Background:
Previous research found that infants who were exposed to high levels of arsenic in utero had an increased risk of infectious disease in the first year of life. This prospective study examined the association between arsenic exposures during gestation, and respiratory, diarrheal, and febrile morbidity in children 4–5 years of age.
Methods:
A cohort of pregnant women was recruited in 2008–2011 in Bangladesh. Their children (N = 989) were followed, and household drinking water samples were collected during pregnancy, toddlerhood (12–40 months of age), and childhood (4–5 years of age). We actively surveyed mothers every 2 weeks regarding their children’s infectious diseases symptoms from 4 to 5 years of age. Poisson regression models were used to estimate the association between arsenic exposure and respiratory and febrile illness.
Results:
Median drinking water arsenic was 4.6, 8.8, and 4.2 µg/L in pregnancy, toddlerhood, and childhood, respectively. We observed 0.01, 1.2, and 1.0 cases per 100 person-days of diarrhea, respiratory, and febrile illness, respectively. The incident rate ratios (IRRs) for each doubling of drinking water arsenic during pregnancy were 1.10 (95% confidence interval [CI] = 1.00, 1.22) and 0.93 (95% CI = 0.82, 1.05) for respiratory and febrile illness, respectively, after adjusting for covariates. The association between arsenic exposure measured during toddlerhood and childhood was attenuated and not significantly associated with either outcome. Diarrheal disease was too infrequent to assess.
Conclusions:
Drinking water arsenic exposure during pregnancy was associated with a higher risk of acute respiratory infections in children 4–5 years old in Bangladesh.
Keywords: Arsenic, Bangladesh, Respiratory infection, Pregnancy, Windows of susceptibility, Developmental immunotoxicity
What this study adds
This prospective study observed an exposure–response relationship between gestational arsenic exposure and a modest increased risk of acute respiratory illness in children 4–5 years old. However, arsenic exposure during toddlerhood and childhood was not associated with an increased risk of respiratory illness suggesting that fetal development may be the most susceptible lifestage to the immunotoxic effects of arsenic.
Introduction
Inorganic arsenic is immunotoxic. Yet few epidemiologic studies have examined the effect of environmental exposure to arsenic and immune-related outcomes in pediatric populations. Bangladesh presents a unique opportunity to examine the association between arsenic exposure from drinking water and acute infectious diseases. This is because arsenic exposure from drinking contaminated groundwater remains widespread in this country even though there have been tremendous efforts to remediate this hazard.1,2 Drinking water surveys conducted in 2012–2013 reported one-eighth of sampled wells had arsenic concentrations above the Bangladeshi national guideline of 50 μg/L,3 and one-quarter were above the World Health Organization (WHO) guideline of 10 μg/L.4,5 These data indicate that there are approximately 57 million people exposed to arsenic concentrations from drinking water that exceed WHO recommendations. Furthermore, infectious diseases remain an important cause of morbidity and mortality. In Bangladesh, infectious diseases in children under 5 years of age account for 17%, 6%, 4.5%, and 2% of deaths from lower respiratory infections, intestinal infections and diarrhea, neonatal sepsis and other infections, and vaccine-preventable illnesses, respectively (2015 estimate6).
The potential for inorganic arsenic to influence immunological functioning is well established in experimental models. In vitro studies demonstrate that arsenic selectively inhibits T-cell proliferation, has dose-dependent effects on natural T regulatory lymphocytes, causes apoptosis of monocytic and lymphoid cells, impairs macrophages,7–15 and causes higher morbidity and mortality from influenza.16–18 Additionally, inorganic arsenic can readily cross the placenta. Thus, gestational exposure may also be immunotoxic.19–21 Epidemiologic studies have shown that arsenic exposure from drinking water contributes to altered DNA methylation of leukocytes in expectant mothers and their fetuses.7,22–24 Data collected in a large prospective study in Bangladesh also reported a positive dose–response between gestational arsenic exposure and increased risk of lower respiratory infection and diarrhea in children during their first year of life.25 In the same cohort, arsenic was also associated with decreased bacillus Calmette-Guerin (BCG, anti-Tb) vaccine response,26 increased total IgG and IgE, and decreased mumps-specific IgG.27 Another prospective study conducted in the United States reported a dose–response association between gestational arsenic exposure and a higher risk of respiratory infections requiring prescription medicine in children during the first year of life.28,29 These studies document an association between gestational arsenic exposure and disease in infancy. However, it is unclear if gestational arsenic exposure is also associated with diseases later in childhood or whether arsenic exposure that occurs after fetal development influences susceptibility to infectious diseases.25,28–34
Therefore, we conducted a follow-up study of an existing prospective birth cohort recruited in Bangladesh. This cohort had repeated arsenic exposure measurements available from pregnancy, when the children were 1–2 years of age and through age 5. We also conducted active disease surveillance among the children when they were 4–5 years old. We hypothesized that higher arsenic exposure, as measured in household drinking water, would be associated with an increased incidence of infectious disease symptoms.
