Abstract
Severe acute respiratory syndrome coranovirus-2 (SARS-CoV-2) infection has become an important health-care issue worldwide. The coronavirus disease 2019 (COVID-19) has also raised concerns among patients with inflammatory rheumatic conditions and their treating physicians. There are emerging data regarding the potential risks of SARS-CoV-2 for this particular patient group. However, less is known with regard to the course of COVID-19 among patients receiving IL-17 inhibitors. The aim of the current article is to review the growing body of knowledge on the course/management of COVID-19 in patients with inflammatory rheumatic diseases by presenting a SARS-CoV-2 infected case with ankylosing spondylitis under secukinumab therapy. A 61-year old patient with ankylosing spondylitis who was on secukinumab therapy for 5 months admitted with newly onset fever and gastrointestinal complaints. After being hospitalized, she developed respiratory manifestations with focal pulmonary ground-glass opacities and multiple nodular densities in both lungs. The patient was tested positive for SARS-CoV-2 infection. Substantial clinical improvement was obtained following a management plan, which included tocilizumab, hydroxychloroquine, prednisolone and enoxaparin sodium. PubMed/MEDLINE and Scopus databases were searched by using relevant keywords and their combinations. The literature search revealed four articles reporting the clinical course of COVID-19 in seven rheumatic patients on secukinumab. The clinical course of SARS-CoV-2 infection was mild in most of these patients, while one of them experienced severe COVID-19. Interleukin-17 has been related to the hyperinflammatory state in COVID-19 and IL-17 inhibitors were presented as promising targets for the prevention of aberrant inflammation and acute respiratory distress in COVID-19. However, this hypothesis still remains to be proved. Further studies are warranted in order to test the benefits and risks of IL-inhibitors in SARS-CoV-2 infected individuals.
Keywords: Ankylosing spondylitis, Biological drugs, COVID-19, Interleukin-17, Rheumatic diseases, Secukinumab, Spondyloarthritis
Introduction
Coronaviruses are a family of zoonotic viruses. Seven of them including the severe acute respiratory syndrome coranovirus-2 (SARS-CoV-2) are known to cause human disease [1]. SARS-CoV-2 related disease is named as the coronavirus disease 2019 (COVID-19). The main SARS-CoV-2 proteins that play role in virus-human cell interaction are the spike, envelope, membrane and nucleocapsid proteins [2]. The host cell-receptor of SARS-CoV-2 is the angiotensin-converting enzyme 2 (ACE2), which is expressed by several human cells such as the pulmonary epithelial cells, endothelial cells, enterocytes, kidney proximal tubular cells, and the liver cells [3–5]. Given this fact, SARS-CoV-2 infection may have a wide range of clinical manifestations, while acute respiratory syndrome is the prominent feature of the disease [3].
Following the outbreak of SARS-CoV-2 infection, a concern has arisen over its impact on immune compromised patients. The maintenance of biological disease modifying anti-rheumatic drugs (bDMARDs) during the pandemic has become a burden. A survey among rheumatology practitioners from India revealed that most of the respondents agreed about the need for a change in the management plans of patients with rheumatic diseases. Besides, the results showed that 47.5% of the participants reduced the use of bDMARDs [6]. Immunobiological agents have been associated with an increased risk of viral, bacterial, granulomatous and opportunistic infections. On the other hand, immune modulation through biological therapy may also prevent from the robust production of immune cytokines and contribute to a less aggressive response to viral infections [7, 8].
Our understanding about the immunological pathways in COVID-19 is improving rapidly. Interleukin (IL)-17 is one of the pro-inflammatory mediators secreted in coronavirus infection [9]. Knowledge on the course of SARS-CoV-2 infection among patients receiving IL-17 inhibitors is increasing. The aims of the present article were (1) to present the management of COVID-19 in a patient with ankylosing spondylitis under secukinumab therapy and (2) to review the growing literature regarding the course/management of COVID-19 in patients with inflammatory rheumatic diseases.
