Abstract
Objectives:
To examine guideline concordance across a national sample and determine the relationship between socioeconomic factors, use of recommended postoperative adjuvant therapy, and outcomes for patients with resected pN1 or pN2 non-small cell lung cancer.
Methods:
All margin-negative pT1–3 N1–2 Mo non-small cell lung cancers treated with lobectomy or pneumonectomy without induction therapy in the National Cancer Database between 2006 and 2013 were included. Use of guideline-concordant adjuvant treatment, defined as chemotherapy for pN1 disease and chemotherapy with or without radiation for pN2 disease, was examined. Multivariable regression models were developed to determine associations of clinical factors with guideline adherence. Survival was estimated using Kaplan-Meier and Cox proportional hazard analyses.
Results:
Of 13,462 patients, 10,113 had pN1 disease and 3349 had pN2 disease. Guideline-concordant adjuvant therapy was used in 6844 (67.7%) patients with pN1 disease and 2622 (78.3%) patients with pN2 disease. After multivariable adjustment, insurance status, older age, pneumonectomy, readmission, and longer postoperative stays were associated with lower likelihood of guideline concordance. Conversely, increased education level, later year of diagnosis, and greater nodal stage were associated with greater concordance. Overall, patients treated with guideline-concordant therapy had superior survival (5-year survival: 51.6 vs 36.0%; hazard ratio, 0.66; 95% confidence interval, 0.62–0.70, P < .001).
Conclusions:
Socioeconomic factors, including insurance status and geographic region, are associated with disparities in use of adjuvant therapy as recommended by National Comprehensive Cancer Network guidelines. These disparities significantly impact patient survival. Future work should focus on improving access to appropriate adjuvant therapies among the under insured and socioeconomically disadvantaged.
Keywords: lung cancer, NSCLC, disparities, socioeconomic factors, adjuvant therapy
Graphical Abstract
Disparities in guideline-concordant adjuvant therapy impact outcomes in lung cancer.
Lung cancer remains the single leading cause of cancer deaths in the United States for both men and women, accounting for an estimated 154,050 deaths in 2018.1 Of the 234,030 newly diagnosed cases each year, 84% are non-small cell lung cancer (NSCLC).1,2 Although survival has improved in the past 2 decades, prognosis remains poor, with only 18.6% of patients surviving to 5 years after diagnosis.2 Since the 1980s, differences in cancer-specific survival have been attributed to clinical factors as well as demographic and socioeconomic factors, such as age, race, place of residence, and health insurance status.1,3–6
The National Comprehensive Cancer Network (NCCN) has established clinical guidelines for the treatment of NSCLC, which have been shown to improve survival.7,8 However, several studies suggest that guideline-concordant therapy is not uniformly delivered.3,7,9,10 Multiple studies have demonstrated disparities in receipt of appropriate care, including surgical treatment, chemotherapy, and radiation, with rates of compliance with guideline-concordant adjuvant therapy ranging from 23% to 71%.4,7,10,11 Factors found to be associated with influencing appropriate receipt of treatment include age, race, and socioeconomic status as well as insurance status and area of residency.10,12–15
Although previous studies provide some insight into disparities in cancer treatment, they are either based on regional or state cohorts, focus on receipt of surgical treatment or patients who have late-stage lung cancer, or do not evaluate receipt of adjuvant therapy for patients with early-stage disease. This study aims to address this knowledge gap by examining guideline concordance across a national sample and to determine the relationship between socioeconomic factors, use of recommended adjuvant therapies, and long-term outcomes for patients with pN1 or pN2 NSCLC.
METHODS
The Duke University Institutional Review Board approved this retrospective review of patients diagnosed with NSCLC in the 2016 National Cancer Database (NCDB) Participant Use Data File, which collects data from more than 1500 centers. The database is estimated to capture 70% of all newly diagnosed cases of cancer in the United States and Puerto Rico and currently contains approximately 34 million patient records. The NCDB records clinical stage using the American Joint Committee on Cancer (AJCC) staging criteria concordant with the year of diagnosis, and the AJCC 6th and 7th editions were therefore used to identify patients for inclusion. As the staging paradigm for regional lymph node metastases did not change between the 6th and 7th editions, no attempts to manually recode clinical staging data were attempted.
Patients were identified by International Classification of Diseases for Oncology, 3rd Edition codes for non-small cell pathologies, and procedure types of lobectomy (Facility Oncology Registry Data Standards code 30–33) and pneumonectomy (Facility Oncology Registry Data Standards code 55–65) between 2006 and 2013 were included. Only patients with clinical T1–3 N0–1 NSCLC who underwent R0 surgical resection without induction therapy and found to have pathologic N1 or N2 disease were selected for analysis. Patients who died within 30 days of surgery or “prior to planned chemotherapy” were excluded a priori, as were patients for whom chemotherapy was “contraindicated due to patient risk factors” (Figure 1, A). Since patient participation in clinical trials is not reliably coded in the NCDB, this status was not a consideration in selecting the study cohort.
FIGURE 1.
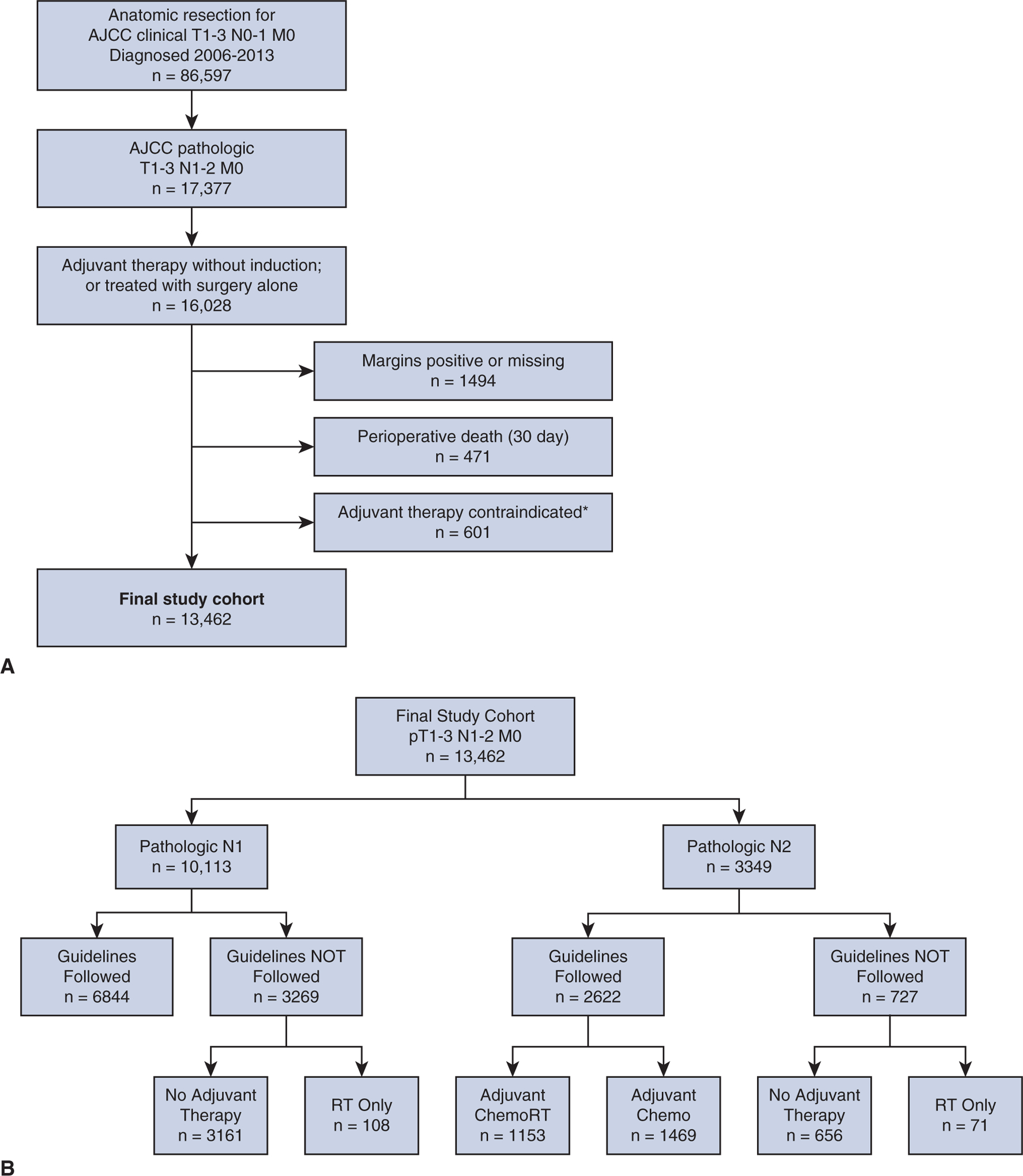
A, Flow diagram showing patient selection and final study cohort using study inclusion and exclusion criteria. B, Flow diagram showing study cohorts by pathologic nodal status, broken down by concordance with appropriate National Comprehensive Cancer Network guidelines for adjuvant therapy following surgery, defined as chemotherapy for pathologic N1 disease, and chemotherapy (chemo) with or without radiation (RT) for pN2 disease. AJCC, American Joint Committee on Cancer.