Methods
Data and study population
Pregnant women were enrolled in a birth cohort study between January 2008 and June 2011 in Sirajdikhan and Pabna Sadar Upazila, Bangladesh. Details about cohort formation are described elsewhere.35–37 Briefly, women were ≥18 years old, had an ultrasound-confirmed singleton pregnancy ≤16 weeks gestational age, and used the same drinking water source for at least 6 months before enrollment. Dhaka Community Hospital (DCH) provided prenatal and primary care to participants. DCH notified participants of the arsenic concentrations in the water from their household’s well after enrollment. If arsenic levels were above 50 μg/L, the Bangladesh national standard, DCH gave participants arsenic awareness training. After the initial enrollment visit, women completed two additional study visits to the clinic and DCH staff provided monthly home visits. Initially, 1,608 eligible pregnant women were enrolled, of which 224 experienced a fetal loss or neonatal death, and 263 withdrew or were lost to follow-up by 1-month postpartum, leaving 1,121 children remaining for study after 1-month postpartum. A subset of these maternal–child pairs were reenrolled to participate in a follow-up study (2010–2013) that collected samples at 12 months of age and/or between 20 and 40 months of age to explore the effects of metals on neurodevelopment.36 In 2015–2017, children and their caregivers were reenrolled into another follow-up study to explore the relationship between metals and immune function.37 In this follow-up study, children needed to be at least 4 years (±3 months) of age to be eligible for the active disease surveillance which ended at their fifth birthday. The active disease surveillance involved a field staff member visiting participants homes every 2 weeks for the duration of time the child was eligible for observation. Of the 1,121 children eligible from the initial birth cohort, we excluded children if they were unable to be contacted or refused to participate (n = 47), were reported dead (n = 1), were too old (n = 78), or were missing outcome data (n = 6), which left a total sample size of 989. Due to the rolling enrollment of the initial cohort and the active disease surveillance follow-up study, children were observed for varying amounts of time, ranging from a single visit to 29 visits over a year (eTable 1; http://links.lww.com/EE/A72).
Consent was obtained from each participant before initiating any study activity. Consent documents were in Bengali and read aloud by study staff to account for varying literacy rates in this population. This study was approved by the Institutional Review Boards at Dhaka Community Hospital, Oregon State University, and Harvard T.H. Chan School of Public Health.
Arsenic exposure assessment
The exposure of interest was arsenic measured in the family’s primary drinking water source. Drinking water samples were collected up to 6 times throughout the cohort study: at ≤16 completed weeks gestational age, within 1 month of birth, at 12 months, 20–40 months, 4 years, and 5 years of age. Concentrations were averaged to summarize these observations into three distinct time periods corresponding to distinct developmental exposure windows: pregnancy, which encompasses transplacental exposure (enrollment and <1-month postpartum); toddlerhood, where breastfeeding provides protection from arsenic ingestion (12 and 20–40 months); and childhood, where exposure results from food and water ingestion (4 and 5 years).37 Water arsenic measurements were available for all children in pregnancy and childhood, but 117 (12%) were missing in toddlerhood. Thus, analyses were conducted using imputed measurements for the missing water samples and using only complete data.
All water samples were collected following the same protocol. Briefly, 50 ml of water was collected and preserved with ultrapure nitric acid. Samples were analyzed following United State Environmental Protection Agency Method 200.8 by Environmental Laboratory Services, as described previously.38 Quality control procedures demonstrated that the average percent recovery of arsenic from PlasmaCAL multielement Quality Control standard #1 solution (SCP Science, Canada) was 101% (range: 92%–110%). The limit of detection (LOD) was 1 μg As/l, and 2%, 19%, and 28% of samples in pregnancy, toddlerhood, and childhood were below the LOD. These samples were subsequently assigned LOD/2 for analysis.
Infectious disease surveillance and outcomes
As part of the active disease surveillance, caregivers were contacted every 2 weeks between children’s fourth and fifth birthdays and administered a structured questionnaire that asked about symptoms experienced by their children in the past 3 days or past 2 weeks. Symptoms included diarrhea (defined as 3 or more loose stools in a 24-hour period), acute respiratory infection (defined as cough or difficulty breathing), and fever (not medically confirmed). Given that 3-day recall is more accurate and less biased than 2-week recall,39 only 3-day recall outcomes were analyzed in this study. This resulted in 8,592 days of observation. Outcomes were analyzed as incidence rates (number of instances of illness per person-time at risk) to account for the variable at-risk observation period of each child that resulted from the rolling enrollment in this follow-up study and that not all children were able to be enrolled on their fourth birthday.
Covariates
Covariates of interest were selected a priori after reviewing literature on arsenic exposure and infectious disease outcomes to improve our ability to replicate previous research findings.31,40 Information on covariates was collected via questionnaires with responses supplied by either caregivers or medical personnel. Demographic variables included child sex, maternal age at time of initial enrollment into the birth cohort (categorized as above or below the median age of 22 years because exact birthdays are often unknown to rural residents of Bangladesh), monthly family income at time of child’s birth (as reported by husband or father), self-reported maternal education, and gravidity (the number of previous pregnancies reported by the mother regardless of the outcome of those pregnancies). Information from birth outcomes was collected by skilled birth attendants: delivery type (vaginal versus Cesarean) and preterm birth (defined as <37 weeks gestation). Information about environmental and behavioral exposures included environmental tobacco smoke (defined as any smoking around the mother during pregnancy or around the child during childhood, ever/never), the number of hours the mother spent a day cooking over an open fire during pregnancy (continuous), biomass fuel use during pregnancy and childhood (defined as kerosene versus dung/crop residue and wood), sanitary latrine (yes/no), and duration of breastfeeding (continuous). Maternal protein consumption was measured using a validated dish-based food-frequency questionnaire41 collected at 28 weeks gestational age and analyzed as tertiles of protein consumption including fish.
Statistical analysis
Graphical and numerical descriptive statistics were used to examine the distributions of water arsenic exposure, infectious disease outcomes, and selected covariates. Water arsenic levels were natural-log transformed for regression analysis. Multicollinearity of drinking water arsenic exposure measurements and covariates was explored by condition indices and variance decomposition proportions in the “perturb” and “car” packages in R. Variance inflation factors (VIF) were all <2.5 indicating no multicollinearity. Poisson regression was used to estimate the association between water arsenic level and incidence of infectious symptoms using the equation ln (θi) = β0 + β1 ln(Aspregnancy) + β2 ln(Astoddlerhood) + β1 ln(Aschildhood) + covariates, where θi is the incidence rate of disease for child i during the person-time at risk for child i and covariates included child sex, maternal age, household income, maternal education, sanitary latrine, maternal protein consumption, preterm birth, Cesarean birth, biomass fuel burning, environmental tobacco smoke, gravidity, duration of breastfeeding, and hours spent over open fire during pregnancy.
