Abstract
Aims
The aim of this systematic review was to identify generic instruments for drug discontinuation in patients with polypharmacy in the primary care setting.
Methods
We systematically searched PubMed and EMBASE, 8 guideline databases (AWMF, NICE, NGC, SIGN, NHMRC, CPG, KCE), the Cochrane Library and grey literature (Google) in 2016 and 2017. Two independent researchers screened and analysed data. The drug discontinuation instruments of the included publications were described and classified.
Results
We identified 16 relevant publications. Here we found complex algorithms as well as instruments composed of distinct sequential steps. Two guidelines are constructed as electronic web‐applications. Instruments revealed diverging emphases on the stages of deprescribing, i.e. preparation, drug evaluation, decision‐making and implementation. Accordingly, 3 types of instruments emerged: general frameworks, detailed drug assessment tools and comprehensive discontinuation guidelines.
Conclusion
Diverse generic instruments exist for different areas of applications in regard to drug discontinuation. However, there is still a need for practical and user‐friendly tools that support physicians in communicational aspects, visualise trade‐offs and also enhance patient involvement.
Keywords: deprescriptions, multimorbidity, polypharmacy, primary health care, systematic review
1.
What is already known about this subject
Polypharmacy is problematic, especially for elderly and frail patients.
General practitioners are expected to integrate guideline recommendations and their patients' individual therapeutic goals.
Despite an abundance of existing polypharmacy guidelines, physicians still face uncertainties in drug discontinuation.
What this study adds
This study identifies 16 generic instruments for drug discontinuation.
Instruments revealed distinct emphases according their area of application.
We provide suggestions for new developments regarding the integration of helpful existing, but also crucial missing elements.
2. INTRODUCTION
In western nations, the proportion of elderly people is steadily rising. 1 A large proportion of older persons are affected by multimorbidity, that is the co‐occurrence of multiple medical conditions. 2 , 3 Most guidelines focus only on 1 single condition, with the result that multimorbid patients frequently experience polypharmacy.
Polypharmacy is defined as the concurrent use of several (mostly 5 or more) long‐term medications. 4 , 5 Polypharmacy is increasingly regarded as problematic. Among the associated risks are interactions and adverse drug events such as bleedings, delirium and falls. Due to age‐related changes in pharmacodynamics and pharmacokinetics, elderly patients are especially susceptive to these risks. 4 , 6 , 7
General practitioners (GPs) are expected to negotiate guideline recommendations and their patients' individual therapeutic goals and preferences. 8 At some point, medication reconciliation involves deprescribing some drugs that the patient is currently taking. This requires the identification of drugs with unfavourable risk–benefit ratios. Ideally, the decision should be shared between clinicians, patients, and perhaps their families. 9
Instruments or tools as understood for this work consist of an evaluative assessment of an individual patient's current long‐term medication (input), as well as recommendations regarding the omission or continuation of drugs (output).
A number of instruments have been developed to support clinicians in the field of polypharmacy. Instruments may derive their input from clinical judgment, such as the Medication Appropriateness Index. 10 These so‐called implicit tools or implicit criteria facilitate drug assessment by providing an array of targeted questions to elicit appropriateness of each drug. 11 However, these tools mostly limit themselves to a mere assessment and do not provide advice for stopping medication, i.e. are not output specified. In this regard, they contrast other tools that are based on defined and measurable criteria (input), rather than on clinical judgement. Examples of these explicit tools 11 are the Beers Criteria, 12 STOPP/START Criteria 13 and STOPPFrail. 14 These tools offer lists of drugs and drug‐classes that should be avoided, replaced or discontinued in elderly or frail patients, i.e. they are output specified. However, they lack implicit input and therefore a universal usability and guidance for deprescribing. We call this approach of an universally applicable guidance generic in contrast to various existing drug‐specific guidelines, which have a restricted scope of application. If deprescribing instruments are to meet their objectives, they must go beyond particular conditions or drugs.
Despite a number of assisting instruments having been developed, clinicians still face multiple uncertainties in regard to deprescribing, and a turnover in the increasing drug load has not been recorded so far. 5 , 8 Attempts to review existing tools for deprescribing are either restricted in scope 15 or lack a systematic approach. 9 , 16 , 17 The aim of this systematic review is to identify generic instruments for drug discontinuation in patients with polypharmacy in the primary care setting.
3. METHODS
3.1. Inclusion criteria
We included publications reporting generic guiding instruments for drug discontinuation in patients with polypharmacy in the primary care setting. We have considered every tool that was designed to systematically assist GPs in evaluating the current patient's medication (input) and that provided advice on drug discontinuation or at least recommended it (output). Instruments were considered generic if they offered implicit assessment criteria and applicability without being restricted to certain drugs or drug classes. Instruments referring to residents of long‐term care facilities were included as long as GPs were involved in patient care. Instruments could be displayed in any format, e.g. algorithm, narration and diagram, or a combination of these. We included all kinds of study designs. An evaluation of the instrument was not mandatory. We considered publications in English, Swedish and German.
3.2. Exclusion criteria
Publications were excluded if the instrument was exclusively developed for settings other than primary care (e.g. secondary or hospital care), targeted prescribing optimization only, focussed on drug evaluation without recommending discontinuation, was confined to special patient subgroups (e.g. pregnant women or children), or if the instrument had been previously published (e.g. reviews or guidelines that only referred to already published instruments).
3.3. Data source and search strategy
We conducted computer‐based searches using PubMed (Medline) and EMBASE database in November 2016 and January 2017, respectively. There was no restriction regarding the date of publication. The search syntax for PubMed included the following terms, linked with an “OR” or “AND” as command code: MESH terms “Potentially Inappropriate Medication List” and “Deprescriptions”, the term “deprescri*” in title or abstract, the term “discontinuation” in the title, the term “medication” in the title, the term “instrument/decision rule” in the title, the term “general practice” (in title and abstract as well as in affiliation of authors), the MESH terms “family practice”, “physicians, family” and “primary health care”. All terms were used in various notations. “Addiction parameters” (smok* and tobacco*) as well as certain publication formats (autobiographies, case reports, portraits etc.) were excluded. For the search in EMBASE the syntax was adapted, if necessary: MeSH terms were replaced by corresponding Emtrees (EMBASE subject heading), and the authors affiliation block for “general practice” had to be removed. The EMBASE search was restricted to EMBASE, without medline (command: AND embase/lim NOT medline/lim). The detailed search syntax is made available as supplementary material (Appendix 1).