Cases were then stratified by pathologic node status as pN1 or pN2. Guideline-concordant adjuvant therapy was defined as receipt of chemotherapy for pN1 disease, and chemotherapy with or without radiation for pN2 disease. For the purposes of this study, the time between resection and receipt of adjuvant therapy was not considered, merely whether it was administered or not. Similarly, for radiation therapy, a minimum dosimetry amount was not considered in the analysis, as the clinical decision to treat was deemed to be more relevant than the dose delivered. Since Commission on Cancer (CoC) programs are required to identify treatment received from all sources regardless of where the treatment was performed, the analysis was not restricted to the site at which surgery was performed. The treatment facility categories designated in the NCDB include community cancer programs, comprehensive community cancer programs, and academic comprehensive cancer programs, which differ in the number of new cancer cases each year, cancer-related research, and participation in resident training.
Baseline univariate comparisons were made using the Pearson χ2 test on categorical variables and Student t test on continuous variables. To estimate the independent effect of various tumor and patient factors on guideline concordance, a multivariable logistic regression model was constructed that included the following variables: patient age, sex, race, Charlson-Deyo comorbidity score, median education and income level in the patient’s zip code, insurance status, AJCC pathologic T and N stage, extent of surgical resection, postoperative length of stay (LOS), unplanned hospital readmission, and type of treating facility. Long-term survival was estimated using the Kaplan-Meier method and Cox proportional hazard analysis adjusting for age, year of diagnosis, sex, race, comorbidity score, education and income levels, insurance status, disease stage, extent of surgery, LOS, readmission, facility type, and receipt of guideline-concordant adjuvant therapy.
To further examine the effect of insurance status, patients were then restratified by type of insurance (private, Medicare/Medicaid, other government, uninsured, or unknown), and univariate comparisons on continuous variables treatment details and outcomes were made using the Pearson χ2 test on categorical variables and Student t test.
Missing data were handled with complete-case analysis, given the completeness of the NCDB data for the years included in this study. An affirmative decision was made to control for type I error at the level of the comparison, and P values less than .05 were considered statistically significant for all comparisons. All analyses were performed using R version 3.4.0 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 13,462 patients were identified who met study inclusion and exclusion criteria. Of these, 10,113 (75.1%) had pN1 disease and 3349 (24.9%) had pN2 disease. Overall, 70.3% of patients received guideline-concordant adjuvant therapy as defined, with 6844 (67.7%) patients with pN1 and 2622 (78.3%) patients with pN2 disease receiving appropriate therapy (Table 1, Figure 1, B). Of the 3269 (32.3%) patients with pN1 disease who did not receive guideline-concordant care, 3161 (96.7%) received no adjuvant therapy after surgery, and 108 (3.3%) received adjuvant radiation therapy only without chemotherapy. Of the 2622 (78.3%) patients with pN2 disease who received guideline-concordant therapy, 1469 (56.0%) received adjuvant chemotherapy alone whereas 1153 (44.0%) received both chemotherapy and radiation. Of the 727 (21.7%) patients with pN2 disease who did not receive guideline-concordant therapy, 656 (90.2%) received no adjuvant therapy after surgery and 71 (9.8%) received adjuvant radiation therapy alone without chemotherapy (Figure 1, B).
TABLE 1.
Baseline characteristics by receipt of guideline-concordant versus guideline-discordant care
| Total (n = 13,462) | Guideline-discordant (n = 3996) | Guideline-concordant (n = 9466) | P value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y | <.001 | |||
| <50 | 783 (5.8%) | 158 (4.0%) | 625 (6.6%) | |
| 50–59 | 2960 (22.0%) | 558 (14.0%) | 2402 (25.4%) | |
| 60–69 | 4844 (36.0%) | 1197 (30.0%) | 3647 (38.5%) | |
| 70–79 | 3946 (29.3%) | 1477 (37.0%) | 2469 (26.1%) | |
| >79 | 929 (6.9%) | 606 (15.2%) | 323 (3.4%) | |
| Female | 6523 (48.5%) | 1855 (46.4%) | 4668 (49.3%) | .002 |
| Race | .093 | |||
| White | 11,728 (87.7%) | 3503 (88.4%) | 8225 (87.4%) | |
| Black | 1213 (9.1%) | 327 (8.3%) | 886 (9.4%) | |
| Other | 432 (3.2%) | 133 (3.4%) | 299 (3.2%) | |
| Charlson Comorbidity Score | <.001 | |||
| 0 | 6939 (51.5%) | 1939 (48.5%) | 5000 (52.8%) | |
| 1 | 4815 (35.8%) | 1489 (37.3%) | 3326 (35.1%) | |
| 2+ | 1708 (12.7%) | 568 (14.2%) | 1140 (12.0%) | |
| Education quartile | <.001 | |||
| Bottom | 2166 (16.3%) | 722 (18.3%) | 1444 (15.4%) | |
| Second | 3723 (28.0%) | 1127 (28.6%) | 2596 (27.7%) | |
| Third | 4454 (33.5%) | 1256 (31.9%) | 3198 (34.1%) | |
| Top | 2963 (22.3%) | 834 (21.2%) | 2129 (22.7%) | |
| Income quartile | .001 | |||
| Bottom | 2482 (18.7%) | 805 (20.5%) | 1677 (17.9%) | |
| Second | 3233 (24.3%) | 974 (24.8%) | 2259 (24.1%) | |
| Third | 3704 (27.9%) | 1075 (27.3%) | 2629 (28.1%) | |
| Top | 3879 (29.2%) | 1081 (27.5%) | 2798 (29.9%) | |
| Insurance | <.001 | |||
| Private | 4895 (36.4%) | 1042 (26.1%) | 3853 (40.7%) | |
| Medicare/Medicaid | 7953 (59.1%) | 2778 (69.5%) | 5175 (54.7%) | |
| Other government | 145 (1.1%) | 51 (1.3%) | 94 (1.0%) | |
| Unknown | 134 (1.0%) | 32 (0.8%) | 102 (1.1%) | |
| Uninsured | 335 (2.5%) | 93 (2.3%) | 242 (2.6%) | |
| Tumor characteristics | ||||
| Tumor size, mm, median (IQR) | 33 (23.0, 48.0) | 34 (24.8, 49.0) | 33 (23.0, 48.0) | .318 |
| Clinical T stage | .018 | |||
| T1 | 5710 (42.4%) | 1672 (41.8%) | 4038 (42.7%) | |
| T2 | 6634 (49.3%) | 2027 (50.7%) | 4607 (48.7%) | |
| T3 | 1118 (8.3%) | 297 (7.4%) | 821 (8.7%) | |
| Clinical N stage | .042 | |||
| N0 | 8076 (60.0%) | 2344 (58.7%) | 5732 (60.6%) | |
| N1 | 5386 (40.0%) | 1652 (41.3%) | 3734 (39.4%) |
IQR, Interquartile range.