We used multiple imputation by chained equations (MICE) to impute the missing predictor variables in the fully adjusted models using all other predictor variables (12% of toddlerhood drinking water arsenic values and <1.5% of all other variables). We used predictive mean matching (PMM) for continuous variables, logistic regression for binary variables, and multinomial logit regression for categorical variables. A total of 20 imputed datasets were produced. We also reran the analysis using complete case (nonimputed) data and explored effect measure modification by including interaction terms for child sex and each time period of arsenic exposure.
Results
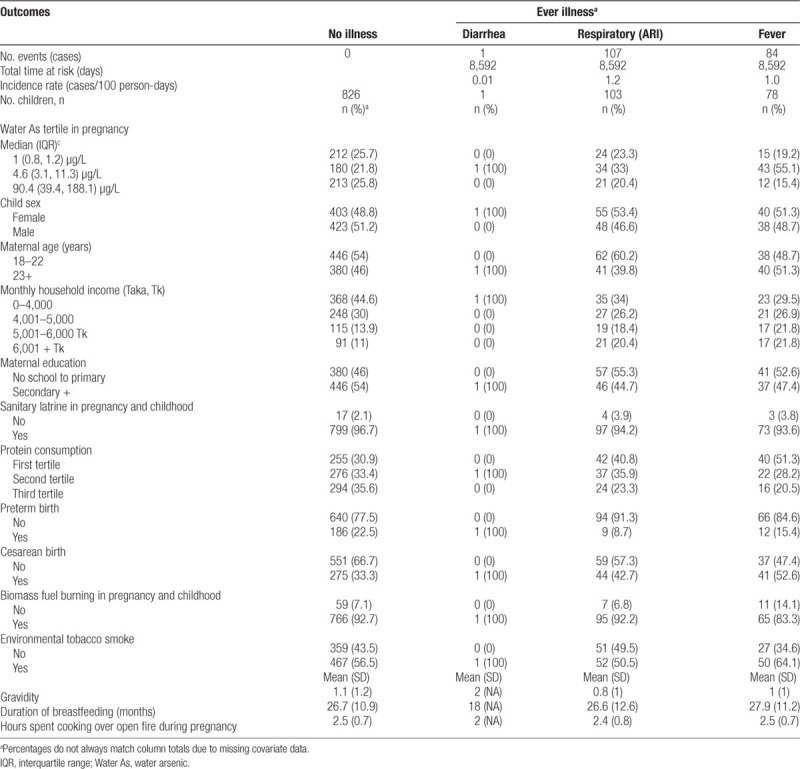
A description of the selected characteristics of the 989 children who were included in this analysis is presented in Table 1. Child sex was evenly distributed between girls and boys. For most mothers (70%), this was their first or second pregnancy, and half of mothers were under 23 years of age. Most mothers received at least a primary education and breastfed for an average of 27 months, and a majority of households had sanitary latrines. Households that never reported illnesses tended to be lower income, have higher maternal protein consumption during pregnancy, have a higher proportion of preterm births but fewer Cesarean births, and more biomass fuel burning in the home. A majority of pregnant women and children were exposed to environmental tobacco smoke, and women spent on average 2.5 hours a day over a cooking fire during pregnancy. Infectious disease symptoms experienced in the last 3 days are reported in Table 1. Symptoms that could indicate acute respiratory illness (ARI) was most frequently observed with 16.5% of children reporting respiratory symptom during the active disease follow-up (4–5 years of age) yielding an incidence rates of 1.2 per 100 person-days. Fever was the next most common symptom with an incidence rate of 1.0 cases per 100 person-days. Only 1 case of diarrhea was reported corresponding to incidence rates of 0.01 cases per 100 person-days. Given the scarcity of diarrhea cases, this outcome was not analyzed further in this study.
Table 1.
Descriptive statistics of selected characteristics by illness status using a 3-day recall period during 12 months of active disease surveillance in a cohort of children age 4–5 years recruited in Bangladesh (N = 989)
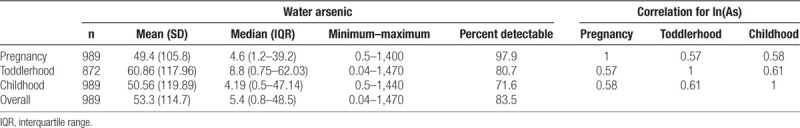
Arsenic levels in drinking water during pregnancy ranged from 1 to 1,400 µg/L. The majority of participants (77%) used wells that met the Bangladesh drinking water action level (Table 1). Generally, drinking water arsenic levels were low. The median arsenic concentration in the household’s drinking water well was 4.6 µg/L when the mother was pregnant with the child (pregnancy), 8.8 µg/L when the child was between the ages of 12 and 40 months (toddlerhood), and 4.2 µg/L when the child was 4–5 years of age (childhood) (Table 2). Water arsenic values were correlated between all three follow-up visits (r = 0.57 to r = 0.61), with an intraclass correlation coefficient of 0.57.
Table 2.
