Abstract
Background
Elevated concentrations of cerebrospinal fluid (CSF) tau, a marker of axonal injury, have been associated with coma in severe malaria (cerebral malaria [CM]). However, it is unknown whether axonal injury is related to long-term neurologic deficits and cognitive impairment in children with CM.
Methods
Admission CSF tau concentrations were measured in 145 Ugandan children with CM and compared to clinical and laboratory factors and acute and chronic neurologic and cognitive outcomes.
Results
Elevated CSF tau concentrations were associated with younger age, increased disease severity (lower glucose and hemoglobin concentrations, malaria retinopathy, acute kidney injury, and prolonged coma duration, all P < .05), and an increased CSF:plasma albumin ratio, a marker of blood–brain barrier breakdown (P < .001). Admission CSF tau concentrations were associated with the presence of neurologic deficits at hospital discharge, and at 6, 12, and 24 months postdischarge (all P ≤ .02). After adjustment for potential confounding factors, elevated log10-transformed CSF tau concentrations correlated with worse cognitive outcome z scores over 2-year follow-up for associative memory (β coefficient, –0.31 [95% confidence interval [CI], –.53 to –.10]) in children <5 years of age, and for overall cognition (–0.69 [95% CI, –1.19 to –.21]), attention (–0.78 [95% CI, –1.34 to –.23]), and working memory (–1.0 [95% CI, –1.68 to –.31]) in children ≥5 years of age (all P < .006).
Conclusions
Acute axonal injury in children with CM is associated with long-term neurologic deficits and cognitive impairment. CSF tau concentrations at the time of the CM episode may identify children at high risk of long-term neurocognitive impairment.
Keywords: cerebrospinal fluid, tau, cerebral malaria, neurologic, cognitive, impairment
In children with cerebral malaria, severe disease and blood–brain barrier breakdown were associated with increased admission concentrations of cerebrospinal fluid tau, a marker of axonal injury, which were in turn associated with long-term neurologic and cognitive impairment.
Cerebral malaria (CM), the most severe complication of Plasmodium falciparum malaria, affects mainly infants and children [1]. The defining feature of CM is malarial parasitemia and unarousable coma, with no other explanation for coma (eg, hypoglycemia, postictal state, bacterial meningitis) [2]. CM is associated with significant long-term neurologic and cognitive impairment in survivors [3], but the factors that contribute to long-term neurocognitive impairment are not fully understood.
The protein tau is an axonal degeneration marker that is abundantly expressed in cortical axons and involved in microtubule assembly and stabilization [4]. Six different isoforms of tau exist in the human brain [5]. Insoluble, hyperphosphorylated tau, a form implicated in neurodegenerative disorders (known as tauopathies), is the main component of neurofibrillary tangles, a pathological marker of Alzheimer disease (reviewed in [6]). Nonphosphorylated, soluble cerebrospinal fluid (CSF) tau is emerging as an important biomarker of brain injury, as seen in animal models [7] and human studies of acute stroke [8]. Elevated CSF tau concentrations have been detected after traumatic brain injury [9] and in brain injury after aortic damage [10], and soluble CSF tau concentrations are increased as a result of cortical damage in Alzheimer disease [11]. CSF tau concentrations are also elevated in human immunodeficiency virus (HIV) and have been related in some studies, but not others, to neurocognitive disorders in this population (reviewed in [12]).
Studies investigating brain injury due to P. falciparum malaria infections have shown axonal damage in brain tissue using immunohistochemistry staining [13], and brain parenchymal damage in adults with severe malaria has been associated with elevated CSF soluble tau concentrations [14]. A study in Kenyan children showed elevated concentrations of CSF tau, but not S-100B, a marker of astrocytic damage, in children with CM [15]. In this study, elevated CSF tau concentrations were seen in the 3 children discharged with neurologic deficits compared to those discharged without deficits, but the levels were not significantly different, possibly due to the small sample size [15]. The study did not assess long-term neurologic or cognitive outcomes. Together, these findings indicate that axonal injury as determined by CSF tau concentrations is seen in many central nervous system (CNS) disorders including CM, but the relationship of CSF tau concentrations to long-term neurologic and cognitive outcomes in children with CM is unknown. Clinical and laboratory predictors of increased CSF tau concentrations are also not well characterized.
In the present study, we assessed the relationship of clinical and laboratory factors to admission CSF tau concentrations. We then related CSF tau concentrations to acute and long-term neurologic deficits and cognitive scores. We hypothesized that clinical and laboratory findings that reflect increased disease severity in CM would be associated with axonal injury, and that axonal injury, as assessed by CSF tau concentrations, would be associated with long-term neurologic and cognitive impairment in children with CM [16].