Patients receiving guideline-discordant and -concordant care were similar in racial background and tumor size but dissimilar in age, sex, comorbidity status, education and income levels, insurance status, and clinical stage (Table 1). Patients receiving discordant care had further median distance to the treating facility, increased number of days to definitive surgery, lower rates of adjuvant radiation, lower pathologic disease stage (T and N), fewer lymph nodes examined, greater rates of readmission, and longer hospital LOS (Table 2).
TABLE 2.
Treatment and outcomes by receipt of guideline-concordant versus guideline-discordant care
| Total (n = 13,462) | Guideline-discordant (n = 3996) | Guideline-concordant (n = 9466) | P value | |
|---|---|---|---|---|
| Facility characteristics | ||||
| Median distance to facility, miles, (IQR) | 11.2 (4.8,27.6) | 12.5 (4.9, 32.2) | 10.8 (4.8, 25.6) | <.001 |
| Facility type | .13 | |||
| Community program | 1034 (8.7%) | 286 (8.0%) | 748 (9.0%) | |
| Comprehensive community program | 6112 (51.2%) | 1834 (51.0%) | 4278 (51.3%) | |
| Research/academic program | 4785 (40.1%) | 1474 (41.0%) | 3311 (39.7%) | |
| Treatment characteristics | ||||
| Extent of resection | .642 | |||
| Lobectomy | 11,589 (86.1%) | 3431 (85.9%) | 8158 (86.2%) | |
| Pneumonectomy | 1873 (13.9%) | 565 (14.1%) | 1308 (13.8%) | |
| Median days to definitive surgery (IQR) | 32 (15,51) | 34 (14,55) | 31 (15,50) | <.001 |
| Adjuvant XRT | 2068 (15.4%) | 179 (4.5%) | 1889 (20.0%) | <.001 |
| Adjuvant chemotherapy | 9466 (70.3%) | 0 (0%) | 9466 (100.0%) | <.001 |
| Surgical endpoints | ||||
| Pathologic T stage | .019 | |||
| T1 | 4154 (30.9%) | 1227 (30.7%) | 2927 (30.9%) | |
| T2 | 7373 (54.8%) | 2249 (56.3%) | 5124 (54.1%) | |
| T3 | 1586 (11.8%) | 430 (10.8%) | 1156 (12.2%) | |
| T4 | 349 (2.6%) | 90 (2.3%) | 259 (2.7%) | |
| Pathologic N stage | <.001 | |||
| N1 | 10,113 (75.1%) | 3269 (81.8%) | 6844 (72.3%) | |
| N2 | 3349 (24.9%) | 727 (18.2%) | 2622 (27.7%) | |
| Median number lymph nodes examined, n (IQR) | 11 (7, 18) | 11 (7,17) | 11 (7, 18) | .004 |
| 30-d unplanned readmission | 563 (4.3%) | 225 (5.7%) | 338 (3.6%) | <.001 |
| Median hospital LOS, d, (IQR) | 6 (4, 8) | 6 (4, 9) | 5 (4, 7) | <.001 |
IQR, Interquartile range; XRT, radiation therapy; LOS, length of stay.
After multivariable adjustment, older age, insurance status, pneumonectomy, longer postoperative stays, and readmission were associated with significantly lower likelihood of guideline concordance, whereas increased level of education and greater pathologic N stage were associated with significantly greater rates of guideline concordance (Table 3). Patients with pN2 disease were more likely to receive guideline-concordant therapy than those with pN1 disease (odds ratio [OR], 1.67; 95% confidence interval [CI], 1.50–1.86, P < .001). The overall group included 36.4% of patients who were privately insured, 59.1% on Medicare or Medicaid, 1.0% with unknown insurance, and 2.5% who were uninsured. Treatment characteristics and outcomes varied between groups stratified by insurance status, including extent of resection, days to definitive surgery, adjuvant chemoradiation and chemotherapy rates, and hospital LOS (Table E1). Compared with private insurance status, there was no significant difference in receipt of guideline-concordant care for patients with Medicare/Medicaid. However, uninsured patients were significantly less likely to receive guideline-concordant therapy (OR, 0.54; CI, 0.40–0.72, P < .001), as were patients with other government insurance (OR, 0.64; CI, 0.42–0.99, P = .04) (Table 3). There was also no difference in the rates of guideline-concordant care among treatment facility category. However, receipt of guideline-concordant therapy was noted to vary by geographical region (Figure 2).
TABLE 3.
Multivariable analysis of factors associated with guideline-concordant adjuvant therapy in patients with resected pathologic T1–3 pN1–2 M0 non-small cell lung cancer
| Odds ratio | 95% confidence interval (lower, upper) | P value | |
|---|---|---|---|
| Age (per decade) | 0.53 | (0.50, 0.57) | <.001 |
| Year of diagnosis (ref = 2006) | 1.11 | (1.09, 1.13) | <.001 |
| Female sex | 1.04 | (0.95, 1.13) | .44 |
| Race (ref = white) | |||
| Black | 1.00 | (0.85, 1.18) | .99 |
| Other | 0.90 | (0.70, 1.15) | .41 |
| Charlson Comorbidity Score (ref = 0) | |||
| 1 | 0.97 | (0.88, 1.07) | .53 |
| 2+ | 0.90 | (0.79, 1.03) | .14 |
| Education (per quartile) | 1.07 | (1.01, 1.14) | .02 |
| Income (per quartile) | 1.05 | (0.99, 1.11) | .09 |
| Insurance status (ref = private) | |||
| Medicare/Medicaid | 0.90 | (0.81, 1.01) | .07 |
| Other government | 0.64 | (0.42, 0.99) | .04 |
| Unknown | 1.23 | (0.76, 1.99) | .40 |
| Uninsured | 0.54 | (0.40, 0.72) | <.001 |
| AJCC pathologic T stage | 1.06 | (0.99, 1.12) | .10 |
| AJCC pathologic N2 (vs Nl) | 1.67 | (1.50, 1.86) | <.001 |
| Pneumonectomy (vs lobectomy) | 0.78 | (0.68, 0.89) | <.001 |
| Length of stay (per day) | 0.96 | (0.95, 0.96) | <.001 |
| Readmission | 0.60 | (0.49, 0.72) | <.001 |
| Facility type (ref = community) | |||
| Comprehensive community program | 1.00 | (0.84, 1.19) | .99 |
| Research/academic program | 0.91 | (0.76, 1.10) | .32 |
AJCC, American Joint Committee on Cancer.
FIGURE 2.