Description of arsenic exposure in participants’ household drinking water during pregnancy, toddlerhood, and childhood
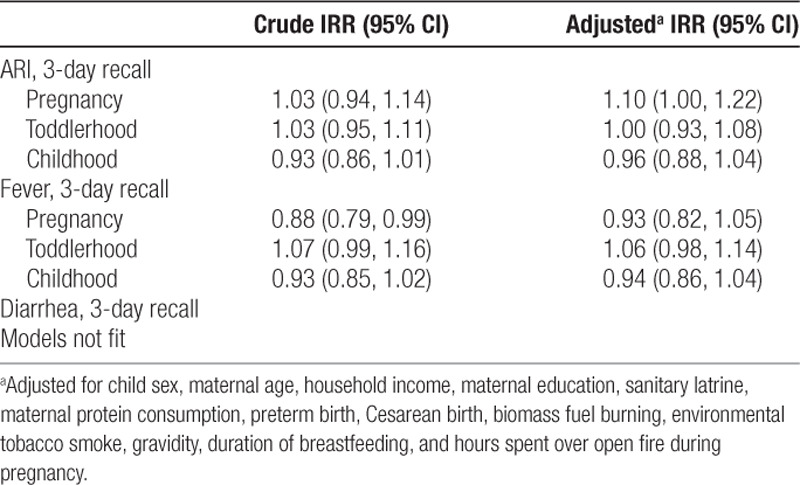
The associations between household drinking water arsenic concentrations at each life stage and disease incidence are presented in Table 3. The association between arsenic exposure during pregnancy and the incidence rate ratio (IRR) for respiratory symptoms in crude models was 1.03 (95% confidence interval [CI] = 0.94, 1.14) only controlling for toddlerhood and childhood arsenic exposures. However, adjusting for covariates strengthened this association, and we observed that each doubling in drinking water arsenic during pregnancy was associated with a 10% increase in ARI (IRR = 1.10, 95% CI = 1.00, 1.22) after controlling for arsenic exposure during toddlerhood and childhood, child sex, maternal age, household income, maternal education, sanitary latrine, maternal protein consumption, preterm birth, Cesarean birth, biomass fuel burning, environmental tobacco smoke, gravidity, duration of breastfeeding, and hours spent over open fire during pregnancy. The association between drinking water arsenic exposure in toddlerhood and childhood and ARI was attenuated (Astoddlerhood IRR = 1.0, 95% CI = 0.93, 1.08; Aschildhood IRR = 0.96, 95% CI = 0.88, 1.04). Arsenic exposure during pregnancy was associated with a lower incidence rate ratio for fever symptoms in models that only adjusted for arsenic exposures at different life stages (IRR = 0.88, 95% CI = 0.79, 0.99). However, after adjusting for other covariates, the associations became null (Aspregnancy IRR = 0.93, 95% CI = 0.82, 1.05). Null associations were also observed in adjusted models between fever and drinking water arsenic in toddlerhood (IRR = 1.06, 95% CI = 0.98, 1.14) and drinking water arsenic in childhood (IRR = 0.94, 95% CI = 0.86, 1.04). These analyses with the complete data produced similar conclusions (eTable 2; http://links.lww.com/EE/A72). The interaction terms for child sex and drinking water arsenic were null at all three time periods of exposure (eTable 3; http://links.lww.com/EE/A72).
Table 3.
Poison models estimated incident rate ratio for respiratory and fever symptoms in children 4–5 years of age per doubling in household drinking water arsenic concentration at the three life stages, Bangladesh, 2008–2017 (N = 989, missing data imputed)
Discussion
In this prospective cohort study in Bangladesh, we observed that exposure to arsenic from drinking water during pregnancy was marginally associated with higher incidence of respiratory systems but not fever in children between the fourth and fifth year of life. However, exposure to arsenic from drinking water during toddlerhood or childhood was not associated with infectious disease symptoms. This attenuation of effect could be due to exposure misclassification from using household water arsenic levels instead of using a biomarker of internal dose. Alternatively, it could also be a function of the timing of the arsenic exposure and the developing fetus being more sensitive to the immunotoxic effects of arsenic.
Our observation that gestational arsenic exposure is associated with higher incidence of respiratory disease symptoms is consistent with other prospective birth cohort studies that showed that higher maternal urinary arsenic levels were associated with more infectious diseases in the first year of life. The Maternal and Infant Nutrition Interventions in Matlab (MINIMat) study in Bangladesh measured arsenic exposure in maternal urine twice during pregnancy and field staff conducted weekly home visits to interview caregivers about disease symptoms during children’s first year of life.25 The authors reported an increased risk of lower respiratory infection among children with highest versus lowest arsenic exposure levels after controlling for mother’s education, asset index, parity, body mass index, gestational age, and infant’s sex. The New Hampshire Birth Cohort Study (NHBCS), another prospective birth cohort study conducted in the United States, also used maternal urinary biomarkers to assess arsenic exposure during pregnancy.28,29 This study also reported that higher arsenic exposure during pregnancy was associated with an increased risk of respiratory infection based on parent recall of infections and doctor’s diagnoses during the first 4 months of life. Our study provides a useful addition to these studies by continuing follow-up into the fifth year of life. Like the MINIMat and NHBCS studies, we also observed a positive exposure–response between arsenic exposure and respiratory infectious disease symptoms, although the magnitude of effect observed in our study is smaller than the studies that utilized urinary arsenic biomarkers. However, our study did differ from the NHBCS and MINIMat study regarding fever. The NHBCS reported a positive association between arsenic exposure and fever resulting in a doctor’s visit in the first year of life,29 and the MINIMat study reported a positive association between arsenic exposure and maternal fever during pregnancy.31 However, we observed a null association between arsenic exposure in drinking water at all three time points and fever.
