Patients with classical Hodgkin lymphoma (cHL) typically receive first-line chemotherapy or chemoradiotherapy, with high cure rates.1 However, some patients may have primary refractory cHL, commonly defined as disease progression during front-line therapy; additionally, disease progression within 90 days of completion of front-line therapy or failure to achieve partial response (PR) to initial therapy has also been referred as primary refractory cHL.2 Patients with primary refractory disease have poor prognoses2 and require new treatment options. Two single-center studies of patients with biopsy-proven primary refractory cHL treated with high-dose chemotherapy and autologous stem cell transplantation (ASCT) reported progression-free and overall survival rates of 49% and 48%, respectively,2 and event-free survival and overall survival rates of 36% and 64% respectively.2,3
Programmed death 1 (PD-1) pathway blockade is an effective treatment option in patients with cHL after failure of ASCT and brentuximab vedotin (BV), failure of BV (ineligible for ASCT), or failure of ASCT without subsequent BV.4–6 Pembrolizumab, a high-affinity, anti–PD-1 antibody, demonstrated high response rates and acceptable safety in patients with relapsed or refractory cHL (RRcHL) in the phase 2 KEYNOTE-087 study, leading to approval in the United States and Europe.4,7,8 However, efficacy and safety of PD-1 inhibitors in patients with chemorefractory cHL are unknown. This exploratory post hoc analysis of KEYNOTE-087 evaluated efficacy and safety of pembrolizumab in a patient subpopulation with primary refractory cHL (ClinicalTrials.gov identifier, NCT02453594).
Detailed methods were published previously and the protocol was approved by the independent institutional review board or ethics committees.4 Patients with RRcHL were enrolled in 1 of 3 cohorts: cohort 1 (ASCT and subsequent BV), cohort 2 (salvage chemotherapy and BV, ineligible for ASCT), and cohort 3 (ASCT but did not receive BV after transplantation). Patients received pembrolizumab 200 mg every 3 weeks for a maximum of 24 months. Efficacy and safety were analyzed in all patients who received ≥1 dose of pembrolizumab.
Primary refractory cHL was determined retrospectively by investigators and defined as progressive disease (PD) or stable disease (SD) as best response to first-line therapy or relapse within 90 days of completion of therapy. Objective response rate (ORR; complete response [CR] + PR) was assessed by blinded independent central review using International Working Group 2007 criteria,9 and 95% CI of ORR was calculated using binomial exact CI method. Duration of response (DOR) was estimated using the Kaplan-Meier method. Patients were censored if they went off protocol to undergo allogeneic stem cell transplantation or ASCT. Adverse events (AEs) were graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Of 210 treated patients in KEYNOTE-087, 71 (33.8%) had primary refractory cHL. At database cutoff (March 21, 2018), 12 (16.9%) patients completed 2 years of treatment and 59 (83.1%) patients discontinued therapy (23, PD; 7, physician decision; 14, CR [per protocol]; 6, AE; 2, bone marrow transplantation; 1, clinical progression [disease progression without substantiation by imaging]; 3, patient withdrawal; 1, pregnancy; 2, loss to follow-up). Median follow-up was 27.9 months (range, 8.5-32.7 months).
Median age of patients was 32 years and almost half were male (Supplemental Table 1). Sixty-three (88.7%) patients previously received ≥3 lines of therapy and 61 (85.9%) were previously treated with BV.
Of 71 patients with primary refractory cHL, 58 (81.7%; 95% CI, 70.7-89.9) achieved ORR (Table 1); 25 (35.2%) experienced CR, and 33 (46.5%) experienced PR. Two (2.8%) patients had SD, and 8 (11.3%) had PD. Most patients (n = 66; 97.1%) experienced reduction in target lesion size (Figure 1A). In 139 patients without primary refractory disease, 93 (66.9%) had ORR (Table 1), 33 (23.7%) had CR and 60 (43.2%) had PR.
Table 1.
Best overall response by blinded independent central review
| All patients N = 210 | ||||
|---|---|---|---|---|
| Patients with primary refractory cHL n = 71 | Remaining patients n = 139 | |||
| n | % (95% CIa) | n | % (95% CIa) | |
| ORR | 58 | 81.7 (70.7-89.9) | 93 | 66.9 (58.4-74.6) |
| CR | 25 | 35.2 (24.2-47.5) | 33 | 23.7 (16.9-31.7) |
| PR | 33 | 46.5 (34.5-58.7) | 60 | 43.2 (34.8-51.8) |
| SD | 2 | 2.8 (0.3-9.8) | 21 | 15.1 (9.6-22.2) |
| PD | 8 | 11.3 (5.0-21.0) | 24 | 17.3 (11.4-24.6) |
| NA | 3 | 4.2 (0.9-11.9) | 1 | 0.7 (0.0-3.9) |
cHL, classic Hodgkin lymphoma; CR, complete response; NA, no assessment; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Based on binomial exact CI.