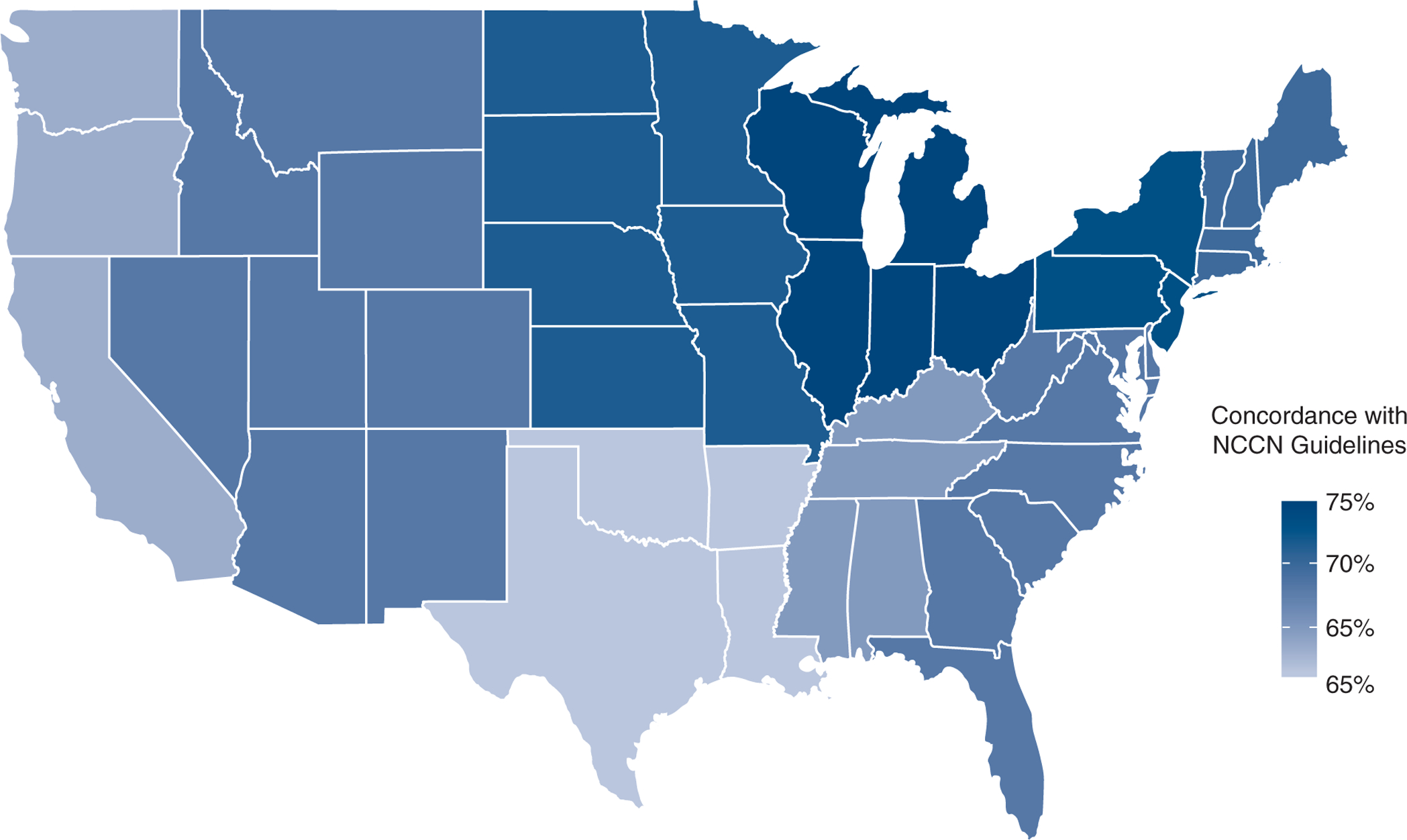
Geographic representation of the percentage of patients receiving National Comprehensive Cancer Network (NCCN) guideline-concordant adjuvant therapy following surgery for pathologic N1 or N2 non-small cell lung cancer in the United States, by state.
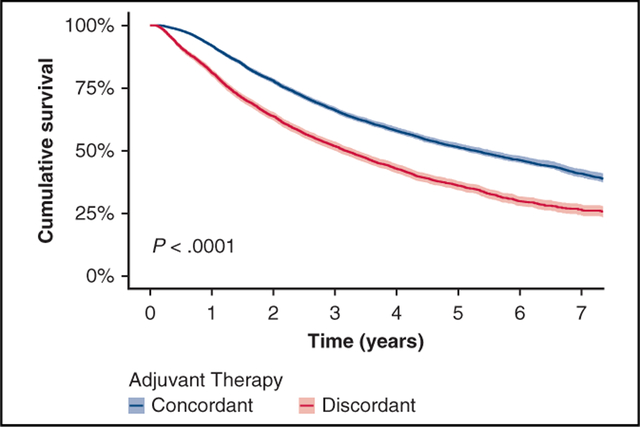
Overall, patients treated with guideline-concordant therapy had superior unadjusted survival (5-year survival: 51.6% vs 36%, median survival: 66.9 vs 51.0 months, P < .001) (Figure 3, A). For patients with pN2 disease, those who received chemotherapy only or chemoradiation had similar survival (5-year survival: 44.7% vs 43.1%, median survival 59.5 vs 60.2 months). Patients who received radiation alone or no adjuvant therapy had significantly worse survival than those who received guideline-concordant treatment (5-year survival: 24.4% vs 26.5%, median survival 36.3 vs 42.7 months, P < .001) (Figure 3, B). Cox proportional hazard analysis also demonstrated a significant survival benefit for patients who received guideline-concordant adjuvant therapy (hazard ratio, 0.66; CI, 0.62–0.70, P < .001) (Table 4).
FIGURE 3.