As part of a complementary search in October 2016, we screened the Cochrane Library as well as the following electronic guideline databases: AWMF (The Association of the Scientific Medical Societies in Germany), GIN (Guidelines International Network), NICE (The National Institute for Health and Care Excellence), NGC (National Guideline Clearinghouse), SIGN (Scottish Intercollegiate Guidelines Network), NHMRC (National Health and Medical Research Council—Australian Government), CPG (Canadian Clinical Practice Guidelines) and KCE (Belgian Health Care Knowledge Centre). Because only few of these databases are searchable by using syntax, we applied the term “discontinuation” in various notations (stop*, discontinu*, withdraw*, cessation, deprescri*, reduc*) and the term “multimedication/polypharmacy” in various notations (polypharm*, multimedic*, pharmac*, drug*, medication*) as well as the term “multimorbidity”; each as single search terms. Where applicable, we searched for the MeSH Terms “Depresciptions” and “Potentially Inappropriate Medication List”. Due to the rapidly increasing number of recommendations that have not yet been certified as standardized guidelines, we furthermore searched for elaborated guidances and toolkits, where indicated.
We performed backward citation tracking (snowballing) and forward citation tracking (reverse snowballing) for relevant articles via Google Scholar. Additionally, grey literature (Google) was screened using the same search strategy that we had used in the guideline databases in October 2016.
3.4. Screening and selection of publications
After excluding duplicates, all identified research articles were subjected to a 2‐step selection process. First, we examined titles and abstracts to see whether the publication covered the topics drug discontinuation, polypharmacy, instrument and primary care setting. In a second step, we analysed the full text of potentially eligible studies regarding inclusion and exclusion criteria. The screening and selection process was undertaken independently by 2 researchers (N.G., M.M.C.) and conflicts were resolved in discussion with the supervisor (A.V.).
By default, guideline databases and the Cochrane Library arrange results by relevance. Searching with single terms in some cases produced an excessive amount of results. Thus in all cases of >100 hits, we pragmatically decided to screen the first 100 most relevant results.
3.5. Data extraction and synthesis
For all publications included, we extracted the bibliographic information (author, corresponding author's speciality, country of corresponding author's institution, publication year, title, journal). Additionally, we collected the instruments' target user groups and patient groups. To be able to describe and compare the instruments, we extracted and classified their single components. Mapping these elements allowed us to identify patterns and types of instruments. Extensive discussions within our research team finally led to the displayed analysis.
3.6. Quality assessment
Since there are no published standardised and accepted quality criteria or reporting guidelines for drug discontinuation instruments, our search revealed a large diversity in methods of development and reporting. We classified the instruments with regard to their development process as formal statements or informal statements. Classification as formal statement required a detailed description of the development process (regardless of its quality) as well as stewardship of a nonprofit professional organisation in development and publication (e.g. official association, medical society or college) and compliance with international recommendations on guideline development. 18 , 19 Consequently, publications that did not meet these criteria were categorised as informal statements.
4. RESULTS
4.1. Search result
Our literature search in the PubMed and EMBASE databases yielded 2834 publications (Figure 1). The Cochrane library generated 1276 relevant hits and electronic guideline databases further 2617 relevant hits. Our search in grey literature resulted in 14 publications and snowball tracking during the whole process resulted in another 33 publications. After excluding duplicates, we had identified 2788 publications and 3893 guidelines. After screening title and abstracts of this initial pool, we screened the full text of 108 publications. Ninety‐two publications were excluded because they did not meet the inclusion criteria. Finally, we included 16 publications in our analysis. None of the included publications had been obtained via guideline databases or Cochrane Library.
FIGURE 1.
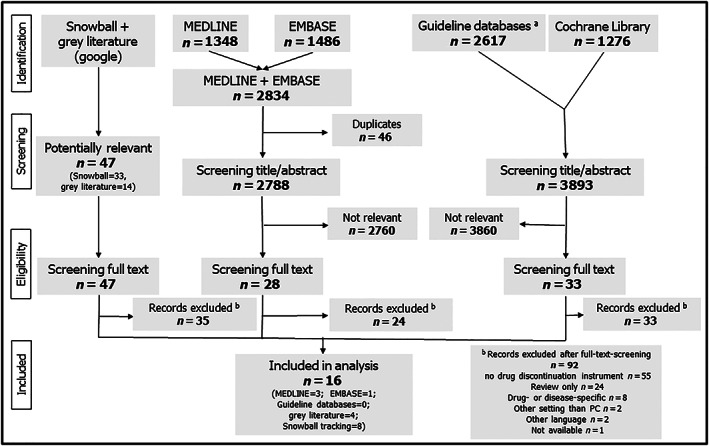
Flow chart; #AWMF, GIN, NICE, NGC, SIGN, NHMRC, CPG, KCE
4.2. Included publications
The articles' basic characteristics are shown in Table 1 and Table A (supplementary material). Publication dates ranged from 1994 until 2016 with an increase in numbers of publication towards 2016. Half of the publications were written by corresponding authors from Australia (8/16), 3 by corresponding authors from the USA, 2 from the UK, and a single 1 each from Israel, the Netherlands, and Spain (see Table A supplementary material). Most of the corresponding authors were pharmacists (7/16) or geriatricians (5/16). The majority of the publications addressed “clinicians” or “clinicians caring for older people” (including clinicians caring for elderly people in long‐term care facilities) as target audience for the respective drug discontinuation instrument (8/16). Four publications explicitly referred to GPs (sometimes framed as practicing physicians).
TABLE 1.