Figure 1. Treatment response and progression-free survival in patients in the primary refractory subgroup.
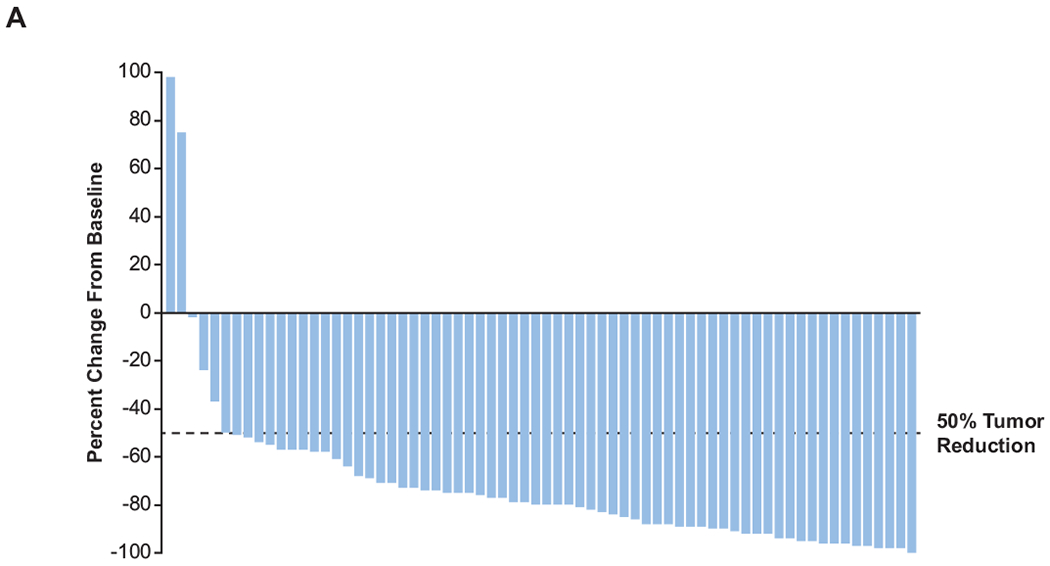
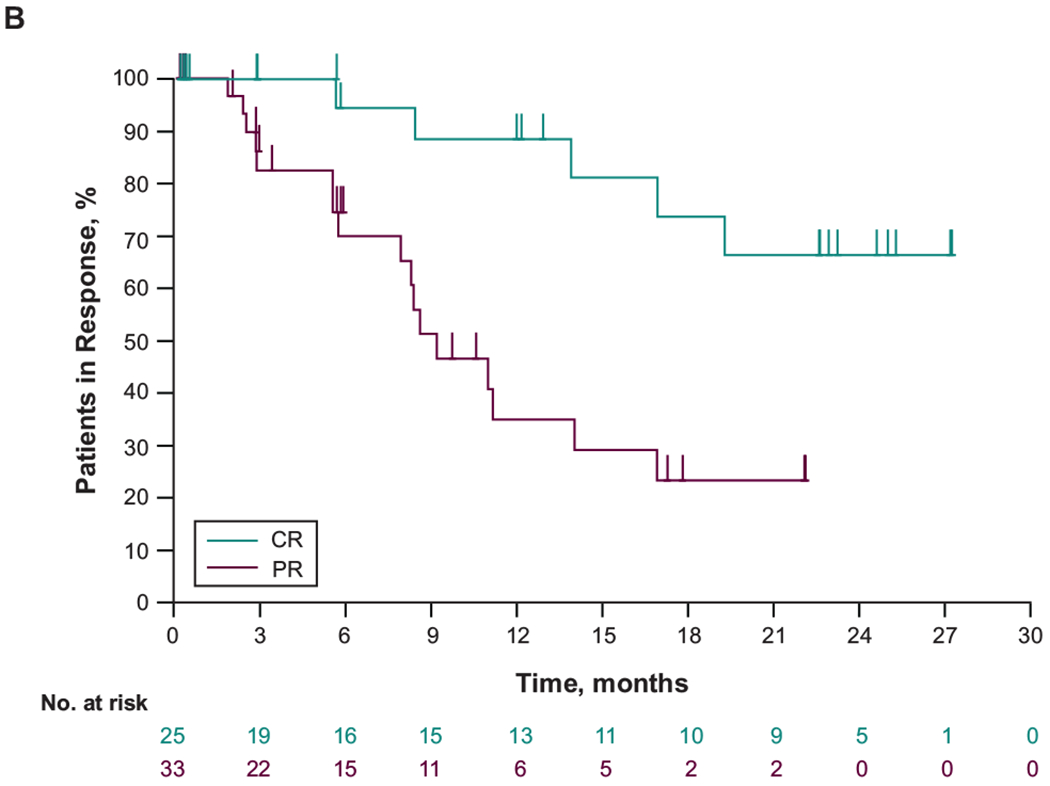
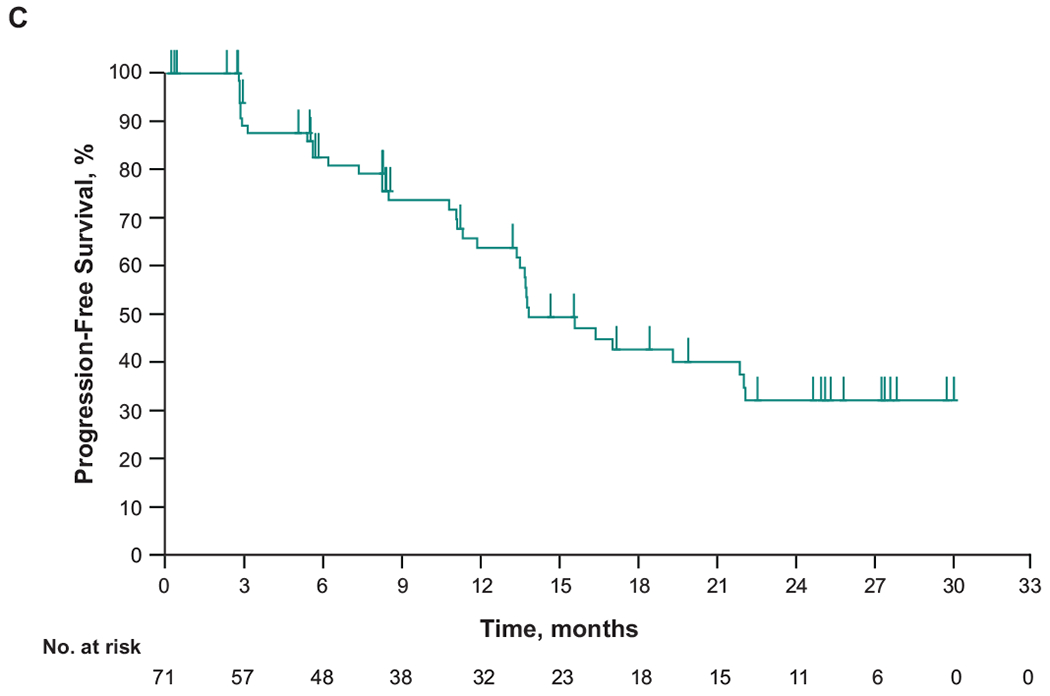
(A) Change from baseline in target lesion size and (B) duration of response in patients with primary refractory cHL who had ≥1 evaluable postbaseline assessment (n=68). (C) Progression-free survival. CR, complete response; PR, partial response
For patients with primary refractory cHL, median time to response was 2.8 months (range, 2.1-8.8 months) (Figure 1B). Responses were durable in all responders, particularly in patients with CR (Figure 1C). Median DOR was 16.8 months (range, 0.0+ to 27.0+ months). Median progression-free survival (PFS) was 13.8 months (95% CI, 11.9-22.0); the 12- and 24-month rates were 63.9% and 32.2%, respectively. Median overall survival was not reached; the 12 and 24-month rates were 98.5%, and 94.0% respectively.
When response was evaluated by cohort in KEYNOTE-087, ORRs of patients with primary refractory disease in cohorts 1, 2, and 3 were 84.6%, 75.0%, and 88.5%, respectively (Supplemental Table 2), whereas ORRs of corresponding cohorts in the overall population were 76.8%, 66.7%, and 73.3%, respectively (R.C. et al., manuscript submitted February 2019). Among patients in cohort 3 with primary refractory disease (n = 26), 16 received BV before ASCT; of those, 13 (81.3%; 95% CI, 54.4-96.0) experienced objective response (CR in 6 [37.5%], PR in 7 [43.8%]), and 3 (18.8%) experienced PD. The other 10 patients in cohort 3 did not receive BV before ASCT and all experienced objective response; 3 (30.0%) experienced CR and 7 (70.0%) experienced PR.