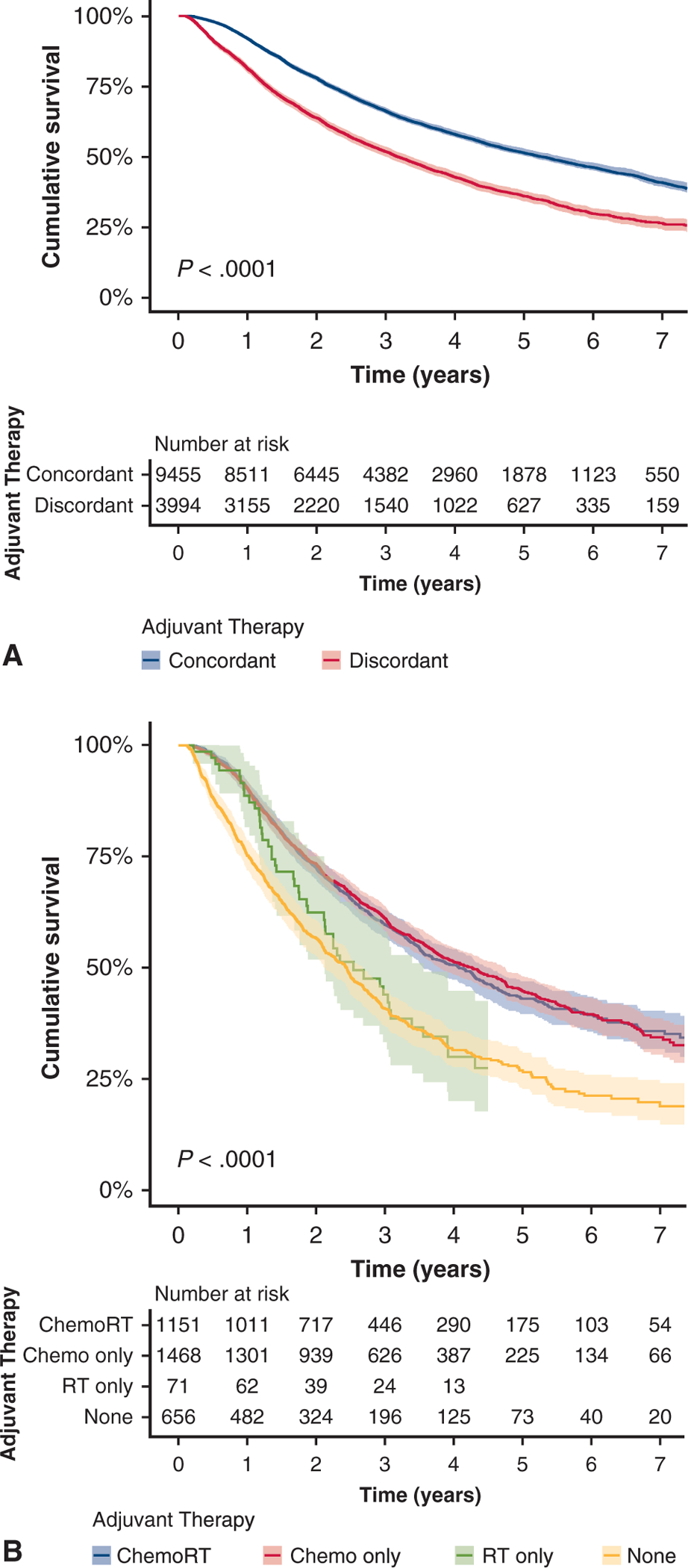
A, Kaplan-Meier unadjusted survival curves for patients with pathologic N1 and N2 non-small cell lung cancer stratified by use of National Comprehensive Cancer Network guideline-concordant adjuvant therapy, defined as chemotherapy for pathologic N1 disease and chemotherapy with or without radiation for pathologic N2 disease, versus guideline-discordant therapy (other therapy or omission of concordant adjuvant therapy). B, Kaplan-Meier unadjusted survival curves for patients with pathologic N2 non-small cell lung cancer, stratified by use of guideline-concordant adjuvant therapy (defined as chemoradiation or chemotherapy only) versus guideline-discordant adjuvant therapy (radiation therapy [RT] only or omission of adjuvant therapy [none]).
TABLE 4.
Multivariable analysis of factors associated risk of death in patients with resected pathologic T1–3 pN1–2 M0 non-small cell lung cancer
| Hazard ratio | 95% confidence interval (lower, upper) | P value | |
|---|---|---|---|
| Age (per decade) | 1.15 | (1.11, 1.20) | <.001 |
| Year of diagnosis (ref = 2006) | 0.98 | (0.97, 0.99) | .005 |
| Female sex | 0.79 | (0.75, 0.84) | <.001 |
| Race (ref = white) | |||
| Black | 0.99 | (0.89, 1.10) | .84 |
| Other | 0.85 | (0.72, 1.01) | .06 |
| Charlson Comorbidity Score (ref = 0) | |||
| 1 | 1.15 | (1.08, 1.22) | <.001 |
| 2+ | 1.26 | (1.16, 1.37) | <.001 |
| Education (per quartile) | 1.00 | (0.96, 1.04) | .97 |
| Income (per quartile) | 0.97 | (0.93, 1.00) | .052 |
| Insurance status (ref = private) | |||
| Medicare/Medicaid | 1.20 | (1.12, 1.29) | <.001 |
| Other government | 1.09 | (0.81, 1.46) | .58 |
| Unknown | 1.08 | (0.81, 1.44) | .61 |
| Uninsured | 1.36 | (1.12, 1.64) | .002 |
| AJCC pathologic T stage | 1.24 | (1.19, 1.28) | <.001 |
| AJCC pathologic N2 (vs Nl) | 1.40 | (1.32, 1.50) | <.001 |
| Pneumonectomy (vs lobectomy) | 1.06 | (0.98, 1.16) | .14 |
| Length of stay (per day) | 1.02 | (1.02, 1.03) | <.001 |
| Readmission | 1.25 | (1.11, 1.41) | <.001 |
| Facility type (ref = community) | |||
| Comprehensive community program | 0.84 | (0.76, 0.93) | .001 |
| Research/academic program | 0.76 | (0.68, 0.84) | <.001 |
| Guideline-concordant adjuvant therapy | 0.66 | (0.62, 0.70) | <.001 |
AJCC, American Joint Committee on Cancer.
DISCUSSION
Disparities in cancer care are common and are seen across multiple cancer types, including lung, breast, colorectal, gastric, and ovarian cancers.3,16–21 Within lung cancer, multiple studies have previously shown disparities in receipt of appropriate care due to differences in age, race, socioeconomic status, geographic region, and insurance status.9–14,22 These barriers to appropriate surgical resection for early-stage NSCLC as well as appropriate chemotherapy and radiation for unresectable NSCLC have been well documented, although disparities in appropriate adjuvant therapy have been underexplored. In this study of patients with resected, pathologic node-positive NSCLC in the United States from 2006 to 2013, we found that lack of insurance and geographic region are associated with disparities in use of adjuvant therapy as recommended by the NCCN (Figure 4). Other factors significantly influencing receipt of adjuvant therapy include increasing age, extent of resection (pneumonectomy vs lobectomy), patient readmission, and longer hospital LOS after surgery. Furthermore, we found that these disparities have significant impacts on patient outcomes, including long-term survival4,7,14 (Figure 4).
FIGURE 4.
