Abstract
Bacterial sexually transmitted infections (STIs) have been increasing over the past 2 decades in gay, bisexual, and other men who have sex with men. With the widespread use of early human immunodeficiency virus (HIV) treatment, which virtually eliminates transmission risk, and the availability of HIV pre-exposure prophylaxis, there have been attitudinal changes regarding HIV infection with resultant increases in sexual contact and declines in condom use. Doxycycline is used for primary prophylaxis in a number of infectious diseases. We conducted a state-of-the-art review to examine the current state of research, knowledge gaps, and challenges around the use of doxycycline prophylaxis to prevent syphilis and other STIs. International academic and government experts met in March 2019 to frame the initial inquiry, which was supplemented by focused literature searches. Two small short-term randomized controlled trials examining doxycycline prophylaxis found high efficacy. Five additional clinical studies are underway or in development. Studies differed in design, population, outcomes, and safety measures. Doxycycline prophylaxis for bacterial STIs shows promise. Better and more robust data are needed on efficacy; target population; community acceptability; behavioral risk compensation; doxycycline dose, regimen, and formulation; long-term safety; antimicrobial resistance; cost-effectiveness; and risk–benefit.
Keywords: doxycycline, prophylaxis, syphilis, chlamydia, men who have sex with men
Sexually transmitted infections have been increasing among men who have sex with men. Doxycycline prophylaxis for syphilis and chlamydia has been effective in initial trials. Future research should focus on populations with high incidence/morbidity, dose and regimen, and antimicrobial resistance.
Bacterial sexually transmitted infections (STIs) have been steadily increasing in gay, bisexual, and other men who have sex with men (MSM) over the past 2 decades [1–4]. While that trend started prior to the introduction of human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP) in 2012 [1, 3], HIV PrEP has been associated with increases in sexual contacts and decreases in condom use with an resultant acceleration in the increase of bacterial STIs such as gonorrhea, syphilis, and chlamydia [5–8]. However, the increasing adoption of HIV PrEP [8] has shown that biomedical interventions for STI prevention can be effective, safe, and highly acceptable.
This state-of-the-art review was conducted to examine the current state of research, knowledge gaps, and challenges around the use of doxycycline prophylaxis to prevent syphilis, caused by Treponema pallidum (TP), and other bacterial STIs such as Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG). International public health and clinical experts from academia, government, and community-based organizations met on 3 March 2019 in Seattle, Washington, to frame the initial inquiry, which was then supplemented by focused literature searches to address specific questions of interest. Findings are summarized using the Grading of Recommendations, Assessment, Development, and Evaluations framework specifically focusing on the quality of evidence and benefits versus harms [9].
CURRENT EVIDENCE ON DOXYCYCLINE PROPHYLAXIS
Doxycycline is a moderate-spectrum, second-generation tetracycline that is generally well tolerated [10]. It is rapidly and almost completely absorbed after oral administration [10]. First introduced commercially in the 1960s, doxycycline has been used by millions to manage acne and as primary prophylaxis for scrub typhus [11], leptospirosis [12], malaria [13], and Lyme disease [14]. There are anecdotal reports of doxycycline used for syphilis prophylaxis among US and Australian military personnel during the Vietnam War. Doxycycline is a first-line agent for treatment of chlamydia and an alternative regimen for syphilis [10, 15].
Several studies examined doxycycline prophylaxis for STI prevention (Table 1). In a small, open-label pilot study, 30 MSM living with HIV with prior syphilis were randomized 1:1 to daily doxycycline 100 mg as pre-exposure prophylaxis (Doxy PrEP) for 48 weeks versus a financial incentive–based behavioral intervention [16]. There was a 73% reduction (P = .02) in syphilis, NG, or CT in the Doxy PrEP group compared with the control group. Most intervention-arm participants maintained blood doxycycline levels of greater than 1 μg/mL. Reported sexual behaviors were similar in both groups.
Table 1.