Case report
A 61-year old female was admitted to the hospital for a 1-day history of fever, vomiting and diarrhea. The anamnesis revealed that she has been complaining of intermittent nausea for almost 1 week. The patient also reported that her sister was diagnosed as COVID-19 the day before her admission. She had a history of ankylosing spondylitis dated back 11 years and over the past 5 months, she had been treated with secukinumab with a good clinical response. The patient has taken the last dose of secukinumab 5 days ago. On physical examination, the body temperature was 38.3 °C, lungs revealed bilateral rales at lower zones. Auscultation of the heart revealed rhythmic tachycardia with no extra heart sounds. The patient was hospitalized for further assessment and management. Laboratory parameters are given in Table 1. After being hospitalized, the patient experienced respiratory manifestations. Computerized tomography scan of the thorax revealed focal pulmonary ground-glass opacities and multiple nodular densities in both lungs. On the first day of hospitalization, the patient was started on favipiravir (loading dose 1600 mg twice on the first day, maintenance dose 600 mg twice daily), oseltamivir (75 mg twice daily) and hydroxychloroquine (loading dose 400 mg twice on the first day, maintenance dose 200 mg twice daily). Sample was collected for SARS-CoV-2 reverse transcription-polymerase chain reaction test (RT-PCR). On the second day of hospitalization, the patient still had fever. She was started on enoxaparin sodium 60 mg daily. On the third day, the patient still had fever, a positive RT-PCR test result for SARS-CoV-2 was obtained and oseltamivir treatment was stopped. Interleukin-6 inhibitor was planned. On the fourth day, the patient showed clinical and radiographical progression. She had constant fever. Intravenous prednisolone (40 mg) and ranitidine (50 mg) were administered. Repeated RT-PCR test for SARS-CoV-2 also resulted positive. IL-6 level was high (13.0 pg/mL). The patient was given a single dose of 400 mg tocilizumab via intravenous infusion. On the fifth day, favipiravir and hydroxychloroquine treatments were stopped. The patient showed substantial clinical improvement shortly after tocilizumab therapy. RT-PCR test for SARS-CoV-2 resulted negative. She was discharged from the hospital on the 8th day of admission. She continued daily enoxaparin sodium 60 mg for 2 weeks till follow-up visit. At the follow-up visit (2 weeks after discharge), she had no complaints and physical examination was normal. Rapid IgM-IgG Combined Antibody Test for COVID-19 was positive. Blood samples were collected for laboratory analysis (Table 1). The patient was not given the maintenance dose of secukinumab therapy. At the second follow up visit (7 weeks after discharge), the patient underwent a CT scan, which revealed significant improvement. Given the two negative RT-PCR test results for SARS-CoV-2 and complete clinical recovery, secukinumab infusion was performed just after the second follow-up visit. The timeline of the disease course is depicted in Fig. 1. Written informed consent was obtained from the patient for the publication of her patient data.
Table 1.
Laboratory parameters during the course of coronavirus disease 2019 in the patient with ankylosing spondylitis
| Pre-infectiona | Hospitalization | Follow-upb | |
|---|---|---|---|
| WBC (µL) (4800–10,800) | 8200 | 5400 | 4900 |
| Neutrophil (µL) (1800–7700) | 4000 | 3200 | 1500 |
| Lymphocyte (µL) (1000–4800) | 3400 | 1400 | 2500 |
| Monocyte (µL) (300–800) | 700 | 800 | 700 |
| Hct (%) (36–46) | 39.5 | 40 | 39.8 |
| Platelets (µL) (130,000–400,000) | 268,000 | 222,000 | 219,000 |
| ESR (mm/h) (0–30) | 23 | 34 ↑ | – |
| CRP (mg/L) (0–8) | 4.71 | 10.6 ↑ | 1.15 |
| ALT (U/L) (7–35) | 16 | 22 | 46 |
| AST (U/L) (15–41) | 19 | 32 | 27 |
| LDH (U/L) (115–248) | – | 258 ↑ | – |
| BUN (mg/dL) (8–20) | 11.9 | 11 | 10.3 |
| CK (U/L) (38–234) | – | 53 | – |
| Creatinine (mg/dL) (0.4–1.0) | 0.58 | 0.54 | 0.56 |
| PT (s) (11–15) | – | 11.6 | 10.7 |
| aPTT (s) (20–35) | – | 21.4 | 21 |
| Fibrinogen (mg/dL) (180–380) | – | 427.63 ↑ | – |
| Ferritin (ng/mL) (11–307) | – | 43.1 | – |
| D-dimer (mg/L) (0–0.55) | – | 0.4 | – |
WBC white blood cell, Hct hematocrit, ESR erythrocyte sedimentation rate, AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactic dehydrogenase, CK creatinine kinase, PT prothrombin time, aPTT activated partial thromboplastin time
a2 months before COVID-19
b2 weeks after discharge
Fig. 1.
Timeline of the disease course
Search strategy
The literature search was conducted through PubMed/MEDLINE and Scopus by using the keywords “coronavirus disease 2019”, “SARS-CoV-2”, “inflammatory rheumatic diseases”, “ankylosing spondylitis”, “spondyloarthropathy”, “secukinumab”, “disease modifying anti-rheumatic drugs”, and “biological drugs”. The inclusion criteria were as follows: (1) observational and/or interventional studies, (2) case series/case reports, (3) brief reports, (4) narrative reviews, systematic reviews, meta-analyses, (5) letters/correspondences, and (6) articles written in English language. Unpublished items and abstracts were excluded [10] (Fig. 2).
Fig. 2.
