Abstract
This chapter is focused on the discussion and review of cytomorphological features of benign and malignant respiratory lesions. The respiratory system can be divided into upper and lower tract, thus, the variety of sampling and preparation methods are also reviewed, since the cytomorphology of the lesion may reveal differently depending on the sampling and preparation techniques. In addition, the critical information regarding molecular tests of lung carcinomas are also reviewed.
Keywords: Epidermal Growth Factor Receptor Mutation, Anaplastic Lymphoma Kinase, Small Cell Carcinoma, Hyperchromatic Nucleus, Goblet Cell Hyperplasia
Table 1.1.
Benign component in lung cytopathology
| Descriptions | Differentials | |
|---|---|---|
| Benign bronchial epithelium | Clusters, sheets, or picket fence arrangement of cells with cilia, may have a mild nuclear atypia | Cells are well organized and with cilia. Nuclei show mild variation in size and multinucleation |
| DD: well-differentiated adenocarcinoma | ||
| Reactive bronchial cells (Creola body) | Three-dimensional clusters of bronchial epithelial cells with cilia, marked variation of nuclear size, and mild to moderate nuclear atypia | Small nucleoli, no chromatin hyperchromasia, or markedly nuclear atypia |
| DD: adenocarcinoma | ||
| Bronchial reserve cells (basal cells) | Tight clusters or sheets of small hyperchromatic cells with mild nuclear crowding | Small “dark” cells with smudgy chromatin, no mitoses or necrosis |
| DD: small cell carcinoma | ||
| Squamous cells | Scattered individual cells with small pyknotic nuclei, and dense cytoplasm, indicating cytokeratin formation (orangeophilic color with Papanicolaou stain) | Cells have a oval or spindle shape, low N/C ratio, and benign nuclear features |
| DD: squamous cell carcinoma | ||
| Bronchoalveolar macrophages | Loose clusters or individual cells with centrically located small round nuclei, benign nuclear features, and foamy cytoplasm | Cells have cytoplasmic pigments (anthracotic pigments in smokers) or vacuoles |
| DD: well-differentiated adenocarcinoma | ||
| Type II pneumocytes | Intermediate-sized cells with large nuclei, coarse chromatin, prominent nucleoli, smooth nuclear membrane, and scant cytoplasm | Single cells and/or cluster of cells with benign nuclear features, may be associated with acute lung injury |
| DD: adenocarcinoma | ||
| Goblet cells | Sheets and clusters of cells with abundant mucin-filled cytoplasm and eccentrically located nuclei with “signet ring” appearance | Cells are without nuclear atypia |
| DD: mucinous adenocarcinoma, signet ring-cell carcinoma | ||
| Cryptococcosis | Yeast-form budding fungus with variable size, mucin capsule, and refractile center | Narrow-based budding yeast with 4–15 μm in diameter |
| DD: histoplasmosis, blastomycosis | ||
| Pneumocystis jiroveci (carinii) pneumonia (PCP) | Extracellular and/or intracytoplasmic amorphous material with Papanicolaou stains | Methenamine silver stain highlights numerous tiny cup-shaped cysts with a black dot in the center of the cyst |
| DD: coccidiomycosis | ||
| Candidiasis | Budding yeast with pseudohyphae | Pseudohyphae are 2–10 μm in diameter and branch at a 90° angle |
| DD: aspergillosis | ||
| Aspergillosis | Hyphae with septate | Hyphae are 10–30 μm in diameter and branch at a 45° angle |
| DD: candidiasis | ||
| Birefringent calcium oxalate crystals | Needle-shaped, polarizable crystals; they may form rosettes or wheat sheaf-like clusters | Associated with Aspergillus infection |
| DD: Charcot–Leyden crystals | ||
| Charcot–Leyden crystals | Needle-shaped orangeophilic color crystal, by-product of eosinophil degranulation | Associated with allergy |
| DD: calcium oxalate crystals | ||
| Ferruginous bodies | Dumbbell-shaped mineral fibers, 5–200 μm in length, golden-yellow to black color with Papanicolaou stain | Associated with Asbestos exposure |
| DD: Charcot–Leyden crystals | ||
| Curschmann spiral | Coils and strands of mucus. Purple helices with Papanicolaou stains | Represent mucus and commonly seen in chronic pulmonary disease. Non-specific finding |
| DD: parasite infection | ||
| Granulomas (sarcoidosis) | Clusters of epithelioid histiocytes with elongated nuclei, admixed with lymphocytes, scattered multinucleated giant cells, and reactive bronchial cells | Nodular aggregates of histiocytes and lymphocytes in a background of scattered multinucleated giant cells |
| DD: well-differentiated adenocarcinoma |
Table 1.2.
Neoplastic lesions in lung cytopathology
| Conditions | Descriptions | Differentials |
|---|---|---|
| Adenocarcinoma | Three-dimensional and acinar/papillae arrangement of columnar cells with high N/C ratio, prominent nucleoli, and “lacy” cytoplasm with vacuolization (cytoplasmic mucin) | Large hyperchromatic nuclei with irregular nuclear membranes, coarse chromatin, prominent nucleoli |
| DD: poorly differentiated non-keratinizing squamous cell carcinoma | ||
| Squamous cell carcinoma | Discohesive and scattered individual polymorphic cells with hyperchromatic smudgy chromatin, dense cytoplasm with or without keratinization | Polygonal cells and elongated or tadpole-shaped cells with large hyperchromatic nuclei and dense cytoplasm |
| DD: poorly differentiated adenocarcinoma | ||
| Small cell carcinoma | Tight clusters of small hyperchromatic cells (two- to threefold of mature lymphocytes), fine (salt-and-pepper) chromatin, nuclear molding and crowding, nuclear stripes (broken down nuclear material), inconspicuous nuclei, scant cytoplasm | Fine chromatin texture and nuclear crowding and molding, mitosis, necrosis, and apoptotic body |
| DD: lymphoma, basaloid squamous cell carcinoma, and poorly differentiated adenocarcinoma | ||
| Undifferentiated large cell carcinoma | Loosely cohesive clusters, syncytial sheets, or scattered polymorphic large cells with coarse chromatin, single or multiple prominent nucleoli, and feathery cytoplasm | Large cells with large prominent nucleoli. Numerous mitoses |
| DD: poorly differentiated adenocarcinoma, sarcoma | ||
| Carcinoid | Loosely cohesive clusters and scattered individual cells arrange in rosette-like structures. Tumor cells are relatively uniform in size, with fine (“salt-pepper”) chromatin, and moderate cytoplasm | Monomorphic appearance of tumor cells, with fine chromatin, inconspicuous nucleoli. Branching capillaries in the background. No mitosis or necrosis |
| DD: small cell carcinoma, atypical carcinoid | ||
| Lymphoma | Discohesive and scattered individual cells with vesicular nuclei, clumped (soccer-ball-like) chromatin, irregular nuclear membrane, and scant cytoplasm. Lymphoglandular body in the background | Monomorphous population of cells, with clumped (soccer-ball-like) chromatin, irregular nuclear membranes, conspicuous nucleoli, and scant cytoplasm |
| DD: small cell carcinoma, carcinoid |
Image-Based Questions 1–82
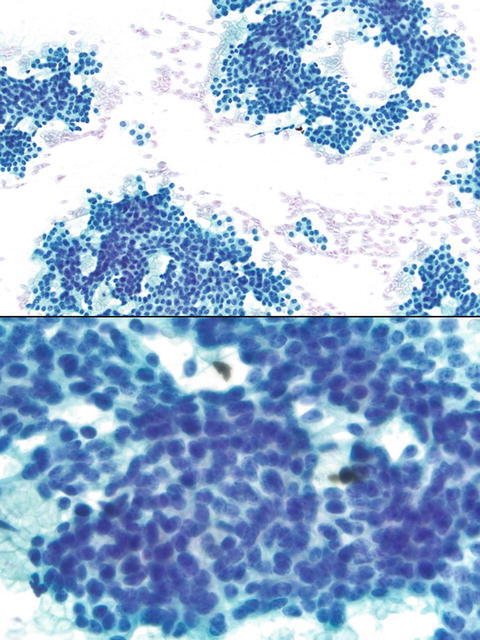
Fig. 1.1
- Q-1. What is the diagnosis of this specimen?
- Lymphoma
- Poorly differentiated adenocarcinoma
- Reactive lymphocytes
- Carcinoid
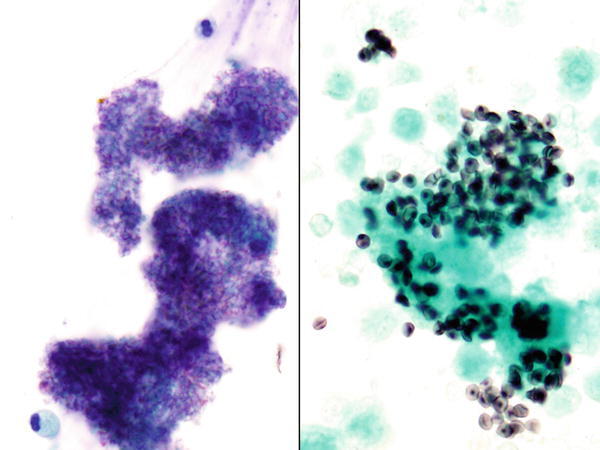 Fig. 1.2
Fig. 1.2 - Q-2. A HIV-positive patient presented with cough and fever. Computed tomography (CT) scan revealed bilateral lung infiltrations. A BAL was performed. What is the diagnosis?
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
- Aggregates of red blood cell (RBC) ghost cells
- Alveolar proteinosis
- Aspergillosis
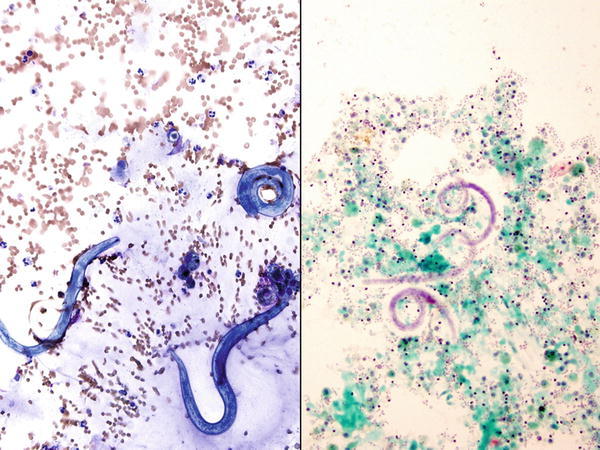 Fig. 1.3
Fig. 1.3 - Q-3. A HIV-positive patient presented with hemoptysis and fever. CT scan revealed bilateral lung infiltrations. A BAL was performed. What is the diagnosis?
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
- Strongyloidiasis
- Alveolar proteinosis
- Aspergillosis
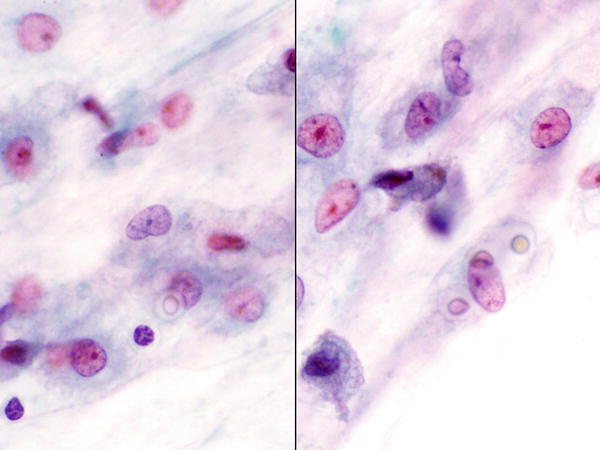 Fig. 1.4
Fig. 1.4 - Q-4. A patient presented with productive cough, fever, and weight loss. CT scan revealed bilateral lung infiltrations. A bronchoalveolar lavage (BAL) was performed. What is the diagnosis?
- Blastomycosis
- Histoplasmosis
- Cryptococcosis
- Aspergillosis
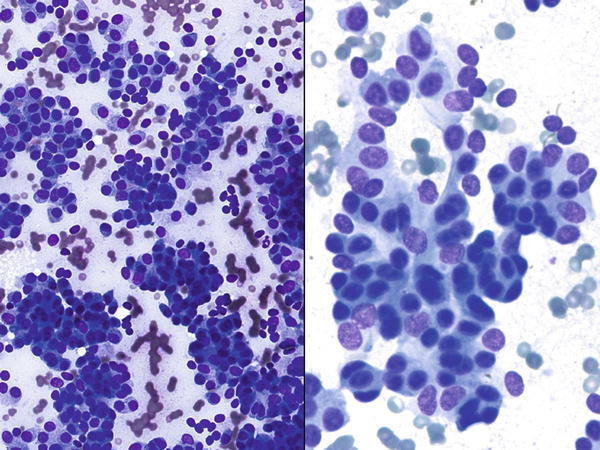 Fig. 1.5
Fig. 1.5 - Q-5. What is the diagnosis of this lung fine-needle aspiration (FNA)?
- Well-differentiated adenocarcinoma
- Carcinoid
- Poorly differentiated adenocarcinoma
- Reactive bronchial cells.
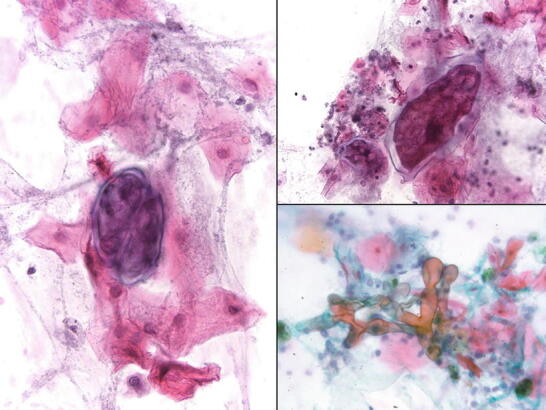 Fig. 1.6
Fig. 1.6 - Q-6. This sputum specimen was collected from a lung cancer patient who presented with neutropenia. What is the diagnosis?
- Blastomycosis
- Mucormycosis (zygomycosis)
- Aspergillosis
- Paragonimus eggs
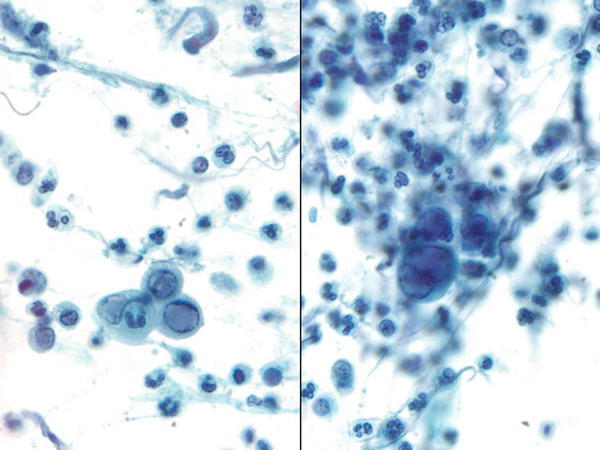 Fig. 1.7
Fig. 1.7 - Q-7. This BAL specimen was collected from a patient with the history of colon adenocarcinoma, who presented with respiratory distress. What is the diagnosis?
- Markedly reactive bronchial cells and alveolar macrophages
- Poorly differentiated adenocarcinoma
- Squamous cell metaplasia
- Herpes simplex virus (HSV) infection
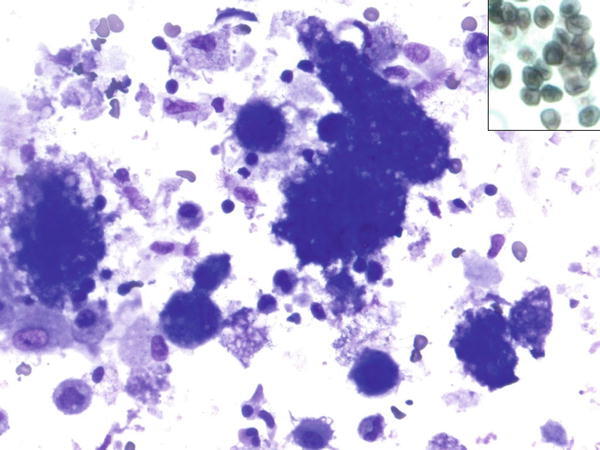 Fig. 1.8
Fig. 1.8 - Q-8. A HIV-positive patient was clinically suspicious for Pneumocystis jiroveci (carinii) pneumonia (PCP) infection of the lung. A BAL was performed. What is the diagnosis?
- Pneumocystis jiroveci (carinii) pneumonia
- Cryptococcus infection
- Alveolar proteinosis
- Lysed red blood cells
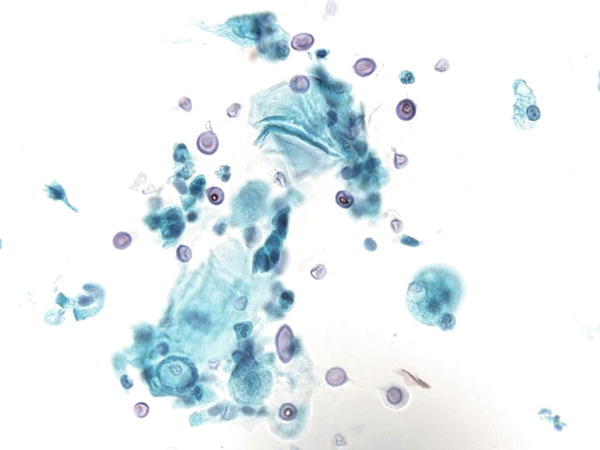 Fig. 1.9
Fig. 1.9 - Q-9. A patient presented with multiple lung nodules and hilar lymphadenopathy. A transbronchial lung FNA of the lung nodule was performed. What is the best diagnosis?
- Reactive bronchial cells and alveolar macrophages
- Poorly differentiated adenocarcinoma
- Histoplasmosis
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
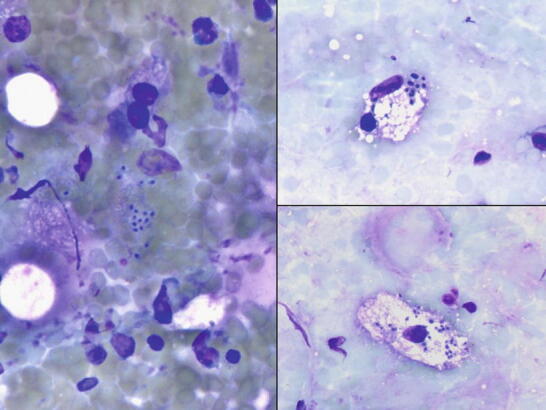 Fig. 1.10
Fig. 1.10 - Q-10. A patient presented with lung and liver cystic masses. The CT-guided FNA of the lung lesion was performed. What is the diagnosis?
- Echinococcosis (Hydatid disease)
- Strongyloidiasis
- Entamoeba histolytica infection
- Paragonimus eggs
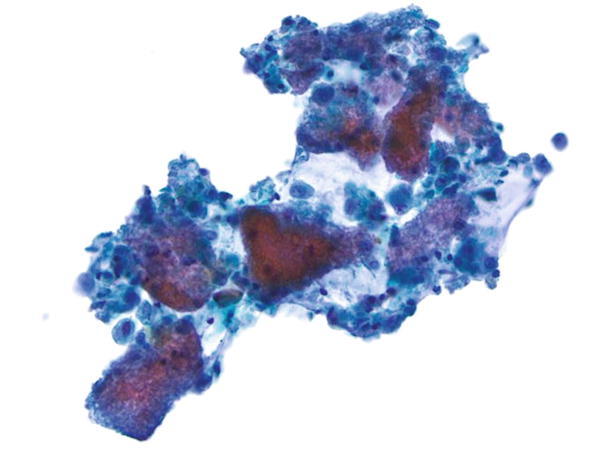 Fig. 1.11
Fig. 1.11 - Q-11. A HIV-positive patient presented with cough and hemoptysis. The chest x-ray revealed a cavitary lung lesion. A FNA of the lung lesion was performed. What is the diagnosis?
- Blastomycosis
- Mucormycosis (zygomycosis)
- Aspergillosis
- Candidiasis
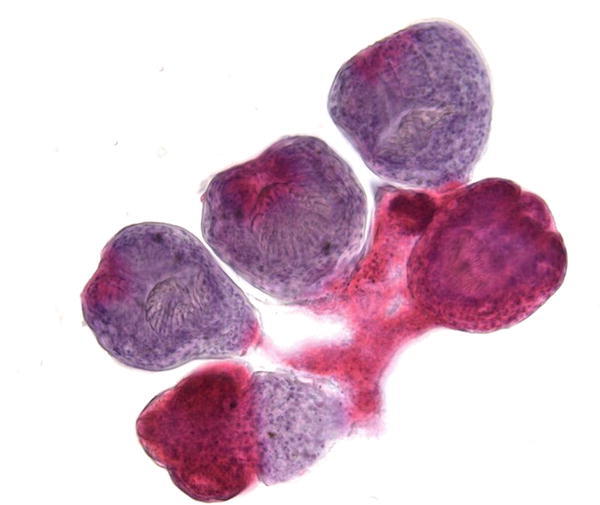 Fig. 1.12
Fig. 1.12 - Q-12. An immunocompromised patients presented with cough, fever, pneumonia, and bilateral lung infiltrations. What is the diagnosis of this BAL specimen?
- Poorly differentiated adenocarcinoma
- Cytomegalovirus (CMV) infection
- Squamous cell metaplasia
- Herpes simplex virus (HSV) infection
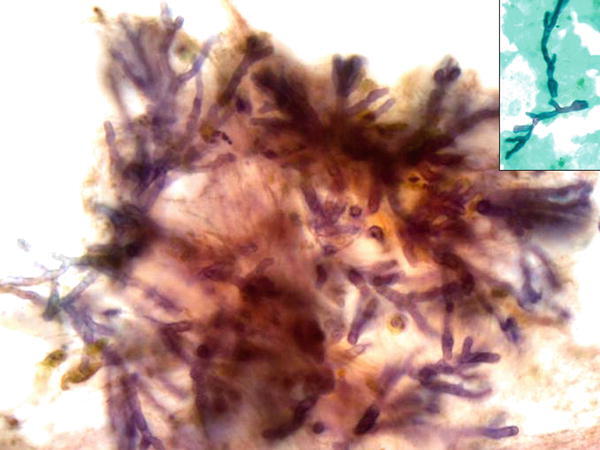 Fig. 1.13
Fig. 1.13 - Q-13. A 32-year-old man presented with chronic cough, hemoptysis, and bilateral lung infiltrations. A sputum specimen was collected. What is the diagnosis?
- Echinococcosis (Hydatid disease)
- Strongyloidiasis
- Entamoeba gingivalis infection
- Paragonimus eggs
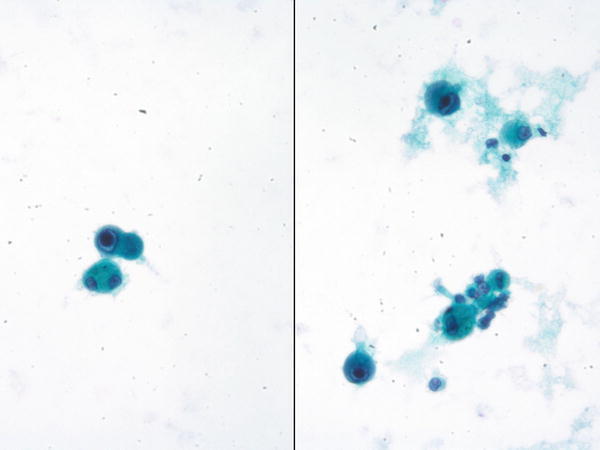 Fig. 1.14
Fig. 1.14 - Q-14. A 67-year-old transplant patient presented with chronic cough and multiple lung nodules. A BAL was performed. What is the best diagnosis?
- Reactive bronchial cells and alveolar macrophages
- Poorly differentiated adenocarcinoma
- Histoplasmosis
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
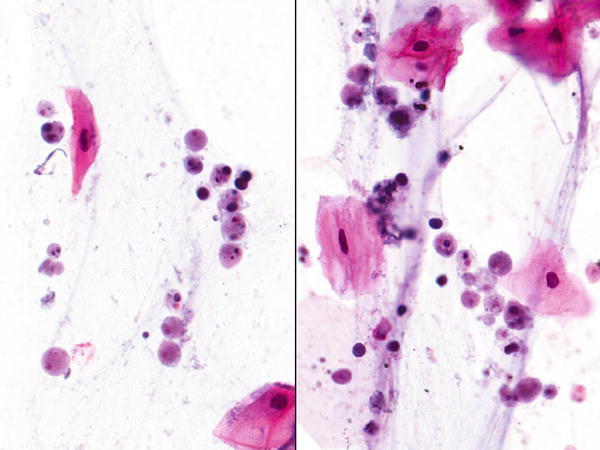 Fig. 1.15
Fig. 1.15 - Q-15. A 33-year-old non-smoking man presented with a 3 cm lung nodule. A transbronchial FNA of the lung lesion was performed. What is the diagnosis?
- Cryptococcosis
- Coccidioidomycosis
- Histoplasmosis
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
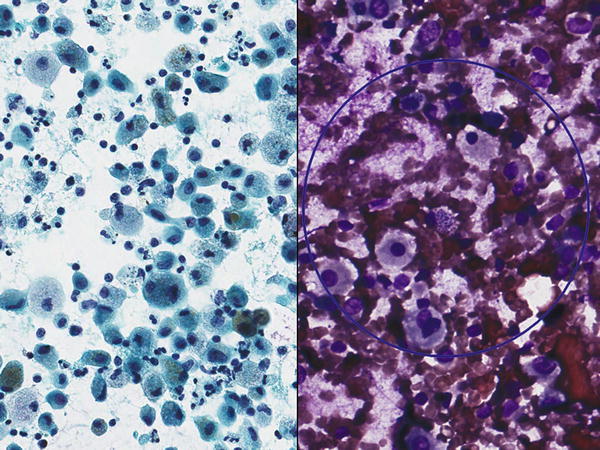 Fig. 1.16
Fig. 1.16 - Q-16. A 33-year-old non-smoking man presented with a 3 cm lung nodule. A transbronchial FNA of the lung lesion was performed. What is the diagnosis?
- Cryptococcosis
- Coccidioidomycosis
- Histoplasmosis
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
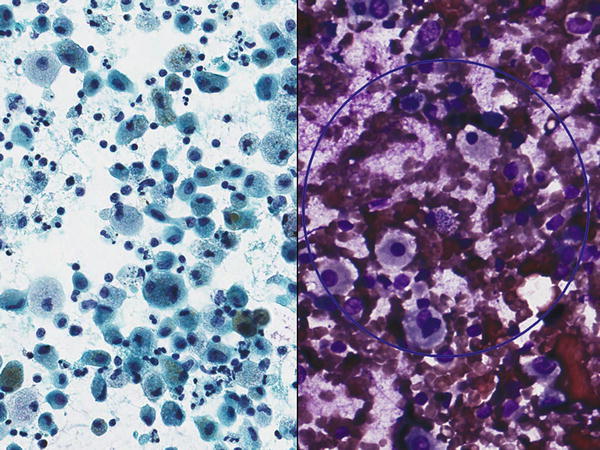 Fig. 1.17
Fig. 1.17 - Q-17. A 53-year-old female presented with chronic cough, night sweats, fever, weight loss, and fatigue. CT scan showed bilateral cavitary lung lesions. A transbronchial FNA of the lung lesion was performed. What is the diagnosis on this Diff-Quik smear?
- Cryptococcosis
- Histoplasmosis
- Tuberculosis
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
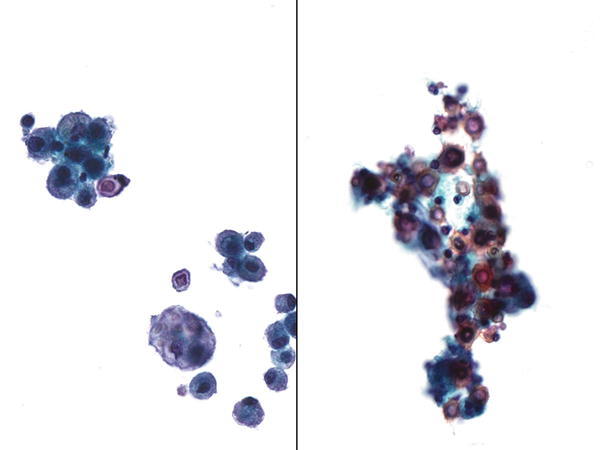 Fig. 1.18
Fig. 1.18 - Q-18. This BAL specimen was collected from a transplant patient. What is the diagnosis of the BAL?