Key Characteristics of Completed Studies on Doxycycline Prophylaxis for Sexually Transmitted Infections
| Study, First Author [Reference] | Design | Sample Size | Intervention | Study Population and Inclusion Criteria | Duration | Findings |
|---|---|---|---|---|---|---|
| Bolan [16] | Open-label RCT; patients randomized 1:1 to intervention and standard of care |
30 | Daily doxycycline hyclate, 100 mg tablet | MSM living with HIV infection; 2 or more treated syphilis diagnoses since HIV diagnosis | 48 weeks | Diagnosis of any bacterial STI at any site: odds ratio 0.27 (0.09–0.83), P = .02; no significant differences in sex behaviors at baseline or follow-up. One patient discontinued doxycycline due to GERD. |
| ANRS IPERGAY Doxy PEP study, Molina [17] | Open-label RCT; patients randomized 1:1 to intervention and no prophylaxis | 232 | Doxycycline hyclate, 200 mg tablet, single dose within 24–72 hours post–condomless sexual encounter; maximum 3/week | MSM and transgender women without HIV on HIV PrEP having condomless sex with men | Median follow-up, 8.7 months | Diagnosis of any bacterial STI at any site: hazard ratio = 0.57 (0.13–0.62), P = .014. No substantive difference in sexual behaviors at baseline or during study; 32 patients discontinued doxycycline, 8 for gastrointestinal side effects. Remainder discontinued for multiple reasons with no discernable pattern. |
| Wilson [18] | Model of sexual behavior | NA | Daily doxycycline, 100 mg | MSM | NA | Assuming 50% adoption and 70% efficacy, ~50% reduction in syphilis after 12 months and 85% reduction after 10 years. Similar effect seen if only MSM with >10 partners in 6 months receiving intervention. |
| Wilson [18] | Survey and focus groups using respondent-driven and convenience sampling | 2095 | NA | MSM | NA | 52.7% (95% confidence interval, 50.6–54.8%) very/slightly likely to use doxycycline to prevent syphilis in themselves; 75.8% (74.0–77.6%) very/slightly likely to use doxycycline to help control syphilis in MSM community. Survey findings supported by focus groups. |
Abbreviations: ANRS IPERGAY, France Recherche Nord & sud SIDA-HIV hépatites Intervention Préventive de l’Exposition aux Risques avec et pour les Gays; Doxy, doxycycline; GERD, gastroesophageal reflux disease; HIV, human immunodeficiency virus; MSM, men who have sex with men; NA, not applicable; PEP, postexposure prophylaxis; PrEP, pre-exposure prophylaxis for HIV; RCT, randomized controlled trial; STI, sexually transmitted infection.
An open-label extension of the French national HIV research agency (France Recherche Nord & sud Sida-hiv hépatites [ANRS]) Intervention Préventive de l’Exposition aux Risques avec et pour les Gays (IPERGAY) HIV-prevention study continued participant access to HIV PrEP and examined doxycycline postexposure prophylaxis (Doxy PEP) in MSM and transgender women without HIV [17]. Participants (n = 232) were randomly assigned 1:1 to the intervention—doxycycline 200 mg within 24–72 hours of condomless sexual encounters up to 3 times per week—or to no prophylaxis. Those taking Doxy PEP had lower STI incidence (hazard ratio, 0.57; P = .014). Chlamydia trachomatis and syphilis diagnoses were significantly lower in the intervention arm, with a relative reduction of 70–73% in the intention-to-treat analysis. Neisseria gonorrhoeae diagnoses did not differ except for fewer urethral cases in those using Doxy PEP. Four of 9 NG-positive cultures, all from the control arm, had high-level tetracycline resistance consistent with the French background rate [10]. Eighty-two percent (n = 31) of participants with NG detected by nucleic acid amplification testing had genotypic markers of tetracycline resistance; there was no difference between study arms (P = .4). All CT culture isolates (n = 5) were doxycycline susceptible. Adherence was high in the Doxy PEP arm; 63% had doxycycline detected in at least 1 plasma specimen. Twenty-nine (21.5%) Doxy PEP patients discontinued doxycycline, 8 for gastrointestinal side effects. Sexual behaviors did not generally differ between the 2 groups.
While not direct clinical evidence, a modeling study examining the impact of Doxy PrEP on syphilis among Australian MSM estimated that if 50% of MSM used Doxy PrEP and it was 70% effective syphilis would decrease by 50% after 12 months and 85% after 10 years [18]. The authors predicted a similar effect if only 50% of men with more than 20 partners in 6 months were taking doxycycline.
STUDIES UNDERWAY OR IN DEVELOPMENT
The meeting participants discussed 5 studies underway or in development on Doxy PEP/PrEP (Table 2). A pilot study, the Dual Daily HIV and Syphilis PrEP (DuDHS) Study in Canada, is examining concurrent daily HIV PrEP and Doxy PrEP in MSM without HIV. The study will randomize 50 participants 1:1 to immediate Doxy PrEP versus delayed initiation after 6 months; all participants will receive 1 year of HIV PrEP. The primary objective is to examine the acceptability, adherence, and tolerability of daily HIV PrEP and Doxy PrEP. The study will also evaluate STI incidence, sexual behavior, tetracycline-class bacterial resistance through culture of the oropharynx and nares, and an evaluation of the rectal microbiome. As of March 2019, investigators have not detected any STIs in the patients receiving Doxy PrEP in contrast to 3 patients with rectal CT in the delayed arm. No serious adverse events have occurred, although participants in the treatment arm have reported more nausea. The Daily Doxycycline in HIV+ for Syphilis PrEP (DaDHS) Study is examining Doxy PrEP in MSM living with HIV; 52 participants with a prior history of syphilis will be randomized 1:1 to daily doxycycline 100 mg or placebo.
Table 2.