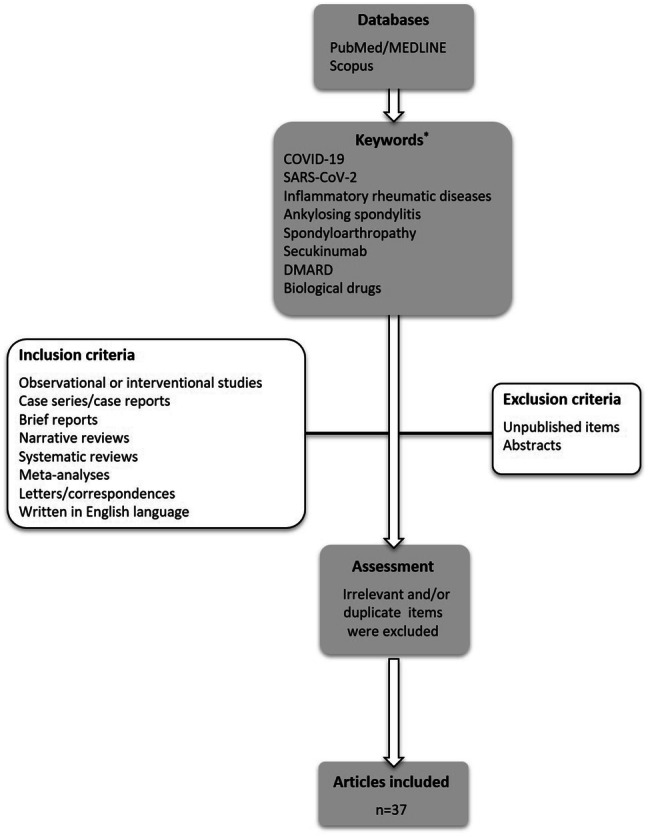
Flowchart of the case-based review. COVID-19: coronavirus disease 2019, SARS-C0V-2: severe acute respiratory syndrome coronavirus-2, DMARD: disease modifying anti-rheumatic drug. * Initial search was done on May 9, 2020 by using the provided keywords. To retrieve more recent articles, a second search was performed on June 12, 2020 by using the keywords “secukinumab”, “coronavirus disease 2019”, and “SARS-CoV-2”. Relevant items were further included
The risk of COVID-19 in patients with inflammatory rheumatic diseases
Immunocompromised patients are at high risk of several infectious diseases including the viral infections. In this regard, SARS-CoV-2 infection emerged as a source of concern for patients with inflammatory rheumatic conditions, particularly for those receiving immunosuppressants including bDMARDs [5]. There are a number of reports examining the risk of COVID-19 in rheumatic conditions. Favalli et al. collected data from patients on bDMARDs [11]. Of the sample (n = 530), 10 reported contact with established COVID-19 cases. However, none developed any symptoms of infection. Of the included patients, three (1 sarcoidosis, 1 axial SpA and 1 PsA) experienced mild COVID-19 with positive nasopharyngeal swab for SARS-CoV-2. All three patients were on bDMARDs (adalimumab, infliximab or secukinumab). Yet, there could be some factors interfered with the results. Firstly, almost 90% of the patients reported that they took preventive measures strictly against COVID-19. Another potential bias is that the survey was unable to identify asymptomatic SARS-CoV-2 infected patients. Therefore, the researchers concluded that the real incidence of SARS-CoV-2 infection among patients with inflammatory rheumatic diseases might be higher [11]. Emmi et al. conducted a survey among patients with systemic autoimmune diseases [8]. Of the 458 patients interviewed, 41% were on bDMARD therapy. Thirteen patients reported symptoms compatible with COVID-19. Seven patients undergone nasopharyngeal swab, one resulted as positive for SARS-CoV-2 infection and developed severe COVID-19. Overall, the prevalence of SARS-CoV-2 infection among patients with systemic autoimmune diseases was reported as 0.22%, which was similar to that observed in the general population (0.20%) [8]. Monti et al. collected information on patients with chronic arthritis treated with bDMARDs or targeted synthetic DMARDs [12]. Of the patients, four reported symptoms compatible with SARS-CoV-2 infection, four had confirmed COVID-19 identified through rhinopharyngeal swabs [12]. A survey by Rosenbaum et al. revealed that IL-17 inhibitors are associated with a rate of 1.3% for COVID-19 infection [13]. Studies from other disciplines did not report any confirmed COVID-19 cases among secukinumab users [14, 15].
Patients at older age, with high rheumatic disease activity and/or systemic comorbidities (coronary heart disease, hypertension, diabetes) would be at higher risk of COVID-19 [5, 16]. Biomarkers predictive of poorer clinical outcomes are lymphopenia, increased C-reactive protein, lactic dehydrogenase, D-dimer and IL-6 levels. However, these risk factors for poorer outcome are not specific to inflammatory rheumatic conditions [17].
Given the data so far, pre-emptively discontinuation of biological therapy is not recommended in patients with no signs of SARS-CoV-2 infection [16, 18, 19]. Following a survey study on 123 adult patients with connective tissue diseases, Favalli et al. concluded as patients should maintain ongoing anti-rheumatic treatments while adhering strictly to the norms of infection prevention [20]. It should be noted that any flare due to drug cessation would put these individuals at higher risk of infection [4].