METHODS
Study Design
This prospective study was performed at Mulago Hospital, Kampala, Uganda, from 2008 to 2015. Children with CM were enrolled if they were between 18 months and 12 years of age. CM was defined as (1) coma (Blantyre Coma Score ≤2); (2) P. falciparum on blood smear; and (3) no other known cause of coma (eg, meningitis, a prolonged postictal state, or hypoglycemia-associated coma reversed by glucose infusion). A lumbar puncture was performed and CSF was obtained on admission to rule out bacterial meningitis or encephalitis in all children whose parents agreed to the procedure and for whom the procedure was not contraindicated. CSF was stored for future testing. Children with CM were managed according to the Ugandan Ministry of Health treatment guidelines, which included intravenous quinine treatment followed by oral quinine for severe malaria while admitted, and artemisinin combination therapy for outpatient follow-up. Exclusion criteria included (1) known chronic illness requiring medical care; (2) known developmental delay; or (3) prior history of coma, head trauma, hospitalization for malnutrition, or cerebral palsy. Children with sufficient CSF for tau testing were included in the present study. CSF samples collected from North American children (n = 5) with prior leukemia, in remission and with no evidence of acute infection or CNS disease, were used as controls.
Ethics Review and Approval
Written informed consent was obtained from parents or guardians of study participants. Ethical approval was granted by the institutional review boards for human studies at the Makerere University School of Medicine, the Uganda National Council for Science and Technology, and the University of Minnesota Medical School.
Clinical and Demographic Assessments
All children underwent a medical history and physical examination at enrollment. Children were assessed for malarial retinopathy using indirect and direct ophthalmoscopy as previously described [17]. Peripheral blood smears were assessed for Plasmodium species by microscopy with Giemsa staining using standard protocols. Nutritional status was assessed by height-for-age and weight-for-age z scores (Epi Info version 3.5.3; Centers for Disease Control and Prevention) and socioeconomic status was measured using a validated scoring system previously published [18]. Duration of coma was defined as time from admission until the child regained full consciousness (Blantyre Coma Score = 5 or Glasgow Coma Scale = 15). Acute kidney injury (AKI) was defined as a 1.5-fold increase in creatinine level from imputed baseline level, using the Kidney Disease: Improving Global Outcomes guidelines based on a single admission creatinine level [19].
Neurologic and Cognitive Assessments
Neurologic and cognitive assessments were performed at discharge (neurologic testing) or 1 week after discharge (cognitive testing), and 6, 12, and 24 months after discharge. A neurologic deficit (ND) was defined as presence of motor or cranial nerve deficit, ataxia, a movement disorder, or clinically detectable behavioral, speech, or visual disorders. The tests used to assess cognition were validated in cohorts of Ugandan children and described in detail in prior publications [16, 20, 21]. In brief, for children <5 years of age, the Mullen Scales of Early Learning were used to measure cognitive ability [22]. Scores from fine motor, visual reception, receptive language, and expressive language scales were summed to give the early learning composite score, a measure of overall cognitive ability. Attention was assessed using the Early Childhood Vigilance Test [23], and associative memory was assessed using the Color Object Association Test [24]. In children ≥5 years, the Kaufman Assessment Battery for Children (K-ABC, second edition) was used to measure overall cognitive ability, with summary mental processing index as the primary outcome [25]. Attention was assessed using the Test of Variables of Attention, with D prime measure as the primary outcome [26]. K-ABC subtests for sequential processing and simultaneous processing were used to assess working memory and problem-solving skills. Age-adjusted z scores for cognitive outcomes were created using scores of Ugandan community children as previously described [27].
Laboratory Testing
CSF (0.5–2 mL) was collected from children with CM and stored at −80°C for later testing. CSF from North American control children was obtained and stored similarly. CSF tau testing was performed at our University of Minnesota laboratory after transport of samples on dry ice. Testing was performed on admission CSF samples and on controls using the Luminex-based Human Tau (total) Singleplex Bead Kit (Invitrogen, Carlsbad, California) and the Human Neuroscience Buffer Reagent Kit (Invitrogen). The lower limit of detection for this kit is reported as <1 pg/mL. Plasma P. falciparum histidine-rich protein (PfHRP-2) quantification was performed using the Malaria Ag CELISA (Cellabs, Brookvale, Australia). Plasma and CSF albumin were quantified at the University of Minnesota Advanced Research and Diagnostic Laboratory using the Bromocresol Purple Albumin Assay (Sigma-Aldrich, St Louis, Missouri).