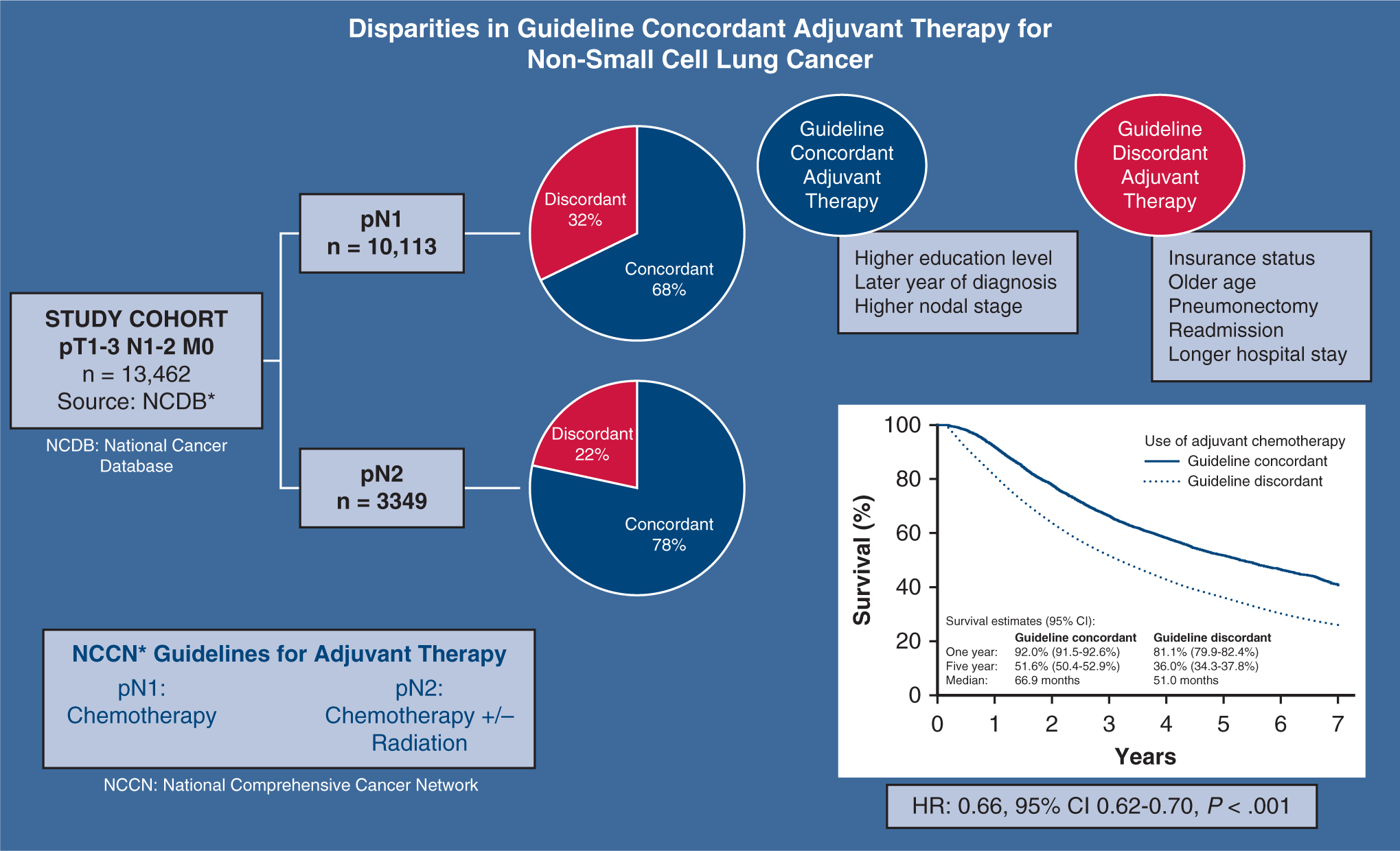
This study identified patients with resected T1–3 N1–2 M0 non-small cell lung cancer in the National Cancer Database (NCDB) between 2006 and 2013, and evaluated guideline-concordance with adjuvant therapy to determine the relationship between socioeconomic factors, use of recommended postoperative adjuvant therapy, and outcomes. Among patients with pN1 disease (n = 10,113) and pN2 disease (n = 3349), guideline-concordant adjuvant therapy was used in 6844 (67.7%) patients with pN1 and 2622 (78.3%) patients with pN2. After multivariable adjustment, insurance status, older age, pneumonectomy, readmission, and longer postoperative stays were associated with lower likelihood of guideline concordance, whereas increased education level, later year of diagnosis, and greater nodal stage were associated with greater concordance. Overall, patients treated with guideline-concordant therapy had superior survival (5-year survival: 51.6 vs 36.0%; hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.62–0.70, P < .001). Socioeconomic factors, including insurance status and geographic region, are associated with disparities in use of adjuvant therapy as recommended by National Comprehensive Cancer Network (NCCN) guidelines, which significantly impact patient survival.
Although there have been previous studies showing disparities in cancer care for patients with NSCLC, including those related to insurance status, geographic area, age, racial and ethnic disparities, and socioeconomic status, previous studies have focused on receipt of initial surgical treatment, chemoradiation therapy for advanced, unresectable disease, or used smaller, institutional, or regional datasets.4,10,13,23,24 Groth and colleagues13 found that in a cohort of almost 11,000 patients in the California Cancer Registry, patients without private insurance were significantly less likely to undergo lobectomy for resectable NSCLC. Ahmed and colleagues7 used the NCDB to show that only 23% of patients with unresectable stage III NSCLC received guideline-concordant chemoradiation, which is similarly influenced by insurance status and geographical region and similarly results in worse survival outcomes. Our study finds similar disparities related to insurance status, but in contrast, focuses on receipt of guideline-appropriate adjuvant therapy following surgical resection for locoregional disease.
Our findings are consistent with others that have shown that a significant portion of patients do not receive the recommended standard of care. However, the overall rates of adjuvant therapy are comparable, or even superior, to other malignancies including breast, colorectal, and gastric cancer.19–21 In this cohort, 70.3% of patients overall received appropriate adjuvant therapy, with 67.7% of patients with pN1 disease receiving adjuvant chemotherapy and 78.3% of patients with pN2 disease receiving either adjuvant chemotherapy alone or chemoradiation. It is reassuring that in this cohort later years of diagnosis were associated with increasing concordance (Table 3: OR, 1.11; CI, 1.09–1.13, P < .001), suggesting that progress has been made in recent years. The reasons for this are likely multifactorial; however, it is possible that wider adoption of minimally invasive resection techniques in recent years has facilitated the provision of adjuvant therapy.25 Although it is not realistic to expect 100% compliance with national guidelines, our study identified opportunities to target certain patient populations who are at high risk for omission of guideline-appropriate care.
Although several other studies have shown racial disparities in delivery of appropriate care and outcomes for patients with NSCLC, this analysis did not demonstrate differences in receipt of guideline-concordant adjuvant therapy or adjusted survival for black patients compared to white patients.3,6,26,27 Interestingly, Soneji and colleagues28 reported on 105,121 patients with early-stage NSCLC in the Surveillance, Epidemiology, and End-Results database and found that although non-Hispanic black patients did experience worse overall survival, the worse survival was attributable to competing causes, such as cardiovascular disease and other cancers, rather than lung cancer-specific survival, suggesting that improvements in racial disparities for these patients may not be mitigated by equalizing access to appropriate lung-cancer care.
However, 2 previous studies using the Veterans Affairs Central Cancer registry have specifically shown disparities in cancer treatment for black patients compared with white patients.29,30 A study of 82,414 veterans with all stages of NSCLC found that although black patients had greater stages of disease, and fewer received guideline-appropriate surgery or chemotherapy, black patients had better overall survival.29 In another analysis of 18,466 veterans with stage I or II NSCLC, although there was no difference in mortality demonstrated, there was a disparity in receipt of surgical resection for black patients compared to white patients, which decreased over time.30 Unlike our study, these analyses were performed at a single-payer, equal-access health care system and were not similarly influenced by different insurance types. Together with our findings, it remains unclear just how race affects appropriate lung cancer care and mortality, and how these differences are related to or influenced by socioeconomic status, insurance or other poorly measurable factors.
The current study demonstrates that lack of insurance, as well as non-Medicare/Medicaid government insurance, is associated with nondelivery of guideline-concordant adjuvant therapy for patients, which is consistent with previous findings in studies of both early and late stages of NSCLC, as well as across perioperative time points.4,7,14,31 One potential reason for this may be the differences in the financial burden borne by the patient between surgery (typically a one-time copay) and the longitudinal nature of adjuvant therapy. In a study of colorectal and lung cancer patients, 48% reported some degree of financial burden, with difficulty living on their current household income.32 Future work should focus on improving access to appropriate adjuvant therapies among the uninsured and socioeconomically disadvantaged. Given that patients with adequate insurance and greater access to care, as shown in the Veterans Affairs population, appear to have fewer disparities in lung cancer care and clinical outcomes, broadening access to care via a single-payor system could potentially improve the disparities seen in this and other studies.29,30 Improving access to care, particularly high-volume and comprehensive centers, which have largely been shown to have improved outcomes, and addressing the disparity in guideline-concordant care delivery to patients who are under- or uninsured may have the added benefit of potentially helping mitigate disparities related to race and socioeconomic status.28–30,33,34 Furthermore, we found that similar to other studies examining disparities in aspects of lung cancer care, there are significant differences in delivery of adjuvant therapy in relationship to geographic region, particularly in the west and south, which is likely related to access to care, density of providers, socioeconomic disparities, or rural versus urban settings.7,14,33
Addressing disparities in cancer care is complex. Race, insurance status, socioeconomic status, and geographic location have all been shown in different studies to impact receipt of guideline-concordant care, which in turn negatively impacts survival. These factors are closely interrelated, in addition to being patient, institution, and region-dependent.33 High-quality, guideline-based cancer care should ideally be accessible, patient-centered, and delivered to patients regardless of their race, geographic location, socioeconomic status, or insurance status. Controlling high health care costs, maximizing value of care, and ensuring adequate health insurance coverage for patients will be critical as the health care system is increasingly rewarding efficient, quality care.