Short description and elements of the drug discontinuation instruments (sorted by format and author)
| Authors | Year | Format a | Name (a), short description (b) and list of elements and questions (as named by instrument; c) |
|---|---|---|---|
| Jansen et al 32 | 2016 | Step‐by‐step (4) |
a: “Process for deprescribing with older adults”: b: Narrative description of the deprescribing process in 4 steps with a clear focus on shared‐decision‐making c: Elements: 1) creating awareness that options exist (triggering situations, patients' attitude, cognitive biases, multidisciplinary) 2) discussing options and their benefits and harms 3) exploring patient preferences 4) decision‐making |
| Reeve et al 16 | 2014 | Step‐by‐step (5) |
a: “The 5‐step patient‐centred deprescribing process”: b: ‐ framework of a 5‐step deprescribing process ‐ utilization and integration of existing tools such as Medication Appropriateness Index and Beers list c: Elements: 1) comprehensive medication history 2) identify potentially inappropriate medication (referring to existing tools) 3) determine if medication can be ceased and prioritized 4) plan and initiate withdrawal 5) monitoring, support and documentation |
| Scott et al 25 | 2012 | Step‐by‐step (10) |
a: “A 10‐step drug minimization framework”: b: ‐ 10 sequential steps guiding process of drug discontinuation including medication assessment ‐ caveats and practical instructions are highlighted for each step c: Elements: A: Constructing a patient profile 1) ascertain all current medications 2) identify patients at high risk for adverse drug reactions 3) estimate life expectancy 4) define care goals in the context of life expectancy, disability and priorities B: Making treatment decisions 5) define and confirm current indications 6) determine time until benefit for disease‐modifying medications 7) estimate magnitude of benefit vs harm 8) review relative utility of different drugs 9) identify drugs that may be discontinued C: Monitoring and reviewing treatment decisions 10) implement and monitor a drug minimization plan |
| Tenni and Dunbabin et al 30 | 2016 | Step‐by‐step (6) |
a: “Deprescribing: a personalised approach”: b: ‐ framework of 6 sequential steps in a “deprescribing cycle” ‐ every step comes with a description including drug assessment ‐ beside this general booklet, different medication‐specific brochures were published by same group c: Elements: 1) consider patient (expectations, frailty, life‐expectancy) 2) medication history (what, how long, why, ADR, interactions), 3) identify potential drug targets (risk/benefit [PIM lists], duplicates, poor risk, risky dose etc.) 4) determine cessation priority (least utility, high risk, adverse impact, patient preference, complicated administration) 5) plan and withdraw, 6) monitor, support and document |
| Woodward et al 34 | 2003 | Step‐by‐step (5) |
a: “Deprescribing principles”: b: ‐ narrative sequence of 5 steps describing the ideal deprescribing process ‐ list of medication‐specific recommendations enclosed c: Elements: 1) review all current medications (including indication for use, compliance, ADR) 2) identify medications to be targeted for cessation 3) plan a Deprescribing regimen (best in team, prioritizing drugs) 4) plan in partnership with patient and Carers (shared decision‐making) 5) frequent review and support |
| Bain et al 20 | 2008 | Algorithm |
a: “The prescribing stage revised ‐ discontinuing medications”: b: Integration of medication discontinuation into the traditional medication‐use process algorithm of starting, changing and continuing medication c: Elements: ‐ indication to discontinue medication? ‐ identification and prioritization of medication to discontinue ‐ plan, communicate and coordinate discontinuation ‐ monitor effects |
| Garfinkel et al 21 | 2007 | Algorithm |
a: “Geriatric‐palliative approach for improving drug therapy in disabled elderly people” b: ‐ complex algorithm with a set of questions culminating in stopping, shifting, reducing or continuing drug: c: Elements: ‐ evidence‐based indication and dosing in patient's age group and disability level? ‐ benefit outweighs possible adverse effects in old and disabled patients ‐ indication seems valid (in patient's age group and disability level) ‐ adverse symptoms/signs ‐ alternative superior drugs ‐ dosing reduction without risk |
| Hardy and Hilmer et al 31 | 2011 | Algorithm |
a: “Algorithm for deprescribing in the last year of life”: b: ‐ algorithm with short questions and hints guiding the process of deprescribing with a focus on last year of life c: Elements: ‐ life expectancy and trajectory of decline ‐ goals of care ‐ list of medication ‐ medication assessment (adherence, adverse reaction, indication, interactions) ‐ immediate cessation vs weaning or continuation on optimal dose ‐ follow‐up (adherence, adverse withdrawal effect, re‐emergence of symptoms, goals of care) ‐ repeat process |
| Jones et al 27 | 2013 | Algorithm |
a: “Medication review process” b: ‐ algorithm with questions guiding medication review leading to continuation, dose reduction or stopping of medicine. c: Elements: ‐ evidence‐based indication and dosage in relevant age group? ‐ balance benefits vs potential adverse effects ‐ replacing vital hormone? ‐ preventing rapid symptomatic deterioration? ‐ medicine for resolved condition or without effect? ‐ dose‐reduction without risk? ‐ in doubtful medication, interprofessional collaboration suggested |
| Meulendijk et al 33 | 2015 | Algorithm |
a: “The Systematic Tool to Reduce Inappropriate Prescribing (STRIP)‐Assistant”: b: ‐ integration of the guideline STRIP on polypharmacy (published 2012 in Dutch) with an electronic web‐based processing ‐ web‐application allows automated analysis of data with output to start, stop or alter medication c: Elements: ‐ STRIP refers to 5 steps: Medication history and assessment; pharmacotherapy review; pharmaceutical care plan; shared decision‐making; follow‐up and monitoring ‐ STRIP‐Assistant integrates this information together with data from guidelines on clinical interactions, double medication, contraindication, dosage, frequency, START/STOPP criteria, physical properties |
| Niehoff et al 23 | 2016 | Algorithm |
a: “Tool to Reduce Inappropriate Medications”: b: ‐ built of 2 applications: First application extracts patient data from electronic health record, second application is a set of clinical algorithms for medication evaluation, allowing an automated assessment ‐ finally a patient‐specific medication management feedback report is generated for clinicians c: Elements: ‐ data from health record: Age, chronic conditions, medication, sex ‐ chart review data: body mass index, renal function, diabetic status, blood pressure ‐ patient assessment: medication history, medication adherence, functional status, executive function, life expectancy, review of systems (falls, dizziness, constipation), side effects, management support ‐ evaluation of overtreatment of diabetes/hypertension, PIM, dosing, patient report of problems |
| Newton et al 22 | 1994 | Algorithm |
a: “The geriatric medication algorithm”: b: ‐ algorithm of questions culminating in discontinuing, substituting, dose‐adjusting, changing schedule/preparation of medication or educating patient/caregiver c: Elements: ‐ obtain medication list and orthostatic blood pressure ‐ question indication ‐ risk assessment (high risk drug, aggravating underlying conditions, atypical side effects, orthostatic hypotension, toxicity) ‐ question dosing ‐ look for drug interactions and side effects; simplify drug regimen ‐ consider patient's compliance (unclear regimen, compliance aid needed?) |
| Poudel et al 24 | 2016 | Algorithm |
a: “Algorithm of medication review in frail older people”: b: ‐ based on the identification of high‐risk medication according a newly synthesised list of PIMs ‐ algorithms culminates in continuation, changing or stopping of medication ‐ advice for specific withdrawal regimens and alternative medical and nonmedical management strategies given c: Elements: ‐ identify a high‐risk medication (newly synthesised PIM list); ‐ ascertain current and valid indication (previous trial of discontinuation?) ‐ assess symptomatic benefit and adverse drug events ‐ consider withdrawing, altering or continuing medications |
| Government of Catalonia, Ministry of Health et al 28 | 2015 | Combination of algorithm and step‐by‐step (3) |
a: “Medication management in the complex chronic patient” b: Built of an algorithm for detailed medication assessment (A) and a simple guiding frame of 3 deprescribing stages (B) c: Elements: A. “algorithm for medication clinical review in the complex chronic patient”: ‐ indication ‐ appropriateness (dose, duration, age, renal/hepatic function) ‐ effectiveness (therapeutic objectives) ‐ safety (overlaps, contraindications, drug related problems, prescribing cascade) ‐ Patient's perspective (management and coping, skills, patient's perception of situation) ‐ final advice to withdraw, adjust, replace or continue the drug B. “Deprescription stages”: 1) acknowledge the necessity and situation 2) prepare patient (evaluate patient, negotiate, reach consensus, plan withdrawal, prepare for adverse event) 3) withdraw medicinal product (prioritise, withdraw, evaluate) |
| Scott et al 26 | 2015 | Combination of algorithm and step‐by‐step (5) |
a: “The deprescribing protocol”: b: ‐ five sequential steps for deprescribing ‐ detailed and practical suggestions for every step ‐ integrated medication assessment algorithm culminating in continuing, discontinuing or restarting medication c: Elements: 1) ascertain all drugs and reasons for each 1 2) consider overall risk of drug‐induced harm 3) assess each drug (indication, prescribing cascade, actual or potential harm vs potential benefit, etc.) 4) prioritize drugs for discontinuation 5) implement discontinuation regimen and monitor patients |
| Starkey et al 29 | 2015 | Set of recommendations |
a: “Deprescribing: a practical guide”: b: Loose collections of narrative and listed recommendations on deprescribing c: Elements: ‐ drug assessment (indication, benefit, appropriateness, duration, adherence, prescribing cascade, evidence, treatment goals, ADR, redundancy, changed condition) ‐ stopping/withdrawal regimens (stepwise vs abruptly vs mixed approach) ‐ elaborative description of target population (elderly, frail, vulnerable, housebound patients with multiple drugs and/or shortened life expectancy and fit patients on polypharmacy but lack of indication) ‐ medication review with practical advice ‐ risk/benefit assessment with help of number needed to treat/harm |
The instruments' format: algorithm, step‐by‐step approach (number of steps), combinations of algorithm and step‐by‐step approach, narrative set of recommendations.
