Abstract
Background
Prior studies suggest that transgender women (TW) with human immunodeficiency virus (HIV) are less likely to be virally suppressed than cisgender women (CW) and cisgender men (CM). However, prior data are limited by small sample sizes and cross-sectional designs. We sought to characterize the HIV care continuum comparing TW to CW and CM in the United States and Canada.
Methods
We analyzed annual HIV care continuum outcomes by gender status from January 2001 through December 2015 among adults (aged ≥18 years) in 15 clinical cohorts. Outcomes were retention in care and viral suppression.
Results
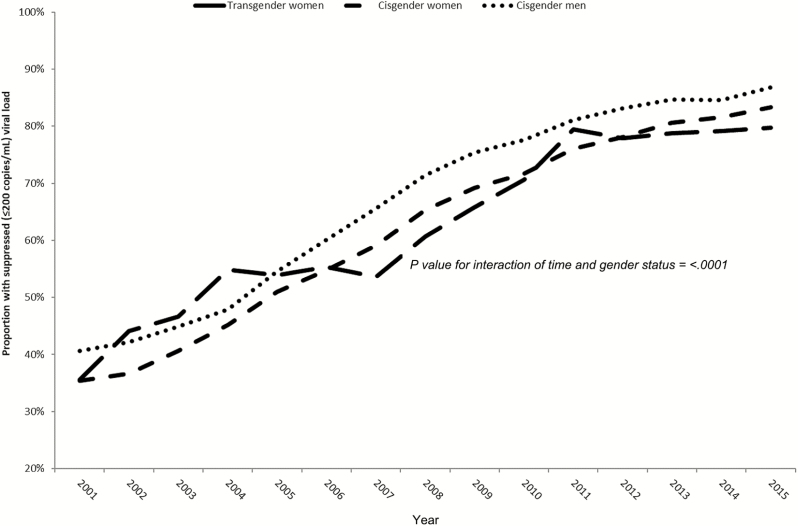
The study population included TW (n = 396), CW (n = 14 094), and CM (n = 101 667). TW had lower proportions retained in care than CW and CM (P < .01). Estimates of retention in care were consistently lower in TW, with little change over time within each group. TW and CW had similar proportions virally suppressed over time (TW, 36% in 2001 and 80% in 2015; CW, 35% in 2001 and 83% in 2015) and were lower than CM (41% in 2001 and 87% in 2015). These differences did not reach statistical significance after adjusting for age, race, HIV risk group, and cohort.
Conclusions
TW experience challenges with retention in HIV care. However, TW who are engaged in care achieve viral suppression that is comparable to that of CW and CM of similar age, race, and HIV risk group. Further research is needed to understand care engagement disparities.
Keywords: transgender women, HIV continuum of care, retention in care, HIV viral suppression
Transgender women (TW) experience challenges with retention in human immunodeficiency virus (HIV) care. However, TW who are engaged in care achieve viral suppression that is comparable to that of cisgender women and cisgender men of similar age, race, and HIV risk group. Further research is needed to understand care engagement disparities.
An estimated 1 in 5 transgender women (TW; individuals assigned male at birth who identify as women) in the United States are living with human immunodeficiency virus (HIV) [1, 2]. The Centers for Disease Control and Prevention (CDC) National HIV Surveillance System reported 1974 newly diagnosed cases of HIV in TW from 2009 through 2014, the most recent years for which data have been published [3]. Approximately 1 in 4 of these women were diagnosed with AIDS within 3 months of their HIV diagnosis, suggesting delayed engagement in HIV testing and care. Successful engagement and retention in HIV care as well as viral suppression are critical to reducing morbidity and mortality among people living with HIV [4] and to reducing onward transmission [5]. The published literature on HIV care engagement among TW is limited. However, patient self-reported data suggest inequities in HIV care engagement [6], antiretroviral therapy adherence [6,7], and viral suppression [7–9].
Cross-sectional studies based on medical records have found that TW have lower rates of viral suppression but similar rates of retention in clinical care compared with cisgender (nontransgender) women (CW) and cisgender men (CM) living with HIV. In their most recent analysis of transgender people living with HIV, the CDC Medical Monitoring Project pooled population-representative medical record data from 2009 through 2011 representing 5729 TW in the United States. They found no difference in receipt of antiretroviral prescriptions among TW compared with CM and CW [10]. However, a significantly lower proportion of TW had 100% self-reported antiretroviral dose adherence relative to CM and CW (78% vs 87% and 83%, respectively) and viral suppression (51% vs 61% and 57%, respectively). More than 8800 transgender individuals living with HIV received Ryan White HIV/AIDS Program services in 2017, including 7837 TW [11]. TW were as likely as cisgender adults to be retained in care (78.2% vs 80.9%), but they were significantly less likely to have a suppressed viral load (80.9% vs 85.9%).