Clinical course of COVID-19 in patients with inflammatory rheumatic diseases
The clinical picture of COVID-19 varies from no symptoms to a severe respiratory tract infection with bilateral pneumonitis [21]. Initial data from the COVID-19 Global Rheumatology Alliance revealed that the most common COVID-19 symptoms at onset among patients with rheumatic diseases were fever and respiratory system manifestations [22]. However, SARS-CoV-2 infected patients may also present with other distinct symptoms including those related to the gastrointestinal tract. The mechanism underlying the digestive symptoms in SARS-CoV-2 infected patients is the expression of ACE2 receptors by the gastrointestinal epithelial cells [3]. The current case was admitted with vomiting, diarrhea and fever. After being hospitalized, she also developed a rapidly progressive pneumonia. A multi-center study by Pan et al. revealed that 50.5% of 204 patients with COVID-19 reported digestive symptoms [23]. Six of the patients had digestive symptoms with no accompanying respiratory tract manifestations. Patients with digestive manifestations revealed longer prothrombin time, higher liver enzymes and monocyte counts than those without digestive symptoms [23]. Compatible with these findings, the monocyte counts of our patient were at the upper limit of the normal range. The liver enzyme levels were in normal ranges at admission, but markedly increased when compared to pre-infection results. Further increase in alanine aminotransferase, lactic dehydrogenase and aspartate aminotransferase was observed during the hospitalization period. On the other hand, the prothrombin time was normal.
There are a number of articles reporting the clinical course of COVID-19 among patients on secukinumab therapy (Table 2) [11, 24–26]. Favalli et al. reported that one patient receiving secukinumab for PsA was managed at home with no any respiratory complication related to COVID-19 [11]. Haberman et al. identified 86 patients with immune-mediated inflammatory disease who had either confirmed or highly suspected COVID-19 infection. As for the confirmed COVID-19 cases (n = 59), four were on secukinumab therapy. Of those patients, one required hospitalization, however discharged at day 3 [26]. Another patient reported by Di Lernia et al. recovered with two negative RT-PCR results following a course of hydroxychloroquine therapy [24]. There are also reports regarding the favorable outcomes of SARS-CoV-2 infected patients who were on guselcumab (IL-23 inhibitor) and ixekizumab (IL-17A inhibitor) [26–28]. Contrary with these reports on mild cases, Sharmeen et al. presented a severe case of COVID-19 who was on secukinumab therapy for 16 months. The 78-year old patient with multiple comorbidities remained intubated with mechanical ventilation [25].
Table 2.
The characteristics of the patients with confirmed coronavirus disease 2019 who were on secukinumab therapy
| Article | Age/sex | Disease | Duration on secukinumab therapy | Medical history | Presenting COVID-19 symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Favalli [11] | 68/female | PsA | NR | NR | NR |
Not hospitalized Treatment details: NR |
Managed at home with no any respiratory complication |
| Di Lernia [24] | 73/female | PsA | 12–13 months |
Hypertension Tachycardia Osteoporosis Fractures Hyperuricemia |
Fever Sore throat Mild cough |
HQ regimen (800 mg/day for 2 days, followed by 400 mg/day for 5 days) | Recovered, 2 negative RT-PCR tests |
| Sharmeen [25] | 78/male | AS | 16 months |
Medications: Amlodipine Hydrochlorothiazide Losartan Nortriptyline Levothyroxine Rosuvastatin Tamsulosin |
Fever Severe dry cough Fatigue Myalgia Shortness of breath Frontal headache Lightheadedness |
Hospitalized HQ and azithromycin (5 days) Mechanical ventilation |
Remained intubated with mechanical ventilation |
| Haberman [26] | 51/male | PsA | NR |
Hypertension BMI > 35 |
Fever Cough Shortness of breath |
Hospitalized HQ and azithromycin |
Discharged at day 3 |
| Other cases with confirmed COVID-19 (n = 3) did not require hospitalization | |||||||
PsA psoriatic arthritis, COVID-19 coronavirus disease 2019, RT-PCR reverse transcription-polymerase chain reaction test, AS ankylosing spondylitis, NR not reported, BMI body mass index
Age is one of the main risk factors for developing severe COVID-19. Although the present case was 61 years old, she was responsive to treatment and did not require intensive care. Duret et al. reported the favorable outcome of a 60-year old COVID-19 case with spondylarthritis receiving etanercept and methotrexate [29]. Similarly, Song et al. reported the full recovery from COVID-19 in a 61-year old patient on conventional synthetic DMARD therapy [30]. Reports on elderly patients treated with secukinumab revealed favorable outcomes for SARS-CoV-2 infection. However, one case developed severe COVID-19 (Table 2).