ADR, adverse drug reaction; PIM, potentially inappropriate medication.
Most publications broadly indicated older people/patients as the instrument's target group (14/16).
4.3. Quality assessment
None of the identified publications met our requirements of a formal statement. Eight publications described their developmental process at least in very general terms, but no official organization had stewardship for development or publication. 16 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Four publications were published by official organisations, but lacked a description of the developmental process. 27 , 28 , 29 , 30 Four publications neither described the instrument's development nor stated an affiliated official organization. 31 , 32 , 33 , 34
Some of the included tools had been evaluated in pilot studies or trials, e.g. Garfinkel et al. 35 and Newton et al. 22 However, here we have limited our review to the description of the respective tools.
4.4. Format of drug discontinuation instruments
Drug discontinuation instruments are presented in varying formats, including step‐by‐step approaches, algorithms or a set of recommendations in narrative form.
In step‐by‐step approaches, users are sequentially led through the process of medication management, evaluation and deprescribing by defined steps. This is typically illustrated by the publication of Scott et al. 25 with 10 sequential steps leading from drug history over assessment to implementation of the regimen. Five publications show this kind of approach (5/16; Table 1). 16 , 25 , 30 , 32 , 34 The number of steps varies from 3 to 10.
Using algorithms includes defined steps and instructions for the deprescribing process as well, but enable a more complex conditional course of drug discontinuation. Newton et al. 22 for example have applied their “geriatric medication evaluation algorithm” in a classical way. Here, questions regarding drug treatment and patient characteristics (input) are interlinked with yes and no, finally leading to a recommendation to discontinue, adjust or continue medication (output). Eight publications are merely based on algorithms (8/16; Table 1). 20 , 21 , 22 , 23 , 24 , 27 , 31 , 33 Two of these instruments have designed their algorithm electronically (web application). 23 , 33
Two publications illustrate their ideas in a combined way, paralleling a step‐by‐step and algorithmic (2/16; Table 1). 26 , 28 In both publications algorithms are used to specify medication assessment, while the step‐by‐step approach gives an overview of all steps in the entire deprescribing process.
One publication by Starkey 29 is characterised by a largely narrative approach comprising a set of recommendations, checklists and practical advice.
4.5. Components and classification of drug discontinuation instruments
In general, a detailed evaluation of the medication is an important cornerstone of the drug discontinuation process. This central stage may be part of a larger frame of a discontinuation management comprising the stages preparation, medication evaluation, decision‐making and implementation. Table 2 illustrates these 4 stages (I‐IV) and their corresponding components (1–12) as covered by the instruments that were included in this review.
TABLE 2.
The ingredients of the drug discontinuation instruments (sorted by type and year)
| Application type | Author | Year | Stages (I‐IV) of the drug discontinuation process and their components (1–12) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I. Preparation | II. Medication evaluation | III. Decision‐making | IV. Implementation | |||||||||||
| 1 Patient assessment | 2 Medication history | 3 Indication | 4 Risk | 5 Benefit assessment | 6 Symptoms vs preventive drugs | 7 Dosing | 8 Interactions/ADRs | 9 Prioritization | 10 SDM | 11 Plan + initiation | 12 Monitoring + support | |||
| 1.Framework | Bain et al 20 | 2008 | ||||||||||||
| Reeve et al 16 | 2014 | |||||||||||||
| Jansen et al 32 | 2016 | |||||||||||||
| 2.Focused drug assessment | Newton et al 22 | 1994 | ||||||||||||
| Garfinkel et al 21 | 2007 | |||||||||||||
| Jones et al 27 | 2013 | |||||||||||||
| Poudel et al 24 | 2016 | |||||||||||||
| 3. Comprehensive guideline | Woodward et al 34 | 2003 | ||||||||||||
| Hardy and Hilmer et al 31 | 2011 | |||||||||||||
| Scott et al 25 | 2012 | |||||||||||||
| Government of Catalonia MoH et al 26 | 2015 | |||||||||||||
| Meulendijk et al 33 | 2015 | |||||||||||||
| Scott et al 26 | 2015 | |||||||||||||
| Starkey et al 29 | 2015 | |||||||||||||
| Niehoff et al 23 | 2016 | |||||||||||||
| Tenni and Dunbabin et al 30 | 2016 | |||||||||||||
 = core component.
= core component.
 = component mentioned peripherally.
= component mentioned peripherally.
□ = not mentioned.
Legend (description of item; shown examples are potentially but not mandatory part of instrument)
Application type: category according type of application
Components:
‐ Patient assessment: patient assessment according e.g. life expectancy, frailty, cognitive impairment, comorbidities, measures (e.g. blood pressure, laboratory) nonadherence, personal perspective and goals
‐ Medication history: review of current medication, sometimes including check of compliance
Indication: check of clinical indication/condition
‐ Risk: assessment of risk/harm of drugs taken, sometimes including check of high‐risk medication and potentially inappropriate medication (PIM)
‐ Benefit: estimation of individual clinical benefit and utility,
‐ Symptomatic vs preventive drug: discrimination between symptomatic and preventive drugs, sometimes including assessment of time until benefit and risk of symptomatic deterioration;
‐ Dosing: check for dosing in consideration of patient's condition and organ functions
‐ Interactions/ADRs: check for symptoms or signs of current interactions or ADRs (adverse drug reactions/side effects)
‐ Prioritization: clinician and patient set medication, care and/or clinical priorities before deprescribing
‐ SDM: informed shared decision‐making process of discussion and consent for action
‐ Plan + initiation: action is planned and medication withdrawal is initiated
‐ Monitoring + support: process of follow‐up for monitoring and support
The majority of instruments describe a preparation stage (I) as prerequisite for the deprescribing process (13/16; Table 2). It assesses the current status of the patient and/or the medication history. Patient assessment may include life expectancy, frailty, cognitive impairment or individual goals of care and is often combined with the history of medication. This first stage is followed by a medication evaluation (stage II) that in turn may comprise up to 6 components: a check for the drug's clinical indication, assessment of its potential risk or manifest harm (sometimes including a check for potentially inappropriate medication), estimation of individual clinical benefit and utility, a discrimination of symptomatic and preventive drugs which may include time to benefit analysis, a check for proper dosing and, lastly, assessment of symptoms or signs of current interactions or adverse drug reactions/side effects. At least 3 of these 6 components are mentioned in each instrument (Table 2). In a third stage (III) of deprescribing, most instruments lead through the decision‐making process by addressing a prioritization of medication, care and/or clinical treatment (11/16). Usually a subsequent process of shared decision‐making (SDM) is suggested. Six of 16 instruments stress the importance of SDM as a core component of the deprescribing process. Four of these 6 instruments offer practical advice and support for the implementation of SDM. 26 , 29 , 32 , 34 Another 4/16 instruments at least mention the involvement of patients and caregivers in decision‐making processes. In the final fourth stage (IV) of deprescribing, guidance on practical implementation is given in most instruments. This usually involves planning and initiation of the deprescribing regimen (mentioned in 15/16 instruments). Nine of the publications place particular emphasis on sustainable implementation by suggesting a monitoring phase and further patient support. Another 3 publications at least mention this component.