Many prior studies have been limited by cross-sectional designs and/or self-reported HIV outcomes. Data on changes in TW’s engagement in care over time are scant. Yehia and colleagues reported data from a 2001–2011 retrospective cohort that included 285 transgender patients from 13 HIV clinics across the United States [12]. They found no difference in the percentage of patient-years retained in HIV care, receiving antiretroviral therapy, or being virally suppressed between transgender and cisgender participants. However, these findings did not disaggregate TW from other transgender participants in the cohort.
In this study, we sought to address gaps in the literature on the HIV care continuum among TW in clinical care using data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). NA-ACCORD is the largest ongoing cohort of people living with HIV in the United States and Canada, with more than 20 collaborating cohorts that contribute data on participants living with HIV. Fifteen of these cohorts were able to contribute data on transgender participants. We named this subcohort the North American Transgender Cohort Collaboration (NA-TRACC). Our objective in this study was to estimate retention in care and viral suppression among TW compared with CM and CW in NA-TRACC. We hypothesized that TW would be less likely to be retained in care and less likely to be virally suppressed compared with CW and CM. Based on known social and structural drivers of HIV infection [13, 14], we also hypothesized that TW who are racial/ethnic minorities, are younger, and engage in injection drug use (IDU) would be less likely to engage in care and achieve viral suppression than white, older, and noninjection drug using TW, respectively.
METHODS
The NA-ACCORD is the North American region of the International epidemiology Databases to Evaluate AIDS (IeDEA) project, supported by the National Institutes of Health. Details on this collaboration have been published previously [15]. Briefly, the NA-ACCORD consists of single- and multisite clinical and interval cohorts that prospectively collect data on more than 180 000 adults living with HIV (aged ≥18 years) from multiple sites in the United States and Canada [16]. Each cohort submits comprehensive data on enrolled participants to the Data Management Core (University of Washington, Seattle), where the data undergo quality control, are harmonized across cohorts, and are transmitted to the Epidemiology/Biostatistics Core (Johns Hopkins University, Baltimore, MD), which conducted the analyses presented here. The human subjects research activities of the NA-ACCORD and each of the participating cohort studies have been reviewed and approved by their respective local institutional review boards and the Johns Hopkins School of Medicine. The Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved the present study.
This retrospective, time-series analysis included adults from the 15 NA-TRACC that contribute US and Canadian clinical cohorts with access to electronic medical record data with transgender status from 1 January 2001 to 31 December 2015 (Supplementary Table). Intersex adults (n = 2) and transgender men (n = 38) were excluded from this analysis as the focus of this study was TW.
Gender Status: Transgender Women, Cisgender Women, and Cisgender Men
Transgender status was captured using different approaches across the contributing cohorts, including presence of diagnosis codes for gender dysphoria, comparison of natal sex with reports of feminizing or masculinizing hormones from medication lists, gender identity queried at intake, and medical provider documentation in the clinical record [17]. These approaches were harmonized into a single variable that categorized gender status as TW, CW, CM, and transgender man. TW included participants who were assigned male sex at birth who had a diagnosis of gender dysphoria, were taking feminizing hormones, and/or were identified as women or TW by self-report or provider report in the medical record.
Outcomes: Retention in Care and HIV RNA Suppression
The proportion of participants retained in care and the proportion with viral suppression were estimated in each calendar year from 2001 through 2015, applying a serial cross-sectional approach to the longitudinal data. Both measures used the same denominator: those who had at least 1 HIV clinic visit in the calendar year, based on encounter data. The numerator for the proportion retained in care was the number of participants with at least 2 HIV clinical visits more than 90 days apart in the calendar year. This measure was shown to be an appropriate surrogate for retention in care in prior NA-ACCORD studies [18] and mirrors definition used by the HIV/AIDS bureau of the US Health Resources Services Administration [19]. The numerator for the proportion of participants with viral suppression was the number of participants with an HIV RNA measurement ≤200 copies/mL at the last measurement of the year.
Covariates
All covariates were collected at enrollment into the NA-ACCORD. Age in years was determined from year of birth. Race/ethnicity was categorized as white, black, Hispanic, or other. HIV risk group classification reflected the suspected mode of HIV acquisition categorized as IDU, sexual behavior, other (eg, hemophilia), and other/unknown.