Currently, there is no suggestion as treatment with biologics may predispose to a more serious or lethal form of SARS-CoV-2 infection [12]. It is even possible that elevated type I interferon in autoimmune diseases and the use of certain conventional synthetic and/or bDMARDs have a potentially protective effect [1]. On the other hand, rheumatic patients with systemic involvements (i.e. arterial hypertension, coronary heart disease, and lung involvement) are at higher risk for a severe disease course [31, 32]. The growing body of evidence will help to improve our understanding on the particular topic.
Management of COVID-19 in patients with inflammatory rheumatic diseases
Several drugs used in rheumatology practice have been proposed as potential treatments for SARS-CoV-2 infected patients [33, 34]. Hydroxychloroquine can increase the endosomal pH, inhibit the toll-like receptor activity and interfere with the terminal glycosylation of ACE-2 [33]. For COVID-19, it was recommended to continue hydroxychloroquine 200 mg twice daily for 4 days following a loading dose of 400 mg twice daily [35]. Nevertheless, its potential risks are important and need to be further studied. Although being on steroid therapy is considered as a risk factor for SARS-COV-2 infection, intravenous use of corticosteroids can be considered for SARS-COV-2 infected patients to manage excessive inflammation and to prevent ARDS development [36, 37]. World Health Organization does not recommend routine use of steroid therapy for viral infections [38]. If steroid therapy is indicated, a close monitoring of the patient in terms of hyperglycemia, hypernatremia, and hypokalemia is required [38]. In COVID-19, persistent viral load contributes to the development of T cell abnormalities and cytokine release syndrome. A significant increase in cytokines such as TNF-α, IL-1β, IL-6 and IL-10, as well as chemokines such as CXC-motif chemokine ligand 10 (CXCL10) and CXC-motif chemokine ligand 2 (CXCL2) is observed in COVID-19. Since IL-6 plays a critical role in cytokine-release syndrome, blocking IL-6 by tocilizumab has gained interest in COVID-treatment [5, 33]. Tocilizumab was shown to improve hypoxemia, symptoms and CT opacities shortly after administration [39]. Janus-kinase and IL-1 inhibitors may have the potential to manage cytokine overproduction in COVID-19 [33, 40]. However, further evidence is required in terms of their efficacy and safety [5]. The present case was started on hydroxychloroquine immediately after admission. As the lung involvement deteriorated, the patient was given a single dose of tocilizumab 400 mg. She was also administered a single dose of prednisolone 40 mg. A dramatic improvement in respiratory symptoms and signs was observed after the use of IL-6 inhibitor.
Another important issue in SARS-CoV-2 infected patients with inflammatory rheumatic conditions is the decision of biological therapy cessation. In the setting of confirmed/presumptive COVID-19, as well as following known SARS-CoV-2 exposure, withholding the immunosuppressant therapy should be considered [17, 19, 40]. In select circumstances, IL-6 inhibitors may be continued [17]. After recovering from COVID-19 and two negative test results for SARS-CoV-2, the course of bDMARD therapy can be planned. This important decision should be given jointly by the rheumatology and infectious diseases teams [1]. SARS-CoV-2 infection might induce disease flares as well as other acquired viral infections in patients with inflammatory rheumatic diseases [6]. The present case did not experience a severe flare despite intercurrent viral stimulus. It could be related to the administration of secukinumab just before the development of COVID-19 related symptoms. Inhibition of IL-17 might also have prevented this case from excessive hyperinflammation during the disease course. This immature point of view will be further discussed below.
The potential role of IL-17A in COVID-19
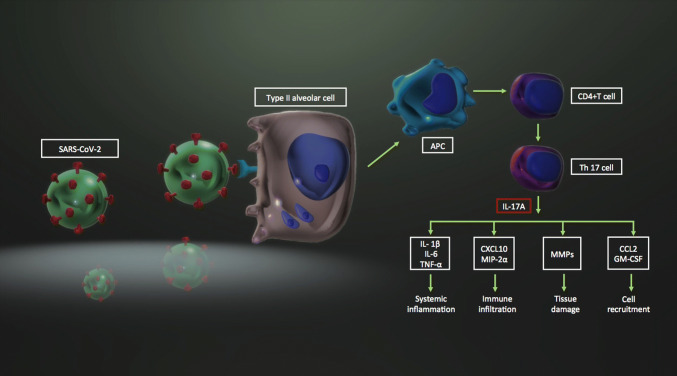
The immunologic response to SARS-CoV-2 is characterized by vigorous production of inflammatory cytokines such as TNF-α, IL-6, IL-10, IFN-ɣ, and IL-1β [9]. Interleukin-17 (formally IL-17A) is another potential cytokine which may have role in SARS-CoV-2 related severe lung inflammation [41–43]. IL-17 is strongly associated with lung injury Murray score in COVID-19 patients. Therefore, IL-17 is proposed as a predictor of disease severity [44]. Supporting this finding, increased number of CC-chemokine receptor 6 + Th17 cells were detected in severe COVID-19 [36]. In response to any viral respiratory infection including the COVID-19, IL-17 is secreted mainly from the T helper 17 (Th17) cells in the lung. Interleukin-17 activates the induction of cytokines (i.e. IL-1β, IL-6, TNF-α), chemokines (i.e. CXCL10, macrophage inflammatory protein 2-alpha), other inflammatory mediators (i.e. granulocyte-colony stimulating factor, monocyte chemoattractant protein-1), matrix metalloproteinases and growth factors [42, 43, 45]. IL-17 may also promote viral persistence by inhibiting the apoptosis of virus-infected cells [41, 46]. This Th17-associated cytokine release syndrome (cytokine storm) might result in systemic inflammation, fever, recruitment of immune infiltrates, neutrophil influx, coagulopathy, tissue damage and pulmonary edema [43] (Fig. 3).
