these psychometric properties in a broader range of clinical settings. A further limitation was the use of convenience, rather than random, sampling, which may have produced results that were unrepresentative of the wider patient population. Despite these limitations, our data provide evidence of reliability and validity, and support the use of the questionnaire to elicit patients' reports of how they actually use their medication.
7. CONCLUSIONS
In summary, MARS‐5 performed well on a number of psychometric indicators and had high internal‐reliability across three different illness groups: hypertension, asthma and diabetes. This data adds to existing data on the utility of MARS‐5 in different countries and settings. MARS‐5 may address some limitations of existing self‐report measures of medication adherence. The questionnaire proved to predict blood pressure control (whether within range or out of range) in a sample of hypertensive patients and also demonstrated a significant association with the treatment Necessity and Concerns scales of the BMQ. The MARS‐5 indicates reasons for poor adherence, which might assist the selection of appropriate interventions, tailored to address the specific reasons for nonadherence. Collectively, the results of this study suggest that MARS‐5 is effective at identifying whether patients are at risk of nonadherence and can also be used to assess adherence reports over the course of the treatment. Based on these findings, MARS‐5 shows promise as a self‐report tool for measuring patients' reports of their use of medication across a range of illnesses.
COMPETING INTERESTS
A.C. reports consultancy fees from Janssen‐Cilag and Spoonful of Sugar Ltd, and speaker fees from Novartis, outside the submitted work. R.H. reports fees from Medical Innovation Academic Consortium (CASMI), AbbVie, Amgen, Biogen, Idec, Gilead Sciences, GlaxoSmithKline, Janssen, Pfizer, Roche, Shire Pharmaceuticals, MSD, Astellas, AstraZeneca, DRSU, Novartis, Universiteatsklinikum Hamburg‐Eppendorf and Teva Pharmaceuticals, and is the Founder and Director of Spoonful of Sugar Ltd, outside the submitted work.
CONTRIBUTORS
A.C. was involved in the data analysis, interpretation and study write up. R.H. was involved in the study design, data collection, interpretation and write up review. M.H. was involved in the study design, data analysis, interpretation and write up review. C.C. was involved in the data analysis, interpretation, write‐up and final review.
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated (historical dataset).
Chan AHY, Horne R, Hankins M, Chisari C. The Medication Adherence Report Scale: A measurement tool for eliciting patients' reports of nonadherence. Br J Clin Pharmacol. 2020;86:1281–1288. 10.1111/bcp.14193
The authors confirm that the Principal Investigator for this paper is Rob Horne and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Sabaté E. Adherence to long‐term therapies: evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 2. Simpson SH, Eurich DT, Majumdar SR, et al. A meta‐analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074‐1090. [DOI] [PubMed] [Google Scholar]
- 4. Chan AHY, Reddel HK, Apter A, Eakin M, Riekert K, Foster JM. Adherence monitoring and e‐health: how clinicians and researchers can use technology to promote inhaler adherence for asthma. J Allergy Clin Immunol Pract. 2013;1(5):446‐454. [DOI] [PubMed] [Google Scholar]
- 5. Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high‐tech best? J Clin Psychol Med Settings. 2002;9(1):25‐34. [Google Scholar]
- 6. Horne R, Weinman J, Barber N, Elliott R, Morgan M, Cribb A. Concordance, adherence and compliance in medicine taking: Report for the National Co‐ordinating Centre for NHS Service Delivery and Organisation R & D (NCCSDO). London: NCCSDO; 2005. [Google Scholar]
- 7. Horne R, Chapman S, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients' adherence‐related beliefs about medicines prescribed for long‐term conditions: a meta‐analytic review of the necessity‐concerns framework. PloS One. 2013;8(12):e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stirratt MJ, Dunbar‐Jacob J, Crane HM, et al. Self‐report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Podsakoff PM, MacKenzie SB, Lee J‐Y, Podsakoff NP. Common method biases in behavioral research: a critical review of the literature and recommended remedies. J Appl Psychol. 2003;88(5):879‐903. [DOI] [PubMed] [Google Scholar]