Statistical Analyses
Differences in covariates among TW, CW, and CM were examined using a χ2 test (for differences in proportions), a t test (for differences in means), and the Kruskal-Wallis test (for a difference in medians). The annual proportions of participants retained in care and virally suppressed were estimated from 2001 through 2015. Because individuals could contribute information to more than 1 calendar year, the P value for trend was estimated using a log-binomial regression model with generalized estimating equations (as the observations in each year are not independent), with an ordinal variable for calendar year, which tests the null hypothesis that there is no change in the indicator over time. To determine if there was statistical evidence that the association of gender status and the outcomes of interest changed over time, we used a nested model approach that allowed us to compare the fit statistics for models with and without the interaction terms using log binomial regression models with generalized estimating equations.
We restricted the analysis of gender status and the outcomes of interest to 2014 (the most recent year in which all 15 cohorts contributed data for the entire year). Log binomial regression for retention in care and modified Poisson regression for viral suppression (due to failed convergence of the log binomial) were used to estimate adjusted prevalence ratios (aPR) and 95% confidence intervals ([,]), accounting for age, race/ethnicity, history of IDU, and cohort.
Finally, we restricted the study population to only TW and used the same models to examine the associations of age, race/ethnicity, and history of IDU with the outcomes of interest.
RESULTS
As listed in Table 1, the study population included TW (n = 396), CW (n = 14 094), and CM (n = 101 667). TW were younger at baseline (median, 36 years; interquartile range [IQR], 29, 43) compared with CW (40 years; IQR, 32, 47) and CM (44 years; IQR, 37, 52), with 36% of TW aged ≥40 years vs 49% of CW and 66% of CM. Among TW, 40% were black compared with 56% of CW and 36% of CM. Sexual behavior was the most common HIV acquisition risk among TW (79%) compared with CW (61%) and CM (45%).
Table 1.
Participant Characteristics in the First Year the Participant Had 1 Human Immunodeficiency Virus Care Visit, by Gender—North American AIDS Cohort Collaboration on Research and Design, 2001–2015
| Characteristic | Transgender Women | Cisgender Women | Cisgender Men | |||
|---|---|---|---|---|---|---|
| (N = 396) | (N = 14 094) | (N = 101 667) | ||||
| Age, y | ||||||
| Mean (standard deviation) | 37 | (10) | 40 | (10) | 44 | (11) |
| Median (interquartile range) | 36 | (29–43) | 40 | (32–47) | 44 | (37–52) |
| Categorical (n, %) | ||||||
| 18–39 | 255 | 64 | 7214 | 51 | 34 755 | 34 |
| 40–49 | 97 | 24 | 4408 | 31 | 34 768 | 34 |
| 50–59 | 42 | 11 | 1961 | 14 | 23 097 | 23 |
| 60–69 | 2 | 1 | 439 | 3 | 7110 | 7 |
| ≥70 | … | 0 | 72 | 1 | 1937 | 2 |
| Race/ethnicity | ||||||
| White | 92 | 23 | 3405 | 24 | 44 632 | 44 |
| Black | 160 | 40 | 7923 | 56 | 36 348 | 36 |
| Hispanic | 85 | 21 | 919 | 7 | 9586 | 9 |
| Other | 44 | 11 | 914 | 6 | 3798 | 4 |
| Unknown | 15 | 4 | 933 | 7 | 7303 | 7 |
| Human immunodeficiency virus acquisition risk | ||||||
| History of injection drug use | 30 | 8 | 2878 | 20 | 18 947 | 19 |
| Sexual | 314 | 79 | 8564 | 61 | 45 347 | 45 |
| Other | 4 | 1 | 804 | 6 | 1137 | 1 |
| Unknown | 48 | 12 | 1848 | 13 | 36 236 | 36 |
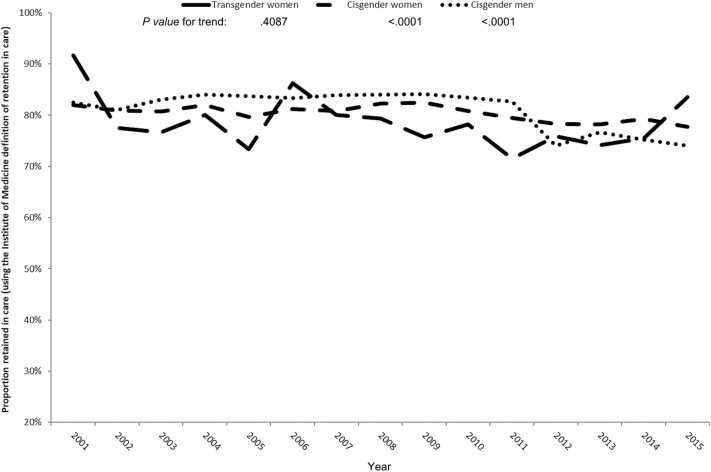
TW demonstrated greater variability in estimates of retention in care, likely due to relatively small sample sizes. A lower proportion of TW were retained in care compared with CW and CM, with statistical evidence of a difference in trends in retention in care by gender (Figure 1; P value for interaction = .0093). The retention in care estimates declined slightly over time for CW and CM (P values for trend: CW, P = .0003 and CM, P < .0001). Estimates of retention in care were consistently lower in TW, with no statistical evidence of a trend (P value for trend: TW, P = .6144). The prevalence of TW retained in care did not differ from CM (aPR = 0.99 [0.90, 1.08]) after adjusting for age, race/ethnicity, HIV risk group, and cohort (Table 2). However, the prevalence of patients retained in care was greater in CW than CM (aPR = 1.03 [1.02, 1.05]).