Fig. 3.
The potential contribution of IL-17A to the hyperinflammatory state in SARS-CoV-2 infection. SARS-C0V-2 severe acute respiratory syndrome coronavirus-2, APC antigen presenting cell, CD cluster of differentiation, Th17 T helper 17, IL interleukin, TNF-α tumor necrosis factor-alpha, CXCL10 CXC-motif chemokine ligand 10, MIP-2α macrophage inflammatory protein 2-alpha, MMP matrix metalloproteinase, CCL2 CC-motif chemokine ligand 2, GM-CSF granulocyte monocyte colony stimulating factor
Given the above-mentioned pathophysiology, inhibition of IL-17 has gained attention as a potential management option in COVID-19 [43, 47, 48]. Researchers suggested the use of fedratinib, which can decrease the expression of IL-17 by murine TH17 cells [43]. In this regard, secukinumab might also have the potential to suppress Th17 related cytokine storm. One could avoid cytokine inhibition which is regarded as the suppression of immune system during a viral pandemic. Nevertheless, targeting certain cytokines (TNF, IL-6, IL-17A, IL-23 or IL-4/IL-13) with antibodies may exert benefits in terms of inhibiting the hyper-inflammatory state in COVID-19. It has been postulated that blocking IL-17 might not impair viral clearance [18]. Therefore, IL-17A stands as a plausible promising target for the prevention of aberrant inflammation and acute respiratory distress in COVID-19 [48]. However, further studies are warranted in order to test this immature hypothesis.
Discussion
We reported the management of COVID-19 in a patient with ankylosing spondylitis treated with secukinumab. The case presented with gastrointestinal symptoms and developed respiratory manifestations following the admission. Substantial improvement was achieved with hydroxychloroquine, prednisolone, tocilizumab and enoxaparin sodium. There are four articles, so far, reporting the outcomes of COVID-19 in patients with inflammatory rheumatic conditions who were on secukinumab treatment. In general, the outcomes are favorable [11, 24–26]. However, there is one report regarding an elderly multi-comorbid patient who remained intubated with severe COVID-19 [25]. Age and systemic comorbidities can increase the risk of developing severe and/or lethal form of COVID-19.
There is emerging knowledge about the risk and/or course of COVID-19 in patients with inflammatory rheumatic diseases treated with bDMARDs. The prevalence of SARS-CoV-2 infection was reported as 1.3% among patients receiving secukinumab therapy [13]. Secukinumab therapy may be continued unless confirmed/suspected COVID-19 or any exposure to a SARS-CoV-2 is present. Otherwise, systemic inflammation and a flare-up due to drug cessation would bear much risk for those patients. On the other hand, in patients infected with SARS-CoV-2, maintenance of secukinumab following drug cessation can be considered after a complete clinical recovery and/or two consecutive negative results of RT-PCR for SARS-CoV-2.
Interleukin-17 is one of the pro-inflammatory mediators that has been related to the hyperinflammatory state in COVID-19 [41–43]. It may induce several cytokines/chemokines, matrix metalloproteinases and growth factors, thereby can trigger cell recruitment, immune infiltration and systemic inflammation. Th-17 related hyperinflammation may be considered as a target in COVID-19 treatment [43, 47, 48]. In this regard, IL-17 inhibitors may serve as potential inhibitors of cytokine storm in SARS-CoV-2 infection. Nevertheless, less is known about their potential benefits and risks for SARS-CoV-2 infected patients with inflammatory rheumatic conditions.
Limitations
As the literature lacks of data on the consequences of COVID-19 among patients receiving IL-17 inhibitors, we were unable to compare the outcomes of the current case with similar reports. Next, the review was based upon a single case. Therefore, it would not be possible to generalize the findings and/or outcomes of the current case to the whole SARS-CoV-2 infected patients with inflammatory rheumatic diseases.
Concluding remarks
Patients with inflammatory rheumatic diseases including those on bDMARD therapy should be advised to apply preventive measures (social isolation, wearing mask, washing hands, etc.).
Biological DMARDs may be continued unless patients have confirmed/presumptive COVOD-19 or any known history of SARS-CoV-2 exposure.
After the recovery from COVID-19, maintenance of bDMARD therapy should be decided jointly by the treating rheumatology and infectious disease physicians.