Statistical Analysis
CSF tau concentrations in children with CM vs control children and in children with CM with vs without ND were compared using Wilcoxon rank-sum test. For the latter, nonparametric receiver operating characteristic (ROC) curves were also generated and the area under the ROC (AUC) was compared. Spearman rank correlation analysis was used to compare CSF tau concentrations and clinical and laboratory characteristics. Linear, logistic, and negative binomial regression analyses were used to compare CSF tau concentrations (log10-transformed) to continuous, dichotomous, and count outcomes, respectively. For cognitive outcomes, a longitudinal mixed-effects model was used to allow for comparison of CSF tau concentrations to testing over all testing time points in the 2-year follow-up. In the model, observations within subject were correlated using a subject-specific intercept and time-points were treated as categorical variables. A banded diagonal covariance matrix was used to model within-subject variance-covariance errors, and the mixed model was fit by restricted maximum likelihood and Kenward-Roger approximations were used to estimate the denominator degrees of freedom. Multivariate models were adjusted for covariates if the correlation between covariates and outcomes had a P < .05 in a bivariate analysis or an a priori hypothesized relationship with the outcome. Analyses were conducted in Stata version 14 (StataCorp LP, College Station, Texas).
RESULTS
Demographic and Clinical Characteristics of Children With Cerebral Malaria
CSF was available from 145 of the 269 children with CM. Figure 1 outlines the reasons CSF tau was not tested in the remaining 124 children with CM. Table 1 summarizes the demographic and clinical characteristics of children with CM who did compared with those who did not have admission CSF tau concentrations tested. Children who had CSF tau concentrations tested had a significantly lower mortality than those who did not have testing, which likely reflects the association of contraindications to lumbar puncture (evidence of increased intracranial pressure, clinical instability) with mortality (Table 1). Among those who had CSF tau testing, neurologic deficits were frequent at discharge (39.2%) but much less frequent at 6, 12, and 24 months (6.1%, 3.8%, and 3.1%). Neurologic deficits did not differ significantly between those with vs without CSF tau testing (Table 1).
Figure 1.
Study participant flow diagram. Abbreviations: CM, cerebral malaria; CSF, cerebrospinal fluid.
Table 1.
Demographic and Clinical Characteristics of 145 Ugandan Children With Cerebral Malaria (CM) in Whom Admission Cerebrospinal Fluid (CSF) Tau Concentrations Were Measured Compared With 124 Uganda Children With CM in Whom CSF Tau Concentrations Were Not Measured
| Characteristic | CSF Tau Measured (n = 145a) | CSF Tau Not Measured (n = 124a) | P Valueb |
|---|---|---|---|
| Age, y, median (IQR) | 3.36 (2.63–4.19) | 3.63 (2.29–5.22) | .48 |
| Female sex, % (no.) | 40.69 (59) | 41.13 (51) | .94 |
| HIV status, % positive (no./No.) | 2.13 (3/141) | 1.89 (2/106) | .89 |
| Hemoglobin, g/dL, median (IQR) | 6.3 (4.9–8.5) | 7 (5.65–8.85) | .06 |
| Glucose, mmol/L, median (IQR) | 6.6 (5.1–9.2) | 6.5 (4.65–8.65) | .55 |
| Parasite density, parasites/µL, median, (IQR), No. | 53 680 (13 980–294 660), 141 | 42 560 (9760–209 220), 121 | .44 |
| Malaria retinopathy present, % (no./No.) | 68.97 (100/145) | 60.91 (67/110) | .18 |
| Acute kidney injury, % (no./No.) | 50.00 (70/140) | 35.83 (43/120) | .02 |
| Mortality, % (no.) | 5.52 (8) | 20.97 (26) | <.001 |
| Total hours in coma, median (IQR), no. | 60 (33–83), 137 | 45.5 (27–80), 98 | .08 |
| Convulsions during hospitalization, % (no.) | 60.00 (87) | 45.97 (57) | .02 |
| No. of convulsions during hospitalization, median (IQR) | 1 (0–2) | 0 (0–2) | .10 |
| Neurologic deficits at discharge, %, (no./No.) | 39.26 (53/135) | 30.93 (30/97) | .19 |
| Neurologic deficits, 6-mo follow-up, %, (no./No.) | 6.11 (8/131) | 3.23 (3/93) | .32 |
| Neurologic deficits, 12-mo follow-up, %, (no./No.) | 3.82 (5/131) | 2.17 (2/92) | .49 |
| Neurologic deficits, 24-mo follow-up, %, (no./No.) | 3.08 (4/130) | 2.22 (2/90) | .70 |
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IQR, interquartile range.
aFor variables for which the no. is less than the total No. listed for the group, the numbers for that variable and group are noted in the table.
bWilcoxon rank-sum test for continuous variables, χ2 test for categorical variables.