All this considered, the instruments' individual components reveal a clear pattern of emphasis that is reflected by the number of core components of each stage (Table 2). Some instruments emphasize the stage of preparation, decision‐making and implementation of a deprescribing trial. 16 , 20 , 32 By contrast, there are a number of instruments that rather neglect these stages and instead stress the stage of medication evaluation. 21 , 22 , 24 , 27 However, the majority of instruments cover all stages in the process. 23 , 25 , 26 , 28 , 29 , 30 , 31 , 33 , 34 We have grouped the instruments accordingly, as displayed in Table 2. The emerging pattern reflects the different types of instruments that correspond to their area of application (see first column of Table 2: application type).
The first type represents general frameworks, which mainly offer behavioural guidance in medication discontinuation. 16 , 20 , 32 Here, the focus lies on the overarching task of medication management, including—at some point—discontinuation. The second type, however, emphasises the detailed assessment and evaluation of medication. 21 , 22 , 24 , 27 These instruments lack or only peripherally mention components up‐ or downstream of the deprescribing process (see Table 2).
The third type of instruments offers both at once–it presents a comprehensive description of the entire deprescribing process, including process‐focused advice as well as detailed instruction to evaluate the medication. 23 , 25 , 26 , 28 , 29 , 30 , 31 , 33 , 34
5. DISCUSSION
5.1. Main findings
Our analysis identified 16 generic instruments for drug discontinuation guidance for physicians treating patients with polypharmacy. These instruments revealed a variety of formats. We found complex algorithms, instructions with sequential single steps as well as instruments that offer a combination of both. Two instruments were constructed as electronic applications. The instruments had different focusses on the respective stages of deprescribing—preparation, drug evaluation, decision‐making and implementation.
From a user's point of view, the instruments may be grouped in 3 types according to their intended application: Frameworks offering behavioural guidance in medication discontinuation, instruments focussed on medication assessment, and instruments offering a comprehensive integration of both approaches into an all‐encompassing medication discontinuation guideline.
5.2. Comparison of literature
There are some earlier published reviews about deprescription instruments. 15 , 16 , 17 , 36 However, these have restricted their exploration of deprescribing tools to frail patients or those with limited life expectancy. 15 None of these reviews focuss on generic instruments or the primary care setting, nor does any of them include a search in guideline databases. 15 , 16 , 17 , 36 Most of these reviews are of narrative nature. 16 , 17 , 36
There is an abundance of guidelines and instruments dealing with polypharmacy in general. At a closer look, however, most of them only summarise and combine existing literature without creating new approaches, and the subject of drug discontinuation is often neglected or just superficially mentioned. 37 , 38 , 39 Furthermore, some guidelines attempt to cover the complete process of medicalisation, mentioning medication withdrawal as potentially indicated, but offer no further elaboration on this task. Some other guidelines give account for the latter, but only as comments on previously published instruments. 40 Neither of these works were included in this systematic review. In contrast, we have concentrated our search on instruments that provide explicit recommendations regarding the input and output of a drug discontinuation decision.
Apart from the sample of instruments analysed here, there are several well described and partly validated explicit instruments that give criterion‐based and drug‐specific advice on potentially inappropriate medication in elderly patients (Beers List, 12 START/STOPP criteria, 13 Priscus, 41 FORTA 42 ). These lists provide details on prespecified high‐risk drugs for frail and multimorbid older patients. Although these explicit instruments are useful to physicians in that they provide drug‐specific information for discontinuation, their focus on single drugs neglects other aspects of a comprehensive drug review, such as drug–drug and drug–disease interactions. Furthermore, their focus on defined drugs disregards the complexity of the deprescribing decision as a whole. Thus, these instruments alone cannot guide practitioners in their management of polypharmacy. Though physicians may benefit from well described implicit, i.e. judgment‐based tools for assessing medication and polypharmacy (e.g. Medication Appropriateness Index, 10 7‐steps, 43 No TEARS, 44 POM, 45 ARMOR 46 ), and there is detailed advice on how to evaluate medication, these tools fail to explicitly inform about the procedure of drug discontinuation; i.e. they are not output specified and thus not included in this review. In contrast, the instruments of focussed drug assessment described in this review (as mentioned second type) follow implicit rules as well, but offer distinct advice for stopping.
Some medication‐specific discontinuation guidelines offer important practical advice on specific withdrawal regimens, such as proton‐pump inhibitors, 47 cholinesterase inhibitors, 48 antihyperglycemic drugs 49 or nonsteroidal anti‐inflammatory drugs. 50 Although useful in this regard, they cannot facilitate decisions on individual discontinuation priorities in polypharmacy nor on contextual factors. Moreover, medication‐specific guidelines focus on the most popular drugs, ignoring other challenging substance classes that physicians require guidance for. The web‐project medstopper.com 51 offers practical withdrawal regimens as well, but offers further support by prioritising medications according to their potential benefit and harm. However, even this useful platform lacks an integration of individual patient preferences, contextual conditions and comorbidities.
Generally, most tools stay vague on how to integrate their time‐consuming approaches into daily practice, as on when and how clinicians should make use of the recommendations. Qualitative studies indicate that time spent on communication and deliberation still is perceived as major barrier to deprescribing practice. 52 The instrument developed by Jansen et al. faces the challenge of communication and shared decision‐making, but still remains unclear on practical issues such as implementation in clinical practice and visualisation of risk–benefit trade‐offs. 32
5.3. Strengths and limitations
The aim of our work was to identify instruments that offer both generic identification of unnecessary drugs (input) and advice in the process of deprescribing (output). Many instruments in the context of polypharmacy focus on only 1 of the 2 aspects (see previous section). Another challenge was to discriminate which instruments were based on genuine new development processes rather than on extraction or composition from earlier works. The main reason for this difficulty was that the developmental process was mostly just outlined. However, we carried out an extensive search based on a comprehensive syntax, searched multiple engines and guideline databases, included a grey literature search and performed snowball tracking to screen all previously published and related work. Furthermore, we used predefined inclusion and exclusion criteria, as well as the screening procedure that was undertaken by 2 independent researchers with controversies being critically discussed within the research team.