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
- Histoplasmosis
- Tuberculosis
- Toxoplasmosis
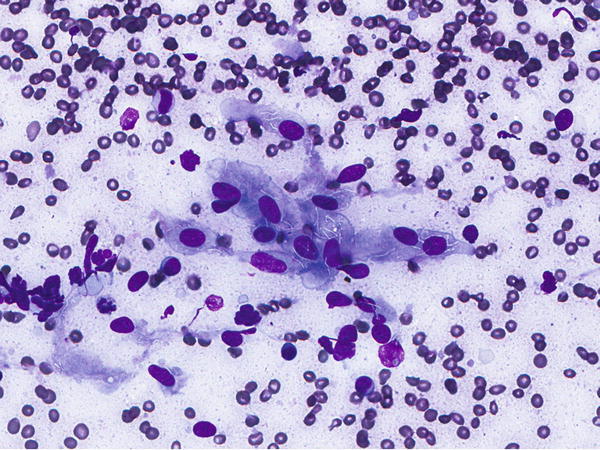 Fig. 1.19
Fig. 1.19 - Q-19. An immunocompromised patients presented with cough, fever, pneumonia, and bilateral lung infiltrations. What is the diagnosis of this BAL specimen?
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
- Cytomegalovirus (CMV) infection
- Co-infection with CMV and PCP
- Herpes simplex virus (HSV) infection
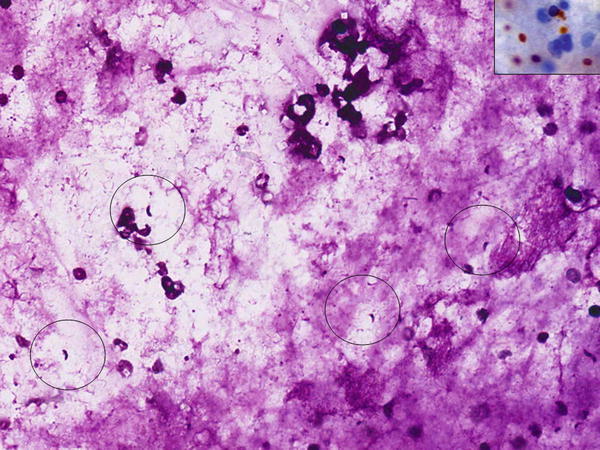 Fig. 1.20
Fig. 1.20 - Q-20. The structures seen in photos are most likely found in a patient with:
- Asthma
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
- Aspergillosis
- Asbestos exposure
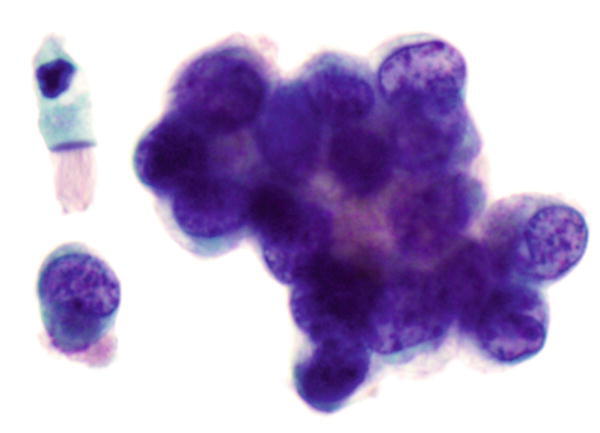 Fig. 1.21
Fig. 1.21 - Q-21. What is the diagnosis of this image?
- Well-differentiated adenocarcinoma
- Reactive bronchial epithelium
- Creola body
- Bronchoalveolar macrophages
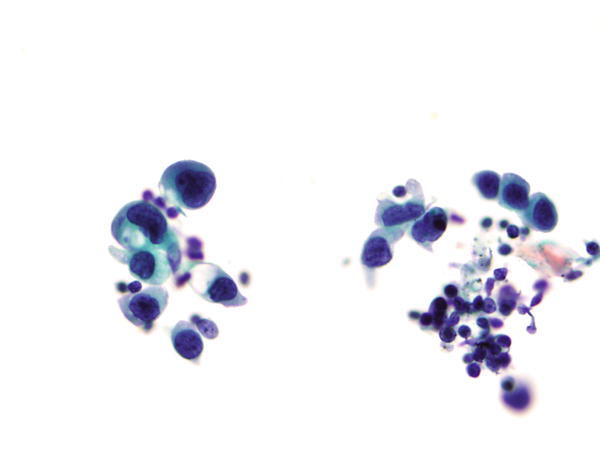 Fig. 1.22
Fig. 1.22 - Q-22. What is the diagnosis of this image?
- Reactive bronchial epithelium
- Creola body
- Bronchoalveolar macrophages
- Adenocarcinoma with cytoplasmic mucin
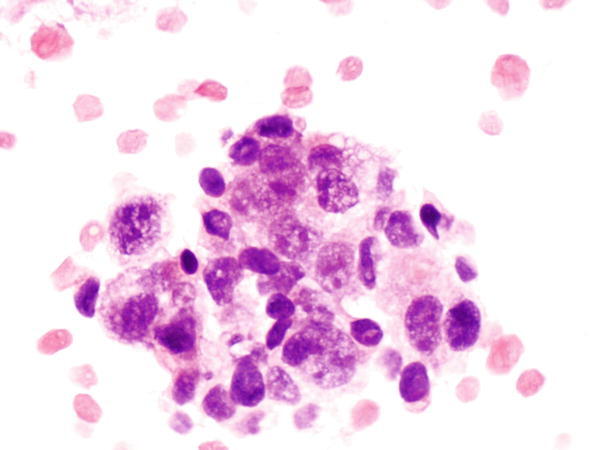 Fig. 1.23
Fig. 1.23 - Q-23. What is the diagnosis of this cell block preparation?
- Bronchoalveolar macrophages
- Goblet cell hyperplasia
- Adenocarcinoma
- Reactive bronchial epithelium
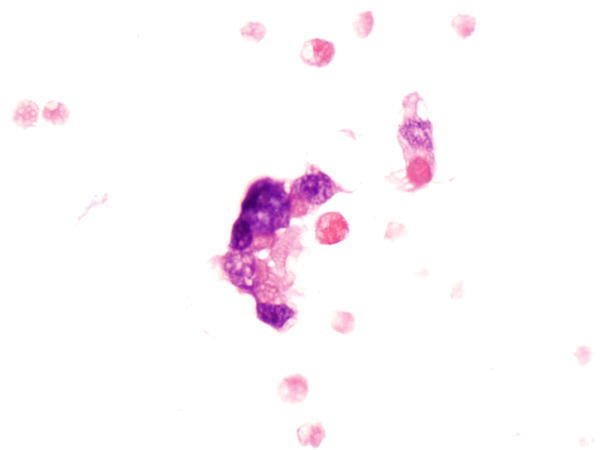 Fig. 1.24
Fig. 1.24 - Q-24. A 67-year-old female smoker had bilateral lung infiltration. What is the diagnosis of this cell block preparation?
- Bronchoalveolar macrophages
- Goblet cell hyperplasia
- Adenocarcinoma
- Reactive bronchial epithelium
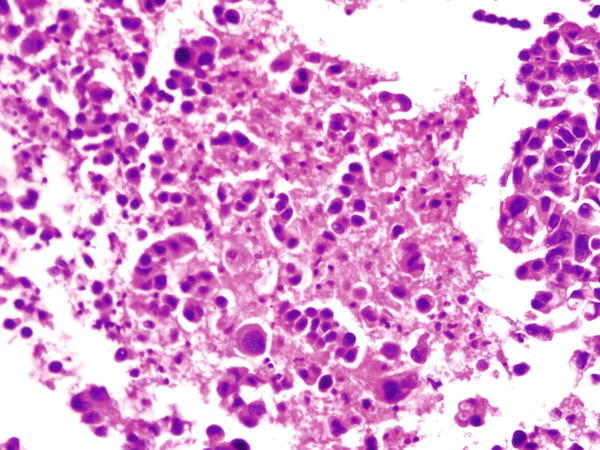 Fig. 1.25
Fig. 1.25 - Q-25. This is a transbronchial fine-needle aspiration from a 78-year-old male smoker who has a right upper lobe lung nodule. What is the diagnosis of this cell block preparation?
- Squamous cell carcinoma
- Bronchoalveolar macrophages hyperplasia
- Adenocarcinoma
- Reactive bronchial epithelium
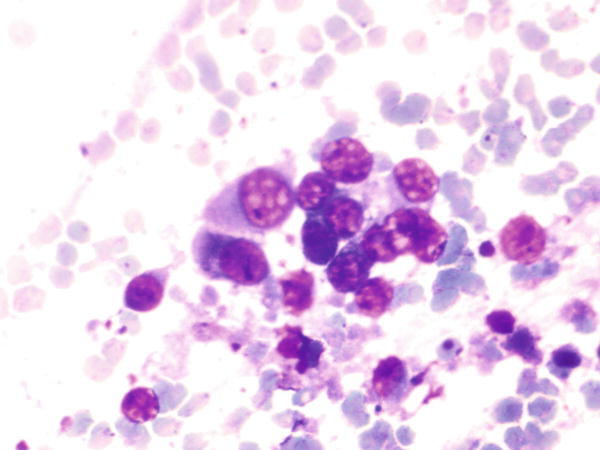 Fig. 1.26
Fig. 1.26 - Q-26. This is an on-site evaluation of a transbronchial fine-needle aspiration. What is the diagnosis of this Diff-Quik preparation?
- Reactive bronchial epithelium
- Squamous cell carcinoma
- Bronchoalveolar macrophages hyperplasia
- Adenocarcinoma
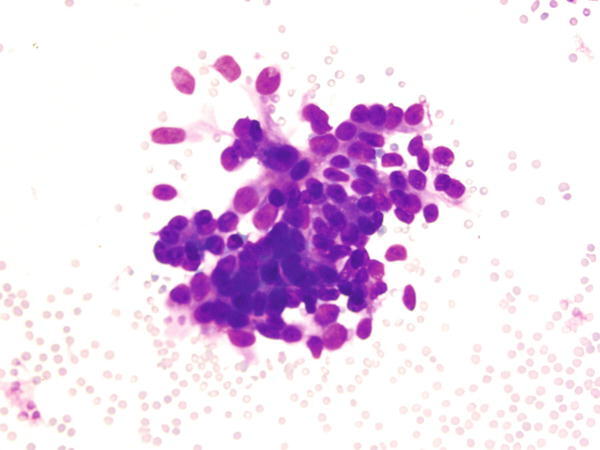 Fig. 1.27
Fig. 1.27 - Q-27. This is an on-site evaluation of a transbronchial fine-needle aspiration from a 52-year-old female with clinical history of a breast cancer, who now has developed bilateral lung nodules. What is the diagnosis of this Diff-Quik preparation?
- Reactive bronchial epithelium
- Squamous cell carcinoma
- Metastatic carcinoma of the breast
- Adenocarcinoma of the lung
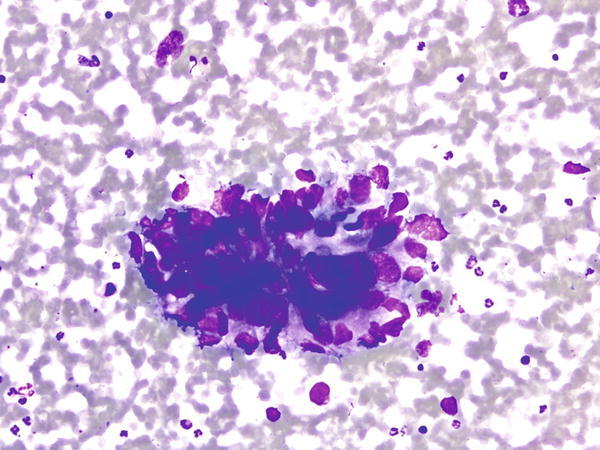 Fig. 1.28
Fig. 1.28 - Q-28. This is an on-site evaluation of a transbronchial fine-needle aspiration from a 68-year-old male with clinical history of a colon cancer, now developed bilateral lung nodules. What is the diagnosis of this Diff-Quik preparation?
- Reactive bronchial epithelium
- Squamous cell carcinoma
- Metastatic adenocarcinoma of the colon
- Adenocarcinoma of the lung
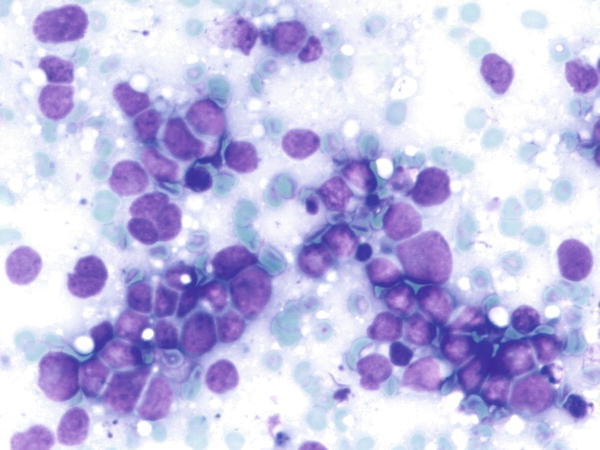 Fig. 1.29
Fig. 1.29 - Q-29. This is an on-site evaluation of a transbronchial fine-needle aspiration from a 68-year-old male smoker with a large hilar mass. What is the diagnosis of this Diff-Quik preparation?
- Reactive bronchial epithelium
- Reactive lymphocytes
- Poorly differentiated squamous cell carcinoma
- Small cell carcinoma
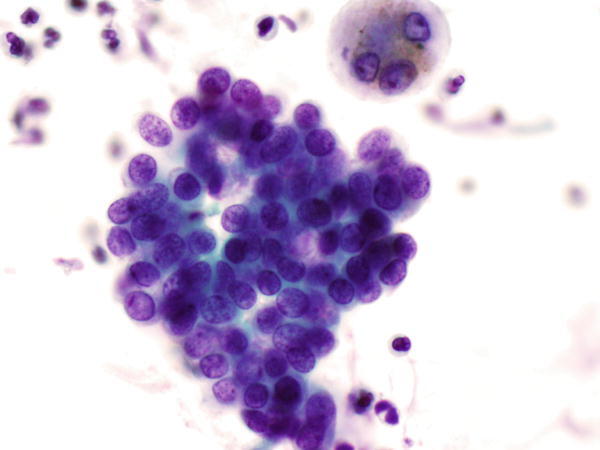 Fig. 1.30
Fig. 1.30 - Q-30. This is a transbronchial fine-needle aspiration from a 74-year-old female with clinical history of a breast cancer, now developed bilateral lung nodules. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial epithelium
- Squamous cell carcinoma
- Metastatic carcinoma of the breast
- Adenocarcinoma of the lung
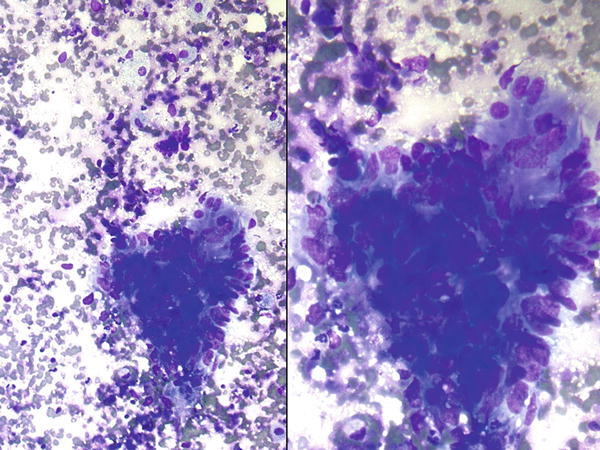 Fig. 1.31
Fig. 1.31 - Q-31. This is a transbronchial fine-needle aspiration from a 68-year-old male with clinical history of a colon cancer, who now has developed bilateral lung nodules. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial epithelium
- Squamous cell carcinoma
- Metastatic adenocarcinoma of the colon
- Adenocarcinoma of the lung
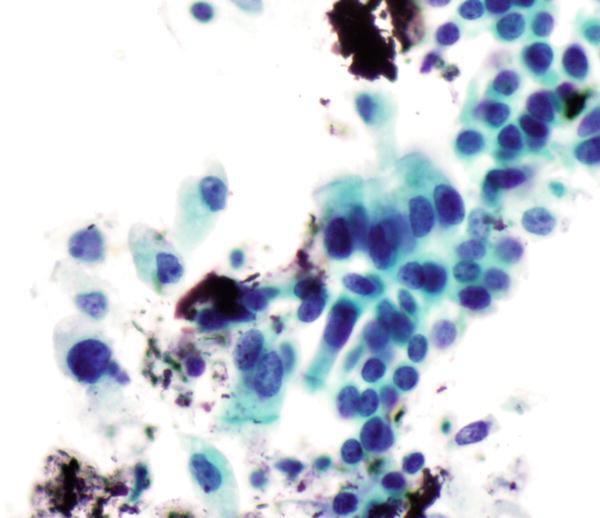 Fig. 1.32
Fig. 1.32 - Q-32. This is a transbronchial fine-needle aspiration from a 70-year-old male with clinical history of a colon cancer, who now has developed bilateral lung infiltration. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial epithelium
- Squamous cell carcinoma
- Metastatic adenocarcinoma of the colon
- Well-differentiated adenocarcinoma of the lung
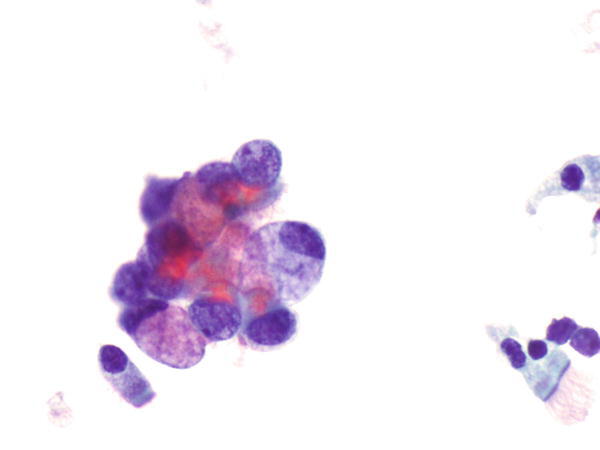 Fig. 1.33
Fig. 1.33 - Q-33. This is a transbronchial fine-needle aspiration from a 70-year-old male smoker with bilateral lung infiltration and productive cough. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial squamous cells
- Goblet cell hyperplasia
- Squamous cell carcinoma
- Adenocarcinoma
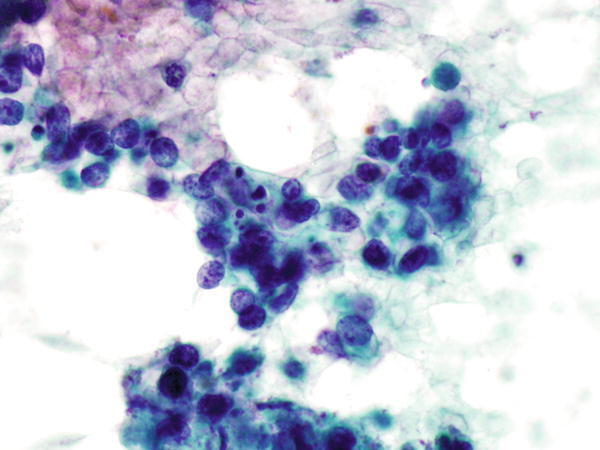 Fig. 1.34
Fig. 1.34 - Q-34. This is a transbronchial fine-needle aspiration from a 66-year-old male smoker with left lung mass and brain metastasis. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial squamous cells
- Small cell carcinoma
- Squamous cell carcinoma
- Adenocarcinoma
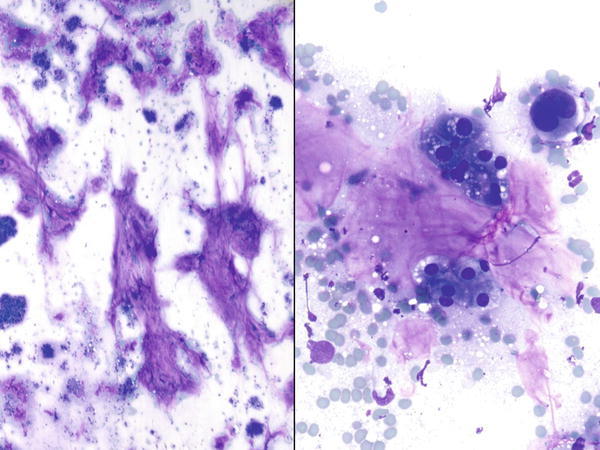 Fig. 1.35
Fig. 1.35 - Q-35. This is a sputum specimen from a 58-year-old male smoker with a lung mass. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial cells
- Small cell carcinoma
- Goblet cells
- Adenocarcinoma with mucinous features
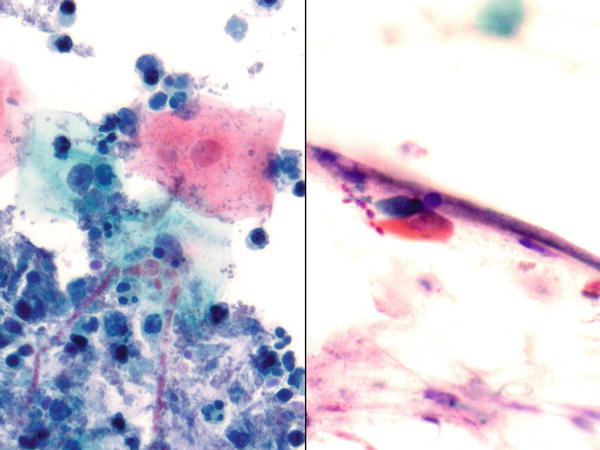 Fig. 1.36
Fig. 1.36 - Q-36. This is a BAL specimen from a 55-year-old female smoker with diabetics, who has developed cough and fever. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial cells and Candida
- Aspergillosis
- Squamous cell carcinoma
- Adenocarcinoma
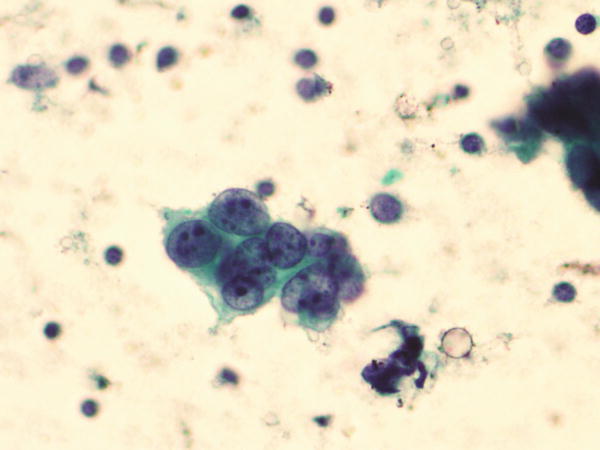 Fig. 1.37
Fig. 1.37 - Q-37. This is a BAL specimen from a 71-year-old female smoker with a right lower lobe lung mass. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial cells
- Small cell carcinoma
- Squamous cell carcinoma
- Adenocarcinoma
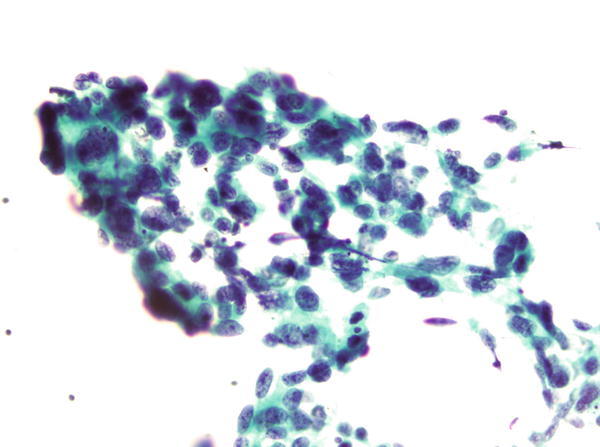 Fig. 1.38
Fig. 1.38 - Q-38. This is a transbronchial fine-needle aspiration specimen from a 58-year-old male smoker with a left lower lobe lung mass. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial cells
- Small cell carcinoma
- Squamous cell carcinoma
- Adenocarcinoma
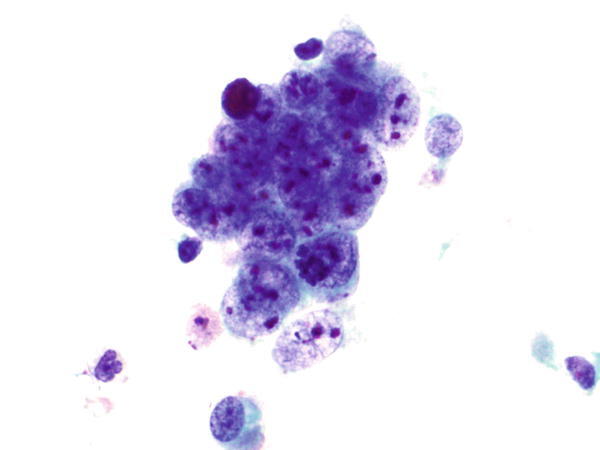 Fig. 1.39
Fig. 1.39 - Q-39. This is a transbronchial fine-needle aspiration specimen from a 58-year-old male smoker with a left lower lobe lung mass. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial cells
- Small cell carcinoma
- Squamous cell carcinoma
- Poorly differentiated adenocarcinoma
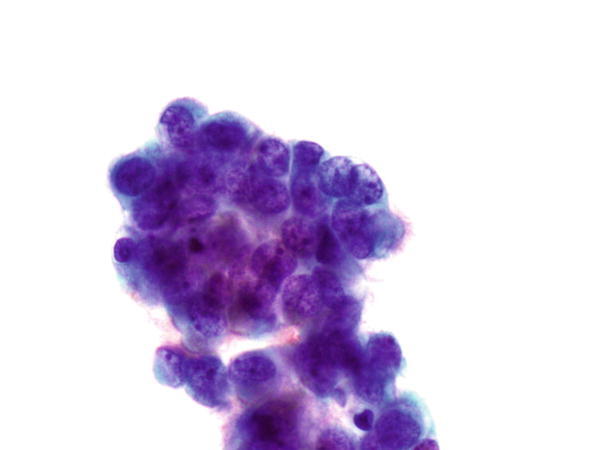 Fig. 1.40
Fig. 1.40 - Q-40. This is a transbronchial fine-needle aspiration specimen from a 20-year-old male smoker with a left lower lobe lung lesion. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial epithelial cells
- Small cell carcinoma
- Squamous cell carcinoma
- Well-differentiated adenocarcinoma
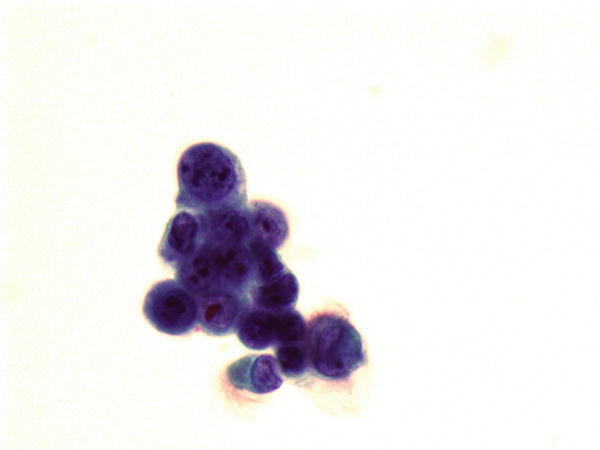 Fig. 1.41
Fig. 1.41 - Q-41. This is a BAL specimen from a 60-year-old male smoker with lower lobe lung lesions. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial epithelial cells
- Small cell carcinoma
- Squamous cell carcinoma
- Well-differentiated adenocarcinoma
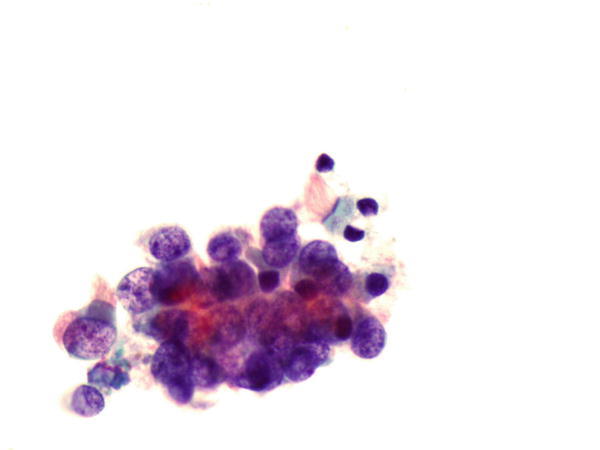 Fig. 1.42
Fig. 1.42 - Q-42. This is a transbronchial fine-needle aspiration specimen from a 70-year-old male smoker with bilateral lung lesion. What is the diagnosis of this Papanicolaou preparation?