CSF Total Tau Concentrations in Children With CM Compared to North American Control Children
Admission CSF tau concentrations in the 145 children with CM (median, 406.99 [interquartile range {IQR}, 238.35–966.13] pg/mL) were 2-fold higher than CSF tau concentrations in the CSF of North American controls (median, 204.13 [IQR, 152.77–226.38] pg/mL; P = .02) (Figure 2). Using thresholds from studies of children with various CNS disorders in which CSF tau was measured [15, 28], 84 (57.9%) children with CM had high tau concentrations (>300 pg/mL) and, of these, 34 (23.5%) had very high tau concentrations (>1000 pg/mL). In comparison, only 1 of 5 control children had an elevated CSF tau, and this elevation was modest (317.94 pg/mL).
Figure 2.
Admission cerebrospinal fluid (CSF) tau concentrations in children diagnosed with cerebral malaria vs control group. Circles represent CSF tau concentration in individual children. Horizontal lines show median and 95% confidence interval. Two-sample Wilcoxon rank-sum (Mann-Whitney) test. Abbreviation: CM, cerebral malaria.
Relationship of Clinical Factors to CSF Tau Concentrations
Admission CSF total tau concentrations correlated inversely with age (Spearman ρ, –0.37; P < .001) (Table 2). Higher CSF tau concentrations were seen in the 116 children with CM <5 years of age (median, 483.12 [IQR, 259.16–1051.66] pg/mL) than in the 29 children with CM aged ≥5 years (median, 217.72 [IQR, 181.48–405.99] pg/mL; P < .001). The mean (standard deviation) age of North American control children was 5.67 (2.66) years, so control children were on average older than the children with CM. CSF tau concentrations correlated inversely with admission glucose (Spearman ρ, –0.25; P = .003), and hemoglobin concentrations (Spearman ρ, –0.27; P < .001), and positively with presence of retinopathy (Spearman ρ, 0.17; P = .04) and AKI (Spearman ρ, 0.29; P < .001) (Table 2). Among the features of retinopathy, CSF tau concentrations correlated most strongly with retinal hemorrhages (Table 3). CSF tau concentrations also correlated positively with the CSF:plasma albumin ratio, a marker of blood–brain barrier (BBB) breakdown (Spearman ρ, 0.37; P < .001; Table 2). Parasite density (PfHRP-2 concentration) and parasite biomass correlated with CSF:plasma albumin ratio (Spearman ρ, 0.39; P < .001 and 0.37; P < .001, respectively), but not with CSF tau concentration (P = .43 and P = .39, respectively; Table 2). There was no difference in the median levels of CSF tau based on HIV status (P = .52).
Table 2.
Relationship of Admission Cerebrospinal Fluid Tau Concentrations With Demographic and Clinical Characteristics in 145 Ugandan Children With Cerebral Malaria
| Clinical Characteristics | Spearman ρ | P Value |
|---|---|---|
| Age | –0.37 | <.001 |
| Lactate level (n = 142) | 0.07 | .38 |
| Glucose level (n = 143) | –0.25 | .003 |
| Hemoglobin | –0.27 | <.001 |
| Presence of malaria retinopathy (n = 144) | 0.17 | .04 |
| Parasite density (PfHRP-2 level) | 0.06 | .43 |
| Sequestered parasite biomass | 0.07 | .39 |
| Acute kidney injury (n = 140) | 0.29 | <.001 |
| CSF:plasma albumin ratio (n = 142) | 0.37 | <.001 |
Abbreviations: CSF, cerebrospinal fluid; PfHRP-2, P. falciparum histidine-rich protein.
Table 3.
