Supplemental Digital Content is available in the text.
Keywords: Green space, Prostate cancer, Built environment, Physical activity, Cohort studies, Causal mediation
Background:
Growing evidence suggests that neighborhood contextual environment could influence risk factors and, therefore, incidence of lethal prostate cancer. We studied the association between neighborhood greenness and lethal prostate cancer incidence and assessed mediation by vigorous physical activity.
Methods:
A total of 47,958 participants were followed in the Health Professionals Follow-up Study from 1986 to 2014. Neighborhood greenness exposure was estimated using normalized difference vegetation index (NDVI) with 1 km resolution, assigned to home or work addresses at start of follow-up. Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were estimated using sequentially adjusted Cox models with individual and contextual prostate cancer risk factors as covariates. Analyses were compared among those whose addresses were constant over follow-up and stratified by population density and address type.
Results:
We observed 898 cases over 1,054,743 person-years. An interquartile range increase in NDVI was associated with 5% lower rate of lethal prostate cancer (aHR = 0.95, 95% CI = 0.88, 1.03), with stronger associations in nonmovers (aHR = 0.92, 95% CI = 0.85, 1.01). Inverse associations were observed among men in high (aHR = 0.90, 95% CI = 0.82, 0.99) but not low (aHR = 1.11, 95% CI = 0.95, 1.29, Phet = 0.086) population density areas, and those reporting from work (aHR = 0.87, 95% CI = 0.75, 1.01) but not home (aHR = 1.04, 95% CI = 0.91, 1.17, Phet = 0.10) addresses. There was no evidence of mediation by vigorous physical activity.
Conclusion:
We report inverse associations between neighborhood greenness and lethal prostate cancer when restricting to nonmovers and in high population density areas. Replication could confirm findings and clarify mechanisms.
What this study adds.
Few effective strategies exist for prostate cancer prevention. Neighborhood greenness could promote higher physical activity, thereby preventing lethal prostate cancer. Using 28 years of prospective cohort data, we linked satellite-derived measures of neighborhood greenness to participants’ home or work addresses. While neighborhood greenness was not associated with lower lethal prostate cancer incidence overall, inverse associations were stronger among those men who remained at the same address over follow-up, among those in high compared with low population density areas, and among those with neighborhood greenness assessed at work compared with home. There was no evidence of mediation by vigorous physical activity.
Introduction
Prostate cancer is the most common noncutaneous malignancy among men in the United States, with an estimated 174,650 new cases and 31,620 deaths in 2019.1 Prostate cancer is considered to be a heterogeneous disease, contrasting indolent, screen-detected cancer with advanced or lethal prostate cancer defined by clinical stage and grade.2,3 Most risk factors for total prostate cancer (age, family history, African American race, height, genetic risk loci) are not modifiable. However, modifiable risk factors, including smoking, obesity, and physical activity, have been identified for lethal prostate cancer.4–7 Focusing purely on individual-level risk factors ignores the broader societal and environmental context in which the individual is embedded.8 Therefore, studying contextual environmental risk factors could help develop a multilevel model of lethal prostate cancer risk,9 as well as identify geographic predictors that can be used to improve prostate cancer risk stratification.10
Natural vegetation in a given area (referred to hereafter as “neighborhood greenness”) is increasingly considered to be a health-promoting contextual environmental factor.11–15 Large observational studies have reported beneficial associations between greenness and health, including all-cause mortality, depression, physical activity, and obesity.16–20 Neighborhood greenness exposure could offer psychological benefits that increase adherence to healthy lifestyles or spaces to exercise which increase physical activity.21–23 In addition, neighborhood greenness is associated with stronger community cohesion and greater social capital, which are associated with increased use of preventive health care services.24–26 Together, these pathways could reduce risk of lethal prostate cancer.27
There are few empirical studies of the association between neighborhood greenness and prostate cancer incidence or mortality. Demoury and colleagues27 reported an inverse association between residential greenness and risk of total prostate cancer risk in an urban setting using a case–control design. Our group reported inverse associations between residential greenness and cause-specific mortality in a US registry-based cohort of Black and White men with prostate cancer.28 Given that neighborhood greenness could be associated with higher levels of physical activity, an established correlate of lethal prostate cancer, use of a prospective design with a lethal prostate cancer endpoint could reveal stronger associations between hypothesized exposure and endpoints and enable exploration of possible mechanisms.