- Well-differentiated adenocarcinoma
- Small cell carcinoma
- Squamous cell carcinoma
- Reactive bronchial epithelial cells
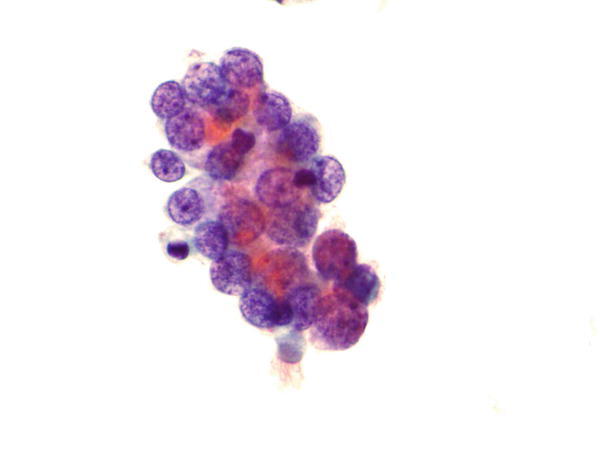 Fig. 1.43
Fig. 1.43 - Q-43. This is a transbronchial fine-needle aspiration specimen from an 80-year-old male smoker with a hilar lung lesion. What is the diagnosis of this Papanicolaou preparation?
- Small cell carcinoma
- Reactive bronchial epithelial cells
- Squamous cell carcinoma
- Well-differentiated adenocarcinoma
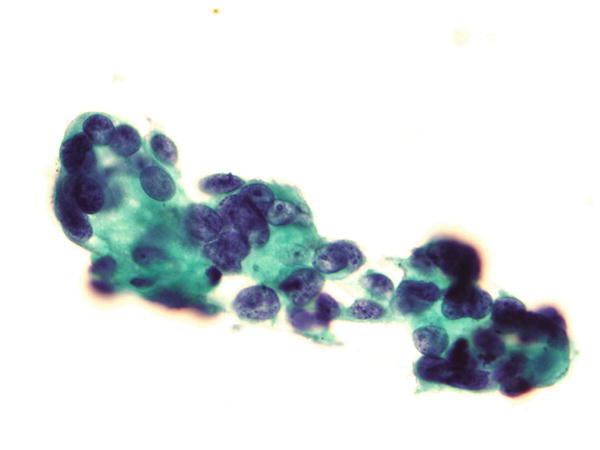 Fig. 1.44
Fig. 1.44 - Q-44. This is a transbronchial fine-needle aspiration specimen from a 79-year-old male smoker with a peripheral right lung lesion. What is the diagnosis of this Papanicolaou preparation?
- Small cell carcinoma
- Reactive bronchial epithelial cells
- Squamous cell carcinoma
- Adenocarcinoma
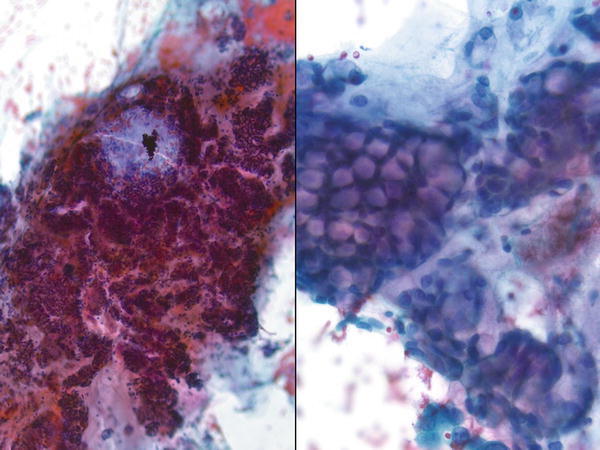 Fig. 1.45
Fig. 1.45 - Q-45. What is the diagnosis of this Papanicolaou preparation from the endobronchial ultrasound (EBUS) specimen?
- Small cell carcinoma
- Reactive bronchial epithelial cells
- Goblet cell hyperplasia
- Adenocarcinoma
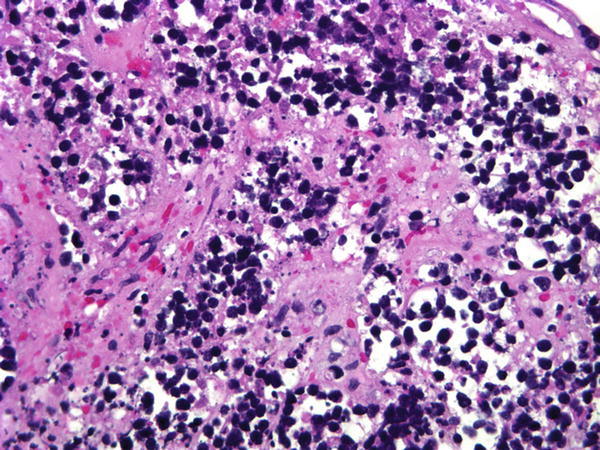 Fig. 1.46
Fig. 1.46 - Q-46. This is a transbronchial fine-needle aspiration specimen from a 69-year-old male smoker with a centrally located lung mass. What is the diagnosis of this cell block preparation?
- Small cell carcinoma
- Reactive bronchial epithelial cells
- Reactive lymphocytes
- Adenocarcinoma
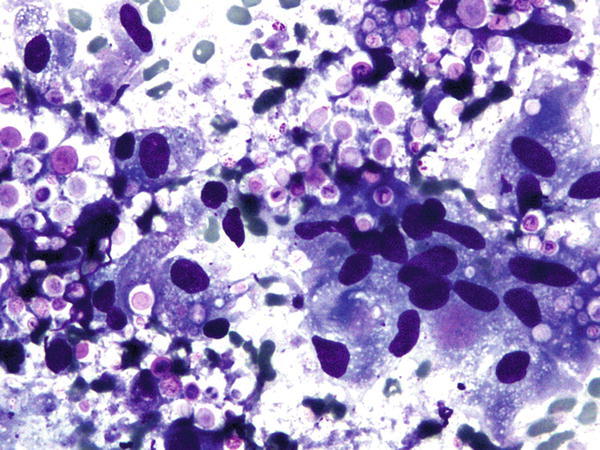 Fig. 1.47
Fig. 1.47 - Q-47. This is a BAL specimen from a 32-year-old male smoker with HIV-positive serum test and lung lesions. What is the diagnosis of this Papanicolaou preparation?
- Reactive goblet cells
- Histoplasmosis
- Cryptococcosis
- Blastomycosis
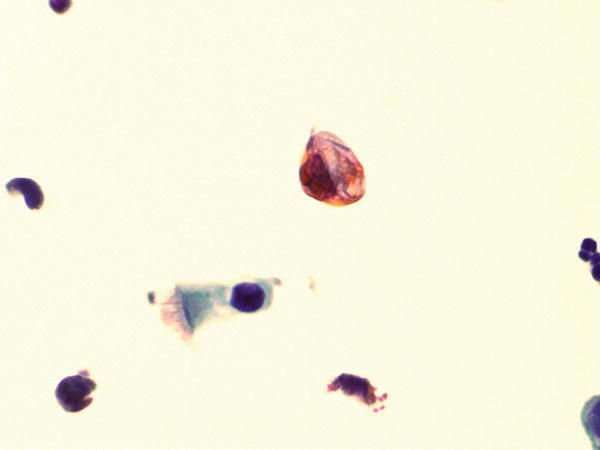 Fig. 1.48
Fig. 1.48 - Q-48. This is a BAL specimen from a 25-year-old male without clinical history of malignancy. What is the diagnosis of this large non-ciliated cell?
- Signet ring cell carcinoma
- Reactive bronchial epithelial cells
- Degenerated alveolar macrophages
- Adenocarcinoma
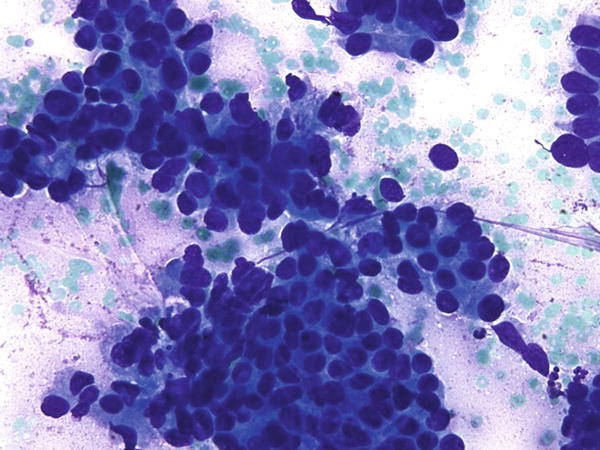 Fig. 1.49
Fig. 1.49 - Q-49. This is a transbronchial fine-needle aspiration specimen from an 80-year-old male smoker with a peripheral lung lesion. What is the diagnosis of this Diff-Quik preparation?
- Small cell carcinoma
- Reactive bronchial epithelial cells
- Squamous cell carcinoma
- Adenocarcinoma
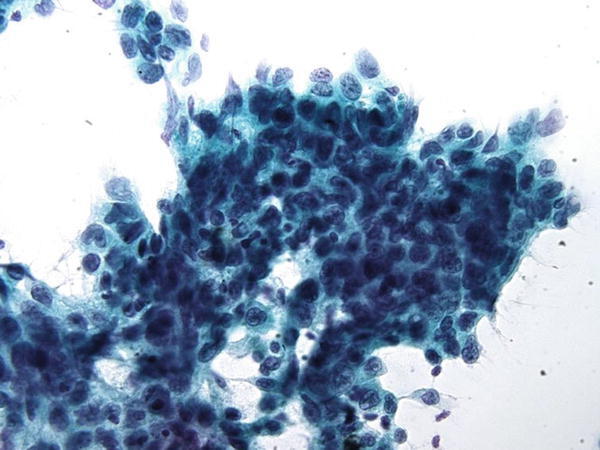 Fig. 1.50
Fig. 1.50 - Q-50. This is a transbronchial fine-needle aspiration from an 84-year-old male smoker with a large hilar mass. What is the diagnosis of this Papanicolaou preparation?
- Reactive bronchial epithelium
- Adenocarcinoma
- Squamous cell carcinoma
- Small cell carcinoma
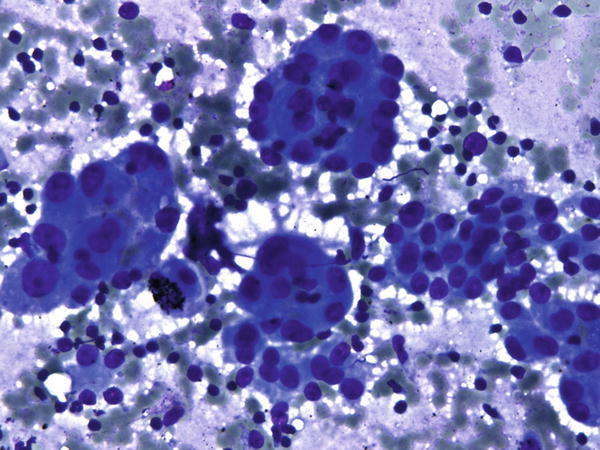 Fig. 1.51
Fig. 1.51 - Q-51. What is the diagnosis of this Diff-Quik preparation?
- Reactive bronchial epithelium
- Adenocarcinoma
- Poorly differentiated squamous cell carcinoma
- Small cell carcinoma
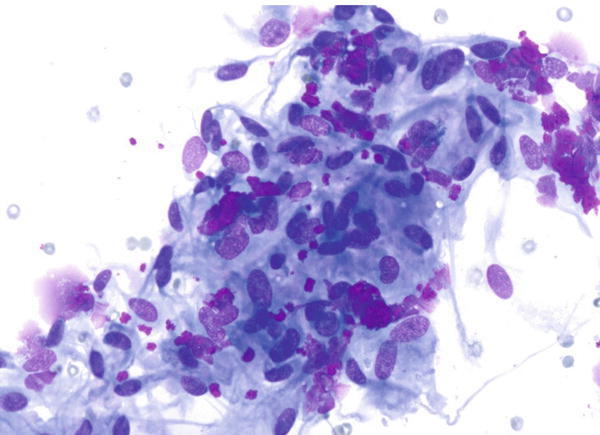 Fig. 1.52
Fig. 1.52 - Q-52. This is a transbronchial fine-needle aspiration from a 48-year-old African American female with bilateral lung infiltration. What is the diagnosis of this Diff-Quik preparation?
- Reactive lymphocytes
- Adenocarcinoma
- Poorly differentiated squamous cell carcinoma
- Granulomatous inflammation
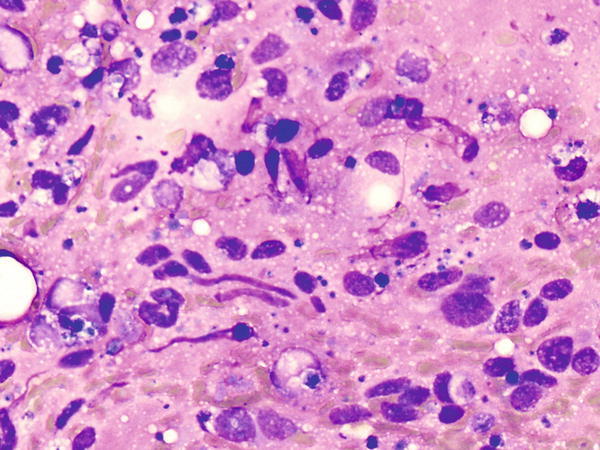 Fig. 1.53
Fig. 1.53 - Q-53. This is a transbronchial fine-needle aspiration from a 55-year-old male with a clinical history of synovial sarcoma, who developed bilateral lung infiltrations. What is the diagnosis of this Diff-Quik preparation?
- Reactive lymphocytes
- Adenocarcinoma
- Poorly differentiated squamous cell carcinoma
- Metastatic synovial sarcoma
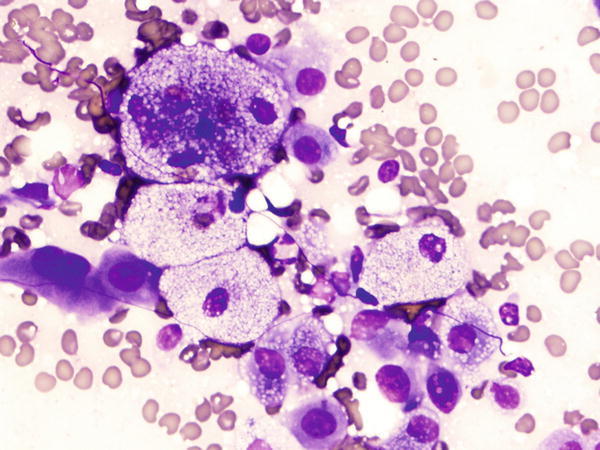 Fig. 1.54
Fig. 1.54 - Q-54. What is the diagnosis of this Diff-Quik smear?
- Goblet cells
- Adenocarcinoma
- Metastatic renal cell carcinoma
- Bronchioalveolar macrophages
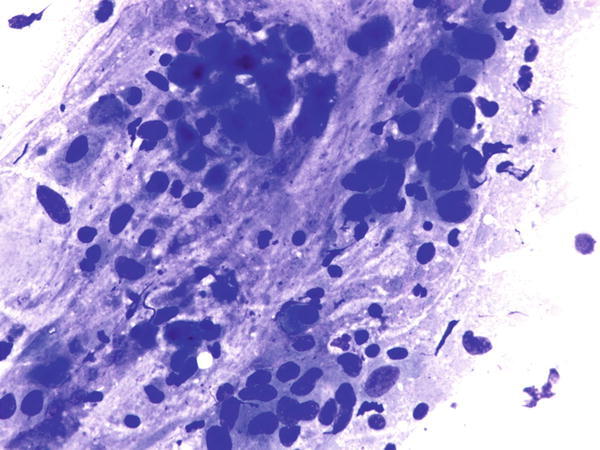 Fig. 1.55
Fig. 1.55 - Q-55. This is an on-site evaluation of a transbronchial fine-needle aspiration from a 74-year-old male smoker with a large left upper lung mass. What is the diagnosis of this Diff-Quik preparation?
- Reactive bronchial epithelium
- Adenocarcinoma
- Poorly differentiated squamous cell carcinoma
- Small cell carcinoma
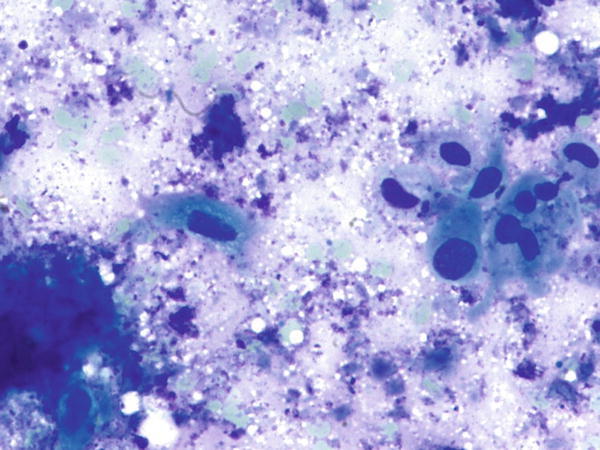 Fig. 1.56
Fig. 1.56 - Q-56. What is the diagnosis of this Diff-Quik smear?
- Goblet cells
- Adenocarcinoma
- Squamous cell carcinoma
- Bronchioalveolar macrophages
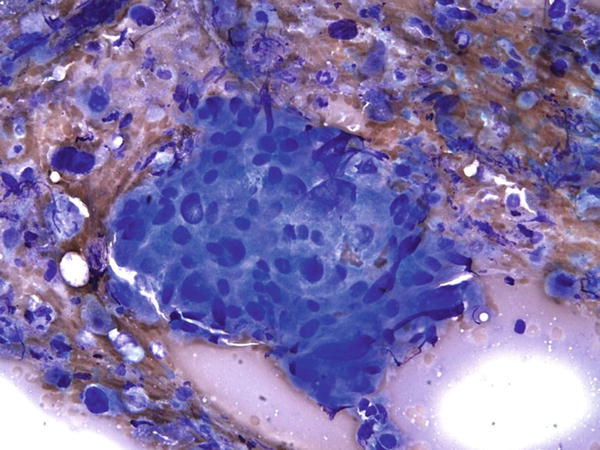 Fig. 1.57
Fig. 1.57 - Q-57. What is the diagnosis of this Diff-Quik smear?
- Granuloma
- Adenocarcinoma
- Squamous cell carcinoma
- Bronchioalveolar macrophages
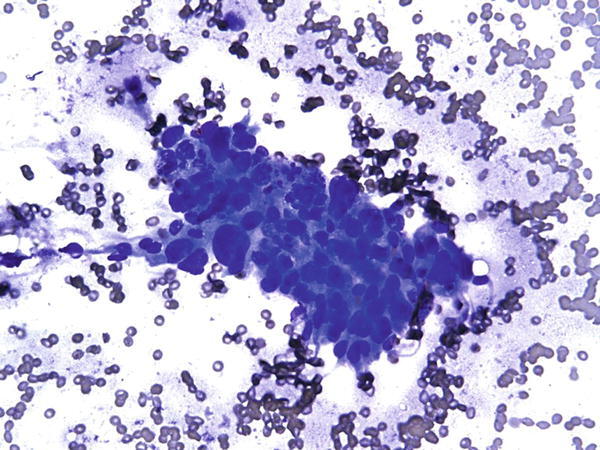 Fig. 1.58
Fig. 1.58 - Q-58. What is the diagnosis of this Diff-Quik smear?
- Granuloma
- Adenocarcinoma
- Squamous cell carcinoma
- Bronchial reserve cell hyperplasia
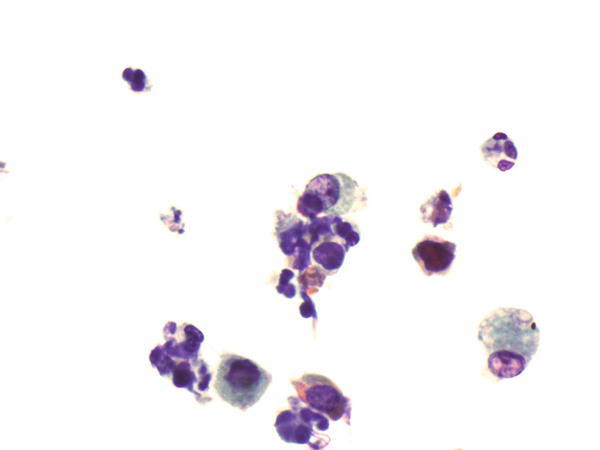 Fig. 1.59
Fig. 1.59 - Q-59. This is a bronchoscopic washing from a 25-year-old female with chronic cough. What is the diagnosis?
- Granuloma
- Adenocarcinoma
- Squamous cell carcinoma
- Degenerated bronchioalveolar macrophages
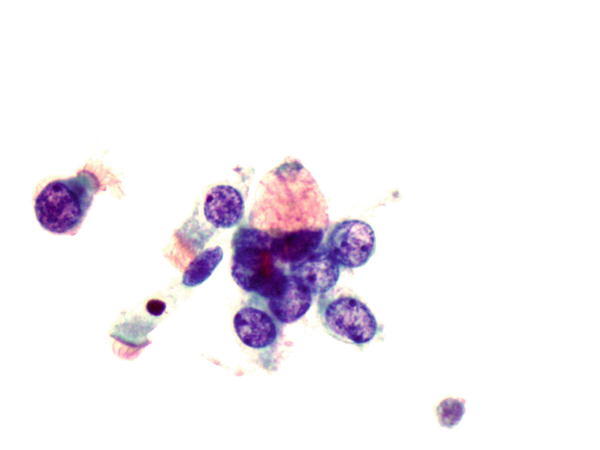 Fig. 1.60
Fig. 1.60 - Q-60. This is a bronchoscopic washing from an 18-year-old female with clinical history of cystic fibrosis. What is the diagnosis?
- Goblet cell
- Adenocarcinoma
- Signet ring cell carcinoma
- Small cell carcinoma
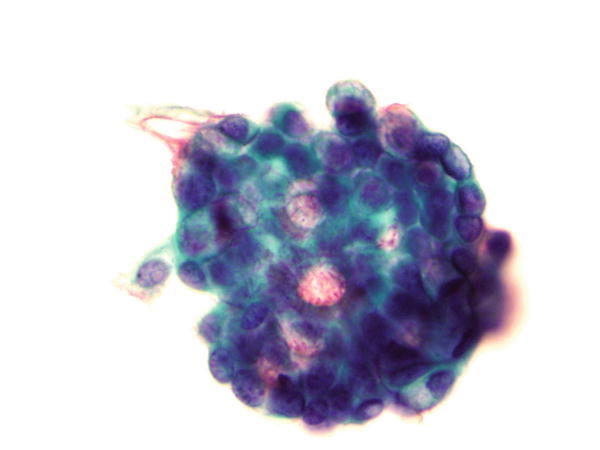 Fig. 1.61
Fig. 1.61 - Q-61. This is a transbronchial fine-needle aspiration from a 35-year-old African American female with bilateral lung infiltration. What is the diagnosis of this Papanicolaou preparation?
- Small cell carcinoma
- Adenocarcinoma
- Granuloma
- Goblet cell hyperplasia
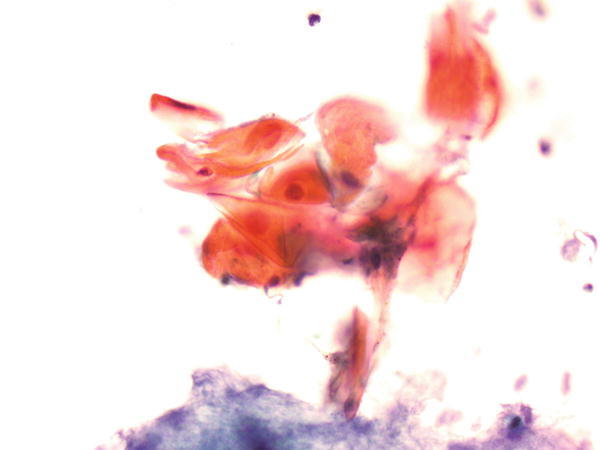 Fig. 1.62
Fig. 1.62 - Q-62. This is a sputum specimen from a 59-year-old smoker with lung lesions. What is the diagnosis?
- Squamous cells with reactive atypia
- Adenocarcinoma
- Squamous cell carcinoma
- Bronchial goblet cell hyperplasia
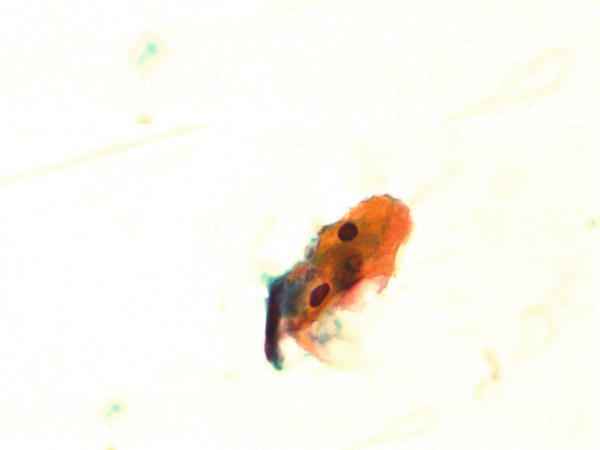 Fig. 1.63
Fig. 1.63 - Q-63. This is a transbronchial fine-needle aspiration from a 62-year-old smoker with lung lesions. What is the diagnosis?
- Adenocarcinoma
- Reactive squamous cells
- Squamous cell carcinoma
- Bronchial macrophages
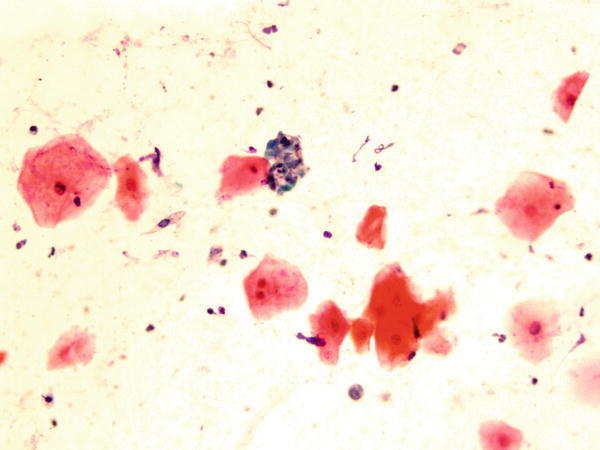 Fig. 1.64
Fig. 1.64 - Q-64. This is a sputum specimen from a 79-year-old smoker with lung lesions. What is the diagnosis?
- Non-diagnostic specimen
- Reactive squamous cells
- Squamous cell carcinoma
- Bronchial macrophages
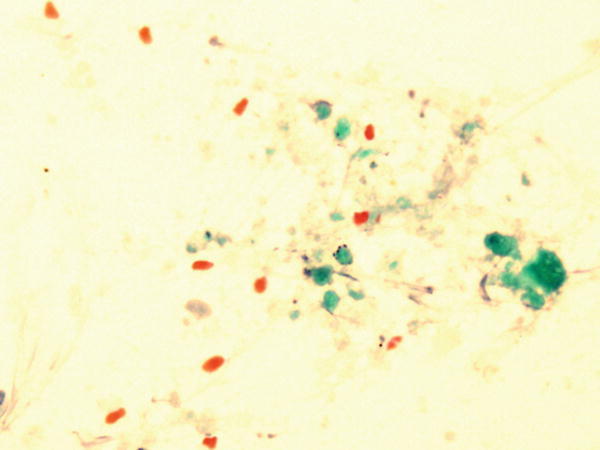 Fig. 1.65
Fig. 1.65 - Q-65. This is a transbronchial fine-needle aspiration from a 67-year-old smoker with a large lung mass, a squamous cell carcinoma is clinically suspected. What is the best interpretation of this slide?