Key Characteristics of Studies in Progress or Development on Doxycycline Prophylaxis for Sexually Transmitted Infections, March 2019
| Study, PI | Design | Sample Size | Intervention | Study Population, Inclusion Criteria | Duration, mo | Endpoints |
|---|---|---|---|---|---|---|
| DuDHS, Grennan | Immediate/deferred initiation single-blind RCT; patients randomized 1:1 to intervention and deferred intervention | 50 | Daily doxycycline hyclate, 100 mg | MSM on HIV PrEP; condomless sex in last 6 months, and syphilis diagnosed and treated within last 3 years | 6/12 | Acceptability, adherence, and tolerability; resistance in oral flora; change in sexual activity; STI diagnosis |
| DaDHS, Grennan | Single-blind RCT; patients randomized 1:1 to intervention and placebo | 52 | Daily doxycycline hyclate, 100 mg | MSM living with HIV infection; condomless sex in last 6 months, and syphilis diagnosed and treated within last 3 years | 12 | Adherence and tolerability; resistance in oral flora; change in sexual activity; bacterial STI diagnosis |
| Syphilaxis, Kaldor | Nonrandomized single-arm trial; before–after comparisons using medical records; STI surveillance data comparing study patients with unenrolled MSM with matching risk profiles | 350 | Daily doxycycline monohydrate tablet, 100 mg | MSM living with HIV and without HIV and transgender persons reporting recent sex with men; diagnosed syphilis in prior 12 months, or any STI in last 12 months and syphilis in last 24 months; at least 2 episodes of STI screening in prior 12 months | 12 | Use and acceptability; NG, CT, and syphilis diagnosis; rectal and oropharyngeal microbiome substudy on antimicrobial resistance (n = 100) |
| ANRS Previnir PrEP study, Molina | RCT open-label; patients randomized 2:1 to intervention Doxy PEP or no PEP combined with RCT on impact of meningococcal type B vaccination on NG incidence (randomization 1:1) | 700 | Doxycycline monohydrate, 200 mg, single dose post–condomless sexual encounter | MSM already enrolled in the ANRS Prevenir PrEP trial with a history of recent STI | 18 | CT, NG, and syphilis incidence; culture and molecular-based resistance testing; rectal and oral microbiome substudy on antimicrobial resistance |
| Doxy PEP study, Celum and Luetkemeyer | Open-label RCT; patients randomized to 2:1 to intervention and no prophylaxis | 780 | Doxycycline hyclate delayed release, 200 mg, single dose within 24–72 hours post–condomless sexual encounter, up to daily use | MSM living with HIV infection and MSM on HIV PrEP; 1 or more bacterial STI and condomless sex with 1 or more male partners in past year | 12 | NG, CT, and syphilis diagnosis; culture and molecular-based resistance testing; commensal flora and gut microbiome resistance testing |
Abbreviations: ANRS, France Recherche Nord & sud SIDA-HIV hépatites; CT, Chlamydia trachomatis; DaDHS, Daily Doxycycline in HIV+ for Syphilis PrEP; Doxy, doxycycline; DuDHS, Dual Daily HIV and Syphilis PrEP; HIV, human immunodeficiency virus; MSM, men who have sex with men; NG, Neisseria gonorrhoeae; PEP, postexposure prophylaxis; PI, principal investigator; PrEP, pre-exposure prophylaxis; RCT, randomized controlled trial; STI, sexually transmitted infection.
The Syphilaxis Study in Australia will be a single-arm study of Doxy PrEP in 350 MSM and transgender persons reporting recent sex with men. MSM with a history of regular STI testing are eligible if they are living with HIV or do not have HIV and are using HIV PrEP. The study will use medical records to measure before–after frequencies of STI diagnoses and an existing STI surveillance database to compare frequencies between study participants and unenrolled MSM. Participants will record doxycycline use daily and complete quarterly questionnaires. The primary goals are to measure acceptability of daily doxycycline and effectiveness in preventing syphilis, NG, and CT.
In France, a substudy within the large ANRS Prevenir PrEP cohort (n = 3000) [19] will use a randomized, open-label, factorial design to examine the efficacy of meningococcal type B vaccine in preventing NG infection and the use of Doxy PEP 200 mg to prevent chlamydia and syphilis in participants with a prior STI diagnosis in the past 18 months. Seven hundred participants will be randomized 2:1 to Doxy PEP or no PEP and 1:1 to meningococcal type B vaccine or no vaccine.