Both the MINIMat and NHBCS studies also observed an association between gestational arsenic exposure and diarrheal disease in the first year of life. We anticipated the same effect in our study; however, almost no diarrhea was reported in our participants and we were not able to assess this outcome. Given that diarrhea is common in Bangladesh, it is unknown why incidence was infrequent in the observed population of children. Such low diarrhea incidence could reflect our public health interventions in the participating communities which included repeated water and health education provided to participating households in this cohort during 5 years of repeated clinic and research staff home visits and training community health care worker. Also, the levels of arsenic exposure in our study were relatively modest, and perhaps there is a threshold related to immunological effects. Finally, it should be noted that almost everyone in this population had sanitary latrines which has been shown to reduce childhood diarrheal illness, although it should be noted that the high prevalence of sanitary latrines in this sample is not representative of all families in rural Bangladesh.
It is biologically plausible that gestational arsenic exposure can increase the risk of respiratory illnesses. Toxicology studies in humans and animals have shown that arsenic accumulates in the lungs.42–45 Numerous studies also report that arsenic exposure leads to chronic inflammation,46–48 including in lung tissues.49–54 Arsenic also leads directly to the generation of reactive oxygen species,55 leading to lipid peroxidation and increased oxidative stress and damage,54,56,57 which contributes to poor lung function.30,58–71 Because lung tissue develops throughout gestation and childhood, impaired lung development at any stage can have long-term consequences on disease risk.72 This is borne out in epidemiologic cohort studies of in utero and childhood arsenic exposure conducted in Chile. These studies showed that early life exposure to arsenic was associated with much higher risk of chronic respiratory disease during adulthood.30,73 Subsequently, fetal development may be a time of unique arsenic susceptibility, possibly due to direct lung tissue damage, or to impaired immune development that in turn inhibits the child’s ability to resist lung infection.34,74,75
Our study had several weaknesses that could explain our inability to replicate the association between arsenic exposure and fever, as well as the attenuation of the effect between postnatal arsenic exposure and respiratory symptoms. Primarily, our analysis relied on household drinking water arsenic levels to assign children’s exposures which could result in exposure misclassification. Arsenic is not readily excreted in breastmilk76,77; thus, arsenic exposure would be reduced during breastfeeding periods. Subsequently, household water arsenic levels would overestimate children’s arsenic exposure during breastfeeding and possibly underestimate their exposure after being weaned because it would not reflect dietary arsenic intake. Additionally, we do not account for seasonal variations in groundwater arsenic levels which could contribute to exposure misclassification. Although previous studies in this cohort have observed that household drinking water arsenic exposure is positively correlated with maternal and infant biomarkers of exposure,78 future studies would be able to minimize postnatal exposure misclassification by using internal biomarkers of exposure such as urine, hair, or nails. Furthermore, urinary arsenic biomarkers would provide an opportunity to examine whether arsenic methylation capacity influences individual susceptibility to infectious disease risk.
Other limitations to our study included reliance on caregiver’s recalling and self-reporting symptoms in their children. Although a parent may observe symptoms more readily, mild diarrheal symptoms may go unnoticed in children 4–5 years old because they may be sufficiently potty trained and not notify their caregivers unless they are severely ill. In addition, both diarrheal and respiratory illnesses are more common at certain times of the year,79–81 and this seasonality was not directly accounted for in this analysis. However, enrollment and active disease follow-up was evenly distributed throughout the year (data not shown) so any seasonality in infectious diseases should be randomly distributed throughout the follow-up period.
Despite these limitations, our study had a number of strengths including repeated measures of arsenic exposure at multiple key developmental periods in a large population-based sample. We also relied on 3-day recall of symptoms which has shown to have the least amount of recall bias.39 Furthermore, our cohort experienced a wide range of arsenic exposure with most occurring below the current World Health Organization drinking water recommended guideline of 10 μg/L which allowed us to examine the effect of low to modest arsenic exposure on infectious disease symptoms in children. Also, we were able to adjust for breastfeeding duration. Given that arsenic does not readily pass the mammary gland, breastfeeding is an important protective behavior against arsenic exposure and is also protective against childhood infection, our main outcomes.82–86
In conclusion, drinking water arsenic during pregnancy was associated with a modest increase in respiratory illness, but not fever, in children between ages 4 and 5 years in Bangladesh. These results add to the growing body of evidence that environmental exposure to arsenic during fetal development is associated with increased risk of infectious diseases during childhood.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
This work was supported by grants R01ES015533, R01 ES023441, and P30ES000002 from the US National Institute of Environmental Health Sciences.
Footnotes
Published online 11 February 2020
References
- 1.Fendorf S, Michael HA, van Geen A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science. 2010;328:1123–1127. doi: 10.1126/science.1172974. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell BK, Caldwell JC, Mitra SN, Smith W. Searching for an optimum solution to the Bangladesh arsenic crisis. Soc Sci Med. 2003;56:2089–2096. doi: 10.1016/s0277-9536(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 3.Arsenic Mitigation in Bangladesh. 2010. [Google Scholar]
- 4.Fund, U.N.C.s. Bangladesh MICS 2012–2013 Water Quality Thematic Report. New York, NY: The United Nations Children’s Fund (UNICEF); 2018. [Google Scholar]
- 5.WHO. Guidelines for Drinking-water Quality. 2011. [Google Scholar]
- 6.IHME. GBD Compare Data Visualization. 2016 Available from: http://vizhub.healthdata.org/gbd-compare. Accessed December 18 2018.