We studied the association between baseline neighborhood greenness and lethal prostate cancer incidence in a nationwide prospective cohort of male health professionals in the United States. We hypothesized that neighborhood greenness would be associated with lower rates of lethal prostate cancer and that this protective association would be mediated in part through higher levels of vigorous physical activity among participants in greener neighborhoods.2,29,30 Because prior studies had focused on urban areas using residential neighborhood greenness as the primary exposure,27 we further sought to evaluate whether associations varied by population density or exposure at home compared with work.
Methods
Study population and design
We used data from the Health Professionals Follow-up Study (HPFS), an ongoing prospective cohort study based at the Harvard T. H. Chan School of Public Health. Since 1986, 51,529 participating male health professionals across the United States have completed biennial questionnaires that record information about lifestyle and health-related factors, as well as diagnosis of new illnesses. Cohort participants could choose to mail their questionnaire to a home or work address over follow-up. Geocoded addresses were available from questionnaire mailing records from 1988 to 2012. In 1988, participants indicated if the address was their home, work, or other address. Upon receipt of a new diagnosis, study personnel conduct a detailed review of medical and pathological information for validation purposes. The questionnaire response rate is 90%, with mortality follow-up over 98%.31 Participants with prior history of prostate cancer or nonmelanoma skin cancer (n = 2,084), missing a geocoded address (n = 1,447) or date of birth (n = 36), or died before returning their first questionnaire (n = 4) were excluded, resulting in a study population of 47,958. The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and Harvard T. H. Chan School of Public Health and those of participating registries as required.
Lethal prostate cancer assessment
Lethal rather than total prostate cancer was chosen as the primary endpoint. Using lethal prostate cancer allowed us to distinguish indolent tumors from aggressive, clinically meaningful tumors with greater public health significance.2,3,32 In addition, due to widespread screening in the United States, the overall prostate tumor burden has shifted over time from largely aggressive to largely indolent tumors,33 which complicates the interpretation of findings if total prostate cancer were used as the endpoint. Finally, indolent tumors and lethal tumors appear to have different metabolic risk factor profiles, with higher body mass index and lower levels of physical activity associated with increased lethal prostate cancer.2,3,32 Because these were hypothesized mechanisms through which neighborhood greenness could influence prostate cancer incidence, we chose to model lethal prostate cancer as our endpoint.
Incident prostate cancer diagnoses were ascertained from biennial questionnaires. Study personnel and clinical staff reviewed medical records and pathology reports to confirm reported diagnosis. Lethal prostate cancer was defined by the presence of distant metastasis (stage M1) or indication that prostate cancer was the primary cause of death for the study participant, over follow-up. Study staff were notified of cohort deaths from family members, as well as linkages with the National Death Index.31
Exposure to neighborhood greenness
Exposure to neighborhood greenness was estimated by linking satellite data on greenness to geocoded participant addresses from the 1988 questionnaire, allowing us to compare greenness exposure measurements at home and work. We used the normalized difference vegetation index (NDVI), calculated by taking a ratio of the difference of near-infrared and visible light divided by the sum of near-infrared and visible light.34 Longitudinal NDVI data were obtained from images produced by the Advanced Very-High-Resolution Radiometer satellite of the National Oceanic and Atmospheric Administration. Images were taken every 16 days at 1,000 m resolution and began in 1989, earlier than other sources. The NDVI scale ranges from −1 to 1, with 1 representing maximal vegetation; values close to 0 representing barren areas of rock, sand ,or snow; and values approaching −1 indicating bodies of water.35 NDVI values of 0 and below were set to missing to restrict our exposure measure to values corresponding to natural green vegetation.