Fifty (70.4%) patients with primary refractory disease experienced treatment-related AEs (TRAEs), most commonly hypothyroidism in 8 (11.3%) patients, followed by diarrhea, nausea, cough, and rash in 6 (8.5%) patients and pyrexia and fatigue in 5 (7.0%) patients. Six patients (8.5%) experienced grade 3/4 TRAEs (neutropenia, thrombocytopenia, cytokine release syndrome, herpes zoster infection, increased amylase, increased lipase, myositis, decreased weight [occurred simultaneously with other AEs], myocarditis, epilepsy, and diarrhea). Incidence of grade 3/4 TRAEs was comparable between the primary refractory subpopulation and the overall population (R.C. et al., submitted February 2019). No deaths were attributed to TRAEs. One patient died of graft-versus-host disease during safety follow-up, but the investigator considered the death unrelated to study treatment.
Five TRAEs resulted in study discontinuation in 4 (5.6%) patients; these were cytokine release syndrome and infusion-related reaction (both occurred in the same patient), myocarditis (n=1, 1.4%), epilepsy (n=1, 1.4%) and pneumonitis (n=1, 1.4%). Regardless of treatment attribution, 22 (31.0%) patients experienced immune-mediated AEs; most common were hypothyroidism (n=9; 12.7%), infusion reactions (n=7; 9.9%), hyperthyroidism (n=4; 5.6%), and pneumonitis (n=2; 2.8%); colitis, iritis, sarcoidosis, myocarditis, and myositis occurred in 1 (1.4%) patient each.
ORRs in the primary refractory subpopulation (81.7%) and overall KEYNOTE-087 population (ORR, 71.9%; 95% CI, 65.3-77.9) were comparable (R.C. et al., submitted February 2019). Pembrolizumab ORR also seemed comparable with that of salvage combination chemotherapy regimens, such as DHAP (89%),10 mini-BEAM (82%),11 ICE (85%),12 and chemotherapy with BV (75%),13 and seemed numerically higher than responses experienced with other second-line monotherapies, such as bendamustine (53%) and everolimus (47%) in RRcHL.13–15 However, such cross-trial comparisons must be interpreted cautiously because of differences in patient populations and trial designs. Pembrolizumab PFS rates in the primary refractory subpopulation were comparable with rates in patients with cHL in the KEYNOTE-013 study (ClinicalTrials.gov identifier, NCT01953692).16 Overall, the safety profile of pembrolizumab in the primary refractory subgroup was similar to that of the overall population and that of nivolumab in patients with RRcHL.5,6
In conclusion, pembrolizumab demonstrated a high response rate and manageable safety in the primary refractory subpopulation of cHL in KEYNOTE-087, similar to results observed in the overall study. Salvage chemotherapy followed by ASCT remains the standard of care for transplantation-eligible patients with primary refractory cHL. However, for patients with primary refractory cHL that does not respond to salvage chemotherapy or for patients who are ineligible for transplantation because of comorbidity, pembrolizumab could be an effective treatment option.
Supplementary Material
Acknowledgments
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. We thank the patients and their families and caregivers for participating in the study. We also thank Mohammed Farooqui for study oversight, Seth Thompson for statistical support and Victoria Fox, Kathryn Fogarty, and Laura Sharon for study team support (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA). Medical writing and/or editorial assistance was provided by Doyel Mitra, PhD, and Matthew Grzywacz, PhD, of the ApotheCom pembrolizumab team (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Conflict of interest disclosure: P.L.Z., and A.T. have nothing to disclose. R.C. reports employment/leadership position/advisory role at Seattle Genetics, Pharmacyclics, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Genentech Inc., Millennium Pharmaceuticals, Inc.; honoraria from Seattle Genetics; research funding from Merck & Co., Inc., Bristol-Myers Squibb, Seattle Genetics, Millennium Pharmaceuticals, Inc., Pharmacyclics. P.A. reports employment/leadership position/advisory role at Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Bristol-Myers Squibb, Infinity Pharmaceuticals; research funding from Merck & Co., Inc., Bristol-Myers Squibb, Pfizer Inc., Affimed, Roche, Serventa, Otsuka, Sigma-Tau; travel fees, gifts, and others from Bristol-Myers Squibb, Merck & Co., Inc. N.A.J. reports employment/leadership position/advisory role at Roche, AbbVie Inc., Lundbeck; honoraria from Roche, AbbVie Inc., Lundbeck, Seattle Genetics; research funding from Roche, AbbVie Inc., Lundbeck; travel fees, gifts, and others from Roche, Lundbeck. P.B. reports honoraria from Takeda France, Bristol-Myers Squibb; consulting or advisory role at Takeda France; research funding from Millennium Takeda. J.R. reports employment/leadership position/advisory role