IL-17 is related to the hyperinflammatory state in SARS-CoV-2 infection. However, the potential role of IL-17 inhibition in the clinical course of COVID-19 still needs to be further studied.
The clinical course of COVID-19 among patients with inflammatory rheumatic conditions treated with secukinumab is mild, in general. However, the reported number of patients are limited and future larger studies are required to have a clear conclusion.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest regarding the publication of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments.
Consent for publication
Written informed consent for print and electronic publication of the case report was obtained from the patient.
Disclaimer
No part of the review is published elsewhere. The case is going to be reported to the TLAR and the EULAR COVID-19 Registry.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ilke Coskun Benlidayi, Email: icbenlidayi@hotmail.com.
Behice Kurtaran, Email: behicekurtaran@gmail.com.
Emre Tirasci, Email: emre_tirasci@hotmail.com.
Rengin Guzel, Email: renginguzel@gmail.com.
References
- 1.Askanase AD, Khalili L, Buyon JP. Thoughts on COVID-19 and autoimmune diseases. Lupus Sci Med. 2020;7:e000396. doi: 10.1136/lupus-2020-000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasparyan AY, Misra DP, Yessirkepov M, Zimba O. Perspectives of immune therapy in coronavirus disease 2019. J Korean Med Sci. 2019;35:e176. doi: 10.3346/jkms.2020.35.e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, Tu L. Implications of gastrointestinal manifestations of COVID-19. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceribelli A, Motta F, De Santis M, Ansari AA, Ridgway WM, Gershwin ME, Selmi C. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun. 2020;109:102442. doi: 10.1016/j.jaut.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev. 2020;19:102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta L, Misra DP, Agarwal V, Balan S, Agarwal V. Management of rheumatic diseases in the time of covid-19 pandemic: perspectives of rheumatology practitioners from India. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217509. [DOI] [PubMed] [Google Scholar]
- 7.Serrato VA, Azevedo VF, Sabatoski V, Gonçalves BP, Machado DM. Influenza H1N1 infection in a patient with psoriatic arthritis in treatment with Adalimumab: a case report. Clin Rheumatol. 2013;32(Suppl 1):S21–S23. doi: 10.1007/s10067-010-1415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmi G, Bettiol A, Mattioli I, Silvestri E, Scala GD, Urban ML, Vaglio A, Prisco D. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409–1417. doi: 10.1007/s00296-011-1999-3. [DOI] [PubMed] [Google Scholar]
- 11.Favalli EG, Ingegnoli F, Cimaz R, Caporali R. What is the true incidence of COVID-19 in patients with rheumatic diseases? Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217615. [DOI] [PubMed] [Google Scholar]
- 12.Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79:667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum JT, Hamilton H, Choi D, Weisman MH, Reveille JD, Winthrop KL. Biologics, spondylitis and COVID-19. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217941. [DOI] [PubMed] [Google Scholar]
- 14.Giulia R, Alice R, Teresa FM, Paolo D, Simone R. Moderate to severe hidradenitis suppurativa under systemic therapy during the COVID-19 outbreak. Dermatol Ther. 2020 doi: 10.1111/dth.13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galluzzo M, D'Adamio S, Silvaggio D, Lombardo P, Bianchi L, Talamonti M. In which patients the best efficacy of secukinumab? Update of a real-life analysis after 136 weeks of treatment with secukinumab in moderate-to-severe plaque psoriasis. Expert Opin Biol Ther. 2020;20:173–182. doi: 10.1080/14712598.2020.1708897. [DOI] [PubMed] [Google Scholar]
- 16.McInnes IB. COVID-19 and rheumatology: first steps towards a different future? Ann Rheum Dis. 2020;79:551–552. doi: 10.1136/annrheumdis-2020-217494. [DOI] [PubMed] [Google Scholar]
- 17.Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, Chatham W, Cohen S, Costenbader K, Gravallese EM, Kalil AC, Weinblatt ME, Winthrop K, Mudano AS, Turner A, Saag KG. American College of Rheumatology guidance for the management of adult patients with rheumatic disease during the COVID-19 pandemic. Arthritis Rheumatol. 2020 doi: 10.1002/art.41301. [DOI] [PubMed] [Google Scholar]
- 18.Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20:271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists' perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020 doi: 10.1007/s10067-020-05073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favalli EG, Agape E, Caporali R. Incidence and clinical course of COVID-19 in patients with connective tissue diseases: a descriptive observational analysis. J Rheumatol. 2020 doi: 10.3899/jrheum.200507. [DOI] [PubMed] [Google Scholar]