- 7.Cardenas A, Koestler DC, Houseman EA, et al. Differential DNA methylation in umbilical cord blood of infants exposed to mercury and arsenic in utero. Epigenetics. 2015;10:508–515. doi: 10.1080/15592294.2015.1046026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemarie A, Morzadec C, Bourdonnay E, Fardel O, Vernhet L. Human macrophages constitute targets for immunotoxic inorganic arsenic. J Immunol. 2006;177:3019–3027. doi: 10.4049/jimmunol.177.5.3019. [DOI] [PubMed] [Google Scholar]
- 9.Lemarie A, Morzadec C, Mérino D, Micheau O, Fardel O, Vernhet L. Arsenic trioxide induces apoptosis of human monocytes during macrophagic differentiation through nuclear factor-kappaB-related survival pathway down-regulation. J Pharmacol Exp Ther. 2006;316:304–314. doi: 10.1124/jpet.105.092874. [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Castro B, Doníz-Padilla LM, Salgado-Bustamante M, et al. Effect of arsenic on regulatory T cells. J Clin Immunol. 2009;29:461–469. doi: 10.1007/s10875-009-9280-1. [DOI] [PubMed] [Google Scholar]
- 11.Seow WJ, Kile ML, Baccarelli AA, et al. Epigenome-wide DNA methylation changes with development of arsenic-induced skin lesions in Bangladesh: a case-control follow-up study. Environ Mol Mutagen. 2014;55:449–456. doi: 10.1002/em.21860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourdonnay E, Morzadec C, Sparfel L, et al. Global effects of inorganic arsenic on gene expression profile in human macrophages. Mol Immunol. 2009;46:649–656. doi: 10.1016/j.molimm.2008.08.268. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Chouly C, Morzadec C, Bonvalet M, Galibert MD, Fardel O, Vernhet L. Inorganic arsenic alters expression of immune and stress response genes in activated primary human T lymphocytes. Mol Immunol. 2011;48:956–965. doi: 10.1016/j.molimm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 14.De Le Fuente H, Portales-Pérez D, Baranda L, et al. Effect of arsenic, cadmium and lead on the induction of apoptosis of normal human mononuclear cells. Clin Exp Immunol. 2002;129:69–77. doi: 10.1046/j.1365-2249.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns LA, Munson AE. Gallium arsenide selectively inhibits T cell proliferation and alters expression of CD25 (IL-2R/p55). J Pharmacol Exp Ther. 1993;265:178–186. [PubMed] [Google Scholar]
- 16.Kozul CD, Ely KH, Enelow RI, Hamilton JW. Low-dose arsenic compromises the immune response to influenza a infection in vivo. Environ Health Perspect. 2009;117:1441–1447. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson R, Vega L, Trouba K, Bortner C, Germolec D. Arsenic-induced alterations in the contact hypersensitivity response in Balb/c mice. Toxicol Appl Pharmacol. 2004;198:434–443. doi: 10.1016/j.taap.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Conde P, Acosta-Saavedra LC, Goytia-Acevedo RC, Calderon-Aranda ES. Sodium arsenite-induced inhibition of cell proliferation is related to inhibition of IL-2 mRNA expression in mouse activated T cells. Arch Toxicol. 2007;81:251–259. doi: 10.1007/s00204-006-0152-7. [DOI] [PubMed] [Google Scholar]
- 19.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci. 1998;44:185–190. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 20.DeSesso JM, Jacobson CF, Scialli AR, Farr CH, Holson JF. An assessment of the developmental toxicity of inorganic arsenic. Reprod Toxicol. 1998;12:385–433. doi: 10.1016/s0890-6238(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 21.Rudge CV, Röllin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JØ. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11:1322–1330. doi: 10.1039/b903805a. [DOI] [PubMed] [Google Scholar]
- 22.Green BB, Karagas MR, Punshon T, et al. Epigenome-Wide assessment of DNA methylation in the placenta and arsenic exposure in the New Hampshire birth Cohort Study (USA). Environ Health Perspect. 2016;124:1253–1260. doi: 10.1289/ehp.1510437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kile ML, Baccarelli A, Hoffman E, et al. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environ Health Perspect. 2012;120:1061–1066. doi: 10.1289/ehp.1104173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kile ML, Houseman EA, Baccarelli AA, et al. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics. 2014;9:774–782. doi: 10.4161/epi.28153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman A, Vahter M, Ekström EC, Persson LÅ. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ Health Perspect. 2011;119:719–724. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed S, Moore SE, Kippler M, et al. Arsenic exposure and cell-mediated immunity in pre-school children in rural Bangladesh. Toxicol Sci. 2014;141:166–175. doi: 10.1093/toxsci/kfu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raqib R, Ahmed S, Ahsan KB, et al. Humoral immunity in arsenic-exposed children in Rural Bangladesh: total immunoglobulins and vaccine-specific antibodies. Environ Health Perspect. 2017;125:067006. doi: 10.1289/EHP318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farzan SF, Korrick S, Li Z, et al. In utero arsenic exposure and infant infection in a United States cohort: a prospective study. Environ Res. 2013;126:24–30. doi: 10.1016/j.envres.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farzan SF, Li Z, Korrick SA, et al. Infant infections and respiratory symptoms in relation to in utero arsenic exposure in a U.S. Cohort. Environ Health Perspect. 2016;124:840–847. doi: 10.1289/ehp.1409282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AH, Yunus M, Khan AF, et al. Chronic respiratory symptoms in children following in utero and early life exposure to arsenic in drinking water in Bangladesh. Int J Epidemiol. 2013;42:1077–1086. doi: 10.1093/ije/dyt120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raqib R, Ahmed S, Sultana R, et al. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol Lett. 2009;185:197–202. doi: 10.1016/j.toxlet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed S, Akhtar E, Roy A, et al. Arsenic exposure alters lung function and airway inflammation in children: a cohort study in rural Bangladesh. Environ Int. 2017;101:108–116. doi: 10.1016/j.envint.