Figure 1.
Proportion retained in care, by gender status, North American AIDS Cohort Collaboration on Research and Design, 2001–2015. Retention in care was defined according to the United States Health Services Resources Administration. The P values for trends in the outcomes over a calendar year were estimated using a log-binomial model with generalized estimating equations (GEE) and a continuous variable for calendar year, which tests the null hypothesis that there is no difference in the outcome over time and allows for repeated measurements from individuals. The P value for interaction of time and gender status was estimated using a nested model approach that allowed us to compare the fit statistics for models with and without the interaction terms using log-binomial regression models with GEE.
Table 2.
Prevalence Ratios for the Associations of Gender With Retention in Care and Viral Suppression—North American AIDS Cohort Collaboration on Research and Design, 2014
| Gender | N | % | PRa | 95% CI | aPRb | 95% CI |
|---|---|---|---|---|---|---|
| Retained in Care (N = 23 354) | ||||||
| Transgender women | 151 | 1 | 1.00 | [.92, 1.10] | 0.99 | [.90, 1.08] |
| Cisgender women | 4731 | 20 | 1.05 | [1.04, 1.07] | 1.03 | [1.02, 1.05] |
| Cisgender men | 18 427 | 79 | Ref | … | Ref | … |
| Suppressed (≤200 copies/mL) Human Immunodeficiency Virus Viral Load (N = 26 355) | ||||||
| Transgender women | 173 | 1 | 0.93 | [.86, .99] | 0.96 | [.89, 1.02] |
| Cisgender women | 5171 | 20 | 0.96 | [.95, .98] | 1.00 | [.98, 1.01] |
| Cisgender men | 21 011 | 80 | Ref | … | Ref | … |
Bold signifies the 95% CI does not include 1.0.
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; PR, prevalence ratio; Ref, Reference category.
aThe PRs and 95% CIs were estimated using a log-binomial regression model.
bThe aPRs and 95% CIs were estimated using a log-binomial regression model (for retention in care) and a Poisson regression with robust variance model (for viral suppression) accounting for age, race/ethnicity, human immunodeficiency virus risk group, and cohort.
All 3 groups showed substantial improvements in viral suppression from 2001 through 2015 (Figure 2; P values for trend: TW, P < .0001; CW, P < .0001; CM, P < .0001). CM had the highest proportion with viral suppression, and TW and CW had proportions that were similar to each other over time. Although differences in viral suppression by gender status were the greatest in 2001, this difference narrowed in more recent years (P for interaction, <.0001). Viral suppression did not differ by gender status in adjusted analyses (TW vs CM aPR = 0.96 [0.89, 1.02]; CW vs CM aPR = 1.00 [0.98, 1.01]; Table 2).
Figure 2.
Proportion with human immunodeficiency virus (HIV) RNA suppression (≤200 copies/mL), by gender status, North American AIDS Cohort Collaboration on Research and Design, 2001–2015. The last HIV RNA measurement was used if there were multiple measurements in a year for an individual. The P values for trends in the outcomes over a calendar year were estimated using a log-binomial model with generalized estimating equations (GEE) and a continuous variable for calendar year, which tests the null hypothesis that there is no difference in the outcome over time and allows for repeated measurements from individuals. The P value for interaction of time and gender status was estimated using a nested model approach that allowed us to compare the fit statistics for models with and without the interaction terms using log-binomial regression models with GEE.