- Metaplastic squamous cells
- Reactive squamous cells
- Karetin debris and it is necessary to repeat the biopsy
- Squamous cell carcinoma
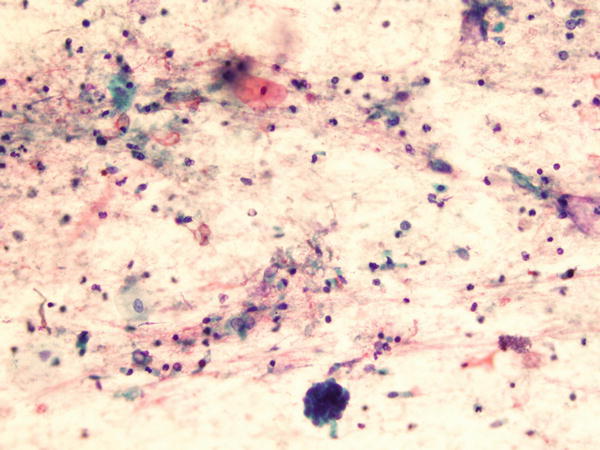 Fig. 1.66
Fig. 1.66 - Q-66. This is a BAL specimen from a 45-year-old smoker with lung lesions. What is the diagnosis?
- Non-diagnostic specimen
- Adenocarcinoma
- Squamous cell carcinoma
- Reactive bronchial epithelium
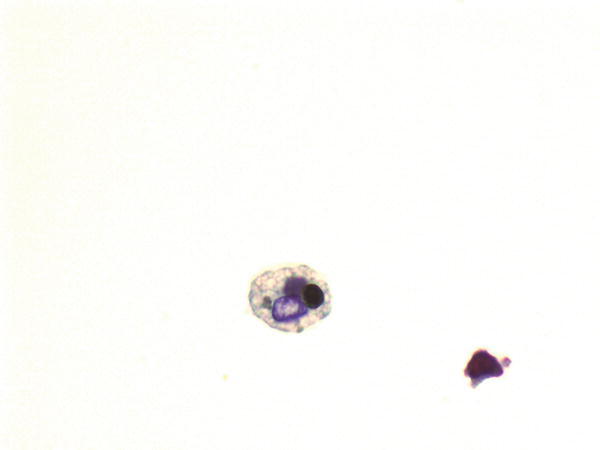 Fig. 1.67
Fig. 1.67 - Q-67. This is a BAL specimen from a 45-year-old smoker with lung lesions. What is the diagnosis?
- Signet ring cell carcinoma
- Adenocarcinoma
- Bronchioalveolar macrophages
- Reactive bronchial epithelium
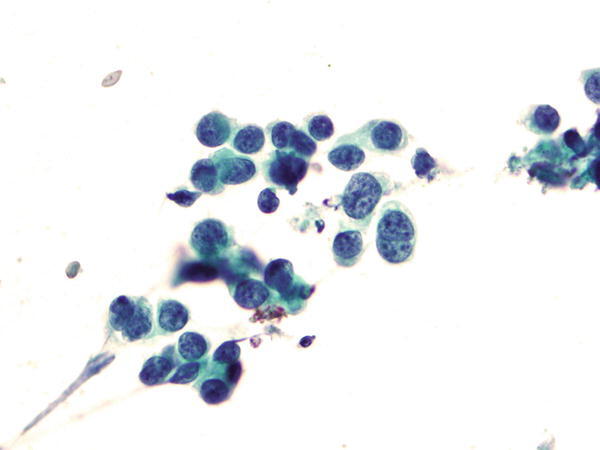 Fig. 1.68
Fig. 1.68 - Q-68. This is a BAL specimen from a 73-year-old smoker with lung lesions. What is the diagnosis?
- Squamous cell carcinoma
- Adenocarcinoma
- Bronchioalveolar macrophages
- Reactive bronchial epithelium
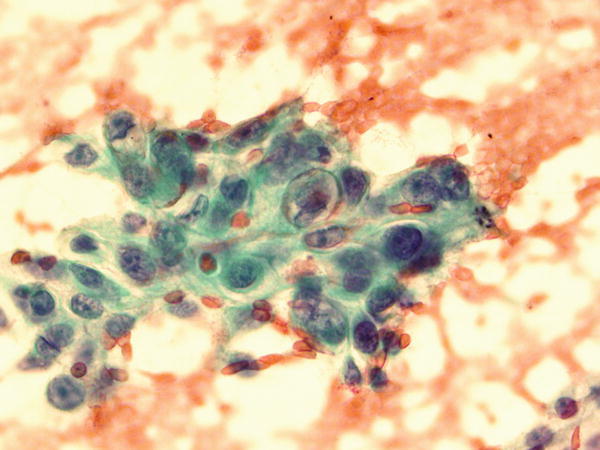 Fig. 1.69
Fig. 1.69 - Q-69. This is a transbronchial fine need aspiration specimen from a 70-year-old smoker with a lung lesion. What is the diagnosis?
- Poorly differentiated squamous cell carcinoma
- Adenocarcinoma
- Bronchioalveolar macrophages
- Reactive bronchial epithelium
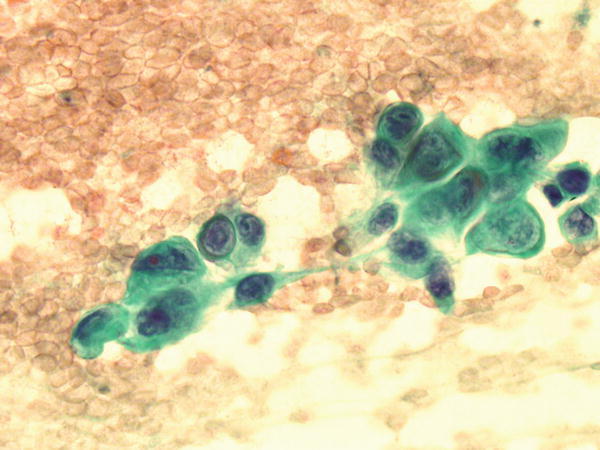 Fig. 1.70
Fig. 1.70 - Q-70. What is the diagnosis of this Papanicolaou preparation?
- Poorly differentiated squamous cell carcinoma
- Adenocarcinoma
- Bronchioalveolar macrophages
- Reactive bronchial epithelium
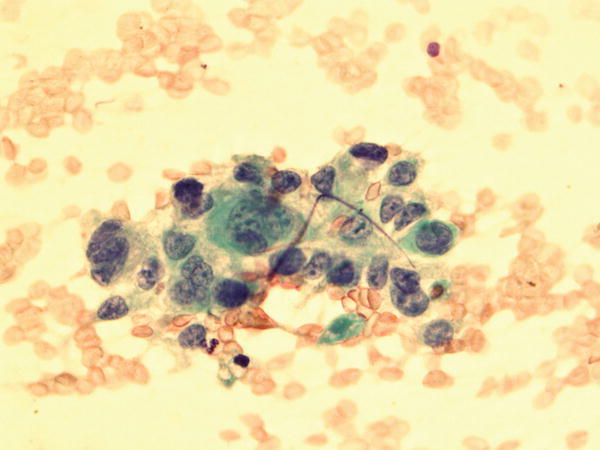 Fig. 1.71
Fig. 1.71 - Q-71. What is the diagnosis of this Papanicolaou preparation?
- Poorly differentiated squamous cell carcinoma
- Adenocarcinoma
- Bronchioalveolar macrophages
- Reactive bronchial epithelium
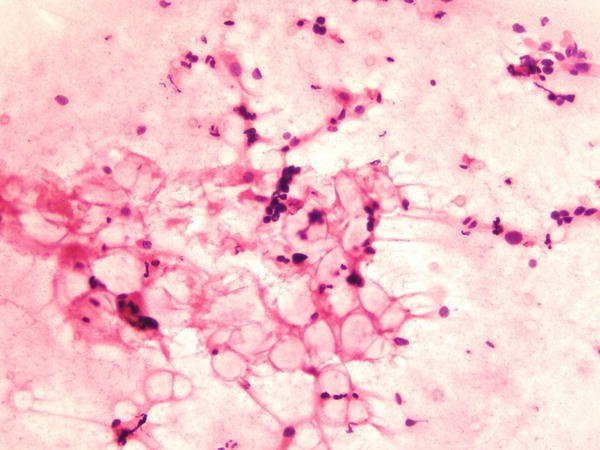 Fig. 1.72
Fig. 1.72 - Q-72. What is the diagnosis of this BAL specimen?
- Vegetable cells
- Goblet cells
- Reactive bronchial cells and mucin vacuole
- Adenocarcinoma
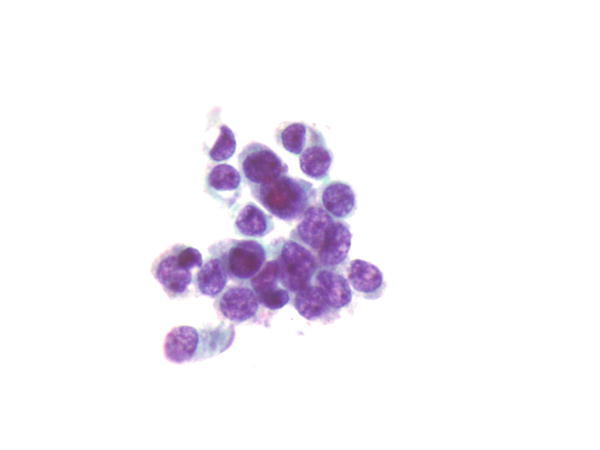 Fig. 1.73
Fig. 1.73 - Q-73. What is the diagnosis of this BAL specimen?
- Small cell carcinoma
- Goblet cells
- Reactive bronchial epithelial cells
- Adenocarcinoma
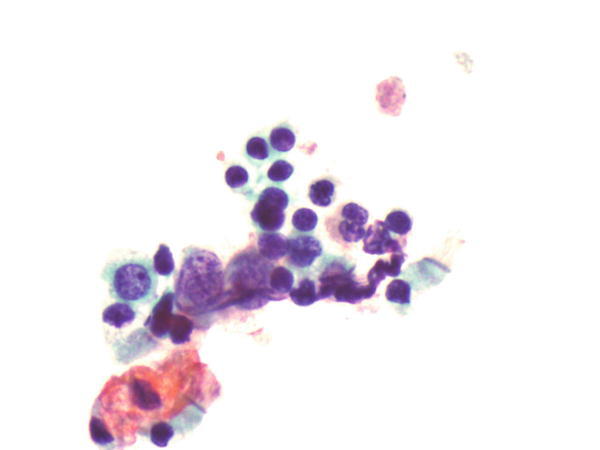 Fig. 1.74
Fig. 1.74 - Q-74. What are these small hyperchromatic cells in this BAL specimen?
- Vegetable cells
- Lymphocytes
- Small cell carcinoma
- Bronchial reserve cells
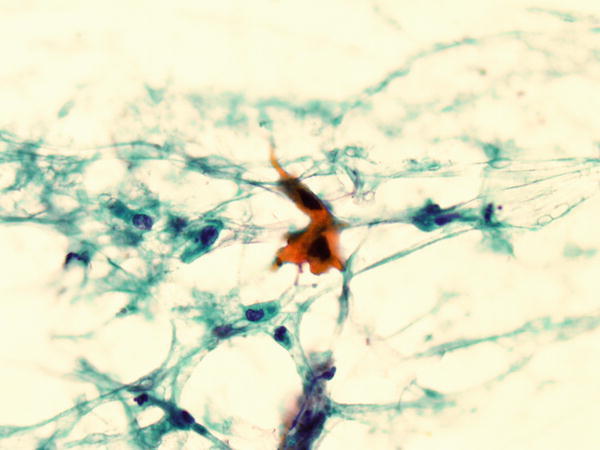 Fig. 1.75
Fig. 1.75 - Q-75. What is the diagnosis of this sputum specimen?
- Reactive squamous cells
- Squamous cell carcinoma
- Reactive bronchial cells
- Adenocarcinoma
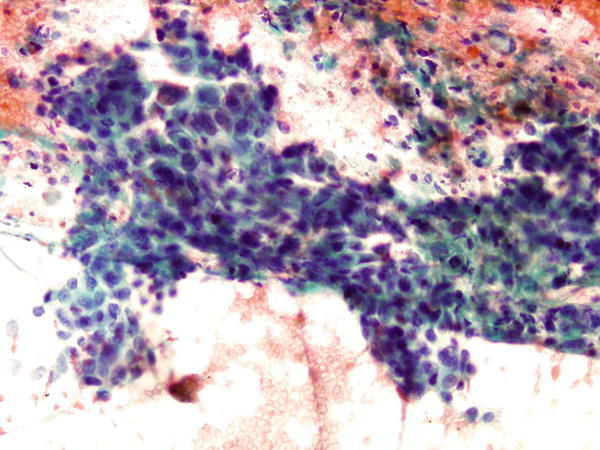 Fig. 1.76
Fig. 1.76 - Q-76. This is a transbronchial fine need aspiration specimen from a 60-year-old smoker with a lung lesion. What is the diagnosis?
- Squamous cell carcinoma
- Adenocarcinoma
- Bronchioalveolar macrophages
- Reactive bronchial epithelium
 Fig. 1.77
Fig. 1.77 - Q-77. This is a transbronchial fine need aspiration specimen from a 48-year-old smoker with a lung lesion. What is the diagnosis?
- Squamous cell carcinoma
- Adenocarcinoma
- Bronchioalveolar macrophages
- Reactive squamous cells
 Fig. 1.78
Fig. 1.78 - Q-78. This is a transbronchial fine need aspiration specimen from a 71-year-old smoker with a lung lesion. What is the diagnosis?
- Reactive squamous cells
- Adenocarcinoma
- Bronchioalveolar macrophages
- Squamous cell carcinoma
 Fig. 1.79
Fig. 1.79 - Q-79. This is a transbronchial fine need aspiration specimen from a 71-year-old smoker with a lung lesion. What is the diagnosis?
- Reactive squamous cells
- Adenocarcinoma
- Bronchioalveolar macrophages
- Poorly differentiated squamous cell carcinoma
 Fig. 1.80
Fig. 1.80 - Q-80. What is the diagnosis of this specimen?
- Reactive squamous cells
- Adenocarcinoma
- Bronchioalveolar macrophages
- Squamous cell carcinoma
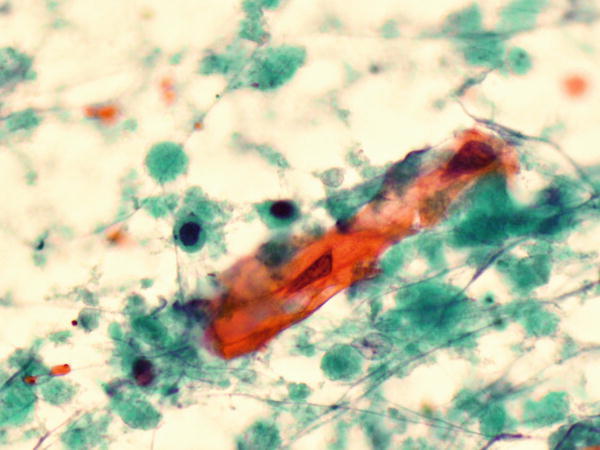 Fig. 1.81
Fig. 1.81 - Q-81. What is the diagnosis of this sputum specimen?
- Reactive squamous cells
- Squamous cell carcinoma
- Reactive bronchial epithelial cells
- Adenocarcinoma
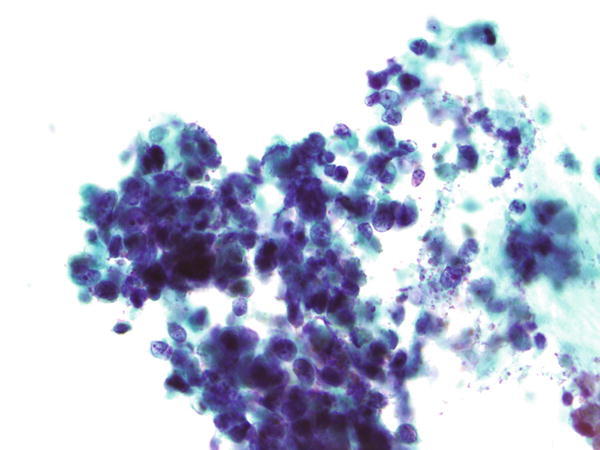 Fig. 1.82
Fig. 1.82 - Q-82. What is the diagnosis of this specimen?
- Reserve cells
- Poorly differentiated adenocarcinoma
- Reactive lymphocytes
- Small cell carcinoma
Text-Based Questions 83–142
- Q-83. For a sputum sample, including both spontaneous and induced specimens, the presence of which of the following cells and/or material is considered to be an adequate specimen?
- Abundant squamous cells and inflammatory cells
- Alveolar macrophages or Curschmann spirals
- Inflammatory cells and bacterial colonies
- Abundant squamous cells and neutrophils
- Q-84. Which pair of technique and adequacy criteria is correct?
- Bronchoscopic brushing and washing—well preserved, properly fixed, and stained respiratory epithelium
- Bronchoscopic fine-needle aspiration (FNA)—representative cells/material of the lesion
- Bronchoalveolar lavage (BAL)—abundant alveolar macrophages
- All of the above
- Q-85. Which type of fine-needle aspirate (FNA) is more often used for staging of lung cancers?
- CT-guided transthoracic
- Ultrasound-guided transcutaneous
- Endoscopic transesophageal FNA with or without ultrasound guidance
- Endoscopic transbronchial FNA with or without ultrasound guidance
- Q-86. Which of following lung cancer patients is more likely to harbor the ALK gene rearrangement?
- A 55-year-old female never smoker
- A 70-year-old male heavy smoker
- A 30-year-old male HIV-positive patient
- A 60-year-old male lung transplant recipient
- Q-87. Which one of the following is the most useful feature for separating reactive bronchial epithelium (reactive atypia) from a well-differentiated lung adenocarcinoma?
- Two- or three-dimensional clusters of bronchial epithelium with nuclear variations
- Multinucleated bronchial epithelium
- Normal N/C ratio
- Clusters of ciliated bronchial epithelium (Creola bodies)
- Q-88. The presence of cartilage on a transbronchial fine-needle aspiration biopsy is diagnostic of lung hamartoma, true or false?
- True
- False
- Q-89. Radiation- and chemotherapy-induced bronchial epithelium changes include all of the following, EXCEPT:
- Cytomegaly with proportionate nuclear enlargement
- Multinucleation and a vesicular chromatin pattern
- Prominent nucleoli
- Hyperchromatic nucleoli and clumped chromatin
- Q-90. Currently EGFR and KRAS mutations are routinely tested in non-small cell lung cancer patients. In addition to adequate tumor cells in the specimen, the following specimens can be used for mutational tests, EXCEPT:
- A small transbronchial FNA biopsy specimen of a large lung mass
- A biopsy specimen of a lymph node with metastatic lung adenocarcinoma
- A surgical resection specimen of lung adenocarcinoma
- A biopsy specimen of a bone lesion with metastatic lung adenocarcinoma
- Q-91. In sputum and bronchial washing specimens, the finding of which type of cells are considered to be an adequate specimen?
- Abundant neutrophils and scattered lymphocytes
- Squamous cells and inflammatory cells
- Scattered ciliated bronchial epithelial cells and inflammatory cells
- Abundant alveolar macrophages
- Q-92. A transthoracic CT-guided fine-needle aspiration is performed on a 65-year-old male who has a large right lower lobe lung mass. Sheets of relatively uniform epithelioid cells with well defined cell borders and intercellular “windows” are identified on aspiration smears. What is the most likely diagnosis?
- Benign mesothelial cells
- Well-differentiated adenocarcinoma of the lung
- Benign bronchial epithelium
- Mesothelioma
- Q-93. All of the following features can be seen in a squamous cell carcinoma, EXCEPT:
- Smudgy chromatin
- Dense cytoplasm
- Prominent nucleoli
- Shared cell borders
- Q-94. A 40-year-old male smoker presents with a 2 cm lung mass. During the on-site evaluation, the smear of a transbronchial fine-needle aspiration reveals “necrotic debris.” What is the best next step?
- Tell the pulmonologist to stop the procedure because no cancer is identified.
- Send the specimen for microbiology culture.
- Send the specimen for microbiology culture and biopsy the peripheral area of the lesion.
- Tell the pulmonologist that the lesion is an “infection.”
- Q-95. Reactive and reparative changes of bronchial epithelium include all of the following features, EXCEPT:
- Prominent nucleoli
- Multinucleation
- Variation of nuclei
- Irregular nuclear membrane and hyperchromasia
- Q-96. Which of the following lung tumors is more likely to shed diagnostic cells in sputum?
- A 3 cm adenocarcinoma involving the pleural surface
- A 3 cm squamous cell carcinoma involving the main bronchi
- A 3 cm adenocarcinoma involving the right lower lobe
- A 3 cm squamous cell carcinoma involving the left upper lobe
- Q-97. All the following tumors have been reported to contain “pseudointranuclear inclusions,” EXCEPT:
- Lung adenocarcinomas with lepidic growth pattern (bronchoalveolar carcinoma)
- Melanomas
- Hepatocellular carcinomas
- Renal cell carcinomas
- Q-98. Cytological features of so-called neuroendocrine tumors may include all of these features, EXCEPT:
- Fine (salt-and-pepper) chromatin pattern
- Nucleoli
- Scant to moderate cytoplasm
- Nuclear grooves
- Q-99. A 60-year-old male patient presents with mediastinal lymphadenopathy. Endoscopic transbronchial fine-needle aspiration (FNA) of a station 7 lymph node was performed. Which of the following features is considered an “abnormal” lymph node?
- Polymorphous population of lymphocytes
- Monomorphous population of lymphocytes
- Tingible body macrophages
- Lymphoglandular bodies
- Q-100. Which of the following tumors is most likely to be mistaken for “reactive atypia” on transbronchial fine-needle aspirations?
- Adenocarcinoma with lepidic growth pattern (bronchoalveolar carcinoma)
- Mucinous adenocarcinoma
- Basaloid squamous cell carcinoma
- Small cell carcinoma
- Q-101. In the sputum, the finding of birefringent calcium oxalate crystals is suggestive of which of the following diagnoses?
- Papillary adenocarcinoma
- Adenocarcinoma with lepidic growth pattern (bronchoalveolar carcinoma)
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
- Aspergillosis
- Q-102. A mediastinal mass was biopsied. The main differentiation diagnosis of the mass is lymphoma verse thymoma. Of the following tests/markers, the single most important diagnostic test/marker is:
- Immunostains of T and B cells
- Flow cytometry for lymphoma markers
- Immunostains of CD45 and CD5
- Immunostains of cytokeratin
- Q-103. Which one of the following bronchoscopic specimens has the lowest sensitivity in the diagnosis of malignancy?
- Bronchoscopic brushing
- Bronchoscopic washing
- Bronchoalveolar lavage
- Transbronchial fine-needle aspiration
- Q-104. The contraindications of percutaneous ultrasound-guided fine-needle aspiration for lung lesions are all the following, EXCEPT:
- Uncontrollable cough
- Severe pulmonary hypertension
- Suspected echinococcal cyst
- Bilateral lung infiltrations
- Q-105. Which of the following findings is not the cytological feature of bronchial reserve cell hyperplasia?
- Mitosis and necrosis
- Tightly packed small-sized cells
- Dark chromatin and scant cytoplasm
- Nuclear molding
- Q-106. Which of the features is NOT seen in benign degenerated squamous cells?
- Smudgy nuclei
- Nuclei with marked variation in size and shape
- Pyknotic nuclei
- Cytokeratin formation
- Q-107. Most squamous cell carcinomas can be distinguished from adenocarcinomas of the lung based on the presence of the following features, EXCEPT:
- Keratinization
- Mucin production (cytoplasmic mucin)
- Pseudoacinar arrangement of tumor cells
- Prominent nucleoli
- Q-108. Cytological preparations from a mucinous adenocarcinoma showed uniform cells with pale nuclei, vesicular chromatin, inconspicuous nucleoli, and foamy cytoplasm, which of the following findings is the most useful feature and can help you to make the diagnosis of adenocarcinoma?
- Nuclear pseudoinclusion
- Multinucleated giant cells
- Three-dimensional cluster of cells
- Clusters of bronchial epithelial cells without cilia
- Q-109. In a well-differentiated adenocarcinoma, tumor cells can be arranged in a variety of architectural patterns, such as two- and three-dimensional clusters, flat sheets, acinar and papillary arrangements.
- True
- False
- Q-110. A bronchoalveolar lavage was performed on a 65-year-old male smoker who presented with an opaque right upper lobe infiltration. On the cytological preparations, large sheets and tight clusters of “signet ring-like” cells are identified. What is the differential diagnosis?
- Adenocarcinoma of the lung
- Goblet cell hyperplasia
- A metastatic adenocarcinoma from stomach
- All of the above
- Q-111. The pulmonary primary epithelioid hemangioendothelioma (EHE) and epithelioid angiosarcoma (EAS) are positive for vascular markers CD31 and CD34, but negative for cytokeratin, true or false?
- True
- False
- Q-112. A transbronchial fine-needle aspiration smear reveal sheets and two-dimensional clusters of spindle cells, the differential diagnosis of the lesion include all of the following, EXCEPT:
- Spindle cell carcinoma
- Leiomyosarcoma
- Spindle cell carcinoid
- Poorly differentiated adenocarcinoma
- Q-113. The presence of numerous small- to medium-sized lymphocytes in a BAL specimen from the patient with autoimmune disease is a suspicious feature of a mucosa-associated lymphoid tissue (MALT) lymphoma. What is the best next step of actions?
- Perform immunostain of B-cell markers
- Perform flow cytometry
- Perform immunostain of T-cell markers
- Perform immunostain of cytokeratin
- Q-114. A transbronchial fine needle biopsy reveals scattered small but well formed non-necrotizing granulomas with little surrounding inflammation. Special stains for microorganisms are all negative. The diagnosis of sarcoidosis is made. Sarcoidosis is generally a self-limiting process, and most of the patients do not progress to pulmonary fibrosis, true or false?
- True
- False
- Q-115. A CT scan from an asymptomatic 50-year-old smoker revealed bilateral ground glass opaque lesions. A BAL specimen from the patient showed numerous alveolar macrophages and eosinophilic acellular material, the most likely diagnosis is:
- Pneumocystis jiroveci (carinii) pneumonia (PCP)
- Alveolar proteinosis
- Amyloid
- Aspergillosis
- Q-116. Which panel of immune markers is most useful for the differential diagnosis of mesothelioma and lung adenocarcinoma?
- Calretinin, CK7, TTF, BerEP4, and CEA
- Desmin, D2-40, WT1, CK7, TTF, BerEP4, and CEA
- Calretinin, p53, WT1, TTF, BerEP4, CEA, and CD15
- P63, p53, WT1, TTF, BerEP4, CEA, and CD15
- Q-117. A 50-year-old female developed a chronic cough, chest x-ray showed bilateral small opaque infiltrates. The on-site evaluation of the transbronchial fine-needle aspiration of the lesion revealed scattered clusters of epithelioid histiocytes. What is the next step of action?
- Microbiology culture and core biopsy of the lesion
- Microbiology culture only
- Core biopsy the lesion
- Do nothing
- Q-118. A HIV-positive patient was clinically suspicious for Pneumocystis jiroveci (carinii) pneumonia (PCP) of the lung. A BAL was performed. What is the best next step of actions?
- Make cell block and perform silver stain
- Make cell block and perform PAS stain
- Make cell block and perform serial H&E stains
- Make cell block only
- Q-119. On a FNA specimen, which one of the following features is commonly seen in a metastatic breast carcinoma to the lung?
- Monotonous appearance of tumor cells
- Tight three-dimensional clusters and/or acini arrangements of tumor cells
- Frequently intracytoplasmic lumen
- All of the above
- Q-120. A primary lung adenocarcinoma intestinal subtype can be reliably differentiated from a metastatic colonic adenocarcinoma to the lung with immunostaining of CK20 and CDX2, because the primary lung adenocarcinoma intestinal subtype is negative for both CK20 and CDX2, true or false?
- True
- False
- Q-121. A transbronchial fine-needle aspiration of a lung mass from a patient with multiple lung nodules was performed and found to be an adenocarcinoma. Tumor cells revealed a distinctively tall columnar “picket fence” appearance, hyperchromatic nuclei, and “dirty” necrosis. Which one is the most likely primary site of the tumor?
- Breast
- Liver
- Colon
- Uterus
- Q-122. Which option is the best one for the diagnosis of a metastatic melanoma involving a hilar lymph node of the lung?