Aside from socioeconomic factors, patients with longer hospital LOS following surgery as well as increasing age were also less likely to receive guideline concordant care. Although this may be due to several factors, we feel that this most likely represents selection bias on the part of the treating provider(s), who may be less likely to offer adjuvant therapy for older patients as well as those with complicated postoperative courses.35 Efforts to minimize postoperative complications, particularly in patients who are frail or have marginal pulmonary function, may facilitate additional improvement in providing guideline concordant therapy. Furthermore, as the use of minimally invasive techniques continues to increase, it may lead to improved administration of adjuvant therapy as well as potentially fewer delays in initiation of therapy given the known benefits of decreased morbidity and LOS.36–39 However, Salazar and colleagues40 demonstrated that adjuvant chemotherapy, provided up to 4 months following resection, was associated with a survival advantage and perhaps thoracic oncologists will consider adjuvant therapy when indicated, even for patients who have had complicated postoperative courses.
It is also notable that female sex, although not significantly associated with guideline-concordant care on our multivariable analysis, was associated with improved survival in our study. The association between female sex and improved long-term survival from NSCLC has been well studied.41,42 In addition, it has been shown that females have fewer postoperative events after lung cancer surgery.43 It is possible that, with fewer postoperative complications, more women are deemed to be appropriate candidates for adjuvant therapy and that this might contribute to the survival differences. However, these associations have yet to be rigorously studied and warrant further consideration.
Finally, although current NCCN guidelines include consideration of adjuvant chemoradiation for patients with pN2 disease, the additional benefit of radiation in this setting is unclear.44–47 In this study, we found that for those patients with pN2 disease, patients who received either chemotherapy alone or chemoradiation had similar, superior survival compared with those patients who received radiation only or no adjuvant therapy. These findings support the notion that radiation may not be necessary or beneficial in all patients with pN2 disease, and future studies should aim to further address this question. Those who received radiation appeared to have an initial, but short-lived, survival benefit, but ultimately had similar or worse 5-year and median survival compared with those who did not receive adjuvant therapy. These results should be interpreted with caution, given the small number of patients who received adjuvant radiation only for pN2 disease, and further studies are needed to further clarify the role of adjuvant radiation therapy and its potential benefits in these patients.
There are several limitations to our study. First, this study is a retrospective review of a national database with incomplete data, which is limited by potential selection bias and confounding. Although the NCDB is extensively validated, it remains subject to potential reporting errors as well as limits in the granularity of the data. Specifically, we were unable to assess at which point within the treatment course the decision to pursue or not pursue appropriate adjuvant therapy was made, or the reasons for those decisions, which could provide important insight into these care disparities. In addition, it is possible that, for certain patients, therapy may have been considered contraindicated due to patient risk factors, age, or other factors but not adequately captured by the coding of the NCDB. Furthermore, as the NCDB draws information from CoC centers, we cannot make any comparisons between the care provided by CoC centers and non-CoC centers. It is also unclear exactly which adjuvant therapies (single- vs multiagent, molecular vs standard chemotherapy) were recommended and provided to patients in the study. Although the NCDB does not provide drug-specific data, it is likely that few, if any, targeted therapies were administered as adjuvant therapies during the study period. Targeted therapies were not recommended in the 2014 NCCN guidelines and thus not likely to be relevant to these data. We were similarly unable to assess many patient-level details that may impact receipt of care, such as patient preferences and beliefs, type and extent of counseling provided, individual income and education levels, preoperative functional status, comorbidities, completion of adjuvant treatment course, and treatment toxicities.
This study is a large, comprehensive analysis of disparities in guideline-concordant adjuvant therapy among a national sample of patients with early-stage NSCLC with pN1 or pN2 disease. The results show a statistically and clinically significant difference in the rate of guideline-concordant adjuvant therapy delivered to patients without insurance, as well as significant variability between geographic regions of the United States. These disparities have impacts on patient outcomes, as patients treated with guideline-concordant therapy had superior survival. Further studies are needed to delineate the factors contributing to this disparity, to ensure high-value, quality care to patients regardless of location, socioeconomic, or insurance status.
Supplementary Material
CENTRAL MESSAGE.
Socioeconomic factors, including lack of insurance and geographic region, are associated with disparities in adjuvant therapy use for node-positive non-small cell lung cancer. These disparities have significant impact on outcomes and survival.
PERSPECTIVE.
Studies examining guideline-concordant therapy in lung cancer are limited in size and to patients with early- or late-stage disease. This study examines nationwide disparities in adjuvant therapy for resected node-positive, non-small cell lung cancer Socioeconomic factors, including insurance status and geographic region, are associated with lower guideline concordance and worse survival.
Abbreviations and Acronyms
- AJCC
American Joint Committee on Cancer
- CI
confidence interval
- CoC
Commission on Cancer
- LOS
length of stay
- NCCN
National Comprehensive Cancer Network
- NCDB
National Cancer Database
- NSCLC
non-small cell lung cancer
- OR
odds ratio
Footnotes
Conflict of Interest Statement
Dr D’Amico serves as a consultant for Scanlan International, Inc. Drs Tong and Hartwig serve on an advisory board for Medtronic, Inc. Dr Farrow is supported by a National Institutes of Health T32 grant (T32 CA 93245). All other authors have nothing to disclose with regard to commercial support.
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Stat Facts: Lung and Bronchus Cancer [National Cancer Institute. web site]. 2018. Available at: https://seer.cancer.gov/statfacts/html/lungb.html. Accessed October 30, 2018.