As hypothesized, older age was associated with a greater likelihood of being retained in care and having a suppressed viral load in unadjusted models among TW (Table 3). While differences by age were no longer significant in adjusted models, TW with an other/unknown HIV acquisition risk were significantly more likely to be retained in care compared with those with sexual risk (aPR = 1.82 [1.24, 2.69]). There were no significant differences by race in either retention in care or viral suppression.
Table 3.
Associations of Age, Race/Ethnicity, and Human Immunodeficiency Virus Risk Group on Retention in Care and Viral Suppression Among Transgender Women in the North American AIDS Cohort Collaboration on Research and Design, 2014
| Characteristics | n | % | PRa | 95% CI | aPRb | 95% CI |
|---|---|---|---|---|---|---|
| Retained in Care (N = 151) | ||||||
| Age, y | ||||||
| 18–39 | 94 | 62 | 0.86 | [.70, 1.05] | 0.81 | [.66, 1.00] |
| 40–49 | 38 | 25 | Ref | … | Ref | … |
| 50–59 | 17 | 11 | 1.08 | [.86, 1.36] | 1.04 | [.81, 1.32] |
| 60–69 | 2 | 1 | 1.23 | [1.05, 1.43] | 1.00 | [.78, 1.30] |
| ≥70 | 0 | 0 | … | … | … | … |
| Race/ethnicity | ||||||
| White | 33 | 22 | Ref | … | Ref | … |
| Black | 61 | 40 | 1.11 | [.85, 1.44] | 1.18 | [.89, 1.56] |
| Hispanic | 41 | 27 | 1.08 | [.82, 1.44] | 1.18 | [.88, 1.57] |
| Other/Unknown | 16 | 11 | 1.17 | [.84, 1.61] | 1.24 | [.87, 1.76] |
| HIV acquisition risk | ||||||
| IDU | 5 | 3 | 1.07 | [.69, 1.68] | 1.06 | [.64, 1.77] |
| Sexual behavior | 133 | 88 | Ref | … | Ref | … |
| Other/Unknown | 13 | 9 | 1.14 | [.88, 1.46] | 1.82 | [1.24, 2.69] |
| Suppressed (≤200 copies/mL) HIV Viral Load (N = 173) | ||||||
| Age (in years) | ||||||
| 18–39 | 112 | 65 | 0.86 | [.74, 1.00] | 0.83 | [.72, .96] |
| 40–49 | 40 | 23 | Ref | … | Ref | … |
| 50–59 | 19 | 11 | 1.12 | [1.00, 1.25] | 1.14 | [.99, 1.32] |
| 60–69 | 2 | 1 | 1.12 | [1.00, 1.25] | 1.31 | [.96, 1.80] |
| ≥70 | … | … | … | … | … | … |
| Race/ethnicity | ||||||
| White | 35 | 20 | Ref | … | Ref | … |
| Black | 76 | 44 | 0.83 | [.69, 1.00] | 0.80 | [.62, 1.03] |
| Hispanic | 45 | 26 | 1.06 | [.91, 1.23] | 1.10 | [.94, 1.27] |
| Other/Unknown | 17 | 10 | 1.00 | [.81, 1.24] | 0.99 | [.78, 1.27] |
| HIV transmission risk | ||||||
| IDU | 7 | 4 | 1.05 | [.77, 1.44] | 0.95 | [.69, 1.30] |
| Sexual behavior | 152 | 88 | Ref | … | Ref | … |
| Other/Unknown | 14 | 8 | 1.14 | [.97, 1.35] | 1.20 | [.83, 1.73] |
Bold signifies the 95% CI does not include 1.0.
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; HIV, human immunodeficiency virus; PR, prevalence ratio; Ref, reference category.
aThe PRs and 95% CIs were estimated using a log-binomial regression model.
bThe aPRs and 95% CIs were estimated using a Poisson regression with robust variance model accounting for age, race/ethnicity, cohort, and HIV risk group.
DISCUSSION
The majority of TW in NA-TRACC were black, younger than cisgender patients, and less likely to be retained in HIV care. Prior research indicates that black TW experience a disproportionate burden of HIV [10, 13]. Therefore, it was not surprising that the majority of TW in NA-TRACC were black. TW were younger than cisgender participants, and sexual contact was the most common risk category. While the younger age of TW in NA-TRACC could be due to earlier diagnosis and linkage to care, prior CDC testing event data suggest that TW acquire HIV infection at earlier ages [20, 21] but may be no more likely than cisgender populations to be tested for HIV [22]. CDC data also indicate that the majority of TW with newly positive HIV test results have been aged <30 years (56.3%), black (53.9%), and had condomless sex (67%) [20]. Given this context, our sociodemographic data suggest that young, black, sexually active TW may be particularly vulnerable to HIV infection and should be prioritized for engagement in HIV testing, prevention, and care.