- Using unstained slides to perform IHC markers S100, HMB45, and Melanin A
- Using unstained slides to perform IHC markers S100, HMB45, and Melanin A with red chromogenics rather than the one with brown chromogenics
- Using unstained slides to perform IHC marker Melanin A only
- Using unstained slides to perform IHC marker S100 only
- Q-123. All of following cytological features can be seen in a small cell carcinoma of the lung, EXCEPT:
- The size of tumor cells are two- to threefold of mature lymphocytes
- Paranuclear blue bodies in the cytoplasm
- Nuclear crowding and molding
- Prominent nucleoli
- Q-124. Which of the following is NOT associated with a small cell carcinoma?
- Lambert–Eaton syndrome
- Cushing’s syndrome
- Hyponatremia
- Pancoast tumor
- Q-125. Which of the following statements is CORRECT?
- Atypical carcinoid can be easily separated from typical carcinoid
- Atypical carcinoid shares many cytological features with typical carcinoid
- Necrosis can be easily identified in an atypical carcinoid
- Increased mitotic figures is the only cytological criterion for diagnosing of atypical carcinoids
- Q-126. Which of the following features can be used to separate typical carcinoids from atypical carcinoids and small cell carcinomas?
- The prominent nucleoli
- Fine (salt-and-pepper) chromatin pattern
- The lower mitotic rate and the absence of necrosis
- Organoid and/or pseudorosette arrangement of tumor cells
- Q-127. Which of the following cytological features is the most helpful one in the differential diagnosis of large cell neuroendocrine tumor?
- Fine (salt-and-pepper) chromatin pattern
- Organoid and/or pseudorosettes arrangement of tumor cells
- The mitotic rate and the focal necrosis
- Carcinoid tumor-like nuclei with markedly atypia and enlargement
- Q-128. Which of the following statements of pulmonary hamartoma is CORRECT?
- Involving the HMGI(Y) gene on chromosome 6p21
- The presence of cartilage on the smear is diagnostic
- It often involves several members from the same family
- It is a infiltrating tumor
- Q-129. In lung transplant patients, bronchoalveolar lavage (BAL) is routinely performed to monitor a patient’s condition. Which statement is INCORRECT?
- Neutrophils are normally seen within the first 3 months after transplantation.
- Increased number of macrophages can be seen for years after transplantation.
- The most common fungal infection is Candida.
- Neutrophils can be seen in 1 year after transplantation.
- Q-130. Anaplastic lymphoma kinase (ALK) gene rearrangement has been detected in a subset tumor of lung adenocarcinomas. ALK gene rearrangement has also been detected in which of the following tumors?
- Leiomyosarcoma
- Inflammatory myofibroblastic tumor (IMT)
- Rhabdomyosarcoma
- Desmoplastic small blue cell tumors
- Q-131. In lung adenocarcinomas, both EGFR and KRAS mutations can occur in the same tumor, true or false?
- True
- False
- Q-132. Which of the following statements is NOT correct?
- Mutation and overexpression of the EGFR gene lead to cancer cell overgrowth, but not tumor progression.
- Mutations of the EGFR gene lead to cancer cell overgrowth and tumor progression.
- Mutations of the EGFR gene stimulate tyrosine kinase activity.
- Erlotinib and gefitinib are tyrosine kinase inhibitors (TKIs) that target the EGFR signaling pathway.
- Q-133. Which of the following statements is NOT correct?
- The KRAS protein stimulates downstream activity of EGFR tyrosine kinase.
- KRAS mutations lead to a constitutive activation of RAS signaling pathway.
- EGFR tyrosine kinase inhibitors (TKIs) cannot block the activity of mutated KRAS proteins.
- KRAS mutations are more likely to be found in non-smokers.
- Q-134. Rearrangements of the gene encoding anaplastic lymphoma kinase (ALK gene rearrangements) have been found in a subset of lung adenocarcinomas. Which of the following statements is CORRECT?
- The most common ALK rearrangement in non-small cell lung carcinoma is EML4-ALK fusion.
- EML4-ALK rearrangements are found in tumor with KRAS mutations.
- EML4-ALK rearrangements are found in tumor with EGFR mutations.
- EML4-ALK rearrangements are more common found in adenocarcinomas of heavy smokers.
- Q-135. In a transthoracic fine-needle aspiration (FNA) of a lung lesion, what is the most important cytological feature to separate reactive methothelial cells from a well-differentiated adenocarcinoma?
- Sheets of intermediate-sized epithelioid cells
- Prominent nucleoli
- Intercellular “windows”
- Three-dimensional clusters of hyperchromatic cells
- Q-136. Which one of the following cytological findings is the most useful feature in the separation of reactive squamous cell atypia from squamous cell carcinoma?
- Prominent nucleoli
- Significant variation of nuclear size and shape
- Dispersed individual cells
- Necrotic debris
- Q-137. In a percutaneous fine-needle aspiration (FNA) of the lung lesion, the presence of mesothelial cells, muscle cells, and adipose tissue is a common finding, true or false?
- True
- False
- Q-138. Numerous benign appearing liver cells were found on a transthoracic fine-needle aspiration (FNA) specimen from a patient with a right lower lobe lung mass. No other type of cells was identified. The differential diagnosis of this FNA includes all of the followings, EXCEPT:
- Benign liver cells
- Small cell carcinoma
- Adenocarcinoma
- Hepatocellular carcinoma
- Q-139. Which of the following crystals/structures is commonly seen in a BAL specimen from an asthma patient?
- Ferruginous bodies
- Psammoma bodies
- Charcot–Leyden crystals
- Corpora amylacea
- Q-140. Which of the following statements regarding EGFR mutations is CORRECT?
- EGFR mutations are often found in adenocarcinoma with micropapillary and/or the lepidic growth pattern.
- EGFR mutations are not found in Asian patients.
- Tumors with EGFR mutation have a poor response to EGFR tyrosine kinase inhibitor therapy.
- EGFR mutation can be detected in a tumor with KRAS mutations.
- Q-141. KRAS mutations are found in non-small cell lung carcinomas. Which of the following statements is NOT correct?
- KRAS mutations are less commonly seen among Asian descents.
- KRAS mutations are more commonly seen among non-smokers.
- Patients with KRAS mutations seem to have a poorer prognosis.
- Patients with KRAS mutations seem to be resistant to EGFR tyrosine kinase inhibitor therapy.
- Q-142. According to the laboratory regulation policy of the Health Care Financing Administration (HCFA), the following items must be included on the requisition form for a BAL specimen, EXCEPT:
- Date and time of the sample collected
- Date of the sample received in the laboratory
- Time of the sample received in the laboratory
- The color and volume of the specimen
Answers and Discussion of Image-Based Questions 1–82
-
A-1. (d) Carcinoid
In carcinoids, tumor cells are arranged in acini and pseudorosette structures, and dispersed individual cells with monomorphic appearance. Tumor cells reveal vesicular nuclei, fine (salt-and-pepper) chromatin, and inconspicuous or small nucleoli. No obvious mitotic activity or tumor necrosis. Although mitotic account plays an important role in the classification, it is not always seen on cytological smears. Therefore, careful evaluation of cytomorphology on slides and immunostain of Ki67 on cell block section may be necessary for an accurate classification. In lymphomas, tumor cells are discohesive and have coarse chromatin, prominent nucleoli and irregular nuclear membranes, and lymphoglandular bodies.
-
A-2. (a) Pneumocystis jiroveci (formerly called carinii) pneumonia (PCP)
The cytological features of PCP on the BAL specimen include predominantly foamy macrophages, amorphous material, and rare inflammatory cells. In foamy macrophages and amorphous material, numerous casts (rounded masses of organisms), staining purple color with Diff-Quik and blue color with Papanicolaou stain, are identified. The spherical or cup-shaped organisms are 4–8 μm in diameter and slightly smaller than RBC. On careful view, ill-defined central or eccentric dots are present and best seen with silver stains such as the Gomori methenamine silver stain, which are revealed black in color. PCP infection usually involves immunocompromised patients. Alveolar proteinosis reveals coarsely granular eosinophilic to cyanophilic debris; they are positive for periodic acid–Schiff stain and negative for sliver stain.
-
A-3. (b) Strongyloidiasis
The infection involves in immunocompromised patients such as HIV infection, transplantation, and chemotherapy; and caused by the nematode Strongyloides strecoralis, which is usually identified in the form of long round worms that are 200–400 μm in length. Infection of the lung is due to the hematogenous migration of the infective larva from the GI tract or skin. The organism can be found in sputum and/or BAL specimens. The strongyloidiasis is differentiated from other hookworms by its short buccal cavity and notched tail.
-
A-4. (c) Cryptococcosis
Cryptococcosis is caused by infection of Cryptococcus neoformans, the inhabitants in the soil with bird droppings. The infection occurs in both immunocompromised and immunocompetent patients worldwide and may involve many body sites; however, lung involvement is more common. In a BAL specimen, the fungus reveals as yeast forms with narrow and pinched buddings, refractile centers, and mucoid capsules (clear zone around the stained organisms). The capsule is positive with periodic acid–Schiff, alcian blue, and mucicarmine stains. In histoplasmosis, numerous small budding yeasts are seen in the cytoplasm of histiocytes. The infection is most commonly seen in the Ohio and Mississippi river valleys. In blastomycosis, specimen reveals large broad-based budding yeasts and thick cell walls. The yeast is bigger than Cryptococcus.
-
A-5. (b) Carcinoid
Typical carcinoid tumors are usually centrally located and may appear as an exophytic endobronchial lesion. In the peripheral location, they may have a spindle cell morphology. The cytopathological features include loosely cohesive clusters with rosette formation or single plasmacytoid cells with uniform nuclei containing “salt-and-pepper” stippled chromatin. In some cases, atypical carcinoid tumors show more mitoses, a higher proliferation index, and associated necrosis than in typical carcinoids, but do not reach the level of pleomorphism seen with small cell carcinoma. The cytological features include findings similar to carcinoid, but with more mitoses than carcinoid and some necrosis. In general, the cells tend to have more cytoplasm and less nuclear atypia than small cell carcinoma. In lymphomas, tumor cells are discohesive and have coarse chromatin, prominent nucleoli and irregular nuclear membranes, and lymphoglandular bodies.
-
A-6. (b) Mucormycosis (zygomycosis)
Pulmonary mucormycosis (zygomycosis) rarely occurs in immunocompetent hosts. The risk factors include diabetes mellitus, neutropenia, sustained immunosuppressive and chronic prednisone therapies, iron chelation therapy, broad-spectrum antibiotic use, severe malnutrition, and primary breakdown in the integrity of the cutaneous barrier such as trauma, surgical wounds, needle sticks, or burns. This invasive fungal infection is caused by mycelia-forming fungi of the Mucorales (e.g., Rhizopus, Mucor spp.) and Entomophthorales (e.g., Conidiobolus and Basidiobolus spp.). In the sputum specimen, it reveals terminal chlamydoconidia and hyphae. Terminal chlamydoconidia are thick-walled spherical structures (Pap stain, intermediate magnification on the left, and high magnification on the right upper images), whereas, hyphae are characterized by irregularly shaped, broad ribbon-like, non-septated hyphae with right-angle branching (Pap stain, high magnification on the right lower image). In aspergillosis, hyphae form fungi are seen, characterized by the presence of true septate and relatively straight branching. The calcium oxalate crystal is also associated with aspergillosis. In blastomycosis, it reveals large round yeast (5–15 μm), characterized by double-contoured thick cell walls and broad-based budding. In parasite infections, the size of Paragonimus eggs is much larger than that of fungi, and reveals a thick, double contour shell with an operculated end.
-
A-7. (d) Herpes simplex virus (HSV) infection
HSV infection commonly occurs in neonates and immunocompromised patients. Clinical manifestations of HSV infection of the lung include necrotizing and/or diffuse interstitial pneumonia. On BAL specimen, it reveals a cellular specimen containing large atypical cells (metaplastic squamous cells) with intranuclear inclusions (cytopathic virus effects). Two forms of characteristic inclusions may be seen, including Cowdry type A inclusions (distinct eosinophilic intranuclear inclusions surrounded by a clear halo due to margination of chromatin material) and Cowdry type B inclusions (eosinophilic ground glass “smudge nuclei” with margination of the chromatin material) (shown in the image). These inclusions can be found within metaplastic squamous cells when the inflammation is centered around airways or in multinucleated giant cells within necroinflammatory debris in the interstitial form of disease. Virus-infected cells may also display prominent nuclear molding. The background of the slide may reveal acute inflammatory cells and necrotic debris. In other conditions, cells may reveal multinucleation and prominent nucleoli; however, no characteristic virus cytopathic inclusion is present. In difficult case such as scant specimen with rare atypical cells, immunostain of HSV may help to confirm the diagnosis of HSV infection.
-
A-8. (a) Pneumocystis jiroveci (carinii) pneumonia
Pneumocystis jiroveci (carinii) pneumonia (PCP) infection usually involves immunocompromised patients, such as HIV infection, organ transplantation, chemotherapy, and cancer patients. The infection typically involves the distal airspaces and is associated with a foamy or frothy exudate. The cytological findings of PCP on the BAL specimen include numerous alveolar macrophages and amorphous/foamy material. Cup-shaped microorganisms (cysts) can be seen in the cytoplasm of alveolar macrophages and in the extracellular amorphous/foamy material by both Diff-Quik and Papanicolaou stains, but organisms are stained poorly or not at all with Papanicolaou method. In silver stains, such as Grocott’s methenamine silver (GMS) stain, the cell wall of the organisms stains with black color. The cysts tend to aggregate together, and should not be confused with Histoplasma or Cryptococcus which typically do not aggregate. Alveolar proteinosis reveals amorphous material without the finding of cup-shaped cysts. The cell block preparation and silver stain may help to identify the cup-shaped microorganisms and confirm the diagnosis of PCP. Other useful ancillary tests include immunocytochemistry using a specific Pneumocystis immunostain and polymerase chain reaction (PCR).
-
A-9. (c) Histoplasmosis
Histoplasmosis is caused by the inhalation of infective spores (microconidia), dimorphic fungus Histoplasma capsulatum, which primarily affect alveolar macrophages. Pulmonary histoplasmosis can present clinically with pneumonia, lung nodule, cavitary lung disease, mediastinal or hilar lymphadenopathy. It is not uncommon for localized infections to mimic cancer. On the cytological preparation, slides reveal numerous intracellular yeasts measuring 3–5 μm, with narrow-based budding, within alveolar macrophages. When cells are disrupted, they may also be seen in the extracellular location. Special stains (GMS and PAS stains) are positive for intra-and extracellular yeasts. In PCP, cytological preparations reveal numerous cup-shaped microorganisms (cysts) in the cytoplasm of alveolar macrophages and in the extracellular amorphous/foamy material. In reactive bronchial epithelium and adenocarcinoma, atypical bronchial cells with enlarged nuclei and prominent nucleoli are usually seen. In difficult cases, fungal culture and antigen detection (enzyme immunoassay using urine, blood, or bronchoalveolar lavage fluid) may confirm the diagnosis.
-
A-10. (a) Echinococcosis (Hydatid disease)
Echinococcosis (Hydatid disease) is the most common cestode (tapeworm species) infection to the lung and is caused by the larvae of Echinococcus granulosus. The infection has a worldwide distribution including South America, the Mediterranean and Middle East countries, Russia, and China. Dogs are the definitive host for E. granulosus and harbor the adult worms in their gut. The eggs shed in dog feces and contaminate food sources. After ingestion of contaminated food, the eggs travel to the liver or lungs and slowly develop into hydatid cysts over a period of several months or years. Although most cysts form in the liver, 20–30 % form in the lung. Occasionally, lung cysts form after transdiaphragmatic spread of parasites following the rupture of liver cysts. Patients may be asymptomatic for many years. FNA of the lesion usually contains hydatid sand with hooklets and detached (free) refractile hooklets (resembling shark’s teeth) and the thick granular material. Cell blocks may contain portions of a cyst wall that consists of three layers: (1) host layer with giant cells, fibroblasts, and eosinophils; (2) middle acellular laminated membrane; and (3) inner germinal layer. A primary hydatid cyst of the chest may mimic a neoplasm. However, it is controversial whether suspected hydatid cysts should be aspirated as fluid leakage could result in anaphylaxis or disseminated disease. In strongyloidiasis, it reveals Strongyloides strecoralis, a long round-worm measuring 200–400 μm in length.
-
A-11. (c) Aspergillosis
Aspergillosis is caused by the inhalation of airborne conidial forms (fruiting body) of fungus Aspergillus. Infections mainly affect individuals with underlying lung disease such as cystic fibrosis and/or immunocompromised patients such as transplant and AIDS patients. There are four types of lung disease caused by Aspergillus: (1) allergic bronchopulmonary aspergillosis, (2) aspergilloma (fungus ball or mycetoma), (3) chronic necrotizing pneumonia, and (4) invasive pulmonary aspergillosis, which may cause lung infarction and dissemination to other organs. On cytological preparations, usually only the hyphal form is seen, characterized by septated hyphae with relatively straight walls and 45° (dichotomous) branching. Conidial forms (fruiting bodies) may be seen when the organism is exposed to air (e.g., abscess cavity or involvement of large airways). Calcium oxalate crystals, which are strongly birefringent under polarized light, may be seen and are highly suggestive of aspergillosis. In mucormycosis (zygomycosis), it reveals terminal chlamydoconidia and hyphae. Terminal chlamydoconidia are thick-walled spherical structures (Pap stain, intermediate magnification on the left, and high magnification on the right upper images), whereas, hyphae are characterized by irregularly shaped, broad ribbon-like, non-septated hyphae with right-angle branching. In blastomycosis, it reveals large round yeast (5–15 μm), characterized by double-contoured thick cell walls and broad-based budding. In candidiasis, it reveals pseudohyphae (elongated yeast joined together) and budding yeasts. Several clinical tests may be used to confirm the diagnosis of aspergillosis, such as immunocytochemistry using an immunostain specific to the Aspergillus genus (but not species-specific), aspergillosis antibody test, and fungal culture.
-
A-12. (b) Cytomegalovirus (CMV) infection
CMV is one of the most common causes of opportunistic infections involving the respiratory tract. In the respiratory tract, CMV mainly targets pulmonary macrophages, endothelial cells, and fibroblasts, but virtually any cell can be infected by the virus. CMV pneumonia is frequently seen in recipients of organ transplants, patients with HIV/AIDS, or elderly patients. The diagnostic features of CMV infection include cytomegaly, large amphophilic intranuclear inclusions with perinuclear halos and chromatin margination (“owl eye” inclusion), and small basophilic cytoplasmic inclusions. The number of cells showing cytopathic changes varies with the severity of infection or as a result of patients receiving prophylactic antiviral therapy. In HSV infection, it reveals intranuclear inclusions, including Cowdry type A inclusions (distinct eosinophilic intranuclear inclusions surrounded by a clear halo due to margination of chromatin material) and Cowdry type B inclusions (eosinophilic ground glass “smudge nuclei” with margination of the chromatin material). In adenocarcinomas, tumor cells form tight three-dimensional clusters with hyperchromatic nuclei, prominent nucleoli, irregular nuclear membrane, and high nucleus:cytoplasm (N:C) ratio. In squamous cell metaplasia, cell reveals normal in size with dark nuclei and dense cytoplasm (cytokeratin formation); no intranuclear inclusion is seen. Most cases of the CMV infection do not need ancillary studies to confirm the diagnosis. In difficult cases, immunocytochemistry or in situ hybridization for CMV, molecular testing (PCR), and viral culture may be performed to confirm the diagnosis.
-
A-13. (c) Entamoeba gingivalis infection
E. gingivalis is a parasitic protozoan of the oral cavity. In patients with poor oral hygiene, aspiration can result in a lung infection. The morphological appearances of E. gingivalis and E. histolytica (causing amebic enteritis and liver abscess) are quite similar, although the trophozoites of E. gingivalis tend to be comparably larger (10–35 vs. 15–20 μm) and, unlike with E. histolytica, there is no associated cyst stage. E. gingivalis is also the only species of amebae that can phagocytose white and red blood cells as well as ingest bacteria. Amebae have a histiocyte-like morphology and typically contain ingested RBCs in their cytoplasm. Trophozoites are, however, slightly larger than macrophages and have smaller nuclei with coarser chromatin than histiocytes. In the Echinococcosis (Hydatid disease), it reveals hydatid sand with hooklets and detached (free) refractile hooklets (resembling shark’s teeth) and the thick granular material. In Paragonimus eggs, the size of Paragonimus eggs is much larger than that of E. gingivalis, and it has a thick, double contour shell with an operculated end.
-
A-14. (c) Histoplasmosis
Histoplasmosis is caused by the inhalation of infective spores (microconidia), dimorphic fungus Histoplasma capsulatum, which primarily affect alveolar macrophages. Pulmonary histoplasmosis can present clinically with pneumonia, lung nodule, cavitary lung disease, mediastinal or hilar lymphadenopathy. It is not uncommon for localized infections to mimic cancer. On the cytological preparation, slides reveal numerous intracellular yeasts measuring 3–5 μm, with narrow-based budding, within alveolar macrophages. When cells are disrupted, they may also be seen in the extracellular location. Special stains (GMS and PAS stains) are positive for intra- and extracellular yeasts. In PCP, cytological preparations reveal numerous cup-shaped microorganisms (cysts) in the cytoplasm of alveolar macrophages and in the extracellular amorphous/foamy material. In reactive bronchial epithelium and adenocarcinoma, atypical bronchial cells with enlarged nuclei and prominent nucleoli are usually seen. In difficult cases, fungal culture and antigen detection (enzyme immunoassay using urine, blood, or bronchoalveolar lavage fluid) may confirm the diagnosis.
-
A-15. (b) Coccidioidomycosis
Coccidioidomycosis is caused by the inhalation of arthroconidia of Coccidioides immitis or Coccidioides posadasii. The infection is endemic in Arizona, California, Nevada, New Mexico, Texas, Utah, and northern Mexico. Most people are asymptomatic following initial respiratory exposure to arthroconidia. Those who become ill typically develop respiratory symptoms, such as cough, fever, and pneumonia. C. immitis infection typically causes a necrotizing granulomatous inflammation, pulmonary nodules, cavities, diffuse reticulonodular pneumonia, and rarely pleural disease. Fungemia can also produce multiple septic pulmonary emboli, especially in immunocompromised patients. Cytological preparations reveal thick walled spherules (measuring 10–80 μm) that contain endospores (measuring 2–5 μm). There are generally very few spherules present in most specimens. Sometimes the spherules may be collapsed and appear as empty structures, surrounded by scattered endospores all over the slide. Wet preparation of fresh samples using saline or potassium hydroxide solution can be utilized to demonstrate spherules. Calcofluor staining is positive. In histoplasmosis, it reveals numerous intracellular yeasts measuring 3–5 μm with narrow-based budding in alveolar macrophages. In cryptococcosis, it reveals round- to oval-shaped yeast (measuring 5–20 μm) with narrow-based budding. Yeasts may resemble Pneumocystis cysts, but tend to be more variable and often larger in size. Encapsulated cryptococcal organisms are surrounded by a thick capsule which is positive with mucicarmine, alcian blue, and colloidal iron stains. In PCP, cytological preparations reveal numerous cup-shaped microorganisms (cysts) in the cytoplasm of alveolar macrophages and in the extracellular amorphous/foamy material.
-
A-16. (a) Cryptococcosis
Humans are infected by Cryptococcus neoformans and Cryptococcus gattii due to the inhalation of basidiospores or yeast. The course and clinical presentation of the disease depends on whether yeast are encapsulated (encapsulated yeast may cause a granulomatous reaction) and the patient’s immune status. Invasive cryptococcosis has become increasingly common among HIV-positive and transplant patients. Clinical manifestation of the lung infection varies from asymptomatic airway colonization to a slowly progressive lung mass (cryptococcoma), pneumonia, and acute respiratory distress syndrome (ARDS), to pleural effusion. On slides, it reveals round- to oval-shaped yeast (measuring 5–20 μm) with narrow-based budding. Yeasts may resemble Pneumocystis cysts, but tend to be more variable and often larger in size. Encapsulated cryptococcal organisms are surrounded by a thick capsule which is positive with mucicarmine, alcian blue, and colloidal iron stains. In coccidioidomycosis, it reveals thick-walled spherules (measuring 10–80 μm) that contain endospores (measuring 2–5 μm). In histoplasmosis, it reveals numerous intracellular yeasts measuring 3–5 μm, with narrow-based budding, within alveolar macrophages. In PCP, cytological preparations reveal numerous cup-shaped microorganisms (cysts) in the cytoplasm of alveolar macrophages and in the extracellular amorphous/foamy material.
-
A-17. (c) Tuberculosis
Pulmonary tuberculosis (TB) is caused by the bacterium Mycobacterium tuberculosis. Pulmonary TB may be due to primary or reactivation (chronic) infection. Manifestations of pulmonary TB include bronchopneumonia, caseating pneumonia, nodular disease (tuberculoma), tracheobronchitis, milliary disease, hilar lymphadenopathy, and pleural disease. Individuals at risk for infection are those who are immunosuppressed, the elderly, and infants. The main cytomorphological findings of pulmonary TB are granulomas that show clusters of epithelioid histiocytes with or without lymphocytes, Langhans and/or foreign body-type multinucleated giant cells, with/without a necrotic background. A negative image of extracellular mycobacteria may be notable with Diff-Quik, Giemsa, or other Romanowsky-type stains. The diagnosis of mycobacterial infection can be made on the basis of the identification of microorganisms with acid-fast (AFB) stains. In cytological preparation, mycobacteria of M. tuberculosis may be rare and require careful and lengthy scrutiny of slides. Mycobacteria may be weakly gram-positive and will stain with GMS. Fluorescence microscopy with fluorochrome dyes such as auramine O or auramine–rhodamine are more sensitive and specific than AFB stains. PCR for diagnosis and subclassification can be done on cell block material. Mycobacteria are slow growing and culture can take weeks (6–8 weeks with conventional Lowenstein-Jensen medium and 3 weeks with Middlebrook liquid and solid media). The differential diagnosis includes other AFB-positive microorganisms that cause necrotizing granulomatous inflammation such as Nocardia, Rhodococcus, and Legionella micdadei, and non-infectious causes of granulomatous lung disease such as sarcoidosis.
-
A-18. (d) Toxoplasmosis
Toxoplasma pneumonia is being recognized with increased frequency, especially in immunocompromised patients. Particularly, lung involvement occurs with severe disseminated infection (toxoplasmosis). Respiratory specimens like BAL require close inspection for crescent or banana-shaped free (extracellular) trophozoites, as they measure only around 5–7 μm in length. These banana-shaped extracellular trophozoites have prominent central nucleus with Giemsa stain. The parasites are barely visible with the Pap stain. Alveolar macrophages may be seen containing several parasites. The trophozoites need to be distinguished from similar-sized Histoplasma (which exhibit narrow-neck budding) and Leishmania (which also contain a kinetoplast). In addition to the Giemsa stain, an immunohistochemical stain for Toxoplasma is also available for the confirmation of the diagnosis. In PCP, cytological preparations reveal numerous cup-shaped microorganisms (cysts) in the cytoplasm of alveolar macrophages and in the extracellular amorphous/foamy material.
-
A-19. (c) Co-infection with CMV and PCP
CMV is one of the most common causes of opportunistic infections involving the respiratory tract. As CMV is frequently found in immunocompromised patients, it may be seen together with other opportunistic pathogens infections such as fungi including P. jirovecii. Co-infection with CMV and P. jirovecii is shown in this BAL specimen. The slides reveal characteristic “owl eye” nuclear inclusions in cells. Multinucleation is rare in CMV infection, but can occur. Pneumocystis infection resulted in the cast of frothy material, containing cup-shaped organisms with a central dot in Pap stain. In HSV infection, it reveals intranuclear inclusions, including Cowdry type A inclusions (distinct eosinophilic intranuclear inclusions surrounded by a clear halo due to margination of chromatin material) and Cowdry type B inclusions (eosinophilic ground glass “smudge nuclei” with margination of the chromatin material).
-
A-20. (a) Asthma
The structures in photos are Curschmann spirals and Charcot–Leyden crystals. Curschmann spirals are coiled strands of mucus, and associated with chronic pulmonary disease. Although non-specific findings, they are commonly seen in asthma patients. Charcot–Leyden crystals are needle-shaped orangeophilic color crystal and by-products of eosinophil degranulation. They can be found in asthma patients. In aspergillosis, birefringent calcium oxalate crystals (needle-shaped, polarizable crystals) are common findings, and they may form rosettes or wheat sheaf-like clusters. In PCP, cytological preparations reveal numerous cup-shaped microorganisms (cysts) in the cytoplasm of alveolar macrophages and in the extracellular amorphous/foamy material. In asbestos exposure, ferruginous bodies are usually seen; they are dumbbell-shaped mineral fibers, 5–200 μm in length, golden-yellow to black color with Papanicolaou stain.