A US study examining Doxy PEP effectiveness and safety/tolerability will enroll 780 MSM and male-to-female transgender individuals (390 living with HIV and 390 without HIV using HIV PrEP) who had 1 or more bacterial STI(s) and 1 or more episode(s) of condomless sexual contact with 1 or more male partner(s) in the previous year. The trial will be open-label with 2:1 randomization to Doxy PEP 200 mg post–condomless sexual contact, up to daily use, versus standard of care with 12 months of follow-up. All positive NG cultures will undergo tetracycline susceptibility testing. Specimens from patients diagnosed with syphilis or CT will undergo molecular tetracycline resistance evaluation using a novel clustered regularly interspaced short palindromic repeats/Cas9 targeted sequencing technique [20]. This will provide a broad-reaching, rapid-throughput method for assessing tetracycline and other antimicrobial resistance genes. Participants will have nasopharyngeal swabs cultured to assess for tetracycline susceptibility in Staphylococcus aureus and Neisseria spp. and rectal swabs and stool samples for metagenomic tests to determine predominant species, species diversity, and changes in the presence of tetracycline resistance genes over time.
KNOWLEDGE GAPS AND CHALLENGES
While the studies described above help address some of the knowledge gaps around Doxy PEP/PrEP, there remain multiple areas for further research, particularly around efficacy and potential benefits and harms.
Quality of the Evidence
Efficacy
Studies on Doxy PEP/PrEP used 2 doxycycline dose/regimen options: 100 mg daily [16] or 200 mg single-dose post–condomless sex event [17]. Investigators selected those regimens from experience with doxycycline prophylaxis in other infectious diseases [12, 14] and the minimum inhibitory concentration (MIC) of TP [21]. The efficacy of doxycycline for pre- or postexposure prophylaxis has yet to be definitively determined. While the 2 randomized controlled trials (RCTs) conducted to date had similar levels of efficacy (~70%), the estimated effect sizes were imprecise because of modest sample sizes [16, 17, 22]. Additionally, it is unknown which specific sex acts were protected. Studies underway will lead to more precise measures of overall efficacy and efficacy for specific sexual behaviors. Those measures are important to inform clinical decision making, cost-effectiveness analyses, community education, and patient counseling.
Population of Focus
Public health experts have long promoted controlling STIs in a core population of individuals with a high number of sexual contacts as an approach to reduce STIs in the general population [23, 24]. Modeling based on Australian parameters [18] suggests that focusing on MSM with higher numbers of sex partners (>20 partners in 6 months) would be almost as effective as broader Doxy PrEP use. Current and planned studies have generally focused on MSM at higher risk of STIs and/or HIV infection, but criteria vary and sample sizes may not be sufficient to stratify results for subpopulations with more elevated risk. Additional modeling studies or pooled analyses may be useful to identify the characteristics of populations most suitable for maximizing the impact of doxycycline prophylaxis.
Benefits and Harms
Safety
Adults generally tolerate doxycycline well [10]. Studies demonstrate the most commonly reported side effects are related to gastrointestinal (<1–55% of patients) and skin (<1–42% of patients), including photosensitivity (6–42%), toxicity over 7 days to 6 months of use [10]. The most severe gastrointestinal effects are esophageal erosion and ulceration; these are most commonly associated with uncoated doxycycline hyclate [25]. Infrequent, more serious side effects in adults, including allergic reactions, exacerbation of systemic lupus erythematosus, anemia, hemolytic anemia, thrombocytopenia, eosinophilia, neutropenia, intracranial hypertension, and tooth staining, are rare [25]. Most serious adverse effects resolve with discontinuation of doxycycline [25]. Despite the known side effects, in clinical trials discontinuation due to side effects has been uncommon [13, 16, 17, 26].
Clinicians routinely prescribe low doses (40–100 mg daily) of doxycycline for weeks to months for acne and rosacea [25] and months to years for malaria prophylaxis [13]. Multiple studies on side effects among patients using doxycycline for malaria prophylaxis have been contradictory or insufficient to draw clear conclusions [13]. Researchers have studied prolonged doxycycline use (3–18 months) for the management of abdominal aortic aneurysm. No serious adverse reactions were seen in those studies and fewer than 10% of patients withdrew because of medication side effects [26–29].
Formulation, Tolerability, and Regimen
Doxycycline monohydrate and doxycycline hyclate are the most commonly used formulations of doxycycline. Due to the pH at which they are soluble, esophageal side effects may be less frequent with doxycycline monohydrate or enteric-coated doxycycline hyclate compared with uncoated doxycycline hyclate [30]. Since the formulation of doxycycline used may impact side effects and patient adherence, this should be tracked closely in future RCTs.
Patient preference is another major consideration. A small qualitative study in Australia (N = 13) found that participants have a strong preference for daily dosing. Patients preferences may vary by HIV infection status, use of HIV PrEP, and how individuals use HIV PrEP (eg, daily or intermittently).
Antimicrobial Resistance
Concern around antimicrobial resistance has been raised by some clinicians and public health organizations, along with a call for more research in this area [31]. In the United States, 23.1% of NG isolates tested in 2017 were resistant to tetracycline [1]; NG resistance to tetracycline is higher in some parts of Europe (France: 45%; England: 49%) [10, 32]. Additionally, gonococcal antimicrobial resistance is frequently higher among MSM [1, 32], the population most likely to use Doxy PEP/PrEP. However, given the existing high rates of tetracycline resistance in NG and the fact that doxycycline is not recommended for treatment, another perspective may be that the additional contribution of prophylactic use to NG resistance in this context is negligible.