- 21.Pope JE. What does the COVID-19 pandemic mean for rheumatology patients? Curr Treatm Opt Rheumatol. 2020 doi: 10.1007/s40674-020-00145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianfrancesco MA, Hyrich KL, Gossec L, Strangfeld A, Carmona L, Mateus EF, Sufka P, Grainger R, Wallace Z, Bhana S, Sirotich E, Liew J, Hausmann JS, Costello W, Robinson P, Machado PM, Yazdany J, COVID-19 Global Rheumatology Alliance Steering Committee Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Lernia V, Bombonato C, Motolese A. COVID-19 in an elderly patient treated with secukinumab. Dermatol Ther. 2020 doi: 10.1111/dth.13580. [DOI] [PubMed] [Google Scholar]
- 25.Sharmeen S, Elghawy A, Zarlasht F, Yao QP. COVID-19 in rheumatic disease patients on immunosuppressive agents. Semin Arthritis Rheum. 2020;50:680–686. doi: 10.1016/j.semarthrit.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haberman R, Axelrad J, Chen A, Castillo R, Yan D, Izmirly P, Neimann A, Adhikari S, Hudesman D, Scher JU. Covid-19 in immune-mediated inflammatory diseases—case series from New York. N Engl J Med. 2020 doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messina F, Piaserico S. SARS-CoV-2 infection in a psoriatic patient treated with IL-23 inhibitor. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balestri R, Rech G, Girardelli CR. SARS-CoV-2 infection in a psoriatic patient treated with IL-17 inhibitor. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duret PM, Sebbag E, Mallick A, Gravier S, Spielmann L, Messer L (2020) Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann Rheum Dis. pii: annrheumdis-2020-217362 [DOI] [PMC free article] [PubMed]
- 30.Song J, Kang S, Choi SW, Seo KW, Lee S, So MW, Lim DH. Coronavirus Disease 19 (COVID-19) complicated with pneumonia in a patient with rheumatoid arthritis receiving conventional disease-modifying antirheumatic drugs. Rheumatol Int. 2020;40:991–995. doi: 10.1007/s00296-020-04584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matucci-Cerinic M, Bruni C, Allanore Y, Clementi M, Dagna L, Damjanov NS, de Paulis A, Denton CP, Distler O, Fox D, Furst DE, Khanna D, Krieg T, Kuwana M, Lee EB, Li M, Pillai S, Wang Y, Zeng X, Taliani G. Systemic sclerosis and the COVID-19 pandemic: World Scleroderma Foundation preliminary advice for patient management. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217407. [DOI] [PubMed] [Google Scholar]
- 32.Nasonov EL. Coronavirus disease (COVID-19): rheumatological prospects/relevance. Rheumatol Sci Pract. 2020;58:123–132. doi: 10.14412/1995-4484-2020-123-132. [DOI] [Google Scholar]
- 33.Benucci M, Damiani A, Infantino M, Manfredi M, Quartuccio L. Old and new antirheumatic drugs for the treatment of COVID-19. Joint Bone Spine. 2020;87:195–197. doi: 10.1016/j.jbspin.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgiev T. Coronavirus disease 2019 (COVID-19) and anti-rheumatic drugs. Rheumatol Int. 2020;40:825–826. doi: 10.1007/s00296-020-04570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu C, Li S, Liu Y. Role of immunosuppressive therapy in rheumatic diseases concurrent with covid-19. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217460. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. https://apps.who.int/iris/handle/10665/331446. Accessed 9 May 2020
- 39.Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cron RQ, Chatham WW. The rheumatologist's role in COVID-19. J Rheumatol. 2020;47:639–642. doi: 10.3899/jrheum.200334. [DOI] [PubMed] [Google Scholar]
- 41.Cafarotti S. Severe acute respiratory syndrome-coronavirus-2 infection and patients with lung cancer: the potential role of interleukin-17 target therapy. J Thorac Oncol. 2020 doi: 10.1016/j.jtho.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tufan A, Avanoğlu Güler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Zhang C, Huang F, Yang Y, Wang F, Yuan J, Zhang Z, Qin Y, Li X, Zhao D, Li S, Tan S, Wang Z, Li J, Shen C, Li J, Peng L, Wu W, Cao M, Xing L, Xu Z, Chen L, Zhou C, Liu WJ, Liu L, Jiang C. Elevated plasma level of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl Sci Rev. 2020 doi: 10.1093/nsr/nwaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muir R, Osbourn M, Dubois AV, Doran E, Small DM, Monahan A, O'Kane CM, McAllister K, Fitzgerald DC, Kissenpfennig A, McAuley DF, Ingram RJ. Innate lymphoid cells are the predominant source of IL-17A during the early pathogenesis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;193:407–416. doi: 10.1164/rccm.201410-1782OC. [DOI] [PubMed] [Google Scholar]
- 46.Megna M, Napolitano M, Fabbrocini G. May IL-17 have a role in COVID-19 infection? Med Hypotheses. 2020;140:109749. doi: 10.1016/j.mehy.2020.109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raucci F, Mansour AA, Casillo GM, Saviano A, Caso F, Scarpa R, Mascolo N, Iqbal AJ, Maione F. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacha O, Sallman MA, Evans SE. COVID-19: a case for inhibiting IL-17? Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]