-
A-21. (a) Well-differentiated adenocarcinoma
The Papanicolaou stain shows three-dimensional clusters or acinar/papillae arrangement of columnar cells with hyperchromatic nuclei and coarse chromatin, irregular nuclear membranes, “lacy” cytoplasm, and cytoplasmic vacuolization. Tumor cells are large in size and have high N:C ratio, and without cilia. Adjacent to tumor cell clusters, there is a benign reactive bronchial cells with cilia. Creola bodies are three-dimensional clusters of reactive bronchial cells with marked variation of nuclear sizes, and without malignant nuclear features, cells still have cilia. Bronchoalveolar macrophages have abundant cytoplasm and eccentrically located nuclei.
-
A-22. (d) Adenocarcinoma with cytoplasmic mucin
The Papanicolaou stain shows predominantly dispersed individual tumor cells with hyperchromatic nuclei and coarse chromatin, irregular nuclear membranes, “lacy” cytoplasm, and cytoplasmic vacuolization (intracytoplasmic mucin). The differential diagnosis includes primary lung adenocarcinoma verse a metastatic adenocarcinoma with mucin production such as gastric and breast carcinoma. IHC study of TTF1 and Napsin A is necessary to confirm the lung primary. The most important differential diagnosis is bronchoalveolar macrophages. Bronchoalveolar macrophages have abundant vacuolated cytoplasm and eccentrically located nuclei, without nuclear atypia.
-
A-23. (c) Adenocarcinoma
The cell block preparation shows loosely formed two-dimensional clusters of large epithelial cells with hyperchromatic nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, “lacy” cytoplasm, and cytoplasmic vacuolization. In reactive bronchial epithelium, cells reveal marked variation of nuclear sizes, without malignant nuclear features, and cells still have cilia. In goblet cell hyperplasia, it reveals clusters of cells in honeycomb arrangement, with abundant mucin-filled cytoplasm and eccentrically located nuclei; no malignant nuclear features are identified. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia.
-
A-24. (c) Adenocarcinoma
In lung adenocarcinomas, particularly in adenocarcinomas with lepidic growth pattern (formerly bronchioalveolar adenocarcinomas), radiographic studies may reveal bilateral infiltrate of the lung. The cell block preparation shows loosely formed clusters and dispersed large epithelial cells with hyperchromatic nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, “lacy” cytoplasm, and cytoplasmic vacuolization. In goblet cell hyperplasia, it reveals clusters of cells in honeycomb arrangement, with abundant mucin-filled cytoplasm and eccentrically located nuclei; no malignant nuclear features are identified. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia.
-
A-25. (c) Adenocarcinoma
The cell block preparation shows loosely formed clusters and dispersed large epithelial cells with hyperchromatic nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, and “lacy” cytoplasm. In reactive bronchial epithelium, cells reveal marked variation of nuclear sizes, without malignant nuclear features, and cells still have cilia. Bronchoalveolar macrophages reveal clusters of cells with abundant vacuolated cytoplasm and nuclei without malignant nuclear features. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen.
-
A-26. (d) Adenocarcinoma
The Diff-Quik stain shows loosely formed clusters with acini arrangement and dispersed large epithelial cells with hyperchromatic nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, and “lacy” cytoplasm. In reactive bronchial epithelium, cells reveal marked variation of nuclear sizes, without malignant nuclear features, and cells still have cilia. Bronchoalveolar macrophages may be arranged in clusters, and cells reveal abundant vacuolated cytoplasm and nuclei without malignant nuclear features. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen.
-
A-27. (c) Metastatic carcinoma of the breast
The Diff-Quik preparation shows tight three-dimensional clusters of cells with acini arrangements. Cells are intermediate in size and relatively uniform in appearance; they may or may not have pseudointranuclear inclusions (best seen on Papanicolaou stain). Tumor cells reveal hyperchromatic nuclei, coarse chromatin, “lacy” cytoplasm and cytoplasmic vacuolization, and high N/C ratio. In adenocarcinomas of the lung, tumor cells are larger in size, and form clusters and dispersed individual cells with hyperchromatic nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, and “lacy” cytoplasm. IHC stains of breast carcinoma markers (ER, PR, GCDFP, mammaglobin, and GATA3) and lung carcinoma markers (TTF1 and Napsin A) may help with the differential diagnosis.
-
A-28. (c) Metastatic carcinoma of the colon
In metastatic colonic adenocarcinoma, tumor cells are large in size and form three-dimensional cluster and acinar arrangements, with elongated nuclei and tumor necrosis. In adenocarcinoma of the lung, tumor cells are arranged in clusters and dispersed individual cells with hyperchromatic nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, and “lacy” cytoplasm. IHC stains of colon carcinoma markers (CK20 and CDX2) and lung carcinoma markers (CK7, TTF1, and Napsin A) may help with the differential diagnosis.
-
A-29. (d) Small cell carcinoma
The Diff-Quik preparation shows tight clusters of small hyperchromatic cells (two to three times of the size of mature lymphocytes) with scant cytoplasm, fine chromatin (“salt-and-pepper” appearance), nuclear molding and crowding, inconspicuous nucleoli (best seen on Papanicolaou stain), nuclear stripes (broken down nuclear material), mitosis, necrosis, and apoptotic bodies. In reactive lymphocytes, cells are discohesive and have coarse chromatin. Lymphoglandular bodies are present on the slide.
-
A-30. (c) Metastatic carcinoma of the breast
Breast carcinoma is a common tumor which metastases to the lung, due to its anatomic location. Metastatic breast carcinomas have many cytological features that overlap with primary lung adenocarcinomas. In metastatic breast carcinomas, tumor cells are intermediate in size and arranged in tight three-dimensional clusters and/or acini. Tumor cells reveal a monotonous appearance, with hyperchromatic nuclei, prominent nucleoli and frequently with intracytoplasmic lumen. In lung adenocarcinomas, tumor cells may have more pleomorphic appearance and variation in size and shape of nuclei. In difficult cases, immunostains of breast cancer markers (estrogen receptor (ER), progesterone receptor (PR), gross cystic disease fluid protein (GCDFP-15), mammaglobin and GATA-3) and lung markers (TTF1 and napsin A) may help for the differential diagnosis.
-
A-31. (c) Metastatic carcinoma of the colon
In metastatic colonic adenocarcinoma, tumor cells are large in size and form three-dimensional clusters and acinar arrangements, with elongated nuclei and tumor necrosis. In adenocarcinoma of the lung, tumor cells are arranged in clusters and dispersed individual cells with hyperchromatic nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, and “lacy” cytoplasm. IHC stains of colon carcinoma markers (CK20 and CDX2) and lung carcinoma markers (CK7, TTF1, and Napsin A) may help with the differential diagnosis.
-
A-32. (a) Reactive bronchial epithelium
It can be difficult to distinguish reactive bronchial epithelial cells (reactive atypia) from a well differentiated adenocarcinoma. In reactive atypia, bronchial epithelial cells form sheets and/or loose cluster of cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, abundant cytoplasm, and normal N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In a well-differentiated adenocarcinoma, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have round nuclei, vesicular chromatin, small nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters.
-
A-33. (b) Goblet cell hyperplasia
In goblet cell hyperplasia, it reveals clusters and/or honeycomb sheets of cells with abundant mucin-filled cytoplasm and eccentrically located nuclei (“signet ring” appearance); no malignant nuclear features are identified. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia. In adenocarcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters.
-
A-34. (b) Small cell carcinoma
The Papanicolaou preparation shows tight clusters of small hyperchromatic cells (two to three times of the size of mature lymphocytes) with scant cytoplasm, fine chromatin (“salt-and-pepper” appearance), nuclear molding and crowding, inconspicuous nucleoli (best seen on Papanicolaou stain), nuclear stripes (broken down nuclear material), mitosis, necrosis, and apoptotic bodies. In reactive squamous, cells are large in size, discohesive, and have pyknotic nuclei with smudgy chromatin. The cytoplasm is dense with cytokeratin. In adenocarcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli and foamy cytoplasm, and high N:C ratio.
-
A-35. (d) Adenocarcinoma with mucinous features
The Papanicolaou stain shows three-dimensional clusters of malignant hyperchromatic cells with extra and intracytoplasmic mucin. The differential diagnosis includes primary lung adenocarcinoma verse a metastatic adenocarcinoma with mucin production, such as adenocarcinoma of stomach, breast, and colon. IHC study (TTF1 and Napsin A) is necessary to confirm the lung primary. In goblet cell hyperplasia, it reveals clusters and/or honeycomb sheets of cells with abundant mucin-filled cytoplasm and eccentrically located nuclei (“signet ring” appearance); no malignant nuclear features are identified. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia.
-
A-36. (a) Reactive bronchial cells and Candida
In candidiasis, budding yeasts with pseudohyphae are identified. Pseudohyphae are 2–10 μm in diameter and branch at a 90° angle. Candida is also a common finding of oral contamination in sputum. Aspergillosis is characterized by the presence of mucus plugs containing fungal organisms admixed with birefringent calcium oxalate crystals, inflammatory cells, and eosinophils. Aspergillus are characterized by true septate hyphae with 45° angle branching. The uniform thickness (3–6 μm in width) and the septa of hyphae differentiate Aspergillus from other fungus such as Mucor and Candida. Aspergillosis can also cause reactive atypia mimicking of non-small cell lung cancers, particularly a squamous cell carcinoma.
-
A-37. (d) Adenocarcinoma
The Papanicolaou preparation shows three-dimensional clusters of cells with acini arrangement. Tumor cells reveal high N/C ratio, hyperchromatic nuclei, “lacy” cytoplasm, and cytoplasmic vacuolization. Nuclei are large with irregular nuclear membranes, coarse chromatin, and prominent nucleoli. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia.
-
A-38. (d) Adenocarcinoma
The Papanicolaou preparation shows clusters of cells with acini arrangement and dispersed individual tumor cells with elongated nuclei. Tumor cells reveal high N/C ratio, hyperchromatic nuclei, “lacy” cytoplasm, and cytoplasmic vacuolization. Nuclei are large with irregular nuclear membranes, coarse chromatin, and prominent nucleoli. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, and marked variation of nuclear size and shape. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Tumor cells of squamous cell carcinomas also reveal dense cytoplasm with cytokeratin formation.
-
A-39. (d) Poorly differentiated adenocarcinoma
The Papanicolaou preparation shows tightly formed three-dimensional clusters of tumor cells with high N/C ratio, “lacy” cytoplasm, and cytoplasmic vacuolization. There is no obvious acini arrangement as seen in a well-differentiated adenocarcinoma. Nuclei of tumor cells are large with irregular nuclear membranes, coarse chromatin, and multiple prominent nucleoli. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia.
-
A-40. (a) Reactive bronchial epithelial cells
It can be difficult to distinguish reactive bronchial epithelial cells (reactive atypia) from a well-differentiated adenocarcinoma. In reactive atypia, bronchial epithelial cells form sheets and/or cluster of cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, abundant cytoplasm, and normal N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In a well-differentiated adenocarcinoma, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have round nuclei, vesicular chromatin, small nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters.
-
A-41. (a) Reactive bronchial epithelial cells
It can be difficult to distinguish reactive bronchial epithelial cells (reactive atypia) from a well-differentiated adenocarcinoma. In reactive atypia, bronchial epithelial cells form sheets and/or cluster of cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, abundant cytoplasm, and normal N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In a well-differentiated adenocarcinoma, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have round nuclei, vesicular chromatin, small nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters.
-
A-42. (d) Reactive bronchial epithelial cells
It can be difficult to distinguish reactive bronchial epithelial cells (reactive atypia) from a well-differentiated adenocarcinoma. In reactive atypia, bronchial epithelial cells form sheets and/or cluster of cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, abundant cytoplasm, and normal N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In a well-differentiated adenocarcinoma, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have round nuclei, vesicular chromatin, small nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters.
-
A-43. (b) Reactive bronchial epithelial cells
It can be difficult to distinguish reactive bronchial epithelial cells (reactive atypia) from a well-differentiated adenocarcinoma. In reactive atypia, bronchial epithelial cells form sheets and/or cluster of cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, abundant cytoplasm, and normal N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In a well-differentiated adenocarcinoma, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have round nuclei, vesicular chromatin, small nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters.
-
A-44. (d) Adenocarcinoma
The Papanicolaou preparation shows clusters of cells with acini arrangement and dispersed individual tumor cells with elongated nuclei. Tumor cells reveal high N/C ratio, hyperchromatic nuclei, “lacy” cytoplasm, and cytoplasmic vacuolization. Nuclei are large with irregular nuclear membranes, coarse chromatin, and prominent nucleoli. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, and marked variation of nuclear size and shape. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Tumor cells of squamous cell carcinomas also reveal dense cytoplasm with cytokeratin formation. In small cell carcinomas, tumor cells are small and with “salt-and-pepper” chromatin, nuclear crowding and molding.
-
A-45. (c) Goblet cell hyperplasia
In goblet cell hyperplasia, it reveals clusters and/or honeycomb sheets of cells with abundant mucin-filled cytoplasm and eccentrically located nuclei (“signet ring” appearance); no malignant nuclear features are identified. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia. In adenocarcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters. In small cell carcinomas, tumor cells are small and with “salt-and-pepper” chromatin, nuclear crowding and molding.
-
A-46. (a) Small cell carcinoma
The cell block preparation shows tight clusters of small hyperchromatic cells with scant cytoplasm, nuclear molding, and crowding. In small cell carcinomas, tumor cells are relatively small in size and approximately two- to threefold of mature lymphocytes. Tumor cells reveal scant cytoplasm and high N:C ratio, large nuclei with fine chromatin (salt-and-pepper) pattern, nuclear crowding and molding, and paranuclear blue bodies in the cytoplasm. Numerous “blue strips” may be seen on smears due to crush artifacts and the breakdown of nuclei DNA material. The nucleoli are inconspicuous in small cell carcinomas. In some cases, tumor cells may reveal rare nucleoli, but they are small in size. In reactive lymphocytes, cells are discohesive and have coarse chromatin. Lymphoglandular bodies are present on the slide. In adenocarcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, and foamy cytoplasm.
-
A-47. (c) Cryptococcosis
Cryptococcosis is caused by infection of Cryptococcus neoformans, the inhabitants in the soil with bird droppings. The infection occurs in both immunocompromised and immunocompetent patients worldwide and may involve many body sites; however, lung involvement is more common. In a BAL specimen, the fungus reveals as yeast forms with narrow and pinched buddings, refractile centers, and mucoid capsules (clear zone around the stained organisms). The capsule is positive with periodic acid–Schiff, alcian blue, and mucicarmine stains. In histoplasmosis, numerous small budding yeasts are seen in the cytoplasm of histiocytes. The infection is most commonly seen in the Ohio and Mississippi river valleys. In blastomycosis, specimen reveals large broad-based budding yeasts (8–20 μm in diameters) with thick cell walls. The yeast is bigger than Cryptococcus.
-
A-48. (c) Degenerated alveolar macrophages
In BAL specimen, alveolar macrophages are arranged in loose clusters or individual cells with benign nuclear features and foamy cytoplasm. Cells may reveal cytoplasmic pigments (anthracotic pigments in smokers) or vacuoles (in the lipid pneumonia). In degenerative changes, cells may reveal hyperchromatic nuclei and slightly irregular nuclear membrane. In adenocarcinomas and signet ring cell carcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, foamy cytoplasm, and high N:C ratio.
-
A-49. (d) Adenocarcinoma
The Diff-Quik preparation shows clusters of cells with acini arrangement and dispersed individual tumor cells. Tumor cells reveal high N/C ratio, hyperchromatic nuclei, “lacy” cytoplasm, and cytoplasmic vacuolization. Nuclei are large with irregular nuclear membranes, coarse chromatin, and prominent nucleoli. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, and marked variation of nuclear size and shape. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Tumor cells of squamous cell carcinomas also reveal dense cytoplasm with cytokeratin formation. In small cell carcinomas, tumor cells are small and with “salt-and-pepper” chromatin, nuclear crowding and molding.
-
A-50. (b) Adenocarcinoma
The Papanicolaou preparation shows predominantly dispersed individual tumor cells and clusters of cells with acini arrangement. Tumor cells reveal high N/C ratio, hyperchromatic nuclei, “lacy” cytoplasm, and cytoplasmic vacuolization. Nuclei are large with irregular nuclear membranes, coarse chromatin, and prominent nucleoli. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, and marked variation of nuclear size and shape. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Tumor cells of squamous cell carcinomas also reveal dense cytoplasm with cytokeratin formation. In small cell carcinomas, tumor cells are small and with “salt-and-pepper” chromatin, nuclear crowding and molding.
-
A-51. (b) Adenocarcinoma
The Diff-Quik preparation shows predominantly clusters and dispersed individual tumor cells with acini arrangement. Tumor cells reveal high N/C ratio, hyperchromatic nuclei, “lacy” cytoplasm, and cytoplasmic vacuolization. Nuclei are large with irregular nuclear membranes, coarse chromatin, and prominent nucleoli. In reactive bronchial epithelium, cells are arranged in three-dimensional clusters, with marked variation of nuclear sizes, without malignant nuclear features, cells still have cilia. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, and marked variation of nuclear size and shape. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. In a poorly differentiated squamous cell carcinoma, tumor cells may have prominent nucleoli, mimicking adenocarcinomas, however, tumor cells of squamous cell carcinomas also reveal dense cytoplasm with cytokeratin formation, separating them from adenocarcinomas. In small cell carcinomas, tumor cells are small and with “salt-and-pepper” chromatin, nuclear crowding and molding.
-
A-52. (d) Granulomatous inflammation
The Diff-Quik preparation shows clusters of epithelioid histiocytes admixed with inflammatory cells, scattered multinucleated giant cells, and reactive bronchial cells. Granulomatous inflammation may be found in both benign such as sarcoidosis and malignant lesions. Sarcoidosis is an idiopathic disease characterized by non-necrotizing granulomas. It typically involves mediastinal lymph nodes and the lung. However, it can be a systemic process and involve other organs, such as the heart, central nerve system, and skin. The disease usually occurs in middle-aged African-American females. The patient may need steroid therapy. In adenocarcinomas, tumor cells reveal high N/C ratio, hyperchromatic nuclei, “lacy” cytoplasm, and cytoplasmic vacuolization. Nuclei are large with irregular nuclear membranes, coarse chromatin, and prominent nucleoli.
-
A-53. (d) Metastatic synovial sarcoma
The Diff-Quik slide shows predominately dispersed individual tumor cells with pleomorphic, spindle- to oval-shaped nuclei, and scant cytoplasm. The nuclei are hyperchromatic with coarse chromatin and small nucleoli. Mitosis is frequent. Tumor cells are positive for AE1/AE3, EMA, CD99, and vimentin. The main differential diagnosis is poorly differentiated carcinoma. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and cytokeratin. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen, however, tumor cells of squamous cell carcinomas also reveal dense cytoplasm with cytokeratin formation. In a poorly differentiated adenocarcinoma, it reveals predominantly dispersed individual tumor cells and clusters of cells with acini arrangement. Tumor cells reveal high N/C ratio, hyperchromatic nuclei, “lacy” cytoplasm, and cytoplasmic vacuolization.
-
A-54. (d) Bronchioalveolar macrophages
In BAL specimen, alveolar macrophages are arranged in loose clusters or individual cells with benign nuclear features and foamy cytoplasm. Cells may reveal cytoplasmic pigments (anthracotic pigments in smokers) or vacuoles (in the lipid pneumonia). In degenerative changes, cells may reveal hyperchromatic nuclei and slightly irregular nuclear membranes. In adenocarcinomas and signet ring cell carcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, foamy cytoplasm, and high N:C ratio.
-
A-55. (c) Poorly differentiated squamous cell carcinoma
In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and cytoplasmic keratin. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. In a poorly differentiated squamous cell carcinoma, tumor cells may have prominent nucleoli, mimicking adenocarcinoma; however, tumor cells of squamous cell carcinomas also reveal dense cytoplasm with cytokeratin formation, separating them from adenocarcinomas. In adenocarcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, foamy cytoplasm, and high N:C ratio. In small cell carcinomas, tumor cells are small and with “salt-and-pepper” chromatin, nuclear crowding and molding.
-
A-56. (c) Squamous cell carcinoma
Diff-Quik stain shows polygonal, rounded, elongated, or tadpole-shaped cells with large hyperchromatic nuclei and dense cytoplasm. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and cytoplasmic keratin. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. In a poorly differentiated squamous cell carcinoma, tumor cells may have prominent nucleoli, mimicking adenocarcinomas, however, tumor cells of squamous cell carcinomas also reveal dense cytoplasm with cytokeratin formation, separating them from adenocarcinomas. In adenocarcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, foamy cytoplasm, and high N:C ratio. In goblet cells and bronchioalveolar macrophages, cells reveal abundant foamy cytoplasm and cytoplasmic vacuoles, without malignant nuclear features.
-
A-57. (c) Squamous cell carcinoma
Diff-Quik stain shows polygonal, rounded, elongated, or tadpole-shaped cells with large hyperchromatic nuclei and dense cytoplasm. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and cytoplasmic keratin. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. In adenocarcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, foamy cytoplasm, and high N:C ratio. In granulomas, it reveals clusters of epithelioid histiocytes admixed with inflammatory cells, scattered multinucleated giant cells, and reactive bronchial cells.
-
A-58. (c) Squamous cell carcinoma
Diff-Quik stain shows polygonal, rounded, elongated, or tadpole-shaped cells with large hyperchromatic nuclei and dense cytoplasm. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and cytoplasmic keratin. In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. In adenocarcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, foamy cytoplasm, and high N:C ratio. In granulomas, it reveals clusters of epithelioid histiocytes admixed with inflammatory cells, scattered multinucleated giant cells, and reactive bronchial cells. In bronchial reserve cell hyperplasia, cells are arranged in cohesive sheets or clusters with nuclear overlapping and crowding. The nuclei reveal dark smudgy chromatin, inconspicuous nucleoli, smooth nuclear membrane, and scant cytoplasm. The N:C ratio is in normal range or slightly increased.
-
A-59. (d) Degenerated bronchioalveolar macrophages
The slide reveals dispersed intermediate cells with normal N:C ratio, cytoplasmic vacuoles, breakdown of nuclei membranes, and smudgy chromatin. In BAL specimen, alveolar macrophages are arranged in loose clusters or individual cells with benign nuclear features and foamy cytoplasm. Cells may reveal cytoplasmic pigments (anthracotic pigments in smokers) or vacuoles (in the lipid pneumonia). In degenerative changes, cells may reveal hyperchromatic nuclei and slightly irregular nuclear membranes. In adenocarcinomas and signet ring cell carcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, foamy cytoplasm, and high N:C ratio.
-
A-60. (a) Goblet cell
In a bronchial washing and/or BAL specimen, goblet cells are arranged in clusters and/or honeycomb sheets of cells with abundant mucin-filled cytoplasm and eccentrically located nuclei (“signet ring” appearance); no malignant nuclear features are identified. In adenocarcinomas and signet ring cell carcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli and foamy cytoplasm, and high N:C ratio. No cilia are identified in tumor cell clusters. In small cell carcinomas, tumor cells are small and with “salt-and-pepper” chromatin, nuclear crowding and molding.
-
A-61. (d) Goblet cell hyperplasia
In goblet cell hyperplasia, it reveals clusters of cells and sheets of cells in honeycomb arrangement, with abundant mucin-filled cytoplasm and eccentrically located nuclei (“signet ring” appearance). Cells are without nuclear atypia. In adenocarcinomas and signet ring cell carcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli and foamy cytoplasm, and high N:C ratio. No cilia are identified in tumor cell clusters. In small cell carcinomas, tumor cells are small and with “salt-and-pepper” chromatin, nuclear crowding and molding. In granulomas, it reveals clusters of epithelioid histiocytes with elongated and curved nuclei admixed with lymphocytes.
-
A-62. (a) Squamous cells with reactive atypia
The slide reveals discohesive clusters and scattered individual cells with keratinization, small pyknotic nuclei, and normal N:C ratio. Reactive squamous cells have several cytological features overlapping with well-differentiated squamous cell carcinomas. In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of the cell is normal or slightly increased. In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cell carcinomas.
-
A-63. (b) Reactive squamous cells
In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of the cell is normal or slightly increased. In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cell carcinomas.
-
A-64. (a) Non-diagnostic specimen
It is a non-diagnostic specimen, due to the lack of bronchoalveolar macrophages. The criteria for an adequate sputum specimen include the presence of alveolar macrophages and/or Curschmann spirals, which indicate a representative collection of the lower respiratory tract material. Other cells and material in specimens such as squamous cells, neutrophils, and chronic inflammatory may represent oral contaminations. Sometimes, ciliated respiratory epithelial cells can be identified in the sputum, but they do not necessarily represent cells that come from the lower respiratory tract, since ciliated respiratory epithelial cells can be found in the lining of sinonasal passage of the upper respiratory tract.
-
A-65. (c) Karetin debris and it is necessary to repeat the biopsy
This is a non-diagnostic specimen; it predominantly contains keratin debris and no tumor cells seen. Prominent keratin and necrotic debris in the background of the slide is a feature commonly seen in a squamous cell carcinomas, however, the feature can also be seen in benign lesions. Therefore, a repeat biopsy of the viable tumor is necessary to establish the diagnosis of a squamous cell carcinoma.
-
A-66. (d) Reactive bronchial epithelium
The slide reveals three-dimensional clusters and dispersed individual columnar cells. It can be difficult to distinguish reactive bronchial epithelial cells (reactive atypia) from a well-differentiated adenocarcinoma. In reactive atypia, bronchial epithelial cells form sheets and/or cluster of cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, abundant cytoplasm, and normal N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In a well-differentiated adenocarcinoma, the tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells are intermediate in size with round nuclei, vesicular chromatin, small nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters.
-
A-67. (c) Bronchioalveolar macrophages
The slide reveals a large cell with round nuclei, normal N:C ratio, vacuolated cytoplasm, and cytoplasmic red blood cells. In BAL specimens, alveolar macrophages are arranged in loose clusters or individual cells with benign nuclear features and foamy cytoplasm. Cells may reveal cytoplasmic pigments (anthracotic pigments in smokers) or vacuoles (in the lipid pneumonia). In degenerative changes, cells may reveal hyperchromatic nuclei and slightly irregular nuclear membranes. In adenocarcinomas and signet ring cell carcinomas, tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells reveal large round to oval nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, foamy cytoplasm, and high N:C ratio.
-
A-68. (b) Adenocarcinoma
The Papanicolaou preparation shows two-dimensional clusters and acini of hyperchromatic tumor cells with high N/C ratio, “lacy” cytoplasm, and cytoplasmic vacuolization. Nuclei are large with hyperchromatic coarse chromatin, irregular nuclear membranes, and prominent nucleoli. In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen.
-
A-69. (a) Poorly differentiated squamous cell carcinoma
Papanicolaou stain shows discohesive and scattered individual polygonal tumor cells with large hyperchromatic nuclei and dense cytoplasm. Some of tumor cells show prominent nucleoli. In a poorly differentiated squamous cell carcinoma, tumor cells may be arranged in clusters and round up with prominent nucleoli, less or no cytokeratin formation. These features may be confused with adenocarcinomas. In adenocarcinomas, tumor cells are arranged in tight three-dimensional clusters. Tumor cells of adenocarcinomas have hyperchromatic nuclei, coarse chromatin, prominent nucleoli and vacuolated and feathery cytoplasm (indication of mucin production).
-
A-70. (a) Poorly differentiated squamous cell carcinoma
Papanicolaou stain shows discohesive and scattered individual polygonal tumor cells with large hyperchromatic nuclei and dense cytoplasm. Some of tumor cells show prominent nucleoli. In a poorly differentiated squamous cell carcinoma, tumor cells may be arranged in clusters and round up with prominent nucleoli, less or no cytokeratin formation. These features may be confused with adenocarcinomas. In adenocarcinomas, tumor cells are arranged in tight three-dimensional clusters. Tumor cells of adenocarcinomas have hyperchromatic nuclei, coarse chromatin, prominent nucleoli and vacuolated and feathery cytoplasm (indicative of mucin production).
-
A-71. (a) Poorly differentiated squamous cell carcinoma
Papanicolaou stain shows discohesive and scattered individual polygonal tumor cells with large hyperchromatic nuclei and dense cytoplasm. Some of tumor cells show prominent nucleoli. In a poorly differentiated squamous cell carcinoma, tumor cells may be arranged in clusters and round up with prominent nucleoli, less or no cytokeratin formation. These features may be confused with adenocarcinomas. In adenocarcinomas, tumor cells are arranged in tight three-dimensional clusters. Tumor cells of adenocarcinomas have hyperchromatic nuclei, coarse chromatin, prominent nucleoli and vacuolated and feathery cytoplasm (indicative of mucin production).