Admission Cerebrospinal Fluid Tau Concentrations by Retinopathy and Associated Subcategories (Retinal Hemorrhages, Vessel Changes, Macular Whitening)
| Category | CSF Tau Concentration, Median (IQR), No. | P Valuea | |
|---|---|---|---|
| Present | Absent | ||
| Retinopathy | 448.64 (266.37–1020.39), 100 | 275.33 (189.06–816.05), 45 | .04 |
| Retinal hemorrhages | 603.35 (287.57–1196.59), 84 | 261.53 (185.63–654.59), 60 | <.001 |
| Peripheral whitening | 460.1 (265.58–1019.81), 39 | 406.99 (234.96–921.34), 105 | .67 |
| Vessel changes | 293.29 (250.44–957.60), 36 | 436.59 (238.13–975.94), 108 | .54 |
| Papilledema | 257.47 (125.45–1411.87), 4 | 417.33 (239.66–971.16), 140 | .39 |
| Macular whitening | 408.14 (262.42–1019.81), 54 | 414.25 (226.75–921.34), 90 | .38 |
Abbreviations: CSF, cerebrospinal fluid; IQR, interquartile range.
aWilcoxon rank-sum test.
CSF Tau Concentrations and In-hospital Outcomes
After adjusting for factors associated with increased CSF tau concentrations (age, hemoglobin and glucose concentrations, retinopathy, and AKI), admission CSF tau concentrations were independently associated with longer coma duration (P = .001) but not with number of convulsions or mortality (Table 4).
Table 4.
Association of Admission Cerebrospinal Fluid Tau Concentrationsa With In-hospital Outcomes in Ugandan Children With Cerebral Malaria
| Clinical Outcome | Value | Unadjusted OR, Rate Ratio, or Risk Difference (95% CI), No. | P Value | Adjusted OR, Rate Ratio, or Risk Difference (95% CI)a, No.b | P Value |
|---|---|---|---|---|---|
| Mortality | OR | 2.12 (.43–10.49), 145 | .36 | 1.18 (.20–6.82), 138 | .85 |
| Coma durationc | β | 0.21 (.09–.33), 137 | .001 | 0.22 (.09–.35), 131 | .001 |
| No. of convulsions in hospital | IRR | 1.53 (.94–2.49), 145 | .08 | 1.32 (.76–2.27), 138 | .32 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; OR, odds ratio.
aLogistic regression (OR), multiple linear regression (β coefficient), or negative binomial regression (IRR) adjusted for age, hemoglobin and glucose concentrations, presence of retinopathy, and acute kidney injury.
bLower no. in adjusted group on account of missing data points in adjusted variables.
cLog-transformed (base 10).
CSF Tau Concentrations and Neurologic and Cognitive Outcomes Over 24-Month Follow-up
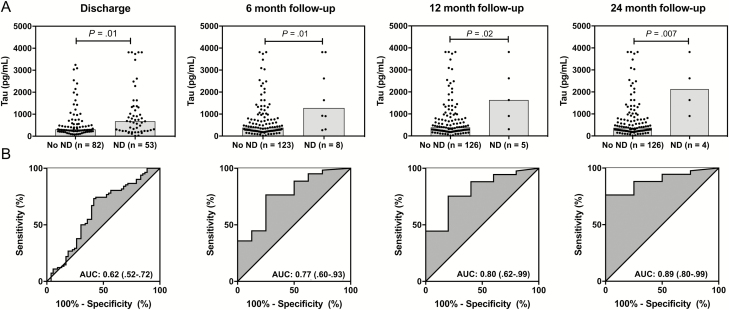
Elevated baseline CSF tau concentrations were observed in children with ND compared to those without ND at discharge and 6, 12, and 24 months after discharge (all P ≤ .02; Figure 3A). All differences remained significant when children with HIV infection (n = 3) were excluded (data not shown). The predictive value of admission CSF tau concentrations increased over time with AUCs of 0.62 (95% confidence interval [CI], .52–.72), 0.77 (95% CI, .60–.93), 0.80 (95% CI, .62–.99), and 0.89 (95% CI, .80–.99), at discharge, 6, 12, and 24 months, respectively (Figure 3B).
Figure 3.
Association with neurologic deficits (NDs). A, Admission cerebrospinal fluid (CSF) tau concentrations (log-transformed [base 10]) in children with cerebral malaria without and with NDs at baseline, 6, 12, and 24 months. Two-sample Wilcoxon rank-sum [Mann-Whitney] test. Symbols represent CSF tau concentration in individual children. Shaded boxes show medians. B, Nonparametric receiver operating characteristic curve from predictive probabilities of logistic regression models with ND and CSF tau concentrations. Abbreviations: AUC, area under the receiver operating characteristic curve.