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Attreed SE, Navas-Acien A, Heaney CD. Arsenic and immune response to infection during pregnancy and early life. Curr Environ Health Rep. 2017;4:229–243. doi: 10.1007/s40572-017-0141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietert RR. Developmental immunotoxicity, perinatal programming, and noncommunicable diseases: focus on human Studies. Adv Med. 2014;2014:867805. doi: 10.1155/2014/867805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kile ML, Rodrigues EG, Mazumdar M, et al. A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh. Environ Health. 2014;13:29. doi: 10.1186/1476-069X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues EG, Bellinger DC, Valeri L, et al. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health. 2016;15:44. doi: 10.1186/s12940-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch BM, Branscum A, Ahmed SM, et al. Arsenic exposure and serum antibody concentrations to diphtheria and tetanus toxoid in children at age 5: a prospective birth cohort in Bangladesh. Environ Int. 2019;127:810–818. doi: 10.1016/j.envint.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agency EP. EPA Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry. Cincinnati, OH: Environmental Protection Agency; 1994. [Google Scholar]
- 39.Feikin DR, Audi A, Olack B, et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol. 2010;39:450–458. doi: 10.1093/ije/dyp374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan MN, B Nurs CZ, Mofizul Islam M, Islam MR, Rahman MM. Household air pollution from cooking and risk of adverse health and birth outcomes in Bangladesh: a nationwide population-based study. Environ Health. 2017;16:57. doi: 10.1186/s12940-017-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin PD, Kile ML, Christiani DC, et al. Validation of a dish-based semiquantitative food questionnaire in rural Bangladesh. Nutrients. 2017;9:49. doi: 10.3390/nu9010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerhardsson L, Brune D, Nordberg GF, Wester PO. Multielemental assay of tissues of deceased smelter workers and controls. Sci Total Environ. 1988;74:97–110. doi: 10.1016/0048-9697(88)90131-3. [DOI] [PubMed] [Google Scholar]
- 43.Marafante E, Rade J, Sabbioni E, Bertolero F, Foà V. Intracellular interaction and metabolic fate of arsenite in the rabbit. Clin Toxicol. 1981;18:1335–1341. doi: 10.3109/00099308109035074. [DOI] [PubMed] [Google Scholar]
- 44.Saady JJ, Blanke RV, Poklis A. Estimation of the body burden of arsenic in a child fatally poisoned by arsenite weedkiller. J Anal Toxicol. 1989;13:310–312. doi: 10.1093/jat/13.5.310. [DOI] [PubMed] [Google Scholar]
- 45.Bertolero F, Marafante E, Rade JE, Pietra R, Sabbioni E. Biotransformation and intracellular binding of arsenic in tissues of rabbits after intraperitoneal administration of 74As labelled arsenite. Toxicology. 1981;20:35–44. doi: 10.1016/0300-483x(81)90103-7. [DOI] [PubMed] [Google Scholar]
- 46.Recio-Vega R, Gonzalez-Cortes T, Olivas-Calderon E, Lantz RC, Gandolfi AJ, Gonzalez-De Alba C. In utero and early childhood exposure to arsenic decreases lung function in children. J Appl Toxicol. 2015;35:358–366. doi: 10.1002/jat.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fry RC, Navasumrit P, Valiathan C, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olivas-Calderón E, Recio-Vega R, Gandolfi AJ, et al. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicol Appl Pharmacol. 2015;287:161–167. doi: 10.1016/j.taap.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De BK, Majumdar D, Sen S, Guru S, Kundu S. Pulmonary involvement in chronic arsenic poisoning from drinking contaminated ground-water. J Assoc Physicians India. 2004;52:395–400. [PubMed] [Google Scholar]
- 50.Nemery B. Metal toxicity and the respiratory tract. Eur Respir J. 1990;3:202–219. [PubMed] [Google Scholar]
- 51.Olsen CE, Liguori AE, Zong Y, Lantz RC, Burgess JL, Boitano S. Arsenic upregulates MMP-9 and inhibits wound repair in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L293–L302. doi: 10.1152/ajplung.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherwood CL, Liguori AE, Olsen CE, Lantz RC, Burgess JL, Boitano S. Arsenic compromises conducting airway epithelial barrier properties in primary mouse and immortalized human cell cultures. PLoS One. 2013;8:e82970. doi: 10.1371/journal.pone.0082970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramsey KA, Foong RE, Sly PD, Larcombe AN, Zosky GR. Early life arsenic exposure and acute and long-term responses to influenza A infection in mice. Environ Health Perspect. 2013;121:1187–1193. doi: 10.1289/ehp.1306748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, Su X, Gao Y, et al. Exposure of low-concentration arsenic-initiated inflammation and autophagy in rat lungs. J Biochem Mol Toxicol. 2019;33:e22334. doi: 10.1002/jbt.22334. [DOI] [PubMed] [Google Scholar]
- 55.Hubaux R, Becker-Santos DD, Enfield KS, Lam S, Lam WL, Martinez VD. Arsenic, asbestos and radon: emerging players in lung tumorigenesis. Environ Health. 2012;11:89. doi: 10.1186/1476-069X-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lantz RC, Hays AM. Role of oxidative stress in arsenic-induced toxicity. Drug Metab Rev. 2006;38:791–804. doi: 10.1080/03602530600980108. [DOI] [PubMed] [Google Scholar]