Since strategies for deprescribing are sometimes developed on local governmental initiatives, we might have missed some national or local deprescribing guidelines. However, we were able to screen and analyse publications in English, German and Swedish.
For some instruments, the precise analysis was challenging. This became obvious in the electronic applications STRIP‐Assistant 33 and Tool to Reduce Inappropriate Medications. 23 These tools were described in publication texts, and further material (pictures and videos) was available online. The original software and its underlying algorithm was not publicly available. Nevertheless, the descriptions at hand gave enough detailed impression of the software to allow the inclusion in this review.
Due to the descriptive character of our systematic review, we were not able to draw any conclusions about the instruments' validation, or estimate effectiveness and utilization rates of the instruments in daily practice. Systematically reviewing every validating study and trial of the included tools is beyond the scope of this review. However, the included instruments were internationally published and available to a broad practical and scientific audience, suggesting that utilisation of at least a few tools can be presumed.
5.4. Implications for future research, policy and practice
Despite the abundance of existing drug discontinuation instruments, there is obviously no tool so far that offers guidance in the face of all uncertainties and barriers in the deprescribing process for all drugs and all patients that may be seen in primary care. 52 In line with this, evidence of patient‐relevant effects of existing deprescribing interventions is still scarce. 53 Further deprescribing tools are under development and validation. 54 , 55 , 56 , 57 Of course, polypharmacy may to some extent be necessary and beneficial in several multimorbid patients. However, GPs, working in the front line of the health‐care system, are responsible to integrate patient preferences and guideline recommendations. Hence, further research focussing on the development of feasible, practical and user‐friendly deprescribing instruments is clearly needed. Until now, implicit medication assessment tools and explicit lists of criteria for deprescribing often go in parallel. However, both approaches are needed for every patient. Implicit strategies can serve as general guides in the evaluation of polypharmacy and identification of target medication, whereas explicit drug‐specific advice and withdrawal regimens are needed for hands‐on implementation of recommendations. Thus, these approaches need to be closely interwoven and integrated to offer true guidance and deploy their whole potential. Electronic applications may offer the solution for such a complex integral approach and may include further important features. By contrast, electronic tools carry the risk of overloading and confusing users with too much information instead of supplying useful information. Tools in development will have to face this conflict.
So far, only 2 of the proposed instruments in this review were of electronic design. Electronic applications may, if properly designed, be of assistance in several areas:
generic guidance through the deprescribing process
provision of medication specific risk–benefit information
informing patients, e.g. by visualisation of complex trade‐offs
integration of patient preferences in an ideally shared decision‐making
consideration of emotional factors and uncertainties of physicians and patients
offering practical advice in withdrawal regimens and implementation strategies
integration of prescribing management between multiple providers, e.g. offering digital data exchange.
From a methodological point of view, applications certainly need a transparent and traceable developmental process and robust validation. 58
Last but not least, complex management of medication and deprescribing requires health political back‐up: a legal environment must be created that allows for the resources that are required in a proper deprescribing process.
6. CONCLUSION
For drug discontinuation in patients with polypharmacy, multiple generic instruments exist. These instruments showed a variety of formats and could be assigned to 3 types according to their area of application. However, there is still a need for an advanced development of practical and user‐friendly tools. These should support physicians in communicational aspects, visualise trade‐offs and encourage patient involvement to meet patients' changing priorities and care goals.
8. COMPETING INTERESTS
As part of the study “Polypharmacy/MediQuit”, the authors themselves are developing a deprescribing tool for GPs and their patients. The authors declare to have no further conflicts of interest.
CONTRIBUTORS
U.J.W. and N.D.B. conceived and designed the study. M.M.C., N.G. and A.V. designed the search syntax and planned the details of the systematic review. M.M.C. and N.G. performed data search, screening and analysis. A.V. supervised the process. All authors discussed results and contributed to the final analysis. M.M.C. wrote the manuscript with support from the research team. All authors contributed to the manuscript. All authors read and approved the final manuscript.
Supporting information
TABLE S1 Characteristics of the included articles
7. ACKNOWLEDGEMENTS
This article originates from the project “Polypharmacy/MediQuit” at the Philipps‐University Marburg, Marburg, Germany and the Hannover Medical School, Hannover, Germany. The authors would like to thank the German Research Foundation DFG (DFG DO 513/11–1; JU 2992/2–1) for their financial support.