There are no established standards for identifying or measuring doxycycline resistance in NG, CT, or TP via culture or molecular techniques, although investigators have developed methods for research purposes and most clinicians apply tetracycline susceptibility data for NG to doxycycline [33–35].
Treatment failure in CT has been reported in 5–23% of persons [36], although these studies did not test for resistance and the causes of treatment failure are unclear. Treatment failure in patients with CT has been associated with a range of in vitro doxycycline MICs of more than 0.125 μg/mL to more than 4.0 μg/mL [33, 37]; however, there is not a strict correlation between treatment failure and tested MIC [33] so the clinical relevance of these findings are unclear. Several small population-level studies in communities with high background doxycycline use or subsequent to mass-treatment programs for trachoma did not find evidence of doxycycline resistance in CT [36].
Two studies have evaluated tetracycline resistance in TP. In China, molecular typing of TP from 438 case-patients [34] with syphilis found no evidence of a mutation in the 16S rRNA gene that is associated with tetracycline resistance in other bacterial species. A similar study of 53 case-patients in Italy [38] also did not identify any doxycycline resistance mutations. Complete genome sequencing of TP [39] has not found genetic elements associated with gene-transfer mechanisms. That suggests that TP is less likely than other bacteria to develop plasmid-mediated antimicrobial resistance. However, macrolide resistance in TP has been documented to occur due to a single point mutation [40], suggesting the potential for tetracycline resistance [41].
Antimicrobial resistance in Mycoplasma genitalium (MG), a frequent cause of nongonococcal urethritis in men, is also a growing concern [42, 43]. Even though tetracyclines have low efficacy against MG [15], doxycycline is a recommended alternative regimen [15] because of emerging resistance to first-line treatments. In a substudy of the ANRS IPERGAY doxycycline extension, 11% of the 210 participants tested positive for MG at baseline and 11 participants acquired MG during the study [43]. Azithromycin and fluoroquinolone resistance was identified in 70% and 15%, respectively, of tested specimens [43]. Broad doxycycline use in populations with high prevalence rates of MG could decrease treatment options for that bacterium. Mycoplasma genitalium prevalence and antibiotic susceptibilities should be examined in future studies.
Finally, Doxy PEP/PrEP could contribute to development of doxycycline resistance in commensal organisms, including those with the potential to transmit resistance. Studies of military members deployed overseas taking doxycycline for malaria prophylaxis have found conflicting data on the impact of doxycycline prophylaxis on antimicrobial resistance in oropharyngeal and intestinal commensal organisms [44–46]. Further study is needed. While it is possible to evaluate the impact of doxycycline on oral and rectal flora by examining the microbiome and resistome in these areas, there are no standard guidelines for interpreting findings.
Community Acceptability and Perceptions
Multiple surveys have demonstrated that doxycycline prophylaxis is acceptable to MSM. In an online survey of 2095 Australian MSM, 53% indicated they would be likely to take doxycycline to prevent syphilis and 76% indicated they would take doxycycline to reduce syphilis in the community [18]. Among 1301 users of a US social-networking app for MSM, 84% were interested in trying Doxy PEP [47]. Interest was higher among African-American and Latino/a respondents [47]. At a joint Australia–New Zealand HIV/STI scientific meeting of clinical and public health experts in 2015, 52% of 63 providers felt that the benefits of doxycycline prophylaxis outweighed the risks, although 88% had some concerns about antimicrobial resistance.
Some MSM in North America and Europe may already be using doxycycline for STI prophylaxis. In a survey of MSM taking HIV PrEP in the United Kingdom [48], 6 of 106 respondents reported taking doxycycline to prevent STIs in the previous 6 months. In the ANRS IPERGAY Doxy PEP study [17] 3–13% of participants in the placebo group had detectable doxycycline blood levels at each study visit. Doxycycline is also frequently available through online companies selling HIV PrEP [31].
Researchers and study participants have expressed concern about the potential for confusion between Doxy PEP/PrEP and HIV PrEP. Both medications can be used daily or intermittently. Individuals living with HIV may believe themselves to be ineligible for Doxy PEP/PrEP. Individuals without HIV may not understand that HIV PrEP does not protect them against STIs and Doxy PEP/PrEP does not protect them from HIV. Effective educational campaigns, designed using evidence from ongoing and future studies, will be critical to address those concerns.
Risk Compensation
Investigators have documented decreased condom use among MSM using HIV PrEP [8]. While reports from completed Doxy PEP/PrEP trials have not identified similar changes in risk behavior [16, 17], findings from the ongoing Canadian DuDHS study suggest some risk compensation might occur. Notably, risk compensation among people taking HIV PrEP was not identified in the initial placebo-controlled trials but only became evident in later uncontrolled trials [8].
