Platelets are the major blood component bridging immunity and thrombosis. Abundant in the circulation, platelets encounter pathogens at a higher rate than any circulating leukocyte. Viral particles of various blood-borne pathogens such as HIV,1 Dengue,2 or even respiratory viruses such as influenza3 are found inside of human platelets. Viral infections with these viruses4 as well as the SARS-CoV-2 can lead to thrombocytopenia5 along with thrombotic complications6–8 in patients. The presence of internalized viral particles, thrombocytopenia and thrombosis implicate platelets as active participants in immunity during viral infections.
Influenza, HIV and SARS-CoV-2 are classified as single-stranded RNA (ssRNA) viruses. Although there are differences in the overall immunity to blood-borne vs. respiratory RNA-viruses, the initial response to these pathogens by the host’s cells is achieved by the endosomal toll-like receptors (TLR).9 In human cells, TLR7 and TLR8 are the receptors that mediate a response to degraded viral ssRNA.9, 10 Endosomal TLRs require the acidic pH of these compartments for proper vRNA-recognition, activation and signaling. Another endosomal receptor, TLR9, is activated by microbial (bacterial or viral) or mitochondrial DNA.9
The involvement of platelets in the initial viral immune response is evident by the expression of functional TLRs. Human platelets can express all ten TLRs11 and platelets mediate the initial response to single-stranded viruses such as influenza or Encephalomyocarditis (EMCV) through TLR7.3, 12 Activation of platelet-TLR7 leads to AKT and p38-MAPK phosphorylation,12 α-granule release,3, 12 P-selectin and CD40L surface expression,12 complement C3 release,3 platelet-neutrophil aggregate formation12 and platelets-mediated neutrophil DNA release.3 Although the ssRNA viral activation of platelet-TLR7 and the consequent neutrophil engagement has been described, the precise mechanism of platelet viral uptake and granule release remains unknown.
In this issue of ATVB, Banerjee et al,13 provide a mechanism by which platelets endocytose viral particles such as HIV and elucidate the downstream TLR7-signaling cascade. Using HIV pseudo particles (HIVpp), the authors elegantly show that the proteins important for the endocytic internalization of HIV are ARF6 and VAMP313; proteins generally responsible for endocytosis in platelets.14, 15 Interestingly, Banerjee et al show that platelets contain early (Rab4) and late endosomes (Rab7) suggesting that lysosomes are not the only organelles of the endocytic membrane transport pathway.13 As with stimulation by the TLR7-specific agonist loxoribine,12 activation of TLR7 by HIVpp required acidic pH.12, 13 Utilizing various genetically modified murine models, the authors show that platelet-TLR7 needs MyD88 to relay the downstream signal in a IRAK4-IKKb-SNAP-23 manner.13 Activation of this signaling cascade resulted in α-granule release, P-selectin surface expression and interaction of platelets with leukocytes.13 Similarly to TLR7, platelet-TLR9 engaged the same signaling mechanism and also led to platelet-leukocyte aggregate formation.13 Since TLR7 and TLR9 mediate a response to different pathogen species (RNA-viruses vs. bacteria or DNA viruses respectively), additional work is needed to clarify the similarities or differences in consequent platelet-leukocyte interactions. Banjaree et al also observed an increase in α2bβ3 activation when platelets were stimulated with HIVpp.13 Interestingly, increase in α2bβ3 activation and aggregation are not observed when human- platelets are stimulated by a TLR7-specific agonist.12 This is not surprising as TLR7 is a dual receptor with distinct ligand-binding sites, the first recognizes guanosine and the second binds to uridine in single-stranded vRNA enhancing the affinity of the first.16 Differences in TLR7-agonist- vs HIVpp- stimulated platelets can also indicate that there are additional receptors, such as TLR8, that may be involved in the mediation of an immune response to different RNA-viruses.
Active participation of platelets in a response to ssRNA-viruses takes on greater importance during the current COVID-19 pandemic. SARS-CoV-2 is also a ssRNA virus but, contrary to HIV, does not incorporate into the host’s DNA, is short lived and, similar to influenza, is classified as a respiratory virus. Despite these differences, if SARS-CoV-2 is internalized by platelets via receptor-mediated endocytosis, the initial response to the vRNA would likely be mediated by TLR7, however, this remains to be investigated. Based on known interactions between influenza and human platelets,3 SARS-CoV-2 would likely crossover into the circulation and become internalized by platelets. Transmission electron micrographs of human platelets in the presence of influenza3 suggest that the endocytic/lysosomal pathway described by Barnjee et al is likely a uniform mechanism of viral endocytosis. As with influenza infection, the internalized SARS-CoV2 could activate platelet TLR7 and initiate granule release of complement C3 (C3)3 which, in turn, could lead to NETosis.3 Although NETosis is beneficial to the host,17 when dysregulated it can become highly thrombotic.18 It is possible that SARS-CoV-2 initiates a similar cascade of events after TLR7 activation (Figure 1). As COVID-19 progresses, NETosis could be amplified through tissue factor (TF) release from the damaged tissue. TF-mediated thrombin generation is known to increase platelet aggregation, C3 release from platelets3, and C3 activation19. Such activation of the complement cascade7 and endothelial compromise are evident in COVID-19 patients.20 However, while receptors for influenza and HIV are expressed on platelets, it remains unclear if the receptor for SARS-CoV-2, ACE2 is present. Studies are necessary to determine if platelet-TLR7 mediates the response to SARS-CoV-2 in a MyD88-IRAK-IKKb-SNAP23-C3 dependent manner and if this virus gets internalized similarly to HIVpp or influenza.