We hypothesized that TW would be less likely than cisgender participants to be retained in care. Consistent with this hypothesis, TW demonstrated lower retention in care over time compared with cisgender patients. Transgender-specific barriers to engagement in care include fear of disclosure of transgender identity; poor treatment by staff, such as using the inappropriate name or pronoun; and provider lack of knowledge about transgender health [23–25]. From 2012 through 2017, the US Health Services Resources Administration (HRSA) funded 9 sites in 4 urban areas to implement innovative interventions designed to improve engagement in HIV care for TW of color [26]. None of these sites were NA-TRACC sites. While outcome data from the HRSA initiative have not yet been published, baseline data suggested that participants who received both their HIV care and gender-affirming care (eg, provision of hormones) from the same healthcare provider were more likely to be retained in care [27]. Emerging data support that TW frequently prioritize gender-affirming care over other health issues [28] and that meeting the gender-affirmation needs of TW living with HIV may be an effective way to improve care engagement [29].
We hypothesized that TW would be less likely to achieve viral suppression compared with cisgender participants. While TW in NA-TRACC were less likely to achieve viral suppression than CM in bivariate analyses, they had viral suppression that was similar to that of CW, and no significant difference was found by gender in adjusted analyses. One possible explanation for this finding could be that NA-TRACC sites provide gender-affirming care. Baseline data from a different multisite, longitudinal study on engaging TW of color in HIV care found that viral suppression was more common among TW whose HIV care provider also prescribed their gender-affirming hormones [30]. A study among transgender people living with HIV found that gender-affirming and supportive attitudes by healthcare providers were associated with viral suppression, while making access to hormone therapy contingent upon antiretroviral therapy adherence was associated with a lower likelihood of viral suppression [31]. In short, clinicians who provide both HIV care and gender-affirming hormones without requiring antiretroviral therapy adherence to get hormones see more success with viral suppression among their TW patients living with HIV. Data on gender-affirming hormone therapy use in NA-TRACC were not available for this analysis. However, these data are currently being collected by participating cohorts, and future analyses should be able to provide insight into the role gender-affirming hormone therapy may play in viral suppression for TW.
Contrary to our hypotheses, we found no significant differences in retention in care and viral suppression among TW based on age or race in adjusted analyses. TW with IDU as their primary HIV risk did not differ significantly in retention in care or viral suppression from TW with sexual risk. However, sample sizes for these analyses were relatively small (n = 151 for retention in care and n = 91 for viral suppression), resulting in limited power to detect statistically significant differences.
Given that the majority of TW in NA-TRACC were racial/ethnic minorities and that TW were significantly younger than cisgender participants, strategies to engage young racial/ethnic minorities should be transgender-inclusive and consider the intersecting barriers to care engagement that may exist for populations with multiple marginalized identities. For example, youth-focused programs to improve access, uptake, and adherence to antiretroviral therapy should incorporate ways to address transgender community concerns about potential drug–drug interactions between antiretroviral therapy and gender-affirming hormones [28]. Emerging data indicate that concentrations of tenofovir (a commonly used antiretroviral agent) are affected by the presence of exogenous estrogen, even when tenofovir is used as part of a fully suppressive combination regimen [32, 33]. While the clinical significance of this interaction is unclear, it highlights the importance of considering transgender-specific issues in the provision of HIV care.
The main limitations of the study are related to measurement of gender status and sample size. Transgender status was ascertained in different ways across the 15 contributing cohorts and typically collected at a single time point. Therefore, some participants may have been misclassified, for example, if they did not disclose their transgender identity, if it was not documented in the medical record, or if they did not identify as transgender until some time after the enrollment period. It is unlikely that such misclassification would significantly impact the findings given the small numbers of TW in comparison to CM and CW. This is one of the largest studies of TW in the United States and Canada; however, the relatively small sample size limited precision and the ability to adjust for numerous potential confounders. It is possible that the sample size will grow over time as more TW are identified in existing cohorts and if additional cohorts are able to collect data on gender identity to contribute to NA-TRACC. With a larger number of transgender participants, estimates will be more stable, and more complex statistical analysis will be possible.