Risk–Benefit and Cost-effectiveness
While the benefits of HIV PREP as a way to prevent a life-threatening infection are clear, the risk-balance of STI prevention may be more uncertain. As Golden and Handsfield [22] suggest, a key issue to consider is which benefits of Doxy PEP/PrEP are most important to prioritize. While STI treatment has significant personal and financial costs, and STIs can cause serious sequelae in men (eg, blindness and hearing loss due to syphilis), much of the direct STI morbidity is in women (infertility and adverse pregnancy outcomes). Primarily using Doxy PEP/PrEP in MSM, a population at high risk of bacterial STIs, might have relatively limited impact on reproductive health outcomes at the population level. The question of which benefits to focus on will also directly impact the cost-effectiveness of Doxy PEP/PrEP as the number of people who would need to receive treatment to avert a negative outcome will vary substantially depending on the outcome selected [22].
CONCLUSIONS
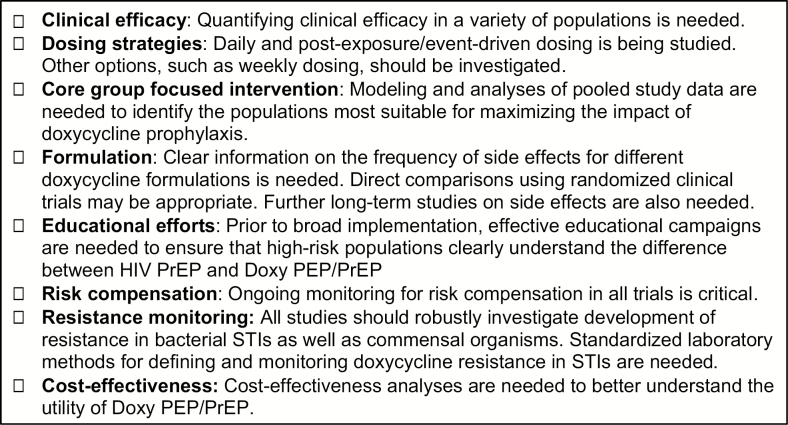
Based on our review of the current evidence and studies underway, doxycycline prophylaxis for bacterial STIs shows promise. However, there are several research priorities that need to be addressed before it is recommended broadly (Figure 1). In addition to increasing the evidence base on the efficacy of Doxy PEP/PrEP, researchers should carefully consider which populations to focus on, the doxycycline formulation and regimen and ensuring that findings can be translated to real-world implementation. Pooled analyses across studies may be helpful in examining issues such as identifying subpopulations most likely to benefit from Doxy PEP/PrEP. There is also a clear and immediate need to develop consistent laboratory methods for evaluating doxycycline resistance in NG, CT, TP, and MG, as well as other common pathogens such as S. aureus and Streptococcus pneumonia, and broader guidance on how to interpret and use microbiome and resistome data. Finally, cost-effectiveness and modeling studies that consider different scenarios and individual- versus population-level impacts of Doxy PEP/PrEP are needed to guide conclusions around the appropriateness of Doxy PEP/PrEP to prevent bacterial STIs.
Figure 1.
Recommendations for research activities. Abbreviations: Doxy, doxycycline; HIV, human immunodeficiency virus; PEP, postexposure prophylaxis; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Notes
Acknowledgments. The authors thank Robert Bolan and Matt Golden for their expertise and assistance in developing this manuscript. We also thank the attendees of the Doxycycline for Prevention of Syphilis in High-Risk Populations preconference leadership meeting for CROI 2019 who contributed to a useful and insightful discussion: Cherie Blair, Robert Bolan, David Burns, Phillip Chan, Vincent Cornelisse, Carlos Del Rio, Julie Dombrowski, Deborah Donnell, Richard Gilson, Matt Golden, Rebecca Guy, Elske Hoornenborg, Jeanne Marrazzo, Tarek Mikati, Sheldon Morris, Vincent Ofori, and Darrell Tan.
Financial support. This work was supported by Team Klausner Saving Lives, University of California Los Angeles Center for AIDS Research (grant number 5P30 AI028697) and the National Institute of Allergy and Infectious Diseases (NIAID grant number 1R01AI139265).
Potential conflicts of interest. A. F. L. has received grants from ViiV Healthcare Limited. J-M. M. has received personal fees from Gilead Sciences, Inc, Merck & Co., ViiV Healthcare Limited, Bristol-Myers Squibb, and Teva Pharmaceutical Industries and grants from Gilead Sciences, Inc. The other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. sexually transmitted disease surveillance 2017. Atlanta, GA: US Department of Health and Human Services, 2018. [Google Scholar]
- 2. Choudhri Y, Miller J, Sandhu J, Leon A, Aho J.. Infectious and congenital syphilis in Canada, 2010–2015. Can Commun Dis Rep 2018; 44:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Centre for Disease Prevention and Control. Surveillance atlas of infectious diseases Available at: https://ecdc.europa.eu/en/syphilis/surveillance-and-disease-data/disease-data-atlas. Accessed 3 October 2019.