-
A-72. (c) Reactive bronchial cells and mucin vacuole
The slide reveals dispersed bronchial epithelial cells admixed with mucin vacuoles. In vegetable cells, it reveals sheets of rectangular shape, uniform cells with cellulose walls. Vegetable cells have large nuclei, resembling tumor cells of squamous cell carcinomas.
-
A-73. (c) Reactive bronchial epithelial cells
The diagnosis is reactive bronchial cells. The Papanicolaou preparation shows two- or three-dimensional clusters of columnar cells with cilia, benign nuclear features, and some variation of nuclear size. It can be difficult to distinguish reactive bronchial epithelial cells (reactive atypia) from a well-differentiated adenocarcinoma. In reactive atypia, bronchial epithelial cells form sheets and/or loose cluster of cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, abundant cytoplasm, and normal N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In a well-differentiated adenocarcinoma, the tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells are intermediate in size with round nuclei, vesicular chromatin, small nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters.
-
A-74. (d) Bronchial reserve cells
The slide reveals loose clusters of small “dark” cells with smudged chromatin and scant cytoplasm. In BAL and/or bronchial washing specimens, bronchial reserve cells are arranged in clusters and sheets. They are small in size and have hyperchromatic nuclei with nuclear crowding. Nuclei are “dark” with smudged chromatin, no mitoses or necrosis. The main differential diagnosis of bronchial reserve cells is small cell carcinoma. In small cell carcinomas, tumor cells are relatively small in size and approximately two- to threefold of mature lymphocytes. Tumor cells reveal scant cytoplasm and high N:C ratio, nuclei with fine chromatin (salt-and-pepper) pattern, nuclear crowding and molding, and paranuclear blue bodies in the cytoplasm. Numerous “blue strips” may be seen on smears due to crush artifacts and the breakdown of nuclear DNA material.
-
A-75. (b) Squamous cell carcinoma
Papanicolaou stain shows discohesive and scattered individual polygonal tumor cells with large hyperchromatic nuclei and cytokeratin. In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cell carcinomas. In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of the cell is normal or slightly increased.
-
A-76. (a) Squamous cell carcinoma
Papanicolaou stain shows discohesive sheets and scattered individual polygonal tumor cells with large hyperchromatic nuclei and cytokeratin. In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cell carcinomas. In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of the cell is normal or slightly increased. In adenocarcinomas, the tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have high N:C ratio, round nuclei with vesicular chromatin, small nucleoli, and foamy cytoplasm.
-
A-77. (a) Squamous cell carcinoma
Papanicolaou stain shows discohesive sheets and scattered individual polygonal tumor cells with large hyperchromatic nuclei and cytokeratin. In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cell carcinomas. In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of the cell is normal or slightly increased. In adenocarcinomas, the tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have high N:C ratio, round nuclei with vesicular chromatin, small nucleoli, and foamy cytoplasm.
-
A-78. (d) Squamous cell carcinoma
Papanicolaou stain shows discohesive sheets and scattered individual polygonal tumor cells with large hyperchromatic nuclei and cytokeratin. In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cells carcinoma. In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of the cell is normal or slightly increased. In adenocarcinomas, the tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have high N:C ratio, round nuclei with vesicular chromatin, small nucleoli, and foamy cytoplasm.
-
A-79. (d) Poorly differentiated squamous cell carcinoma
Papanicolaou stain shows discohesive and scattered individual polygonal tumor cells with large hyperchromatic nuclei. Some of the tumor cells show prominent nucleoli. In a poorly differentiated squamous cell carcinoma, tumor cells may be arranged in clusters and round up with prominent nucleoli, less or no cytokeratin formation. These features may be confused with adenocarcinomas. In adenocarcinomas, tumor cells are arranged in tight three-dimensional clusters. Tumor cells of adenocarcinoma have hyperchromatic nuclei, coarse chromatin, prominent nucleoli, and vacuolated and feathery cytoplasm (indication of mucin production).
-
A-80. (d) Squamous cell carcinoma
Papanicolaou stain shows clusters/sheets and scattered individual polygonal tumor cells with large hyperchromatic nuclei and dense cytoplasm (cytokeratin formation). In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cell carcinomas. In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of the cell is normal or slightly increased. In adenocarcinomas, the tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have high N:C ratio, round nuclei with vesicular chromatin, small nucleoli, and foamy cytoplasm.
-
A-81. (b) Squamous cell carcinoma
The sputum specimen shows scattered individual polygonal tumor cells with large hyperchromatic nuclei and dense cytoplasm (cytokeratin formation). In squamous cell carcinomas, tumor cells are predominately arranged in clusters and dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cell carcinomas. In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of the cell is normal or slightly increased. In adenocarcinomas, the tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells have high N:C ratio, round nuclei with vesicular chromatin, small nucleoli, and foamy cytoplasm.
-
A-82. (d) Small cell carcinoma
The slide reveals tight clusters of small hyperchromatic cells with nuclear molding and crowding, mitosis, necrosis, and apoptotic body. In small cell carcinomas, tumor cells are relatively small in size and approximately two- to threefold of mature lymphocytes. Tumor cells reveal scant cytoplasm and high N:C ratio, nuclei with fine chromatin (salt-and-pepper) pattern, nuclear crowding and molding, and paranuclear blue bodies in the cytoplasm. Numerous “blue strips” may be seen on smears due to crush artifacts and the breakdown of nuclear DNA material. In BAL and/or bronchial washing specimens, bronchial reserve cells are arranged in clusters and sheets. They are small in size and have hyperchromatic nuclei with nuclear crowding. Nuclei are “dark” with smudged chromatin, no mitoses or necrosis. The main differential diagnosis of bronchial reserve cells is small cell carcinoma. In reactive lymphocytes, cells reveal coarse chromatin, scant cytoplasm, and lymphoglandular bodies, without nuclear crowding and molding.
Answers and Discussion of Text-Based Questions 83–151
-
A-83. (b) Alveolar macrophages or Curschmann spirals
Sputum specimens, including both spontaneous and induced specimens, are routinely used for the evaluation of respiratory tract lesions. A “good” sputum specimen is considered a specimen containing cellular material from the lower respiratory tract. Although they are easy to obtain, they are often contaminated with oral cells and material. Therefore, the first step in the cytological evaluation of sputum is to determine the adequacy of the specimen. The criteria for an adequate sputum specimen include the presence of alveolar macrophages and/or Curschmann spirals, which indicate a representative collection of the lower respiratory tract material. Other cells and material in sputum specimens such as squamous cells, neutrophils, mucus, bacteria, and Candida are considered oral contents, and they represent an unsatisfactory specimen. Sometimes, ciliated respiratory epithelial cells can be identified in the sputum, but they do not necessarily represent cells that come from the lower respiratory tract, since ciliated respiratory epithelial cells can be found in the lining of sinonasal passage of the upper respiratory tract.
-
A-84. (d) All of the above
For bronchoscopic procedures, including brushings, lavages/washings, and FNA specimens, an adequate specimen is crucial for an accurate diagnosis. Several factors are considered to play important roles in the process for accurate diagnosis, including (1) representative cells and material from the lesion, (2) a proper fixation of the specimen, and (3) crushing and drying artifacts. Furthermore, abundant ciliated bronchial epithelial cells may be present in the bronchoscopic specimen in a lung cancer patient, but the specimen should still be considered an inadequate and/or a non-diagnostic specimen due to lack of diagnostic tumor cells. In the BAL and washing specimens, as in sputum specimens, the finding of abundant alveolar macrophages is considered to be a satisfactory specimen.
-
A-85. (d) Endoscopic transbronchial FNA with or without ultrasound guidance
Endoscopic transbronchial FNA with (EBUS-TBNA) or without ultrasound guidance (TBNA) are commonly used procedures for the diagnosing and staging of lung cancers. Although both of them are useful for sampling mediastinal lymph nodes, EBUS-TBNA has a higher sensitivity and specificity than conventional TBNA in the staging of lung cancer patients. EBUS-TBNA can sample small lung lesions and small lymph nodes with the guidance of a built-in ultrasound probe. Otherwise, those small lesions and/or small mediastinal lymph nodes cannot be sampled by conventional TBNA. Endoscopic transesophageal FNA with (EUS-FNA) or without ultrasound guidance is also used for staging of lung cancer patients, but it can only sample certain locations of mediastinal lymph node. Both CT-guided transthoracic and ultrasound-guided transcutaneous fine needle biopsy (FNA) are more commonly used for diagnosis of lung lesions.
-
A-86. (a) A 55-year-old female never smoker
Recently, it has been reported that genomic abnormalities in the gene of anaplastic lymphomakinase (ALK) are detectable in a subset of 4 % (range: 0.9–13 %) of non-small cell lung cancer (NSCLC). In these tumors, a small paracentric inversion within the short arm of chromosome 2 results in an ALK gene rearrangement, causing a fusion gene EML4-ALK gene, which leads to a consecutive expression of the ALK kinase protein. Tumors containing ALK rearrangements are almost exclusive of adenocarcinomas without mutations of epidermal growth factor receptor (EGFR) gene or V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) gene, and preferentially involve in female non-smokers. Crizotinib, the ALK inhibitor, has been used as a targeted therapy in patients who have ALK gene rearrangement and shown to improve survival rates of patients. ALK gene rearrangement can be tested/detected by an FDA-approved ALK-FISH assay.
-
A-87. (d) Clusters of ciliated bronchial epithelium (Creola bodies)
Reactive bronchial epithelium hyperplastic changes (reactive atypia) can be caused by a variety of reasons, such as chemo- and radiation therapy, inflammation, chronic irritation, smoke, and others. Bronchial epithelium hyperplasia results in papillary-like mucosal folds. Bronchial epithelial cells are arranged in two- and three-dimensional clusters. The most characteristic finding is the presence of cells with cilia and/or Creola bodies, which are three-dimensional clusters of ciliated bronchial epithelium with slight variation of nuclei. Cells of Creola bodies have mild to moderate enlarged nuclei, vesicular chromatin, small nucleoli, and smooth nuclear membrane. The important differential diagnosis of reactive atypia is a well-differentiated adenocarcinoma. In adenocarcinomas, tumor cells form tight three-dimensional clusters with hyperchromatic nuclei, prominent nucleoli, irregular nuclear membrane, and high N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells.
-
A-88. (b) False
Fragments of cartilage on a transbronchial fine-needle aspiration biopsy are common findings, they are not indicative of a lung hamartoma. During the procedure of transbronchial fine-needle aspiration biopsy, a wire stylet is placed within the lumen of the aspiration needle to prevent and to remove possible intraluminal bronchial epithelium and cartilage, that may obstruct the needle lumen. Then, the stylet is removed, and a VacLok syringe is attached to the instrument in order to aspirate biopsy material under a negative suction pressure. Therefore, the presence of cartilage should not be interpreted as a hamartoma. In hamartomas, in addition to cartilage, other diagnostic features include the presence of ciliated bronchial epithelium, bland spindle cells, adipocytes, and background of myxoid material.
-
A-89. (d) Hyperchromatic nucleoli and clumped chromatin
In reactive/reparative bronchial epithelium, including radiation and chemotherapy-induced changes, epithelial cells form cohesive sheets and/or two-dimensional clusters. These cells can be big (cytomegaly), but the nuclear enlargement is proportional to the cytoplasm with a normal N:C ratio. Multinucleation and prominent nucleoli are common. However, hyperchromatic nuclei, clumped chromatin, and irregular nuclear membrane are not features seen in reactive atypia. The presence of these features indicates malignant lesions. The differential diagnosis of reactive changes includes non-small cell lung carcinomas, particularly a well-differentiated adenocarcinoma. In adenocarcinomas, tumor cells form tight three-dimensional clusters with hyperchromatic nuclei, prominent nucleoli, irregular nuclear membrane, and high N:C ratio. The important clue for adenocarcinoma is the loss of cilia on bronchial epithelial cells.
-
A-90. (d) A biopsy specimen of a bone lesion with metastatic lung adenocarcinoma
Any type of formalin-fixed and paraffin-embedded specimens can be used for molecular and mutational analyses. However, bone biopsy material of a metastatic carcinoma is usually not a good sample for molecular analyses/tests, because the decalcification procedure is usually needed in order to cut blocks for hematoxylin and eosin (H&E) stain. The decalcification may destroy tumor cell DNA, and may potentially affect the sensitivity and accuracy of molecular tests. Therefore, decalcified bone biopsy material is not a good specimen for molecular tests. For all types of specimens, the diagnosis and the presence of adequate tumor cells must be confirmed by a pathologist prior to sending the specimen for any molecular tests.
-
A-91. (d) Abundant alveolar macrophages
The finding of abundant alveolar macrophages is considered an adequate specimen, particularly for a sputum specimen. The criteria for an adequate specimen include the presence of alveolar macrophages and/or Curschmann spirals, which indicate a representative collection of the lower respiratory tract material. Other cells and material in specimens such as squamous cells, neutrophils, and chronic inflammatory may represent oral contaminations. Sometimes, ciliated respiratory epithelial cells can be identified in the sputum, but they do not necessarily represent cells coming from the lower respiratory tract, since ciliated respiratory epithelial cells can be found in the lining of sinonasal passage of the upper respiratory tract. In bronchial washing specimens, scattered inflammatory cells, including neutrophils and lymphocytes, are also identified. An increased number of inflammatory cells is considered abnormal. Abundant neutrophils may be suggestive of an acute pneumonia. Numerous lymphocytes may be indicative of a chronic inflammation or a lymph proliferative disorder.
-
A-92. (a) Benign mesothelial cells
In a transthoracic CT-guided fine-needle aspiration, the presence of benign mesothelial cells is a common finding. They should not be confused with mesothelioma and/or adenocarcinoma. Benign mesothelial cells are arranged in flat sheets. The cells have round and/or oval nuclei, vesicular chromatin, abundant cytoplasm, and “windows” between cells. In case of mesothelioma, tumor cells form three-dimensional clusters with scalloped (knobby) edges. The high power view reveals that tumor cells have centrally placed round nuclei, coarse chromatin, prominent nucleoli, dense cytoplasm with peripheral “halo” and intercellular “windows.” In adenocarcinomas, tumor cells are arranged in three-dimensional clusters with smooth edges. Tumor cells have hyperchromatic nuclei, irregular nuclear membranes, prominent nucleoli, scant cytoplasm, and without intercellular “windows.” In difficult cases, immunostains of mesothelial cell marker (calretinin, D2-40, WT1, p53) and adenocarcinoma markers (TTF, Napsin A, BerEP4) could be performed to separate mesothelial cells from adenocarcinoma.
-
A-93. (d) Shared cell borders
Tumor cells of squamous cell carcinomas form loose clusters or dispersed individual cells with hyperchromatic nuclei, smudgy chromatin, dense cytoplasm and cytokeratin formation, and a distinct cell border. In a well-differentiated tumor, numerous spindle-shaped malignant cells with prominent cytoplasmic keratin are identified. Cytokeratin stains purple to blue color with Diff-Quik method and orangeophilic with Papanicolaou method. In a poorly differentiated squamous cell carcinoma, tumor cells may be arranged in clusters and round up with prominent nucleoli, and less or no cytokeratin formation. In adenocarcinomas, tumor cells are arranged in tight three-dimensional clusters. Tumor cells of adenocarcinomas have hyperchromatic nuclei, coarse chromatin, prominent nucleoli, and vacuolated and feathery cytoplasm (indication of mucin production). Therefore, shared cell borders are not a feature of squamous cell carcinomas, and it is more often seen in adenocarcinomas.
-
A-94. (c) Send the specimen for microbiology culture and biopsy the peripheral area of the lesion.
During the transbronchial biopsy, the identification of necrotic debris warrants a microbiology culture. A variety of benign and malignant lesions can present with necrosis. For example, squamous cell carcinoma is often associated with tumor necrosis. Benign lesions such as granulomas may also present as a mass with or without central necrosis. Therefore, biopsy of peripheral area of the lesion is crucial for the establishment of the diagnosis. The cytological findings should be corrected with radiographic findings. If an image study is suggestive of a malignant lesion, necrotic debris are found on the FNA specimen during the on-site evaluation, several passes from the peripheral area of the lesion are definitely needed to make an accurate diagnosis.
-
A-95. (d) Irregular nuclear membrane and hyperchromasia
In reactive and reparative cellular changes, bronchial epithelial cells reveal a mild to moderate atypia. Cytological features include sheets and/or loose clusters of epithelial cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, and abundant cytoplasm. N:C ratio is normal in reactive cells. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In adenocarcinomas, tumor cells form acini and/or three-dimensional clusters or many discohesive individual tumor cells with large hyperchromatic nuclei, coarse chromatin, irregular nuclear membranes, prominent nucleoli, overlapping nuclei, and high N:C ratio. Therefore, careful evaluation of nuclear features is crucial in the differential diagnosis of reactive atypia from adenocarcinoma.
-
A-96. (b) A 3 cm squamous cell carcinoma involving the main bronchi
Squamous cell carcinoma accounts for 30–40 % of non-small cell lung cancers. The majority of these tumors are located at main bronchi/hilar area (central area) of the lung; therefore, tumors are more likely to shed diagnostic cells into the sputum. Patients with a squamous cell carcinoma often present with hemoptysis and obstructive pneumonia. On image studies, a cavitary lesion is often identified. Tumor cells of squamous cell carcinomas form loose clusters or dispersed individual cells with hyperchromatic nuclei, smudgy chromatin, dense cytoplasm and cytokeratin formation, and a distinct cell border. In a well-differentiated tumor, numerous spindle-shaped malignant cells with prominent cytoplasmic keratin are identified. Cytokeratin stains purple to blue color with Diff-Quik method and orangeophilic with Papanicolaou method. In a poorly differentiated squamous cell carcinoma, tumor cells may be arranged in clusters and round up with prominent nucleoli, and less or no cytokeratin formation. These features should not be confused with adenocarcinoma.
-
A-97. (d) Renal cell carcinoma
“Pseudointranuclear inclusions” are formed by the protrusion of cytoplasm into the nuclei, and they have been described in a variety of malignant tumors, such as hepatocellular carcinoma, melanoma, a subset of lung adenocarcinomas, papillary thyroid carcinoma, meningioma, and others. Therefore, the finding of “pseudointranuclear inclusion” is non-specific. However, it is usually not seen in renal cell carcinomas. The cytological features of renal cell carcinomas (clear cell renal cell carcinomas) include cohesive clusters of tumor cells with centrally located nuclei, vesicular chromatin, and abundant clear cytoplasm. The presence of nucleoli is variable depending on the Fuhrman grade. The nucleoli is inconspicuous in a low grade tumor, whereas the nucleoli is prominent in a high grade tumor.
-
A-98. (d) Nuclear grooves
Neuroendocrine tumors (NET) are a spectrum of neoplasms. The World Health Organization (WHO) classifies NET into three main categories: (1) well-differentiated neuroendocrine tumors with benign behavior; (2) well-differentiated (low grade) neuroendocrine carcinomas with low-grade malignant behavior; and (3) poorly differentiated (high grade) neuroendocrine carcinomas with aggressive clinical course, such as the large cell neuroendocrine and small cell carcinomas. All NET have common phenotypic characteristics, despite differing embryological origin. The cytological features of tumor cells are characterized by fine granular chromatin (salt-and-pepper chromatin) pattern, inconspicuous nucleoli, and scant cytoplasm. Mitosis may be rare or abundant depending on the differentiation of the tumor. Nuclear crowding and molding are often seen in small cell carcinomas; however, nuclear groove is not a feature of neuroendocrine tumors. In addition, cellular proliferative rate (Ki67 labeling) has been considered as a useful marker for the classification of NET.
-
A-99. (b) Monomorphous population of lymphocytes
Reactive lymphoid hyperplasia contains a heterogeneous mixture of lymphocytes, including small lymphocytes, centrocytes/centroblasts, immunoblasts, tangible-body macrophages, and other cells. There is a great variation of cellular sizes and appearances, characteristic of polymorphous population of cells. Monomorphous population of lymphocytes is usually seen in lymph proliferative disorders and/or lymphomas, particularly in small lymphocytic lymphomas/chronic lymphcytic leukemia (SLL/CLL). It represents a monoclonal proliferation of lymphoma cells. Other cytological features of SLL/CLL include discohesive tumor cells with hyperchromatic nuclei, clumped (soccer-ball-like) chromatin, irregular nuclear membranes, inconspicuous nucleoli, and scant cytoplasm and rare or absence of tangible body macrophages.
-
A-100. (a) Adenocarcinoma with lepidic growth pattern (bronchoalveolar carcinoma)
Adenocarcinoma with lepidic growth pattern (formerly bronchoalveolar carcinoma), particularly the minimal invasive variant, is considered a well-differentiated adenocarcinoma. It can be difficult to distinguish reactive bronchial epithelial cells (reactive atypia) from a well-differentiated adenocarcinoma. In reactive atypia, bronchial epithelial cells form sheets and/or loose cluster of cells with mildly to moderately enlarged nuclei, vesicular chromatin, single or multiple small nucleoli, abundant cytoplasm, and normal N:C ratio. The important clue for reactive atypia is the presence of cilia on bronchial epithelial cells. In a well-differentiated adenocarcinoma, the tumor cells are arranged in two- or three-dimensional clusters or in disorganized sheets. Tumor cells are intermediate in size with round nuclei, vesicular chromatin, small nucleoli and foamy cytoplasm, and high N:C ratio. However, no cilia are identified in tumor cell clusters. In mucinous adenocarcinomas, large tumor cells reveal hyperchromatic nuclei and cytoplasmic mucin. In basaloid squamous cell carcinomas, tumor cells form tight three-dimensional clusters with smudgy and/or coarse chromatin and scant dense cytoplasm. In small cell carcinomas, tumor cell reveals fine (salt-and-pepper) chromatin with nuclear crowding, molding, apoptosis, necrosis, and blue strips of DNA material. The tumor also has a high N:C ratio.
-
A-101. (d) Aspergillosis
Aspergillosis is characterized by the presence of mucus plugs containing fungal organisms admixed with birefringent calcium oxalate crystals, inflammatory cells, and eosinophils. Calcium oxalate crystals are needle-shaped, polarizable crystals; they may form rosettes or wheat sheaf-like clusters. Fungus microorganisms are characterized by true septate hyphae with 45° angle branching. The uniform thickness (3–6 μm in width) and the septa of hyphae differentiate Aspergillus from other fungus such as Mucor and Candida. Aspergillosis can also cause reactive atypia mimicking of non-small cell lung cancers, particularly a squamous cell carcinoma. Aspergillosis may be found in cancer patients, too. In Pneumocystis jiroveci (carinii) pneumonia (PCP) infection, extracellular and intracytoplasmic amorphous material containing cup-shaped microorganisms are seen.
-
A-102. (d) Immunostains of cytokeratin
Thymoma is the most common primary mediastinal neoplasm. Most patients are asymptomatic. When present, symptoms of cough, chest pain, and dyspnea are commonly seen. There are two major subtypes of thymomas (type A and type B) by WHO classification. In type A thymoma, tumor epithelioid cells reveal bland spindle or oval-shaped nuclei and without lymphocytic infiltrates, whereas, in type B thymoma, tumor epithelioid cells reveal polygonal appearance and admixed with different extent of lymphocytic infiltrates. The cytological features of thymoma vary widely, ranging from lymphocyte predominant to a biphasic population of epithelial cells and lymphocytes, to exclusively epithelial cells. The neoplastic epithelial cells are in spindle and oval shape, or polygonal with abundant cytoplasm and well-defined cell borders. The nuclei are bland with evenly distributed chromatin and small inconspicuous nucleoli. In type B tumors, neoplastic epithelial cells present as clusters or as scattered individual tumor cells in a background of lymphocytes. Therefore, the most important single test/marker for the differential diagnosis of thymoma from lymphoma is cytokeratin stain. Cytokeratin is positive for thymoma and negative for lymphoma. Other lymphoid markers such as CD45, CD5, and T/B cell markers could be positive in thymomas and lymphoma. Finally, it is difficult to distinguish benign from malignant thymomas by cytomorphology alone. The presence of pleomorphism, markedly nuclear atypia and increased mitotic figures may be indicative of a thymic carcinoma.
-
A-103. (b) Bronchoscopic washing
Bronchoscopic washing has the lowest diagnostic sensitivity among all procedures. Its sensitivity ranges from 30 to 70 % depending on the location and the size of the lesion. In BAL, the sensitivity is 40–80 %. Bronchial brushing has a higher sensitivity than washing and BAL. Transbronchial fine-needle aspiration has a sensitivity of 60–90 %. Cell block preparations during these procedures can markedly increase the detection sensitivity, since it may provide additional diagnostic cells and/or material.
-
A-104. (d) Bilateral lung infiltrations
Bilateral lung infiltrations can be seen in both benign disease and malignant tumors. In benign lesions, such as infections, granulomas, and interstitial lung diseases, the clinical presentation and radiographic findings can present as bilateral lung infiltrations. The malignant tumors, such as adenocarcinomas with lepidic growth pattern (formerly bronchoalveolar adenocarcinoma) and advanced stages of lung cancers, can also present as bilateral lung infiltrations. The condition is not a contraindication for lung fine-needle aspiration. Other conditions, such as uncontrollable cough, severe pulmonary hypertension, and suspected echinococcal cysts are contraindications for fine-needle aspiration of the lung, due to the potential risk of damage to the lung parenchyma and the dissemination of the disease.
-
A-105. (a) Mitosis and necrosis
All features can be seen in bronchial reserve cell hyperplasia, except mitosis and necrosis. In reserve cell hyperplasia, cells are arranged in cohesive sheets or clusters with nuclear overlapping and crowding. The nuclei reveal dark smudgy chromatin, inconspicuous nucleoli, smooth nuclear membrane, and scant cytoplasm. The N:C ratio is slightly increased. There is no mitosis or necrosis seen. Bronchial reserve cell hyperplasia is a mimic of small cell carcinomas. In small cell carcinomas, tumor cell reveals fine (salt-and-pepper) chromatin with nuclear crowding, molding, apoptosis, necrosis, and blue strips of DNA material. The tumor also has a high N:C ratio.
-
A-106. (b) Nuclei with marked variation in size and shape
Degenerated squamous cells can be seen in a variety of lung diseases and almost any type of injury to the lung, such as infection, pneumonia, chemotherapy, sepsis, and diffuse alveolar damage. The cytological features of degenerated squamous cells reveal single and loosely formed clusters of cells with slightly enlarged pyknotic nuclei, smudgy chromatin, cytoplasmic keratin, and normal N:C ratio. The marked variation of size and shape of the nuclei is the feature of squamous cell carcinomas. In squamous cell carcinomas, numerous spindle-shaped and polygonal malignant cells with hyperchromatic nuclei, smudgy chromatin, cytokeratin, and high N:C ratio are identified.
-
A-107. (d) Prominent nucleoli
In squamous cell carcinomas, tumor cells form loosely clusters or dispersed individual cells with hyperchromatic nuclei, smudgy chromatin, dense cytoplasm and cytokeratin formation, and a distinct cell border. In a well-differentiated tumor, numerous polygonal and spindle-shaped malignant cells with hyperchromatic nuclei and high N:C ratio are identified. Cytokeratin stains purple to blue color with Diff-Quik method and orangeophilic with Papanicolaou method. When tumors are poorly differentiated, the distinction between squamous cell carcinomas and adenocarcinomas may become difficult. In a poorly differentiated squamous cell carcinoma, tumor cells may be arranged in clusters and round up with prominent nucleoli, and with less or no cytokeratin formation. These features should not be confused with adenocarcinomas. In adenocarcinomas, tumor cells form acini and/or three-dimensional clusters or many discohesive individual tumor cells with large hyperchromatic nuclei, coarse chromatin, irregular nuclear membranes, prominent nucleoli, and vacuolated cytoplasm (cytoplasmic mucin production).
-
A-108. (d) Clusters of bronchial epithelial cells without cilia
The cytological features of a mucinous adenocarcinoma include relatively uniform tumor cells with pale nuclei, vesicular chromatin, inconspicuous nucleoli, and foamy cytoplasm. Tumor cells are arranged in tight clusters and dispersed individual cells with inconspicuous cytological atypia, and resemble macrophages. Therefore, careful evaluation of nuclear features and the presence or absence of cilia is critical for the diagnosis. Features such as nuclei enlargement with irregular nuclear membrane and absence of cilia in cellular clusters should be considered malignancy.
-
A-109. (a) True.