(((((((((((deprescri*[tiab]) OR Deprescriptions[MeSH Terms]) OR Potentially Inappropriate Medication List[MeSH Terms])) OR ((((((((((stop*[ti]) OR discontinu*[ti]) OR withdraw*[ti]) OR cessation[ti]) OR ceas*[ti]) OR reduc*[ti]) OR minimi*[ti]) OR priorit*[ti])) AND (((((((((((((((((((((instrument*[ti]) OR decision*[ti]) OR standard*[ti]) OR strateg*[ti]) OR criteria[ti]) OR criterion[ti]) OR tool*[ti]) OR guide*[ti]) OR rule*[ti]) OR list*[ti]) OR catalog*[ti]) OR algorithm*[ti]) OR policy[ti]) OR manual[ti]) OR procedure*[ti]) OR proceeding*[ti]) OR practice*[ti]) OR recommendation*[ti]) OR approach[ti]) OR pathway*[ti]) OR statement*[ti]))) OR ((((((((((stop*[ti]) OR discontinu*[ti]) OR withdraw*[ti]) OR cessation[ti]) OR ceas*[ti]) OR reduc*[ti]) OR minimi*[ti]) OR priorit*[ti])) AND (((((((((((medication*[ti]) OR medicine*[ti]) OR drug*[ti]) OR pharmaceutical*[ti]) OR pharmacological[ti]) OR pharmacotherapy[ti]) OR pill*[ti]) OR multimedication*[ti]) OR multi‐medication*[ti]) OR polypharmac*[ti]) OR poly‐pharmac*[ti]))) AND ((((((((((((((("general practitioner"[TIAB]) OR "general practitioners"[TIAB]) OR "general practice"[TIAB]) OR "family practitioners"[TIAB]) OR "family practitioner"[TIAB]) OR "family medicine"[TIAB]) OR "family physician"[TIAB]) OR "family physicians"[TIAB]) OR "family doctor"[TIAB]) OR "family doctors"[TIAB]) OR "primary care"[TIAB]) OR "family practice"[TIAB])) OR ((((("general practice"[Affiliation]) OR "family practice*"[Affiliation]) OR "family medicine"[Affiliation]) OR "primary care"[Affiliation]) OR community[Affiliation])) OR (((((("Family Practice"[Mesh]) OR "Physicians, Family"[Mesh]) OR "Primary Health Care"[Mesh]) OR "General Practice"[Mesh]) OR "General Practitioners"[Mesh]) OR "Physicians, Primary Care"[Mesh]))) NOT ((smok*[ti]) OR tobacco*[ti]))))) NOT (((((((((((deprescri*[tiab]) OR Deprescriptions[MeSH Terms]) OR Potentially Inappropriate Medication List[MeSH Terms])) OR ((((((((((stop*[ti]) OR discontinu*[ti]) OR withdraw*[ti]) OR cessation[ti]) OR ceas*[ti]) OR reduc*[ti]) OR minimi*[ti]) OR priorit*[ti])) AND (((((((((((((((((((((instrument*[ti]) OR decision*[ti]) OR standard*[ti]) OR strateg*[ti]) OR criteria[ti]) OR criterion[ti]) OR tool*[ti]) OR guide*[ti]) OR rule*[ti]) OR list*[ti]) OR catalog*[ti]) OR algorithm*[ti]) OR policy[ti]) OR manual[ti]) OR procedure*[ti]) OR proceeding*[ti]) OR practice*[ti]) OR recommendation*[ti]) OR approach[ti]) OR pathway*[ti]) OR statement*[ti]))) OR ((((((((((stop*[ti]) OR discontinu*[ti]) OR withdraw*[ti]) OR cessation[ti]) OR ceas*[ti]) OR reduc*[ti]) OR minimi*[ti]) OR priorit*[ti])) AND (((((((((((medication*[ti]) OR medicine*[ti]) OR drug*[ti]) OR pharmaceutical*[ti]) OR pharmacological[ti]) OR pharmacotherapy[ti]) OR pill*[ti]) OR multimedication*[ti]) OR multi‐medication*[ti]) OR polypharmac*[ti]) OR poly‐pharmac*[ti]))) AND ((((((((((((((("general practitioner"[TIAB]) OR "general practitioners"[TIAB]) OR "general practice"[TIAB]) OR "family practitioners"[TIAB]) OR "family practitioner"[TIAB]) OR "family medicine"[TIAB]) OR "family physician"[TIAB]) OR "family physicians"[TIAB]) OR "family doctor"[TIAB]) OR "family doctors"[TIAB]) OR "primary care"[TIAB]) OR "family practice"[TIAB])) OR ((((("general practice"[Affiliation]) OR "family practice*"[Affiliation]) OR "family medicine"[Affiliation]) OR "primary care"[Affiliation]) OR community[Affiliation])) OR (((((("Family Practice"[Mesh]) OR "Physicians, Family"[Mesh]) OR "Primary Health Care"[Mesh]) OR "General Practice"[Mesh]) OR "General Practitioners"[Mesh]) OR "Physicians, Primary Care"[Mesh]))) NOT ((smok*[ti]) OR tobacco*[ti])))) AND ((Addresses[ptyp] OR Autobiography[ptyp] OR Biography[ptyp] OR Case Reports[ptyp] OR Comment[sb] OR Dictionary[ptyp] OR Festschrift[ptyp] OR Historical Article[ptyp] OR Interactive Tutorial[ptyp] OR Portraits[ptyp] OR Scientific Integrity Review[ptyp] OR Twin Study[ptyp])))
Michiels‐Corsten M, Gerlach N, Schleef T, Junius‐Walker U, Donner‐Banzhoff N, Viniol A. Generic instruments for drug discontinuation in primary care: A systematic review. Br J Clin Pharmacol. 2020;86:1251–1266. 10.1111/bcp.14287
PI statement: The authors confirm that the Principal Investigator for this paper is Annika Viniol and that she had direct clinical responsibility.
Since no interventions were applied, nor human participants or animals were involved in this study, we did not request the university's ethics committee for approval.
REFERENCES
- 1. United Nations, Department of Economic and Social Affairs, Population Division . World Population Prospects: The 2017 Revision, Volume I: Comprehensive Tables. 2017. https://esa.un.org/unpd/wpp/Publications/Files/WPP2017_Volume-I_Comprehensive-Tables.pdf. Accessed 25 June 2019.
- 2. van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity. Eur J Gen Pract. 1996;2:65‐70. 10.3109/13814789609162146 [DOI] [Google Scholar]
- 3. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross‐sectional study. Lancet. 2012;380(9836):37‐43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 4. Masnoon N, Shakib S, Kalisch‐Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230 10.1186/s12877-017-0621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao L, Maidment I, Matthews FE, Robinson L, Brayne C, Medical Research Council Cognitive Function and Ageing Study . Medication usage change in older people (65+) in England over 20 years: findings from CFAS I and CFAS II. Age Ageing. 2018;47(2):220‐225. 10.1093/ageing/afx158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999‐2012. JAMA. 2015;314(17):1818‐1830. 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging. 1999;14(2):141‐152. 10.2165/00002512-199914020-00005 [DOI] [PubMed] [Google Scholar]
- 8. Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4(12):e006544 10.1136/bmjopen-2014-006544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254‐1268. 10.1111/bcp.12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045‐1051. 10.1016/0895-4356(92)90144-C [DOI] [PubMed] [Google Scholar]
- 11. Spinewine A, Schmader KE, Barber N, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173‐184. 10.1016/S0140-6736(07)61091-5 [DOI] [PubMed] [Google Scholar]
- 12. By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel . American Geriatrics Society 2019 updated AGS Beers criteria® for potentially inappropriate medication use in older adults: 2019 AGS BEERS CRITERIA® UPDATE EXPERT PANEL. J Am Geriatr Soc. 2019;67(4):674‐694. 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 13. O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213‐218. 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lavan AH, Gallagher P, Parsons C, O'Mahony D. STOPPFrail (screening tool of older persons prescriptions in frail adults with limited life expectancy): consensus validation. Age Ageing. 2017;46:600‐607. 10.1093/ageing/afx005 [DOI] [PubMed] [Google Scholar]
- 15. Thompson W, Lundby C, Graabæk T, et al. Tools for Deprescribing in frail older persons and those with limited life expectancy: a systematic review. J Am Geriatr Soc. 2018;0(1):172‐180. 10.1111/jgs.15616 [DOI] [PubMed] [Google Scholar]
- 16. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014;78(4):738‐747. 10.1111/bcp.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scott I, Anderson K, Freeman C. Review of structured guides for deprescribing. Eur J Hosp Pharm. 2017;24(1):51‐57. 10.1136/ejhpharm-2015-000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NICE ‐ National Institute for Health and Care Excellence . The guidelines manual. 2012. [PubMed]
- 19. World Health Organization . WHO handbook for guideline development. 2014.