- 4. Kirby Insitute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2018. Sydney: Kirby Institute, 2018. [Google Scholar]
- 5. Nguyen VK, Greenwald ZR, Trottier H, et al. . Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS 2018; 32:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montaño MA, Dombrowski JC, Dasgupta S, et al. . Changes in sexual behavior and STI diagnoses among MSM initiating PrEP in a clinic setting. AIDS Behav 2019; 23:548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Traeger MW, Cornelisse VJ, Asselin J, et al. ; PrEPX Study Team Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA 2019; 321:1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Traeger MW, Schroeder SE, Wright EJ, et al. . Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018; 67:676–86. [DOI] [PubMed] [Google Scholar]
- 9. Schünemann HJ, Mustafa R, Brozek J, et al. ; GRADE Working Group GRADE guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol 2016; 76:89–98. [DOI] [PubMed] [Google Scholar]
- 10. Peyriere H, Makinson A, Marchandin H, Reynes J. Doxycycline in the management of sexually transmitted infections. J Antimicrob Chemother 2018; 73:553–63. [DOI] [PubMed] [Google Scholar]
- 11. Twartz JC, Shirai A, Selvaraju G, Saunders JP, Huxsoll DL, Groves MG. Doxycycline propylaxis for human scrub typhus. J Infect Dis 1982; 146:811–8. [DOI] [PubMed] [Google Scholar]
- 12. Takafuji ET, Kirkpatrick JW, Miller RN, et al. . An efficacy trial of doxycycline chemoprophylaxis against leptospirosis. N Engl J Med 1984; 310:497–500. [DOI] [PubMed] [Google Scholar]
- 13. Gaillard T, Madamet M, Pradines B. Tetracyclines in malaria. Malar J 2015; 14:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nadelman RB, Nowakowski J, Fish D, et al. ; Tick Bite Study Group Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med 2001; 345:79–84. [DOI] [PubMed] [Google Scholar]
- 15. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:1–137.25590678 [Google Scholar]
- 16. Bolan RK, Beymer MR, Weiss RE, Flynn RP, Leibowitz AA, Klausner JD. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: a randomized, controlled pilot study. Sex Transm Dis 2015; 42:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molina JM, Charreau I, Chidiac C, et al. ; ANRS IPERGAY Study Group Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis 2018; 18:308–17. [DOI] [PubMed] [Google Scholar]
- 18. Wilson DP, Prestage GP, Gray RT, et al. . Chemoprophylaxis is likely to be acceptable and could mitigate syphilis epidemics among populations of gay men. Sex Transm Dis 2011; 38:573–9. [DOI] [PubMed] [Google Scholar]
- 19. Molina JM, Ghosn J, Béniguel L, et al. . Incidence of HIV-infection in the ANRS Prevenir study in Paris region with daily or on-demand PrEP with TDF/FTC. In: AIDS 2018 Amsterdam, The Netherlands, 2018. Abstract 13278. [Google Scholar]
- 20. Quan J, Langelier C, Kuchta A, et al. . FLASH: a next-generation CRISPR diagnostic for multiplexed detection of antimicrobial resistance sequences. bioRxiv 2018:426338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Norris SJ, Edmondson DG. In vitro culture system to determine MICs and MBCs of antimicrobial agents against Treponema pallidum subsp. pallidum (Nichols strain). Antimicrob Agents Chemother 1988; 32:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Golden MR, Handsfield HH. Preexposure prophylaxis to prevent bacterial sexually transmitted infections in men who have sex with men. Sex Transm Dis 2015; 42:104–6. [DOI] [PubMed] [Google Scholar]
- 23. Gunn RA, Fitzgerald S, Aral SO. Sexually transmitted disease clinic clients at risk for subsequent gonorrhea and chlamydia infections: possible “core” transmitters. Sex Transm Dis 2000; 27:343–9. [DOI] [PubMed] [Google Scholar]
- 24. Ellen JM, Hessol NA, Kohn RP, Bolan GA. An investigation of geographic clustering of repeat cases of gonorrhea and chlamydial infection in San Francisco, 1989–1993: evidence for core groups. J Infect Dis 1997; 175:1519–22. [DOI] [PubMed] [Google Scholar]
- 25. Sloan B, Scheinfeld N. The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin Drug Saf 2008; 7:571–7. [DOI] [PubMed] [Google Scholar]
- 26. Mosorin M, Juvonen J, Biancari F, et al. . Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg 2001; 34:606–10. [DOI] [PubMed] [Google Scholar]
- 27. Hackmann AE, Rubin BG, Sanchez LA, Geraghty PA, Thompson RW, Curci JA. A randomized, placebo-controlled trial of doxycycline after endoluminal aneurysm repair. J Vascu Surg 2008; 48:519–26; discussion 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baxter BT, Pearce WH, Waltke EA, et al. . Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (phase II) multicenter study. J Vasc Surg 2002; 36:1–12. [DOI] [PubMed] [Google Scholar]