- 57.Rashid K, Sinha K, Sil PC. An update on oxidative stress-mediated organ pathophysiology. Food Chem Toxicol. 2013;62:584–600. doi: 10.1016/j.fct.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 58.Ramsey KA, Bosco A, McKenna KL, et al. In utero exposure to arsenic alters lung development and genes related to immune and mucociliary function in mice. Environ Health Perspect. 2013;121:244–250. doi: 10.1289/ehp.1205590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramsey KA, Larcombe AN, Sly PD, Zosky GR. In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC Pharmacol Toxicol. 2013;14:13. doi: 10.1186/2050-6511-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazumder DN, Haque R, Ghosh N, et al. Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India. Int J Epidemiol. 2000;29:1047–1052. doi: 10.1093/ije/29.6.1047. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez TR, Perzanowski M, Graziano JH. Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: a systematic review. Environ Res. 2016;147:537–555. doi: 10.1016/j.envres.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Ehrenstein OS, Mazumder DN, Yuan Y, et al. Decrements in lung function related to arsenic in drinking water in West Bengal, India. Am J Epidemiol. 2005;162:533–541. doi: 10.1093/aje/kwi236. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh A. Evaluation of chronic arsenic poisoning due to consumption of contaminated ground water in West Bengal, India. Int J Prev Med. 2013;4:976–979. [PMC free article] [PubMed] [Google Scholar]
- 64.Das D, Bindhani B, Mukherjee B, et al. Chronic low-level arsenic exposure reduces lung function in male population without skin lesions. Int J Public Health. 2014;59:655–663. doi: 10.1007/s00038-014-0567-5. [DOI] [PubMed] [Google Scholar]
- 65.Nafees AA, Kazi A, Fatmi Z, Irfan M, Ali A, Kayama F. Lung function decrement with arsenic exposure to drinking groundwater along River Indus: a comparative cross-sectional study. Environ Geochem Health. 2011;33:203–216. doi: 10.1007/s10653-010-9333-7. [DOI] [PubMed] [Google Scholar]
- 66.Dauphiné DC, Ferreccio C, Guntur S, et al. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. Int Arch Occup Environ Health. 2011;84:591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinmaus C, Ferreccio C, Acevedo J, et al. High risks of lung disease associated with early-life and moderate lifetime arsenic exposure in northern Chile. Toxicol Appl Pharmacol. 2016;313:10–15. doi: 10.1016/j.taap.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parvez F, Chen Y, Brandt-Rauf PW, et al. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS). Thorax. 2010;65:528–533. doi: 10.1136/thx.2009.119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parvez F, Chen Y, Yunus M, et al. Arsenic exposure and impaired lung function. Findings from a large population-based prospective cohort study. Am J Respir Crit Care Med. 2013;188:813–819. doi: 10.1164/rccm.201212-2282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liao CM, Chio CP, Cheng YH, Hsieh NH, Chen WY, Chen SC. Quantitative links between arsenic exposure and influenza A (H1N1) infection-associated lung function exacerbations risk. Risk Anal. 2011;31:1281–1294. doi: 10.1111/j.1539-6924.2010.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henderson MW, Madenspacher JH, Whitehead GS, et al. Effects of orally ingested arsenic on respiratory epithelial permeability to bacteria and small molecules in mice. Environ Health Perspect. 2017;125:097024. doi: 10.1289/EHP1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soto-Martinez M, Sly PD. Relationship between environmental exposures in children and adult lung disease: the case for outdoor exposures. Chron Respir Dis. 2010;7:173–186. doi: 10.1177/1479972309345929. [DOI] [PubMed] [Google Scholar]
- 73.George CM, Brooks WA, Graziano JH, et al. Arsenic exposure is associated with pediatric pneumonia in rural Bangladesh: a case control study. Environ Health. 2015;14:83. doi: 10.1186/s12940-015-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grandjean P, Barouki R, Bellinger DC, et al. Life-Long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology. 2015;156:3408–3415. doi: 10.1210/EN.2015-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr Opin Pediatr. 2015;27:248–253. doi: 10.1097/MOP.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Concha G, Vogler G, Nermell B, Vahter M. Low-level arsenic excretion in breast milk of native Andean women exposed to high levels of arsenic in the drinking water. Int Arch Occup Environ Health. 1998;71:42–46. doi: 10.1007/s004200050248. [DOI] [PubMed] [Google Scholar]
- 77.Fängström B, Moore S, Nermell B, et al. Breast-feeding protects against arsenic exposure in Bangladeshi infants. Environ Health Perspect. 2008;116:963–969. doi: 10.1289/ehp.11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodrigues EG, Kile M, Dobson C, et al. Maternal-infant biomarkers of prenatal exposure to arsenic and manganese. J Expo Sci Environ Epidemiol. 2015;25:639–648. doi: 10.1038/jes.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Das SK, Begum D, Ahmed S, et al. Geographical diversity in seasonality of major diarrhoeal pathogens in Bangladesh observed between 2010 and 2012. Epidemiol Infect. 2014;142:2530–2541. doi: 10.1017/S095026881400017X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhuyan GS, Hossain MA, Sarker SK, et al. Bacterial and viral pathogen spectra of acute respiratory infections in under-5 children in hospital settings in Dhaka city. PLoS One. 2017;12:e0174488. doi: 10.1371/journal.pone.0174488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, Reeves RM, Wang X, et al. RSV Global Epidemiology Network; RESCEU investigators. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. 2019;7:e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 82.Victora CG, Bahl R, Barros AJ, et al. Lancet Breastfeeding Series Group. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 83.Duijts L, Ramadhani MK, Moll HA. Breastfeeding protects against infectious diseases during infancy in industrialized countries. A systematic review. Matern Child Nutr. 2009;5:199–210. doi: 10.1111/j.1740-8709.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bachrach VR, Schwarz E, Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med. 2003;157:237–243. doi: 10.1001/archpedi.157.3.237. [DOI] [PubMed] [Google Scholar]
- 85.Hornell A, Lagström H, Lande B, et al. Breastfeeding, introduction of other foods and effects on health: a systematic literature review for the 5th Nordic Nutrition recommendations. Food Nutr Res. 2013;57 doi: 10.3402/fnr.v57i0.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horta BL, Victora CG. Geneva, Switzerland: World Health Organization; 2013. Short-term effects of breastfeeding: a systematic review on the benefits of breastfeeding on diarrhoea and pneumonia mortality. [Google Scholar]