- 20. Bain KT, Holmes HM, Beers MH, Maio V, Handler SM, Pauker SG. Discontinuing medications: a novel approach for revising the prescribing stage of the medication‐use process. J Am Geriatr Soc. 2008;56(10):1946‐1952. 10.1111/j.1532-5415.2008.01916.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garfinkel D, Zur‐Gil S, Ben‐Israel J. The war against polypharmacy: a new cost‐effective geriatric‐palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J IMAJ. 2007;9(6):430‐434. [PubMed] [Google Scholar]
- 22. Newton PF, Levinson W, Maslen D. The geriatric medication algorithm. J Gen Intern Med. 1994;9(3):164‐167. 10.1007/BF02600035 [DOI] [PubMed] [Google Scholar]
- 23. Niehoff KM, Rajeevan N, Charpentier PA, Miller PL, Goldstein MK, Fried TR. Development of the tool to reduce inappropriate medications (TRIM). Pharmacotherapy. 2016;36(6):694‐701. 10.1002/phar.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poudel A, Ballokova A, Hubbard RE, et al. Algorithm of medication review in frail older people: focus on minimizing the use of high‐risk medications. Geriatr Gerontol Int. 2016;16(9):1002‐1013. [DOI] [PubMed] [Google Scholar]
- 25. Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations. Am J Med. 2012;125:529‐37.e4. 10.1016/j.amjmed.2011.09.021 [DOI] [PubMed] [Google Scholar]
- 26. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of Deprescribing. JAMA Intern Med. 2015;175(5):827‐834. 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 27. Emyr Jones for the NHS Wales . Polypharmacy‐ Guidance for prescribing in the frail adults. 2013.
- 28. Government of Catalonia (Ester Amado). Ministry of Health . Rational drug use. Medication management in the complex chronic patient: reconciliation, revision, deprescription and adherence.2015.
- 29. Starkey V, NHS Southern Derbyshire CCG Medicines Management Team . Deprescribing: A Practical Guide. 2015.
- 30. Tenni P, Dunbabin D. A Guide To Deprescribing ‐ General Information. Prim Health Tasamania ‐ Deprescribing Clin Ref Group. 2016.
- 31. Hardy JE, Hilmer SN. Deprescribing in the last year of life. J Pharm Pract Res. 2011;41:146‐151. 10.1002/j.2055-2335.2011.tb00684.x [DOI] [Google Scholar]
- 32. Jansen J, Naganathan V, Carter SM, et al. Too much medicine in older people? Deprescribing through shared decision making. BMJ. 2016;353:i2893 10.1136/bmj.i2893 [DOI] [PubMed] [Google Scholar]
- 33. Meulendijk MC, Spruit MR, Drenth‐van Maanen AC, et al. Computerized decision support improves medication review effectiveness: an experiment evaluating the STRIP Assistant's usability. Drugs Aging. 2015;32(6):495‐503. 10.1007/s40266-015-0270-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woodward MC. Deprescribing: achieving better health outcomes for OlderPeople through reducing medications. J Pharm Pract Res. 2003;33:323‐328. 10.1002/jppr2003334323 [DOI] [Google Scholar]
- 35. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648‐1654. [DOI] [PubMed] [Google Scholar]
- 36. Page AT, Potter K, Clifford R, Etherton‐Beer C. Deprescribing in older people. Maturitas. 2016;91:115‐134. 10.1016/j.maturitas.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 37. MacBride‐Stewart S. NHS GG&C Polypharmacy Strategy. 2012.
- 38. NICE . Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. 2015. [PubMed]
- 39. Scottish Government Model of Care Polypharmacy Working Group . Polypharmacy Guidance. 2015.
- 40. Leitliniengruppe Hessen . Hausärztliche Leitlinie Multimedikation (S2e; Version 1.09). 2014.
- 41. Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Ärztebl Int. 2010;107(31‐32):543‐551. 10.3238/arztebl.2010.0543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pazan F, Weiss C, Wehling M, FORTA . The EURO‐FORTA (fit fOR the aged) list: international consensus validation of a clinical tool for improved drug treatment in older people. Drugs Aging. 2018;35(1):61‐71. 10.1007/s40266-017-0514-2 [DOI] [PubMed] [Google Scholar]
- 43. Scottish Government Polypharmacy Model of Care Group . Polypharmacy Guidance, Realistic Prescribing. 3rd ed. Edinburgh: Scottish Government; 2018. https://www.therapeutics.scot.nhs.uk/wp-content/uploads/2018/04/Polypharmacy-Guidance-2018 [Google Scholar]
- 44. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434 10.1136/bmj.329.7463.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maanen ACD, van Marum RJ, Knol W, et al. Prescribing optimization method for improving prescribing in elderly patients receiving polypharmacy. Drugs Aging. 2009;26(8):687‐701. 10.2165/11316400-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 46. Haque R. ARMOR: a tool to evaluate polypharmacy in elderly persons. Ann Long‐Term Care. 2009;17:26‐30. [Google Scholar]
- 47. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence‐based clinical practice guideline. Can Fam Physician. 2017;63(5):354‐364. [PMC free article] [PubMed] [Google Scholar]
- 48. Office P. Evidence‐based Clinical Practice Guideline for Deprescribing Cholinesterase Inhibitors and Memantine. 2018. http://sydney.edu.au/medicine/cdpc/documents/resources/deprescribing-guideline.pdf. Accessed 15 March 2019.
- 49. Farrell B, Black C, Thompson W, et al. Deprescribing antihyperglycemic agents in older persons: evidence‐based clinical practice guideline. Can Fam Physician. 2017;63(11):832‐843. [PMC free article] [PubMed] [Google Scholar]
- 50. A Guide to deprescribing NSAIDs. 2016. https://www.primaryhealthtas.com.au/wp-content/uploads/2018/09/A-Guide-to-Deprescribing-NSAIDs.pdf. Accessed 15 March 2019.
- 51. James McCormack . et al. MedStopper.com. http://medstopper.com/. Accessed 15 March 2019.
- 52. Wallis KA, Andrews A, Henderson M. Swimming against the tide: primary care Physicians' views on Deprescribing in everyday practice. Ann Fam Med. 2017;15(4):341‐346. 10.1370/afm.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Page AT, Clifford RM, Potter K, Schwartz D, Etherton‐Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2016;82(3):583‐623. 10.1111/bcp.12975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sönnichsen A, Trampisch US, Rieckert A, et al. Polypharmacy in chronic diseases–reduction of inappropriate medication and adverse drug events in older populations by electronic decision support (PRIMA‐eDS): study protocol for a randomized controlled trial. Trials. 2016;17:57 10.1186/s13063-016-1177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Charissa Ee . Deprescribing of Symptomatic Medications in Rehabilitative or Subacute Care Patients ‐ Full Text View ‐ ClinicalTrials.gov. 2017. https://clinicaltrials.gov/ct2/show/NCT03354845. Accessed 13 June 2019.
- 56. Gerlach N, Michiels‐Corsten M, Viniol A, et al. Development of the MediQuit deprescribing tool for polypharmacy ‐ results of a panel‐test [Entwicklung der MediQuit‐Absetzhilfe bei Polypharmazie – Ergebnisse eines Panel‐Tests]. German Medical Science GMS Publishing House, p Doc18degam035. 2018.
- 57. Team Approach to Polypharmacy Evaluation and Reduction ‐ Full Text View ‐ ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02942927. Accessed 17 January 2020.
- 58. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Characteristics of the included articles