- 3.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–57. [DOI] [PubMed] [Google Scholar]
- 4.Yorio JT, Yan J, Xie Y, et al. Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center. Clin Lung Cancer. 2012;13:448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg ER, Chute CG, Stukel T, Baron JA, Freeman DH, Yates J, et al. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. N Engl J Med. 1988;318:612–7. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald HP, Polissar NL, Borgatta EF, McCorkle R, Goodman G. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health. 1998;88:1681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed HZ, Liu Y, O’Connell K, Ahmed MZ, Cassidy RJ, Gillespie TW, et al. Guideline-concordant care improves overall survival for locally advanced non-small-cell lung carcinoma patients: a National Cancer Database analysis. Clin Lung Cancer. 2017;18:706–18. [DOI] [PubMed] [Google Scholar]
- 8.Wood DE, Kazerooni EA, Baum SL, Eapen GA, Ettinger DS, Hou L, et al. Lung Cancer Screening, Version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:412–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sineshaw HM, Wu XC, Flanders WD, Osarogiagbon RU, Jemal A. Variations in receipt of curative-intent surgery for early-stage non-small cell lung cancer (NSCLC) by State. J Thorac Oncol. 2016;11:880–9. [DOI] [PubMed] [Google Scholar]
- 10.Salloum RG, Smith TJ, Jensen GA, Lafata JE. Factors associated with adherence to chemotherapy guidelines in patients with non-small cell lung cancer. Lung Cancer. 2012;75:255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshy M, Malik R, Spiotto M, Mahmood U, Weichselbaum R, Sher D. Disparities in treatment of patients with inoperable stage I non-small cell lung cancer: a population-based analysis. J Thorac Oncol. 2015;10:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osuoha CA, Callahan KE, Ponce CP, Pinheiro PS. Disparities in lung cancer survival and receipt of surgical treatment. Lung Cancer. 2018;122:54–9. [DOI] [PubMed] [Google Scholar]
- 13.Groth SS, Al-Refaie WB, Zhong W, Vickers SM, Maddaus MA, D’Cunha J, et al. Effect of insurance status on the surgical treatment of early-stage non-small cell lung cancer. Ann Thorac Surg. 2013;95:1221–6. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AM, Hines RB, Johnson JA III, Bayakly AR. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer. 2014;83:401–7. [DOI] [PubMed] [Google Scholar]
- 15.Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood-level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118:5117–23. [DOI] [PubMed] [Google Scholar]
- 16.Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979–2003. Cancer. 2011;117:3242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed Z, Kujtan L, Kennedy KF, Davis JR, Subramanian J. Disparities in the management of patients with stage I small cell lung carcinoma (SCLC): a surveillance, epidemiology and end results (SEER) analysis. Clin Lung Cancer. 2017;18:e315–25. [DOI] [PubMed] [Google Scholar]
- 18.Parikh AA, Robinson J, Zaydfudim VM, Penson D, Whiteside MA. The effect of health insurance status on the treatment and outcomes of patients with colorectal cancer. J Surg Oncol. 2014;110:227–32. [DOI] [PubMed] [Google Scholar]
- 19.Churilla TM, Egleston B, Bleicher R, Dong Y, Meyer J, Anderson P. Disparities in the local management of breast cancer in the US according to health insurance status. Breast J. 2017;23:169–76. [DOI] [PubMed] [Google Scholar]
- 20.Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karagkounis G, Squires MH 3rd, Melis M, Poultsides GA, Worhunsky D, Jin LX, et al. Predictors and prognostic implications of perioperative chemotherapy completion in gastric cancer. JGastrointestSurg. 2017;21:1984–92. [DOI] [PubMed] [Google Scholar]
- 22.Cykert S, Dilworth-Anderson P, Monroe MH, Walker P, McGuire FR, Corbie-Smith G, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang R, Cheung MC, Byrne MM, Huang Y, Nguyen D, Lally BE, et al. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010;116: 2437–47. [DOI] [PubMed] [Google Scholar]
- 24.Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non-small cell lung cancer among black and white Medicare beneficiaries, 2000–2003. J Natl Med Assoc. 2011;103:711–8. [DOI] [PubMed] [Google Scholar]
- 25.Blasberg JD, Seder CW, Leverson G, Shan Y, Maloney JD, Macke RA. Video-assisted thoracoscopic lobectomy for lung cancer: current practice patterns and predictors of adoption. Ann Thorac Surg. 2016;102:1854–62. [DOI] [PubMed] [Google Scholar]
- 26.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–205. [DOI] [PubMed] [Google Scholar]
- 27.Hunt B, Balachandran B. Black:white disparities in lung cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2015;39:908–16. [DOI] [PubMed] [Google Scholar]
- 28.Soneji S, Tanner NT, Silvestri GA, Lathan CS, Black W. Racial and ethnic disparities in early-stage lung cancer survival. Chest. 2017;152:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganti AK, Subbiah SP, Kessinger A, Gonsalves WI, Silberstein PT, Loberiza FR Jr. Association between race and survival of patients with non-small-cell lung cancer in the United States veterans affairs population. Clin Lung Cancer. 2014;15:152–8. [DOI] [PubMed] [Google Scholar]
- 30.Williams CD, Salama JK, Moghanaki D, Karas TZ, Kelley MJ. Impact of race on treatment and survival among U.S. veterans with early-stage lung cancer. J Thorac Oncol. 2016;11:1672–81. [DOI] [PubMed] [Google Scholar]
- 31.Maguire FB, Morris CR, Parikh-Patel A, Cress RD, Keegan THM, Li CS, et al. Disparities in systemic treatment use in advanced-stage non-small cell lung cancer by source of health insurance. Cancer Epidemiol Biomarkers Prev. 2019;28:1059–66. [DOI] [PubMed] [Google Scholar]
- 32.Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DR, Kontos EZ, Viswanath K, Haas JS, Lathan CS, MacConaill LE, et al. Integrating multiple social statuses in health disparities research: the case of lung cancer. Health Serv Res. 2012;47:1255–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kommalapati A, Tella SH, Appiah AK, Smith L, Ganti AK. Association between treatment facility volume, therapy types, and overall survival in patients with stage IIIA non-small cell lung cancer. JNatl Compr Canc Netw. 2019;17:229–36. [DOI] [PubMed] [Google Scholar]
- 35.Agostini PJ, Lugg ST, Adams K, Smith T, Kalkat MS, Rajesh PB, et al. Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy. J Cardiothorac Surg. 2018;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao C, Manganas C, Ang SC, Peeceeyen S, Yan TD. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg. 2013;16:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang CF, Sun Z, Speicher PJ, Saud SM, Gulack BC, Hartwig MG, et al. Use and outcomes of minimally invasive lobectomy for stage I non-small cell lung cancer in the National Cancer Data Base. Ann Thorac Surg. 2016;101:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattaneo SM, Park BJ, Wilton AS, Seshan VE, Bains MS, Downey RJ, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–5; discussion 235–6. [DOI] [PubMed] [Google Scholar]
- 39.Rajaram R, Mohanty S, Bentrem DJ, Pavey ES, Odell DD, Bharat A, et al. Nationwide assessment of robotic lobectomy for non-small cell lung cancer. Ann Thorac Surg. 2017;103:1092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salazar MC, Rosen JE, Wang Z, Arnold BN, Thomas DC, Herbst RS, et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. 2017;3:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25:1705–12. [DOI] [PubMed] [Google Scholar]
- 42.Sagerup CM, Smastuen M, Johannesen TB, Helland A, Brustugun OT. Sex-specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax. 2011;66:301–7. [DOI] [PubMed] [Google Scholar]
- 43.Tong BC, Kosinski AS, Burfeind WR Jr, Onaitis MW, Berry MF, Harpole DH Jr, et al. Sex differences in early outcomes after lung cancer resection: analysis of the society of thoracic surgeons general thoracic database. J Thorac Cardiovasc Surg. 2014;148:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen WY, Ji J, Zuo YS,Pu J,Xu YM, Zong CD, et al. Comparison of efficacy for postoperative chemotherapy and concurrent radiochemotherapy in patients with IIIA-pN2 non-small cell lung cancer: an early closed randomized controlled trial. Radiother Oncol. 2014;110:120–5. [DOI] [PubMed] [Google Scholar]
- 45.Douillard JY, Rosell R, De Lena M, Riggi M, Hurteloup P, Mahe MA, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. [DOI] [PubMed] [Google Scholar]
- 46.Billiet C, Peeters S, Decaluwe H, Vansteenkiste J, Mebis J, Ruysscher D. Postoperative radiotherapy for lung cancer: is it worth the controversy? Cancer Treat Rev. 2016;51:10–8. [DOI] [PubMed] [Google Scholar]
- 47.Yang CF, Meyerhoff RR, Stephens SJ, Singhapricha T, Toomey CB, Anderson KL, et al. Long-term outcomes of lobectomy for non-small cell lung cancer after definitive radiation treatment. Ann Thorac Surg. 2015;99:1914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