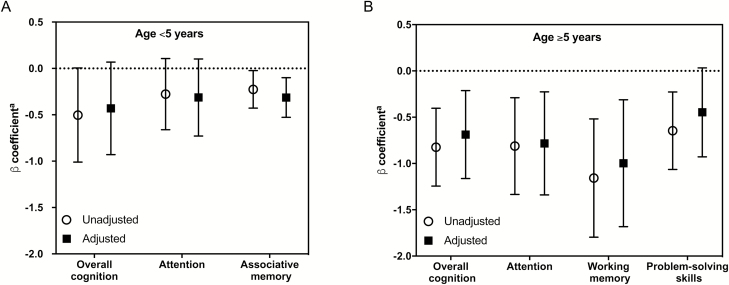
Comparisons to cognitive outcome scores were performed using cognitive z score measurements from all study follow-up time points (1 week after discharge, and 6, 12, and 24 months after discharge) for each child. After adjustment for age, hemoglobin, and glucose concentrations, retinopathy, AKI, and number of convulsions, admission CSF tau concentrations correlated inversely with associative memory z scores but not overall cognitive ability or attention z scores in children <5 years of age (P = .004; Figure 4A, Supplementary Table 1). In children ≥5 years of age, CSF tau concentrations correlated inversely with overall cognition (P = .005), attention (P = .006), and working memory (P = .005) over the 2-year follow-up period (Figure 4B, Supplementary Table 1). The relationships between CSF tau and attention in children aged <5 years, and cognition, attention, and memory in children ≥5 years of age were unchanged when the 3 children with HIV were excluded from analysis (Supplementary Table 2).
Figure 4.
Relationship of admission cerebrospinal fluid (CSF) tau concentrations (log-transformed [base 10]) with cognitive outcomes over a 24-month follow-up period in Ugandan children with cerebral malaria aged <5 years (A) and ≥5 years (B). Plot represents a longitudinal mixed-effects model adjusted for age, hemoglobin and glucose concentrations, presence of retinopathy, acute kidney injury, and number of convulsions, and includes a child-specific intercept with testing visits treated as a categorical variable. aLog10 CSF tau concentrations at admission compared with cognitive z scores.
DISCUSSION
In the present study, we demonstrate that clinical factors reflecting increased disease severity in CM, including young age, low glucose and hemoglobin concentrations, presence of retinopathy and AKI, and prolonged coma duration, are associated with elevated admission concentrations of CSF tau, a marker of CNS axonal injury. We also demonstrate that elevated admission CSF tau concentrations are associated with increased CSF:plasma albumin ratio, an indicator of BBB breakdown, and with neurologic deficits and cognitive impairment over 2 years of follow-up, independent of the clinical factors. Together the findings suggest that factors involved in increased disease severity and BBB breakdown may relate to acute axonal injury in CM, and that this acute axonal injury may be associated with long-term neurocognitive impairment.
While the association of CSF tau with acute neurologic deficits in children with severe malaria has been investigated before [15], the association of CSF tau with long-term neurologic and cognitive impairment in children with CM is a novel finding of our study. Indeed, the few studies of CSF tau in children have assessed only acute outcomes [15, 28]. Studies in adults have shown an association of increased CSF total tau with worse long-term cognition in Alzheimer disease [29], and with neurologic deficits and impaired activities of daily living 1 year after traumatic brain injury [30]. In the present study, increased admission concentrations of CSF tau were associated with a large difference in z scores in multiple areas of cognition in children ≥5 years of age (almost a full standard deviation lower z score for overall cognitive ability, attention, and working memory with each log10 increase in CSF tau), but with modest differences (about one-third of a standard deviation worse z scores) in only one area (associative memory) in children <5 years of age. These differences are present despite children <5 years of age with CM having overall cognitive scores that are significantly lower than their community peers [21], similar to children ≥5 years of age [16], and having higher CSF tau concentrations than children ≥5 years of age. We have shown similar findings (ie, association of CSF biomarker with worse cognition only in children ≥5 years of age) with CSF tumor necrosis factor alpha (TNF-α) [31] and kynurenic acid [32], suggesting that the higher-level cognitive functions affected by these factors manifest and can be tested only at or after 5 years of age [33, 34]. This finding of potentially “growing into deficits” [35] is important, because it suggests that CNS damage occurring early in life may not be clear until later in life, and that studies of correlations of baseline biomarkers to cognitive outcomes should therefore take a long-term follow-up approach.