While our definition for retention in care was consistent with the Department of Health and Human Services definition, alternative ways of operationalizing care engagement (eg, based on missed visits vs kept visits) may have yielded different results [34]. Viral suppression was likely overestimated in all 3 groups as the denominator was limited to those who had an HIV primary care visit in the calendar year. The study is limited by lack of contextual information on the timing of gender transition, the prescription and/or use of gender-affirming hormone therapy, colocation of HIV and gender-affirming medical care, and structural factors such as poverty that may contribute to disparities in HIV outcomes. Future studies in NA-TRACC should collect this information systematically.
In the face of mounting data on the burden of HIV among TW, it is important to ensure equitable access to HIV care and treatment. The HIV care continuum is an important epidemiologic tool that allows us to monitor disparities and identify where interventions are needed. The creation of NA-TRACC and analysis of data disaggregated by gender have been important steps toward ascertaining and addressing inequities in HIV outcomes among TW. Further research with larger numbers of transgender participants and inclusion of contextual data are necessary to inform effective interventions that ensure all people living with HIV have the opportunity to achieve their best health.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Aimee Freeman for her support in project management.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Center for Advancing Translational Sciences (KL2TR001077 to T. P.); the NIH (U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, M01RR000052, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA03629, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214, and Z01CP010176); the Centers for Disease Control and Prevention (CDC-200-2006-18797 and CDC-200-2015-63931); the Agency for Healthcare Research and Quality (90047713), from the Health Resources and Services Administration (90051652); the Canadian Institutes of Health Research (CBR-86906, CBR-94036, HCP-97105, and TGF-96118); the Ontario Ministry of Health and Long Term Care; and the government of Alberta, Canada. Additional support was provided by the National Cancer Institute, the National Institute for Mental Health, and the National Institute on Drug Abuse.
Potential conflicts of interest. T. P. has received research funding from Viiv Healthcare and Gilead Sciences. M. K. has received research grants from ViiV Healthcare, Merck, and Gilead and personal fees from ViiV Healthcare, Bristol-Myers Squibb, AbbVie, and Merck. M. J. S. has received research grants from Merck and Gilead. J. J. E. has received personal fees from Merck, research grants and personal fees from Janssen, Gilead Sciences, and ViiV Healthcare. J. T. has received personal fees from Gilead Science. K. N. A. has received personal fees from TrioHealth. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Becasen JS, Denard CL, Mullins MM, Higa DH, Sipe TA. Estimating the prevalence of HIV and sexual behaviors among the US transgender population: a systematic review and meta-analysis, 2006–2017. Am J Public Health 2018: e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:214–22. [DOI] [PubMed] [Google Scholar]
- 3. Clark H, Babu AS, Wiewel EW, Opoku J, Crepaz N. Diagnosed HIV infection in transgender adults and adolescents: results from the national HIV surveillance system, 2009–2014. AIDS and Behavior 2016. 21:2774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen SM, Hu X, Sweeney P, Johnson AS, Hall HI. HIV viral suppression among persons with varying levels of engagement in HIV medical care, 19 US jurisdictions. J Acquir Immune Defic Syndr 2014; 67:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalichman SC, Hernandez D, Finneran S, Price D, Driver R. Transgender women and HIV-related health disparities: falling off the HIV treatment cascade. Sex Health 2017; 14:469–76. [DOI] [PubMed] [Google Scholar]
- 7. Baguso GN, Gay CL, Lee KA. Medication adherence among transgender women living with HIV. AIDS Care 2016; 28:976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santos GM, Wilson EC, Rapues J, Macias O, Packer T, Raymond HF. HIV treatment cascade among transgender women in a San Francisco respondent driven sampling study. Sex Transm Infect 2014; 90:430–3. [DOI] [PubMed] [Google Scholar]
- 9. Bukowski LA, Chandler CJ, Creasy SL, Matthews DD, Friedman MR, Stall RD. Characterizing the HIV care continuum and identifying barriers and facilitators to HIV diagnosis and viral suppression among black transgender women in the United States. J Acquir Immune Defic Syndr 2018; 79:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizuno Y, Frazier EL, Huang P, Skarbinski J. Characteristics of transgender women living with HIV receiving medical care in the United States. LGBT Health 2015; 2:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Health Resources and Services Administration. Ryan White HIV/AIDS program annual client-level data report 2017. Published Available at: http://hab.hrsa.gov/data/data-reports. Accessed 24 March 2017.