Figure 1: Activation of platelets by HIVpp and influenza and proposed activation of platelet-Toll like receptor 7 (TLR7) by SARS-CoV-2.
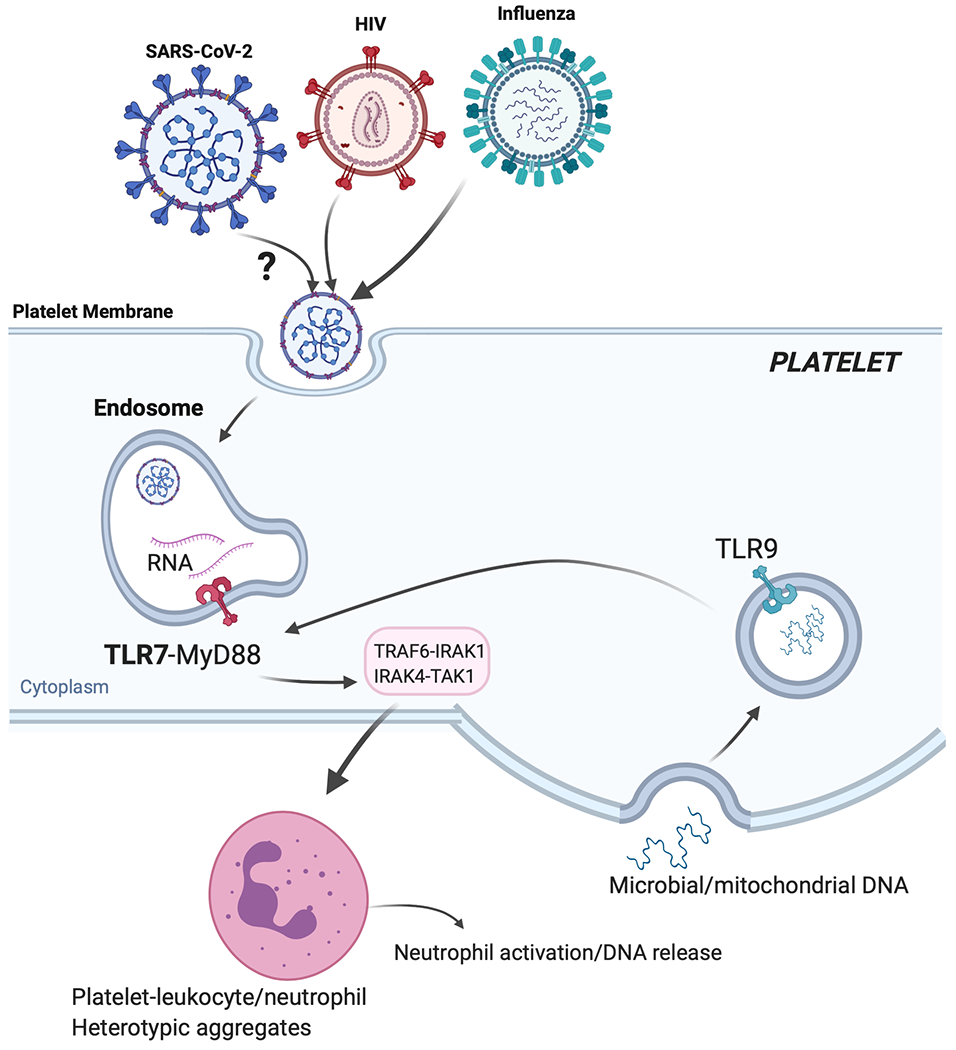
If SARS-CoV-2 (similarly to influenza or HIVpp) can be endocytosed by platelets, its degraded ssRNA would be first recognized by TLR7. Activation of TLR7 in a MyD88-IRAK-IKKb-SNAP23 dependent signaling would lead to P-selectin/CD40L surface expression and complement C3 release form α-granules. P-selectin and CD40L would mediate platelet-leukocyte interactions (predominantly neutrophils). C3 release would increase neutrophil-DNA release and ensures viral capture and removal. As viral infection progresses, C3-mediated netosis may be augmented by thrombin generated as a result of TF released from damaged tissue; thrombin will also directly increase thrombosis. During COVID19, TLR9, could also contribute to platelet activation and possible C3 release. TLR9 can be activated by mitochondrial-DNA and mitochondrial-DNA levels can be increased with tissue damage (or in the setting of obesity). Platelet-TLR9, similarly to TLR7, signals through the MyD88-IRAK-IKKb-SNAP23 pathway leading to granule release. It is not currently known if platelets can release C3 or netosis in a TLR9-dependent manner.