The biological pathways leading to BBB breakdown and axonal injury are not well defined in experimental CM models or human CM. Parasitized red blood cell sequestration has been proposed as a mechanism underlying BBB dysfunction in CM (reviewed in [36]), and the present study did find an association of PfHRP-2 concentrations, a measure of parasite biomass, and the CSF:plasma albumin ratio, a marker of BBB breakdown. However, parasite biomass did not correlate directly with CSF tau concentrations, suggesting that it is not associated with BBB breakdown and axonal injury. Hypoglycemia, another mechanism linked with BBB dysfunction in CM [37], and with axonal injury in other disease processes (reviewed in [38]), was associated with increased CSF tau concentrations, suggesting that metabolic dysfunction may play a role leading to axonal damage in CM.
A number of other pathways could lead to axonal injury, including systemic inflammation, endothelial dysfunction, endothelial vasospasm, local ischemia from sequestration, damage from oxidative stress, and production and release of metabolites, like those of the kynurenine pathway [32], or cytokines, such as TNF-α [31], within the CNS. These pathways may relate to BBB breakdown and allow for passage of factors through the damaged BBB [37, 39], or may cause axonal injury independently of BBB dysfunction. Future research should assess how specific pathways lead to axonal injury.
This study has limitations common to human studies of CM. We were unable to collect CSF from 69 children due to lack of parental consent or clinical contraindications to lumbar puncture, so we cannot comment on associations between CSF tau and mortality. CSF tau concentrations were assessed at a single time point, so the extent to which these concentrations may change over time or how these changes relate to long-term neurocognitive function is unknown. Finally, study control children were North American, and not Ugandan, children. However, the study focus is on Ugandan children with CM, so the findings in control children are for comparison and do not affect the primary study finding that elevated CSF tau concentrations are associated with long-term neurologic and cognitive impairment in children with CM.
In summary, the study findings suggest that markers of increased disease severity and BBB dysfunction are associated with axonal injury in CM, and shows for the first time that axonal injury, as demonstrated by elevated admission CSF tau concentrations during the episode of CM, is associated with long-term neurologic and cognitive impairment in children with CM. Research on the mechanisms that lead to axonal injury in CM could lead to interventions that can prevent or decrease long-term neurocognitive impairment in children with CM.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D. D. analyzed and interpreted the data and drafted the manuscript. G. S. P. and C. C. J. supervised testing of study biomarkers. A. L. C., R. I., P. B., and A. J. S. helped with interpretation of study data. A. L. C., P. F. C., and J. M. S. assisted with statistical analysis. R. O. O. and C. C. J. designed the study and supervised its conduct. C. C. J. designed the research and edited the manuscript. All authors contributed to critical revision of the manuscript.
Acknowledgments. The authors thank the children and their parents who participated in this study and the study team for their dedicated effort in treating the children and collecting the data.
Financial support. This work was supported by funding from the National Institute of Neurological Disorders and Stroke and the Fogarty International Center (grant numbers R01NS055349 and D43 NS078280, respectively). A. J. S. acknowledges support from the Indiana Alzheimer Disease Center (grant number P30 AG010133).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Severe malaria. Trop Med Int Health 2014; 19:7–131. [DOI] [PubMed] [Google Scholar]
- 2. Molyneux ME. The clinical features of cerebral malaria in children. Med Trop (Mars) 1990; 50:65–8. [PubMed] [Google Scholar]
- 3. Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol 2005; 4:827–40. [DOI] [PubMed] [Google Scholar]
- 4. Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 1975; 72:1858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 2000; 33:95–130. [DOI] [PubMed] [Google Scholar]
- 6. Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron 2011; 70:410–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bi M, Gladbach A, van Eersel J, et al. Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat Commun 2017; 8:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hesse C, Rosengren L, Andreasen N, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett 2001; 297:187–90. [DOI] [PubMed] [Google Scholar]
- 9. Zemlan FP, Jauch EC, Mulchahey JJ, et al. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res 2002; 947:131–9. [DOI] [PubMed] [Google Scholar]
- 10. Shiiya N, Kunihara T, Miyatake T, Matsuzaki K, Yasuda K. Tau protein in the cerebrospinal fluid is a marker of brain injury after aortic surgery. Ann Thorac Surg 2004; 77:2034–8. [DOI] [PubMed] [Google Scholar]