- 12. Yehia BR, Fleishman JA, Moore RD, Gebo KA. Retention in care and health outcomes of transgender persons living with HIV. Clin Infect Dis 2013; 57:774–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr 2016; 72(Suppl 3):S210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poteat T, Reisner SL, Radix A. HIV epidemics among transgender women. Curr Opin HIV AIDS 2014; 9:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS cohort collaboration on research and design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.North American AIDS Cohort Collaboration on Research and Design. Available at: www.naaccord.org . Accessed 2 January 2019. [Google Scholar]
- 17.Poteat T, Hanna D, Althoff K. Feasibility and acceptability of developing a multi-site clinical cohort of transgender people with HIV infection. AIDS Research and Human Retroviruses 2015; 31: 870–2. doi: 10.1089/aid.2015.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rebeiro PF, Althoff KN, Lau B, et al. ; North American AIDS Cohort Collaboration on Research and Design Laboratory measures as proxies for primary care encounters: implications for quantifying clinical retention among HIV-infected adults in North America. Am J Epidemiol 2015; 182:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr 2012; 61(5): 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Habarta N, Wang G, Mulatu MS, Larish N. HIV testing by transgender status at Centers for Disease Control and Prevention–funded sites in the United States, Puerto Rico, and US Virgin Islands, 2009–2011. Am J Public Health 2015: e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mulatu M, Wang G, Zhang H, Song W, Wan C, Gilford J. HIV testing, seropositivity, and linkage to care among transgender persons in CDC-funded testing sites in the United States, 2012–2013 National HIV Prevention Conference Atlanta, Georgia: Centers for Disease Control and Prevention, 2015. [Google Scholar]
- 22. Pitasi MA, Oraka E, Clark H, Town M, DiNenno E. HIV testing among transgender women and men— 27 states and Guam, 2014–2015. MMWR Morb Mortal Wkly Rep 2017; 66:883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Logie CH, James L, Tharao W, Loutfy MR. “We don’t exist”: a qualitative study of marginalization experienced by HIV-positive lesbian, bisexual, queer and transgender women in Toronto, Canada. J Int AIDS Soc 2012; 15:17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melendez RM, Exner TA, Ehrhardt AA, et al. Health and health care among male-to-female transgender persons who are HIV positive. Am J Public Health 2006; 96:1034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poteat T, German D, Kerrigan D. Managing uncertainty: a grounded theory of stigma in transgender health care encounters. Soc Sci Med 2013; 84:22–9. [DOI] [PubMed] [Google Scholar]
- 26. Rebchook G, Keatley J, Contreras R, et al. ; SPNS Transgender Women of Color Study Group The Transgender Women of Color Initiative: implementing and evaluating innovative interventions to enhance engagement and retention in HIV care. Am J Public Health 2017; 107:224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reisner SL, Radix A, Deutsch MB. Integrated and gender-affirming transgender clinical care and research. J Acquir Immune Defic Syndr 2016; 72(Suppl 3):S235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braun HM, Candelario J, Hanlon CL, et al. Transgender women living with HIV frequently take antiretroviral therapy and/or feminizing hormone therapy differently than prescribed due to drug–drug interaction concerns. LGBT Health 2017; 4:371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sevelius JM. Gender affirmation: a framework for conceptualizing risk behavior among transgender women of color. Sex Roles 2013; 68:675–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chakravarty D, Rebchook G, Keatley J, et al. The association of healthcare empowerment and structural factors with HIV care outcomes among HIV-positive transgender women of color. National HIV Prevention Conference. Atlanta, Georgia: Centers for Disease Control and Prevention, 2015. [Google Scholar]
- 31. Chung C, Kalra A, McBride B, Roebuck C, Sprague L.. Some kind of strength: findings on health care and economic wellbeing from a national needs assessment of transgender and gender non-conforming people living with HIV. Oakland, CA: Transgender Law Center, 2016. [Google Scholar]
- 32. Cottrell ML, Prince H, Maffuid K, et al. Altered TDF/FTC pharmacology in a transgender female cohort: implications for PrEP. In: AIDS 2018. Amsterdam, The Netherlands: Abstract 11225. [Google Scholar]
- 33. Hiransuthikul A, Himmad K, Kerr S, et al. Drug–drug interactions between the use of feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. In: AIDS 2018. Amsterdam, The Netherlands: Abstract 13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Risher KA, Kapoor S, Daramola AM, et al. Challenges in the evaluation of interventions to improve engagement along the HIV care continuum in the United States: a systematic review. AIDS Behav 2017; 21:2101–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.