at Takeda Pharmaceutical Company, Seattle Genetics, Novartis; stock ownership or options from GlaxoSmithKline, AstraZeneca; research funding from Takeda Pharmaceutical Company. V.R. reports employment/leadership position/advisory role at Gilead, Infinity, Bristol-Myers Squibb, Laboratoires Servier, NanoString Technologies, Incyte Corporation; research funding from Amgen; travel fees, gifts, and others from Roche, Bristol-Myers Squibb. D.M. reports honoraria from Roche Holding AG, Merck & Co., Inc., Bristol-Myers Squibb, Takeda Pharmaceutical Company. T.P.V. reports employment/leadership position/advisory role at Roche, Takeda Pharmaceutical Company, Genesis Pharmaceutical, Inc., Novartis, Bristol-Myers Squibb, Servier; honoraria from Roche, Takeda Pharmaceutical Company, Genesis Pharmaceutical, Inc., Novartis, Bristol-Myers Squibb, Merck & Co., Inc., Winmedica; travel fees, gifts, and others from Roche, Takeda Pharmaceutical Company, Genesis Pharmaceutical, Inc. B.v.T. reports grants, personal fees, and non-financial support from Merck Sharp & Dohme Corp., Novartis, and Takeda. M.A.S. reports employment/leadership position/advisory role at AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Gilead Sciences, Inc., Takeda Pharmaceutical Company; honoraria from AstraZeneca, Bristol-Myers Squibb, Merck & Co., Inc., Gilead Sciences, Inc., Takeda Pharmaceutical Company; research funding from Bristol-Myers Squibb, Bayer. J.L. and A.N. report employment at Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. A.B. reports employment/leadership position/advisory role at Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, stock ownership at Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Amgen. C.H.M. reports employment/leadership position/advisory role at Celgene, Genentech Inc, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Seattle Genetics; research funding from Pharmacyclics, Genentech Inc, Merck & Co., Seattle Genetics.
Study support: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Footnotes
Category: Clinical Trials and Observations
Data sharing statement: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized patient-level data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. The company is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The process includes submission of data requests to the MSD data sharing website (available at http://engagezone.msd.com/ds_documentation.php). Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing the requested data.
References
- 1.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): Hodgkin lymphoma (Version 3.2018). April 16, 2018. https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. Accessed March 8, 2019.
- 2.Moskowitz CH, Kewalramani T, Nimer SD, Gonzalez M, Zelenetz AD, Yahalom J. Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin’s disease. Br J Haematol. 2004;124(5):645–652. [DOI] [PubMed] [Google Scholar]
- 3.Akhtar S, El Weshi A, Abdelsalam M, et al. Primary refractory Hodgkin’s lymphoma: outcome after high-dose chemotherapy and autologous SCT and impact of various prognostic factors on overall and event-free survival. A single institution result of 66 patients. Bone Marrow Transplant. 2007;40(7):651–658. [DOI] [PubMed] [Google Scholar]
- 4.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35(19):2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin lymphoma after autologous stem-cell transplantation and brentuximab vedotin failure: a prospective phase 2 multi-cohort study. Lancet Oncol. 2016;17(9):1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp Merck & Corp Dohme., Keytruda (pembrolizumab) for injection, for intravenous use. Whitehouse Station, NJ, USA; 02/2019. [Google Scholar]
- 8.Keytruda (pembrolizumab) 50 mg powder for concentrate for solution for infusion [summary of product characteristics]. Hoddesdon, UK: Merck Sharpe & Dohme Limited; 09/November/2018. [Google Scholar]
- 9.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 10.Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann Oncol. 2002;13(10):1628–1635. [DOI] [PubMed] [Google Scholar]
- 11.Martin A, Fernandez-Jimenez MC, Caballero MD, et al. Long-term follow-up in patients treated with Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin’s disease. Br J Haematol. 2001;113(1):161–171. [DOI] [PubMed] [Google Scholar]
- 12.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616–623. [DOI] [PubMed] [Google Scholar]
- 13.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston PB, Inwards DJ, Colgan JP, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85(5):320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskowitz AJ, Hamlin PA Jr., Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31(4):456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016;34(31):3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