- 11. Vandermeeren M, Mercken M, Vanmechelen E, et al. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem 1993; 61:1828–34. [DOI] [PubMed] [Google Scholar]
- 12. Brown LA, Scarola J, Smith AJ, Sanberg PR, Tan J, Giunta B. The role of tau protein in HIV-associated neurocognitive disorders. Mol Neurodegener 2014; 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medana IM, Day NP, Hien TT, et al. Axonal injury in cerebral malaria. Am J Pathol 2002; 160:655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medana IM, Lindert RB, Wurster U, et al. Cerebrospinal fluid levels of markers of brain parenchymal damage in Vietnamese adults with severe malaria. Trans R Soc Trop Med Hyg 2005; 99:610–7. [DOI] [PubMed] [Google Scholar]
- 15. Medana IM, Idro R, Newton CR. Axonal and astrocyte injury markers in the cerebrospinal fluid of Kenyan children with severe malaria. J Neurol Sci 2007; 258:93–8. [DOI] [PubMed] [Google Scholar]
- 16. John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 2008; 122:e92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Villaverde C, Namazzi R, Shabani E, Opoka RO, John CC. Clinical comparison of retinopathy-positive and retinopathy-negative cerebral malaria. Am J Trop Med Hyg 2017; 96:1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bangirana P, John CC, Idro R, et al. Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS One 2009; 4:e7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. et al. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012;2:19–36. doi:10.1038/kisup.2011.32. [Google Scholar]
- 20. Boivin MJ, Bangirana P, Byarugaba J, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics 2007; 119:e360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bangirana P, Opoka RO, Boivin MJ, et al. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis 2014; 59:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mullen EM. Mullen Scales of Early Learning manual. Vol ix Circle Pines, MN: American Guidance Services, Inc., 1995:85. [Google Scholar]
- 23. Goldman DZ, Shapiro EG, Nelson CA. Measurement of vigilance in 2-year-old children. Dev Neuropsychol 2004; 25:227–50. [DOI] [PubMed] [Google Scholar]
- 24. Jordan CM, Johnson AL, Hughes SJ, Shapiro EG. The Color Object Association Test (COAT): the development of a new measure of declarative memory for 18- to 36-month-old toddlers. Child Neuropsychol 2008; 14:21–41. [DOI] [PubMed] [Google Scholar]
- 25. Kaufman AS.Kaufman NL. KABC-II: Kaufman assessment battery for children. 2nd ed Circle Pines, MN: AGS Publications, 2004. [Google Scholar]
- 26. Leark RA, Greenberg LM, Kindschi CL, Dupuy TR, Hughes SJ.. Test of variables of attention continuous performance test. Los Alamitos, CA: TOVA Company, 2007. [Google Scholar]
- 27. Bergemann TL, Bangirana P, Boivin MJ, Connett JE, Giordani BJ, John CC. Statistical approaches to assess the effects of disease on neurocognitive function over time. J Biom Biostat 2012; Suppl 7. pii: 7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shahim P, Darin N, Andreasson U, et al. Cerebrospinal fluid brain injury biomarkers in children: a multicenter study. Pediatr Neurol 2013; 49:31–9 e2. [DOI] [PubMed] [Google Scholar]
- 29. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ost M, Nylén K, Csajbok L, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 2006; 67:1600–4. [DOI] [PubMed] [Google Scholar]
- 31. Shabani E, Ouma BJ, Idro R, et al. Elevated cerebrospinal fluid tumour necrosis factor is associated with acute and long-term neurocognitive impairment in cerebral malaria. Parasite Immunol 2017; 39. doi: 10.1111/pim.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holmberg D, Franzén-Röhl E, Idro R, et al. Cerebrospinal fluid kynurenine and kynurenic acid concentrations are associated with coma duration and long-term neurocognitive impairment in Ugandan children with cerebral malaria. Malar J 2017; 16:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sattler JM. Assessment of children: cognitive foundations. Jerome M. Sattler, 2008. [Google Scholar]
- 34. Semrud-Clikeman M, Ellison AT. Child neuropsychology: assessment and interventions for neurodevelopmental disorders. Child Neuropsychol Springer; 2nd ed, 2009. [Google Scholar]
- 35. Aarsen FK, Paquier PF, Reddingius RE, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer 2006; 106:396–402. [DOI] [PubMed] [Google Scholar]
- 36. Hora R, Kapoor P, Thind KK, Mishra PC. Cerebral malaria–clinical manifestations and pathogenesis. Metab Brain Dis 2016; 31:225–37. [DOI] [PubMed] [Google Scholar]
- 37. Medana IM, Turner GD. Human cerebral malaria and the blood-brain barrier. Int J Parasitol 2006; 36:555–68. [DOI] [PubMed] [Google Scholar]
- 38. Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain 2003; 126:515–30. [DOI] [PubMed] [Google Scholar]
- 39. Rénia L, Howland SW, Claser C, et al. Cerebral malaria: mysteries at the blood-brain barrier. Virulence 2012; 3:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.