- 10. Hahn SR, Park J, Skinner EP, et al. Development of the ASK‐20 adherence barrier survey. Curr Med Res Opin. 2008;24:2127‐2138. [DOI] [PubMed] [Google Scholar]
- 11. Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self‐reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011;11:149 10.1186/1471-2288-11-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horne R, Weinman J. Self‐regulation and self‐management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non‐adherence to preventer medication. Psychol Health. 2002;17(1):17‐32. [Google Scholar]
- 13. Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self‐reported medication adherence among inner‐city asthmatic adults: the medication adherence report scale for asthma. Ann Allergy Asthma Immunol. 2009;103:325‐331. [DOI] [PubMed] [Google Scholar]
- 14. Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555‐567. [DOI] [PubMed] [Google Scholar]
- 15. Vluggen S, Hoving C, Schaper NC, De Vries H. Psychological predictors of adherence to oral hypoglycaemic agents: an application of the ProMAS questionnaire. Psychology & health. 2019;1‐8. [DOI] [PubMed] [Google Scholar]
- 16. Tommelein E, Mehuys E, Van Tongelen I, Brusselle G, Boussery K. Accuracy of the Medication Adherence Report Scale (MARS‐5) as a quantitative measure of adherence to inhalation medication in patients with COPD. Ann Pharmacother. 2014;48(5):589‐595. [DOI] [PubMed] [Google Scholar]
- 17. Bäck A., Sundell KA, Horne R, Landén M. & Maring;rdby AC. The medication adherence report scale (MARS‐5) in a Swedish sample with bipolar disorder‐a pilot study. Int J Pers Cent Med. 2012;2(2):263‐270. [Google Scholar]
- 18. Lin CY, Ou HT, Nikoobakht M, Broström A, Årestedt K, Pakpour AH. Validation of the 5‐item medication adherence report scale in older stroke patients in Iran. J Cardiovasc Nurs. 2018;33(6):536‐543. [DOI] [PubMed] [Google Scholar]
- 19. Anthoine E, Moret L, Regnault A, Sébille V, Hardouin J‐B. Sample size used to validate a scale: a review of publications on newly‐developed patient reported outcomes measures. Health Qual Life Outcomes. 2014;12:176 10.1186/s12955-014-0176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boateng GO, Neilands TB, Frongillo EA, Melgar‐Quiñonez HR, Young SL. Best practices for developing and validating scales for health, social, and behavioral research: a primer. FrontPublic Health. 2018;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1‐24. [Google Scholar]
- 22. Kravitz RL, Hays RD, Sherbourne CD, et al. Recall of recommendations and adherence to advice among patients with chronic medical conditions. ArchInt Med. 1993;153:1869‐1878. [PubMed] [Google Scholar]
- 23. Heale R, Twycross A. Validity and reliability in quantitative studies. Evidence‐based nursing. 2015:ebnurs‐2015‐102129. [DOI] [PubMed]
- 24. Rose AJ, Glickman ME, D'Amore MM, Orner MB, Berlowitz D, Kressin NR. Effects of daily adherence to antihypertensive medication on blood pressure control. J Clin Hypertens. 2011;13:416‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brandstetter S, Finger T, Fischer W, et al. Differences in medication adherence are associated with beliefs about medicines in asthma and COPD. Clin Transl Allergy. 2017;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phillips LA, Diefenbach M, Kronish IM, Negron RM, Horowitz CR. The necessity‐concerns‐framework: a multidimensional theory benefits from multidimensional analysis. Ann Behav Med. 2014;48:7‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. West LM, Borg Theuma R, Cordina M. The ‘necessity–concerns framework’ as a means of understanding non‐adherence by applying polynomial regression in three chronic conditions. Chronic Illn. 2018;174239531879984. [DOI] [PubMed] [Google Scholar]
- 28. Tran N, Coffman JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. J Asthma. 2014;51:536‐543. [DOI] [PubMed] [Google Scholar]
- 29. Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. Jama. 2014;312:1237‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31:213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated (historical dataset).