- 29. Meijer CA, Stijnen T, Wasser MN, Hamming JF, van Bockel JH, Lindeman JH; Pharmaceutical Aneurysm Stabilisation Trial Study Group Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial. Ann Intern Med 2013; 159:815–23. [DOI] [PubMed] [Google Scholar]
- 30. Tan KR, Magill AJ, Parise ME, Arguin PM; Centers for Disease Control and Prevention Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg 2011; 84:517–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Public Health England, British Association for Sexual Health and HIV. Position statement on doxycycline as post-exposure prophylaxis for sexually transmitted infections. London: Public Health England, 2017. [Google Scholar]
- 32. Public Health England. Surveillance of antimicrobial resistance in Neisseria gonorrhoeae in England and Wales: key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP). London: Public Health England, 2018. [Google Scholar]
- 33. Pitt R, Alexander S, Ison C, et al. . Phenotypic antimicrobial susceptibility testing of Chlamydia trachomatis isolates from patients with persistent or successfully treated infections. J Antimicrob Chemother 2018; 73:680–6. [DOI] [PubMed] [Google Scholar]
- 34. Xiao Y, Liu S, Liu Z, et al. . Molecular subtyping and surveillance of resistance genes in Treponema pallidum DNA from patients with secondary and latent syphilis in Hunan, China. Sex Transm Dis 2016; 43:310–6. [DOI] [PubMed] [Google Scholar]
- 35. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0, 2019. Available at: http://www.eucast.org [Google Scholar]
- 36. Borel N, Leonard C, Slade J, Schoborg RV. Chlamydial antibiotic resistance and treatment failure in veterinary and human medicine. Curr Clin Microbiol Rep 2016; 3:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Somani J, Bhullar VB, Workowski KA, Farshy CE, Black CM. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J Infect Dis 2000; 181:1421–7. [DOI] [PubMed] [Google Scholar]
- 38. Giacani L, Ciccarese G, Puga-Salazar C, et al. . Enhanced molecular typing of Treponema pallidum subspecies pallidum strains from 4 Italian Hospitals shows geographical differences in strain type heterogeneity, widespread resistance to macrolides, and lack of mutations associated with doxycycline resistance. Sex Transm Dis 2018; 45:237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fraser CM, Norris SJ, Weinstock GM, et al. . Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 1998; 281:375–88. [DOI] [PubMed] [Google Scholar]
- 40. Lukehart SA, Godornes C, Molini BJ, et al. . Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med 2004; 351:154–8. [DOI] [PubMed] [Google Scholar]
- 41. Stamm LV. Global challenge of antibiotic-resistant Treponema pallidum. Antimicrob Agents Chemother 2010; 54:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pitt R, Fifer H, Woodford N, Alexander S. Detection of markers predictive of macrolide and fluoroquinolone resistance in Mycoplasma genitalium from patients attending sexual health services in England. Sex Transm Infect 2018; 94:9–13. [DOI] [PubMed] [Google Scholar]
- 43. Berçot B, Charreau I, Rousseau C, et al. . High prevalence and antibiotic resistance of M. genitalium infections in MSM on PrEP. In: Conference on Retroviruses and Opportunistic Infections Seattle, Washington, 2019. Abstract 966. [Google Scholar]
- 44. Vento TJ, Calvano TP, Cole DW, et al. . Staphylococcus aureus colonization of healthy military service members in the United States and Afghanistan. BMC Infect Dis 2013; 13:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vento TJ, Cole DW, Mende K, et al. . Multidrug-resistant gram-negative bacteria colonization of healthy US military personnel in the US and Afghanistan. BMC Infect Dis 2013; 13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lesens O, Haus-Cheymol R, Dubrous P, et al. ; Working Group on Cutaneous Infections in the Army Methicillin-susceptible, doxycycline-resistant Staphylococcus aureus, Côte d’Ivoire. Emerg Infect Dis 2007; 13:488–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spinelli MA, Scott HM, Vittinghoff E, Liu AY, Coleman K, Buchbinder SP. High interest in doxycycline for sexually transmitted infection post-exposure prophylaxis (doxycycline-PEP) in a multi-city survey of men who have sex with men (MSM) using a social-networking app. Sex Trans Dis 2019; 46:e32–e34. Available at: https://www.ncbi.nlm.nih.gov/pubmed/30870327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carveth-Johnson T, Stingone C, Nwokolo N, Whitlock G. Doxycycline use in MSM taking PrEP. Lancet HIV 2018; 5:e482. [DOI] [PubMed] [Google Scholar]