In summary, the study by Banerjee et al adds to the understanding of functional platelet TLR73, 12, 13 and provides evidence for the endocytic membrane transport pathway in platelets.13 Activation of TLR7 by ssRNA from various viruses leads to platelet-neutrophil aggregates12, 14, netosis,3 and eventual thrombocytopenia.12 Although removal of viral particles from the circulation by netosis is a vital part of the immune process, under settings of dysregulation, it can lead to pathological thrombosis and vessel occlusion. The ability of platelets to carefully balance immunity and thrombosis demonstrates their increasingly important role during viral infections.
ACKNOWLEDGEMENTS
Sources of Funding: MK and JEF are funded by an AHA COVID-19 Rapid Response Award
Footnotes
Disclosure: None
References:
- 1.Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: Engulfment of hiv and staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029 [DOI] [PubMed] [Google Scholar]
- 2.Noisakran S, Gibbons RV, Songprakhon P, Jairungsri A, Ajariyakhajorn C, Nisalak A, Jarman RG, Malasit P, Chokephaibulkit K, Perng GC. Detection of dengue virus in platelets isolated from dengue patients. Southeast Asian J Trop Med Public Health. 2009;40:253–262 [PubMed] [Google Scholar]
- 3.Koupenova M, Corkrey HA, Vitseva O, Manni G, Pang CJ, Clancy L, Yao C, Rade J, Levy D, Wang JP, Finberg RW, Kurt-Jones EA, Freedman JE. The role of platelets in mediating a response to human influenza infection. Nat Commun. 2019;10:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. 2018;122:337–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, Shang Y. Thrombocytopenia and its association with mortality in patients with covid-19. Journal of Thrombosis and Haemostasis. 2020;n/a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Gandet FF, FafiKremer S, Castelain V, Schneider F, Grunebaum L, Eduardo Angles-Cano, Sattler L, Mertes P-M, Meziani F. High risk of thrombosis in patients in severe sars-cov-2 infection: A multicenter prospective cohort study. Intensive Care Medicine. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe covid-19 infection: A report of five cases. Transl Res. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satoh T, Akira S. Toll-like receptor signaling and its inducible proteins. Microbiol Spectr. 2016;4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Ohto U, Shibata T, Taoka M, Yamauchi Y, Sato R, Shukla NM, David SA, Isobe T, Miyake K, Shimizu T. Structural analyses of toll-like receptor 7 reveal detailed rna sequence specificity and recognition mechanism of agonistic ligands. Cell Rep. 2018;25:3371–3381 e3375 [DOI] [PubMed] [Google Scholar]
- 11.Koupenova M, Mick E, Mikhalev E, Benjamin EJ, Tanriverdi K, Freedman JE. Sex differences in platelet toll-like receptors and their association with cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2015;35:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, Freedman JE. Platelet-tlr7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banjaree M, Whiteheart S. Platelets endocytose viral particles and are activated via toll-lke receptor signaling. ATVB. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee M, Joshi S, Zhang J, Moncman CL, Yadav S, Bouchard BA, Storrie B, Whiteheart SW. Cellubrevin/vesicle-associated membrane protein-3-mediated endocytosis and trafficking regulate platelet functions. Blood. 2017;130:2872–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Joshi S, Xiang B, Kanaho Y, Li Z, Bouchard BA, Moncman CL, Whiteheart SW. Arf6 controls platelet spreading and clot retraction via integrin alphaiibbeta3 trafficking. Blood. 2016;127:1459–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Ohto U, Shibata T, Krayukhina E, Taoka M, Yamauchi Y, Tanji H, Isobe T, Uchiyama S, Miyake K, Shimizu T. Structural analysis reveals that toll-like receptor 7 is a dual receptor for guanosine and single-stranded rna. Immunity. 2016;45:737–748 [DOI] [PubMed] [Google Scholar]
- 17.Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA, McFadden G, Kubes P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180 [DOI] [PubMed] [Google Scholar]
- 18.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr., Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of c5a in the absence of c3: A new complement activation pathway. Nat Med. 2006;12:682–687 [DOI] [PubMed] [Google Scholar]
- 20.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in covid-19. Lancet. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