In lung adenocarcinoma, a significant morphological heterogeneity can be seen in tumors. The WHO classification of adenocarcinoma of the lung includes many subtypes, such as acinar, papillary, solid, mucinous, clear, and mixed subtypes. Cytological arrangements of adenocarcinomas may be seen as three-dimensional clusters, acinar and papillary groups, honeycomb-like sheets, and/or dispersed individual tumor cells. In general, tumor cells reveal large round or oval-shaped nuclei, coarse chromatin, irregular nuclear membranes, prominent nucleoli, and cytoplasmic vacuolization. The N:C ratio of tumor cells is high. Increased mitosis and tumor necrosis may also present on the slide.
-
A-110. (d) All of the above
The differential diagnosis of so-called “signet ring” cells in cytological specimens is broad, including both benign (goblet cells) and malignant (adenocarcinomas of the lung and stomach) conditions. A careful evaluation of the nuclear features is important for distinguishing between benign and malignant lesions. The nuclear features of carcinomas include large nuclei, hyperchromatic chromatin, irregular nuclear member, and large prominent nucleoli. Although signet ring cell carcinomas are more commonly seen in stomach carcinomas, a subset of lung adenocarcinomas may have signet ring cell features. Therefore, the finding of signet ring morphology in a lung lesion may be mistaken as a metastatic carcinoma of the stomach. Immunostains of TTF and CDX2 may not help in the differential diagnosis of primary from metastatic adenocarcinoma, since the TTF may be negative and CDX2 may be focally positive in a primary lung mucinous adenocarcinoma. Thus, cytological findings should be correlated with clinical and radiographic findings in order to render the correct diagnosis.
-
A-111. (b) False
Vascular tumors EHE and EAS can occur as a primary tumor of the lung. EHE is a low-to-intermediate grade tumor, whereas EAS is a high-grade tumor. In EHE, tumor cells are arranged in small clusters and cords and reveal epithelioid appearance with abundant eosinophilic cytoplasm. The nuclei are round in shape with hyerchromatic chromatin, prominent nucleoli, and irregular nuclear membranes. In EAS, tumor cells are arranged in dispersed individual cells with marked nuclear pleomorphism. Increased mitotic activity, focal spindle shaped cells, and tumor necrosis are also present. Both tumors have intracytoplasmic vacuoles, representing small vascular lumina, which may mimic cytoplasmic mucin. Therefore, they may be confused with adenocarcinoma due to the epithelioid appearance and the cytoplasmic vacuoles. The diagnosis of both EHE and EAS often requires the immunostain of vascular markers CD31 and CD34. In addition, about one-third of the vascular tumors are immunoreactive with cytokeratin.
-
A-112. (d) Poorly differentiated adenocarcinoma
The differential diagnosis of a spindle cell lesion in FNA cytology is broad, including spindle cell carcinoma, leiomyoma, leiomyosarcoma, melanoma, nerve tumors, fibrous tumors, and others. In a leiomyosarcoma, tumor cells have “cigar-shaped” nuclei and finely granular cytoplasm. Naked nuclei and increased mitosis are also common in the tumor. IHC stains of tumor cells are positive for desmin and myogenin. In spindle cell carcinomas, tumor cells form clusters with epithelioid appearance, such as large hyperchromatic nuclei, coarse chromatin, irregular nuclear membranes, prominent nucleoli, and scant cytoplasm. In spindle cell carcinoids, tumor cells reveal neuroendocrine cell features with uniform oval nuclei, fine chromatin, inconspicuous nucleoli, and abundant cytoplasm. The cytological features of a poorly differentiated adenocarcinoma include predominantly dispersed individual pleomorphic tumor cells and three-dimensional clusters. Tumor cells reveal large round or oval-shaped nuclei with hyperchromatic coarse chromatin, irregular nuclear membranes, prominent nucleoli, and vacuolated cytoplasm. The spindle cell appearance is not the feature of a poorly differentiated adenocarcinoma.
-
A-113. (b) Perform flow cytometry
The FNA of a MALT lymphoma reveals a discohesive population of lymphoma cells, resembling small lymphocytes, centrocytes, or monocytes. Lymphoma cells have round or irregular nuclei, coarse granular chromatin, inconspicuous nucleoli, and scant cytoplasm. In the background of the slide, a few or no tingible-body macrophages are identified. There is no specific IHC marker for diagnosing MALT lymphoma. CD20 stain is diffusely positive for tumor cells, and aberrant coexpression of CD43 by neoplastic B cells is found in up to 50 % of cases. Immunophenotyping such as flow cytometry study is necessary in a suspicious patient to establish the diagnosis. The MALT lymphoma can be confused with a reactive lymphoid hyperplasia and/or reactive lymph node. In reactive lymphoid hyperplasia, in addition to small lymphocytes, centrocytes, and tingible-body macrophages, the dendritic lymphocytic aggregate can be identified. The dendritic lymphocytic aggregate is a loose cluster of small lymphocytes and dendritic cells, dendritic cells reveal vesicular (pale) nuclei, fine chromatin, and delicate cytoplasm.
-
A-114. (a) True
Sarcoidosis is an idiopathic disease characterized by non-necrotizing granulomas. It typically involves mediastinal lymph nodes and the lung. However, it can be a systemic process and involve other organs, such as the heart, central nerve system, and skin. The disease usually occurs in middle-aged African-American females. The patient may need steroid therapy. Only a small percentage of patients progress to pulmonary fibrosis and require lung transplantation.
-
A-115. (b) Alveolar proteinosis
Alveolar proteinosis is an idiopathic disease; however, it can occur in a variety of conditions such as infections, occupational exposures, and malignancies. BAL is often performed for the therapeutic purpose. The patient usually has an excellent prognosis. On BAL slides, numerous alveolar macrophages and eosinophilic granular material are revealed by the Papanicolaou stain. In amyloid, it reveals acellular dense waxy material which has a characteristic birefringent “apple green” appearance by the Congo-red stain under the polarized microscope. PCP infection occurs in immunocompromised patients, and reveals intracytoplasmic cup-shaped microorganisms. In aspergillosis, fungal hyphae are identified.
-
A-116. (c) Calretinin, p53, WT1, TTF, BerEP4, CEA, and CD15
The cytomorphological distinction between mesotheliomas and adenocarcinomas can be difficult due to overlapping cytological features. Therefore, immunostains are necessary to establish the diagnosis. A panel of immunomarkers, including both adenocarcinoma markers and mesothelioma markers, is usually performed. Commonly used adenocarcinoma markers include TTF, BerEP4, CEA, B72.3, MOC-31, and CD15 (Leu-M1), whereas commonly used “mesothelioma markers” include calretinin, p53, D2-40, and WT1. Mesothelioma is positive for Calretinin, p53, and WT1, whereas, adenocarcinoma of the lung is positive for TTF, BerEP4, CEA, B72.3, MOC-31, and CD15.
-
A-117. (a) Microbiology culture and core biopsy of the lesion
The cytological finding of this on-site evaluation indicates a granulomatous inflammation. The granuloma can be found in both benign and malignant lesions. Therefore, the best action is to send specimens for microbiology culture and to perform a core needle biopsy. The microbiology culture will be helpful for the differential diagnosis of infections. The core biopsy material is important for the microscopic evaluation of the lesion and to perform immunostudies of the lesion if it is needed.
-
A-118. (a) Make cell block and perform silver stain
Pneumocystis jiroveci (carinii) pneumonia (PCP) infection usually involves immunocompromised patients, such as HIV infection, organ transplantation, chemotherapy and cancer patients. The cytological findings of PCP infection on the BAL specimen include numerous alveolar macrophages and amorphous material. Cup-shaped microorganisms can be seen in the cytoplasm of the macrophages and in amorphous material by Papanicolaou stain, but organisms are stained poorly or not at all with Papanicolaou method. In silver stains, such as Grocott’s methenamine silver (GMS) stain, the cell wall of the organisms stains with black color. The cell block preparation and silver stain may help to identify the cup-shaped microorganisms and confirm the diagnosis.
-
A-119. (d) All of the above
Breast carcinoma is a common tumor which metastases to the lung, due to its anatomic location. Metastatic breast carcinomas have many cytological features that overlap with primary lung adenocarcinomas. In metastatic breast carcinomas, tumor cells are arranged in tight three-dimensional clusters and/or acini. Tumor cells reveal a monotonous appearance, with hyperchromatic nuclei, prominent nucleoli, and frequently intracytoplasmic lumen. In lung adenocarcinomas, tumor cells may have more pleomorphic appearance and variation in size and shape of nuclei. In difficult cases, immunostains of breast cancer markers (estrogen receptor (ER), progesterone receptor (PR), gross cystic disease fluid protein (GCDFP-15), mammaglobin and GATA-3), and lung markers (TTF1 and napsin A) may help for the differential diagnosis.
-
A-120. (b) False
In a primary lung adenocarcinoma intestinal subtype and/or mucinous subtype, tumors reveal tall glandular or columnar cells with abundant cytoplasmic mucin. In this subtype, tumor cells frequently stain negative for TTF1, but positive for CK20 and CDX2. Therefore, this subtype of primary lung adenocarcinoma cannot be reliably differentiated from a metastatic colonic adenocarcinoma by immunomarkers of CK20 and CDX2. For differential diagnoses, the patient’s clinical history (presence or absence of malignant history or colon lesions), radiographic information (single verse multiple lung nodules), and colonoscopic evaluation (presence or absence of colonic lesions) should be considered and correlated with cytological findings.
-
A-121. (c) Colon
The metastatic colonic adenocarcinoma has characteristic cytological features. Tumor cells are arranged in three-dimensional clusters and acini structures with distinctive tall columnar and glandular cells, showing a “picket fence” appearance. Tumor cells reveal oval hyperchromatic nuclei, coarse chromatin, prominent nucleoli, irregular nuclear membranes, high N:C ratio, and “dirty” necrosis. In metastatic breast carcinomas, tumor cells are arranged in tight three-dimensional clusters and/or acini with a monotonous appearance. In metastatic hepatocellular carcinomas, tumor cells are predominately arranged in dispersed individual cells with polygonal appearance, large nuclei, prominent nucleoli, and dense granular cytoplasm. In metastatic uterine carcinomas, tumor cells may reveal several different cytomorphologies, ranging from malignant glandular cells to clear cells, depending on the subtype of the primary tumor.
-
A-122. (b) Using unstained slides to perform IHC markers S100, HMB45, and Melanin A with red chromogenics rather than the one with brown chromogenics.
Tumor cells of a metastatic melanoma produce dark brown to black color melanin pigment. Macrophages in lymph nodes of the lung contain anthracotic pigment, they can be confused with melanin pigment. Therefore, routine IHC marker using brown color chromogenics will cause false-positive staining. Therefore, using IHC markers with red color chromogenics is more specific for the detection of a metastatic melanoma in the lymph node containing anthracotic pigment. Alternatively, one can use bleached slides to perform routine IHC markers S100, HMB45, and Melanin A. Recently, a nuclear transcript factor SOX10 has been used for the detection of melanomas. It has been reported that SOX10 has a 100 % sensitivity and specificity for the identification of melanomas, particularly in desmorplastic melanoma which is negative for many melanoma markers.
-
A-123. (d) Prominent nucleoli
In small cell carcinomas, tumor cells are relatively small in size and approximately two- to threefold of mature lymphocytes. However, in small cell carcinoma large cell variant, the size of tumor cells can be much larger. Tumor cells show scant cytoplasm and high N:C ratio, large nuclei with fine chromatin (salt-and-pepper) pattern, nuclear crowding and molding, and paranuclear blue bodies in the cytoplasm. Numerous “blue strips” are seen on the slides due to crush artifacts and the breakdown of nuclear DNA material. The nucleoli are inconspicuous in small cell carcinomas. In some cases, tumor cells may reveal rare nucleoli, but they are small in size. The prominent nucleolus is not usually seen in a small cell carcinoma, and it can be seen in atypical carcinoid and/or large cell neuroendocrine tumors.
-
A-124. (d) Pancoast tumor
An apical squamous cell carcinoma in the superior sulcus is called the “Pancoast tumor” and characterized by rib destruction, neural invasion, and Horner syndrome (enophthalmos, ptosis, miosis, and ipsilateral decreased sweating). Other syndromes, such as Lambert–Eaton syndrome (weakness or loss of movement of muscles, vision changes, and nerve system symptoms), Cushing’s syndrome, and hyponatremia are usually associated with a small cell carcinoma.
-
A-125. (b) Atypical carcinoid shares many cytological features with typical carcinoid
Atypical carcinoid shares many cytological features with typical carcinoid, including cellular arrangement and tumor cell morphology. In atypical carcinoids, tumor cells are arranged in acini and pseudorosette structures. Tumor cells may have more pleomorphic appearance and are larger in size than that of typical carcinoids. Atypical carcinoids also reveal vesicular nuclei, fine (salt-and-pepper) chromatin, and slightly increased mitotic activity and focal tumor necrosis. The presence of nucleoli can be seen in both atypical and typical carcinoids. In general, the mitotic figures range from 2 to 10 per 10 high power fields in atypical carcinoids. Although mitotic account plays an important role in the classification, it is not always seen on cytological smears. Therefore, careful evaluation of cytomorphology on slides and immunostain of Ki67 on cell block sections may be necessary for an accurate classification. The distinction between atypical and typical carcinoids usually requires a surgical specimen.
-
A-126. (c) The lower mitotic rate and the absence of necrosis
All neuroendocrine tumors share many cytological features, such as fine (salt-and-pepper) chromatin and acini and pseudorosette arrangement of tumor cells. The prominent nucleoli can be seen in large cell neuroendocrine tumors and atypical carcinoids, but is inconspicuous in small cell carcinomas, and typical carcinoids can have a small nucleoli. The separation from typical carcinoids from small cell carcinomas is easier than from atypical carcinoids. In small cell carcinomas, tumor cells reveal hyperchromatic nuclei with nuclear crowding and molding, scant cytoplasm, numerous apoptotic bodies and mitoses, and tumor necrosis. Typical carcinoid is separated from atypical carcinoid by several criteria, such as a lower mitotic rate, absence of tumor necrosis, and uniformity of tumor cells. The presence of nucleoli can be seen in both atypical and typical carcinoid. It has been considered that the mitotic rate is less than 2, and 2–10 per 10 high power field in a typical and atypical carcinoid, respectively. Although mitotic account plays an important role in the classification, it is not always seen on cytological smears. Therefore, careful evaluation of cytomorphology on slides and immunostain of Ki67 on cell block sections may be necessary for an accurate classification. The distinction between typical and atypical carcinoids usually requires a surgical specimen.
-
A-127. (d) Carcinoid tumor-like nuclei with markedly atypia and enlargement
In large cell neuroendocrine tumors (LCNEC), tumor cells are large in size with more pleomorphic appearance and moderate to abundant cytoplasm. They are arranged in nests, acini, and pseudorosettes. Tumor cells also have carcinoid tumor-like nuclei with markedly atypia and enlargement, vesicular or salt-and-pepper chromatin, and prominent nucleoli. The mitotic rate is very high and typically more than 11 (average of 50–70) per 10 high power fields. A large area of tumor necrosis is also a common finding.
-
A-128. (a) Involving the HMGI(Y) gene on chromosome 6p21
Pulmonary hamartoma is a benign neoplasm and involves the HMGI(Y) gene on chromosome 6p21. The cytological features include benign glandular cells, fibromyxoid material, spindle cells (smooth muscle), cartilage, and adipocytes. There is no evidence of familiarity with this tumor. Clinically, the tumor is a well-circumscribed lesion and usually presents as an incidental finding. Surgical resection of the tumor can cure the disease and the patient has an excellent prognosis.
-
A-129. (d) Neutrophils can be seen in 1 year after transplantation.
In the lung transplant patient, BAL procedure is routinely performed to monitor the patient. In such type of specimens, neutrophils are normally seen within the first 3 months after the transplantation. After this period, the finding of neutrophils in the BAL specimen should be carefully evaluated to determine the presence of potential infections. It is also important to look for fungal, viral, and PCP infections, since transplant patients are immunocompromised, whereas an increased number of macrophages in BAL specimens can be seen for years after transplantation.
-
A-130. (b) Inflammatory myofibroblastic tumor (IMT)
IMT is a myofibroblastic tumor in children and young adults involving lung, liver, as well as retroperitoneal and pelvic soft tissues. It has a low malignant potential and indolent clinical course. In the past, a variety of names have been used to describe this lesion, including inflammatory pseudotumor, plasma cell granuloma, inflammatory myofibroblastic proliferation, and pseudosarcomatous myofibroblastic proliferation. Currently, it has been recognized by WHO classification as IMT. The tumor consists of bland spindle cell proliferation in a background of plasma cells, lymphocytes, neutrophils, and eosinophils. Positive immunoreactivity with antibodies against ALK protein and molecular study of ALK gene arrangement can be detected in approximately 40–50 % of patients, and help in the differential diagnosis.
-
A-131. (b) False
EGFR and KRAS mutational rates are found in 10–20 % and 15–30 % of lung adenocarcinomas in the United States, respectively. EGFR and KRAS mutations are mutually exclusive in non-small cell lung carcinomas, and have not been found to occur in the same tumor. In lung adenocarcinomas, patients with EGFR mutations are associated with a good response to tyrosine kinase inhibitors therapy, whereas KRAS mutations are associated with a resistance to tyrosine kinase inhibitor therapy, and with poor prognosis. Therefore, the tests of EGFR, KRAS, and other genetic mutations in lung cancer patients are critical for targeted therapies.
-
A-132. (a) Mutation and overexpression of the EGFR gene lead to cancer cell overgrowth, but not tumor progression.
In non-small cell lung carcinomas, mutation of EGFR gene induces intrinsic protein tyrosine kinase activity of EGFR receptor and constitutive activation of EGFR signaling pathway, which leads to cell proliferation and uncontrolled cell division. Therefore, activation mutations and overexpression in the tyrosine kinase domain of the EGFR gene involve in tumor overgrowth and progression. Currently, EGFR signaling pathway has been successfully targeted by several tyrosine kinase inhibitors, such as Erlotinib and gefitinib. These tyrosine kinase inhibitors of the EGFR signaling pathway have been used to treat non-small cell lung cancer patients, particularly in lung adenocarcinoma patients. A marked improvement of overall survival rate has been shown in patients with EGFR mutations. Finally, EGFR mutations are more often found in tumors with micropapillary morphology and the lepidic growth pattern (formerly known as bronchioloalveolar carcinoma).
-
A-133. (a) The KRAS protein stimulates downstream activity of EGFR tyrosine kinase
KRAS belongs to the RAS family of oncogenes. KRAS mutations have been detected in approximately 15–30 % of non-small cell lung carcinomas, especially in lung adenocarcinomas. The mutations cause an impaired GTPase activity and subsequently a constitutive activation of RAS signaling pathway, which is located downstream of EGFR signaling. The constitutive activation of RAS signaling pathway leads to activation of anti-apoptotic pathways and cell proliferation. KRAS mutations have been associated with primary resistance to EGFR tyrosine kinase inhibitor therapy in non-small cell carcinomas. It has been found that KRAS mutations occur more commonly in adenocarcinomas from elderly patients and heavy smokers. Patients with KRAS mutations are unlikely to respond to EGFR tyrosine kinase inhibitor therapy and are associated with a poor prognosis.
-
A-134. (a) The most common ALK rearrangement in non-small cell lung carcinoma is EML4-ALK fusion
The most common ALK gene rearrangement in non-small cell lung carcinomas is EML4-ALK rearrangement, which involves the 5′ end of the EML4 gene and the 3′ end of the ALK gene on chromosome 2p23. ALK gene rearrangements occur in non-small cell carcinoma patients with a younger age, who are never smokers. Females are more likely to have the mutation than males. EML4-ALK gene rearrangements have not been found to be associated with EGFR and KRAS mutations, therefore, patients with ALK gene rearrangements do not benefit from EGFR-specific tyrosine kinase inhibitor therapy. Crizotinib (Xalkori®, Pfizer) is the FDA-approved ALK tyrosine kinase inhibitor therapy for patients whose tumors are positive for ALK rearrangements.
-
A-135. (c) Intercellular “windows”
The cytological features of mesothelial cells reveal sheets and loosely formed clusters of intermediate-sized cells with large centrally located nuclei, vesicular chromatin, small prominent nucleoli, dense cytoplasm, and intercellular “windows.” The nuclei of mesothelial cells may reveal mild to moderate variation in size and shape, particularly in reactive mesothelial cells; therefore, they may be confused with a well-differentiated adenocarcinoma. In adenocarcinomas, tumor cells are commonly arranged in tight three-dimensional clusters and acinar structures. Tumor cells are large in size, with high N/C ratio, and hyperchromatic nuclei. The nuclei contain coarse chromatin, prominent nucleoli, and irregular nuclear membranes. The cytoplasm of tumor cells is “lacy” and with cytoplasmic vacuolization. The finding of intercellular “windows” is a reliable feature for the separation between mesothelial cells and adenocarcinomas.
-
A-136. (b) Significant variation of nuclear size and shape
Reactive squamous cells have several cytological features overlapping with squamous cell carcinomas. In reactive squamous cells, cells may be arranged in small two-dimensional clusters and dispersed individual cells. They reveal mildly enlarged nuclei with minimal variation in size and shape, and abundant dense cytoplasm. The N:C ratio of cells is normal or slightly increased. In squamous cell carcinomas, tumor cells are predominately arranged in dispersed individual cells, with large nuclei, hyperchromatic smudgy chromatin, marked variation of nuclear size and shape, and dense cytoplasm (cytokeratin formation). In addition, spindle-shaped cells and bizarre tumor cells are also commonly seen. Prominent necrotic debris in the background is also a feature for squamous cell carcinomas.
-
A-137. (a) True
Percutaneous FNA is a transthoracic procedure and usually performed under CT and/or ultrasound guidance. Therefore, it is important to recognize that several types of cells and tissue fragments can be found on the cytological slides, and they should not be misinterpretated as a lesional component. For example, mesothelial cells from pleura, smooth muscle, and adipose tissue from chest walls are quite common findings. The benign appearances of these cells separate them from a potential lesion. The most important differential diagnosis of mesothelial cells is adenocarcinoma. In adenocarcinomas, tumor cells reveal tight three-dimensional clusters and acinar structures, with high N/C ratio, hyperchromatic nuclei, coarse chromatin, prominent nucleoli, and irregular nuclear membranes. No intercellular “windows” characteristic of mesothelial cells is identified in adenocarcinomas.
-
A-138. (b) Small cell carcinoma
In this situation, several differential diagnoses should be considered in a transthoracic FNA of a right lower lobe lung mass, including lung adenocarcinoma with hepatocellular features, benign liver cells, and/or a hepatocellular carcinoma. In a lung adenocarcinoma of hepatocellular variant, tumor cells reveal prominent nucleoli and dense granular cytoplasm, mimicking hepatocellular carcinomas; however, tumor cells are arranged in tight three-dimensional clusters, with hyperchromatic nuclei, irregular nuclear membrane, and a high N:C ratio. In hepatocellular carcinoma, numerous dispersed individual tumor cells and naked nuclei are characteristic findings. Benign liver cells are arranged in honeycomb sheets and small clusters; cells reveal centrally located nuclei with small nucleoli and a normal N:C ratio. Small cell carcinomas with nuclear crowding and molding should not be confused with liver cells.
-
A-139. (c) Charcot–Leyden crystal
Charcot–Leyden crystal is a needle-shaped, orangeophilic structure derived from degenerated esosinophils. It can be found in a variety of lung diseases associated with increased eosinophils; however, it is more commonly seen in specimens from asthma patients. Ferruginous bodies are dumbbell-shaped, golden yellow to black-colored mineral fibers, and associated with asbestos exposure. Psammoma bodies are structures with concentrically laminated calcification and a circumferential thin layer of epithelial cells; it is commonly seen in both carcinomas and benign conditions. Corpora amylacea are round structures with circumferential and radiating lines; they are a non-specific finding in BAL and sputum specimens.
-
A-140. (a) EGFR mutations are often found in adenocarcinoma with micropapillary and/or the lepidic growth pattern.
Based on the new adenocarcinoma classification proposed by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society, EGFR mutations have been found to be frequently associated with the micropapillary subtype and the lepidic growth pattern (formerly known as bronchioloalveolar carcinoma). Although EGFR mutations are only identified in 10–20 % of surgically resected lung adenocarcinomas in the United States and Europe, they are identified in approximately 50 % of Asians. EGFR and KRAS mutations are mutually exclusive in non-small cell lung carcinomas, and have not been found to occur in the same tumor. In lung adenocarcinomas, patients with EGFR mutations are associated with a good response to EGFR tyrosine kinase inhibitors therapy, and a better survival rate than patients without EGFR mutations. Therefore, the tests of EGFR, KRAS, and other genetic mutations in lung cancer patients are critical for targeted therapies
-
A-141. (b) KRAS mutations are more commonly seen among non-smokers.
KRAS mutations are found in 15–30 % of adenocarcinomas in the United States and Europe. However, they are less commonly seen in tumors of Asian descents. It has been found that KRAS mutations occur more commonly in adenocarcinomas from elderly patients and heavy smokers. Patients with KRAS mutations are unlikely to respond to EGFR tyrosine kinase inhibitor therapy; thus, they are associated with a poor prognosis.
-
A-142. (d) The color and volume of the specimen
According to the laboratory regulation policy of the Health Care Financing Administration (HCFA), the time and date of the sample collected, and the time and date of the sample received by the laboratory must be included and/or documented on the requisition form. The color and volume of the specimen is not required to include on the requisition form by the HCFA.
Footnotes
An erratum to this chapter can be found at http://dx.doi.org/10.1007/978-1-4939-1477-7_14
Contributor Information
Walid E. Khalbuss, Email: walid.khalbuss@ge.com
Qing Kay Li, Email: qli23@jhmi.edu.
Qing Kay Li, Email: qli23@jhmi.edu.
Walid E. Khalbuss, Email: Walid.khalbuss@ge.com.
Reading List
- Bibbo M, Wood MD, Fitzpatrick BT. Peritoneal washings and ovary. In: Bibbo M, Wilbur D, editors. Comprehensive cytopathology. 3. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- Cibas ES. Peritoneal washings. In: Cibas ES, Ducatman BS, editors. Cytology: diagnostic principles and clinical correlates. Philadelphia: Saunders/Elsevier; 2009. [Google Scholar]
- DeMay RM. The art and science of cytopathology, exfoliative cytology. 2. Chicago: ASCP Press; 2012. [Google Scholar]
- Feller-Kopman D, Yung RCW, Burroughs F, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). A study of 135 cases with histology correlation. Cancer Cytopathol. 2009;117:482–90. doi: 10.1002/cncy.20049. [DOI] [PubMed] [Google Scholar]
- Khalbuss WE, Yang H, Lian Q, Elhosseiny A, Pantanowitz L, Monaco SE. The cytomorphologic spectrum of small-cell carcinoma and large-cell neuroendocrine carcinoma in body cavity effusions: a study of 68 cases. Cytojournal. 2011;8:18. doi: 10.4103/1742-6413.86816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalbuss WE, Monaco SE, Pantanowitz L. The ASCP Quick Compendium (QC) of cytopathology. Chicago: ASCP Press; 2013. Chapter 12. Lung and respiratory cytopathology; pp. 212–57. [Google Scholar]
- Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, Thunnissen E, Ladanyi M. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn. doi:10.1016/j.jmoldx.2013.03.001. pii: S1525-1578(13)00041-X. [Epub ahead of print]. 2013;15:415–53. [DOI] [PubMed]
- Monaco SE, Pantanowitz L, Khalbuss WE. Comparing endobronchial ultrasound-guided fine needle aspiration specimens with and without rapid on-site evaluation. Cytojournal. 2012;9:2. doi: 10.4103/1742-6413.92414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munfus-McCray D, Adams C, Harada S, Askin F, Clark DP, Gabrielson E, Li QK. EGFR and KRAS mutations in metastatic lung adenocarcinomas. Hum Pathol. 2011;42:1447–53. doi: 10.1016/j.humpath.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Munfus-McCray D, Cui M, Zhang Z, Askin F, Gabrielson E, Li QK. Comparison of EGFR and KRAS mutations in primary and unpaired metastatic lung adenocarcinoma with potential chemotherapy effect. Hum Pathol. 2013;44:1286–92. doi: 10.1016/j.humpath.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Stoll LM, Johnson MW, Burroughs F, Li QK. Cytological diagnosis and differential diagnosis of lung carcinoid tumors: a retrospective study of 63 cases with histological correlation. Cancer Cytopathol. 2010;118:457–67. doi: 10.1002/cncy.20105. [DOI] [PubMed] [Google Scholar]