The HPFS follows a biennial questionnaire cycle, and cohort participants reside across the United States, reflecting a broad range of regional and seasonal variation in neighborhood greenness. Because prostate cancer has a long natural history, we modeled associations between neighborhood greenness at the start of follow-up and lethal prostate cancer. We took an average of measurements of NDVI corresponding to different seasons (January, April, July, and September) to account for seasonal changes in and geographic differences in duration of greenness. Seasonal average NDVI measurements from 1989, the earliest year that NDVI data were available, were assigned to participant’s geocoded address within a 1,000 m buffer. We chose to use 1 km resolution NDVI to capture possible benefits arising from physical activity, which could occur within a larger area around one’s address, along with more proximal hypothesized mechanisms like mental health and social cohesion.36 Seasonal NDVI allowed us to preserve the marked variability in climate zones across the United States, which represent different weather patterns.11 In addition, preserving seasonal variability in NDVI allowed us to account for geographic differences in seasonal behavioral patterns that could be possible mechanisms, such as physical activity.37,38
We modeled baseline neighborhood greenness as our primary exposure rather than cumulative updated average because we felt that earlier exposure to neighborhood greenness, rather than duration and intensity of exposure up to diagnosis, would be more likely to occur during the etiologic window for lethal prostate cancer. As a secondary exposure, we estimated cumulative updated average NDVI, incorporating four seasonal images per year over follow-up (eMethods S1; http://links.lww.com/EE/A82). We also performed the analysis using maximum baseline NDVI as a sensitivity analysis to reflect maximal intensity of greenness.
Longitudinal measures of physical activity
Physical activity was reported by participants on biennial questionnaires. Participants were asked questions about the average time spent each week engaging in different types of physical activity (walking or hiking outdoors, jogging, running, bicycling, lap swimming, tennis, squash or racquetball, and calisthenics or rowing). In subsequent questionnaire cycles, additional activities were included: heavy outdoor work (from 1988), weightlifting (1990), moderate outdoor work (2004), and lower intensity exercise and other aerobic exercise (2010). Additional activities included flights of stairs traversed daily and usual walking pace. Each activity was assigned a metabolic equivalent of task (MET).39 Nonvigorous activities were classified as those with MET <6, while vigorous activities were classified as MET ≥6. Total physical activity was reported in MET-hours per week, calculated by summing the product of MET-hours and average hours per week for all physical activity reported by participants. Validation studies comparing MET-hours per week in questionnaires to weekly diaries found generally high correlations.40
Statistical analysis
Participant follow-up began with return of the first questionnaire (1986) until diagnosis of lethal prostate cancer, death from another cause, or administrative censoring on 1 January 2014, whichever came first. We used Cox proportional hazards models with study follow-up as the primary time scale to estimate hazard ratios and 95% confidence intervals for the association between rate of lethal prostate cancer and NDVI. We modeled NDVI as quintiles and estimated P values for linear trend using the median value for each NDVI quintile. We also estimated the change in rate of lethal prostate cancer associated with a linear interquartile range (IQR) unit increase in continuous NDVI (0.11 units). We tested for nonlinearity of continuous NDVI using splines. In addition, to more precisely examine long-term exposure to neighborhood greenness, we repeated the main analysis restricting to participants who did not move during follow-up.
To assess the impact of covariate adjustment on effect estimates, we fit sequentially adjusted models (model 1: age [continuous], calendar time at 2-year questionnaire cycle [continuous] included as covariates in the baseline hazard; model 2: All covariates included in model 1, plus race [categorical: White, African American, Other], diabetes mellitus, body mass index [BMI] at age 21 [kg/m2, <20, 20–<22.5, 22.5–<25, ≥25], height [inches, <66, 66–<68, 68–<70, 70–<72, ≥72], smoking [never smokers, current and/or quit smoking ≤10 years ago, quit >10 years ago], family history of prostate cancer, prostate-specific antigen [PSA] testing over follow-up using two variables: ever had PSA screening before diagnosis [lagged to reflect screened, rather than diagnostic PSA test] and intensity of PSA screening before diagnosis [defined as having reported having PSA screening in over half of prior visits since 1994], census tract median income [USD, continuous], census tract median home value [USD, continuous] and population density; and model 3: all covariates in model 2, plus vigorous physical activity, nonvigorous physical activity [quintiles], and current BMI [ kg/m2, <21, 21–<23, 23–<25, 25–<27.5, 27.5–<30, ≥30]). Baseline measures of all lifestyle covariates described above were used in our primary analysis. Vigorous physical activity was modeled as a five-level variable, with the lowest level corresponding to 0 METs of vigorous physical activity, and the remaining levels modeled as quartiles of nonzero vigorous METs.2 Model 2 corresponds to a confounding-adjusted model, and model 3 corresponds to the controlled direct effects model specified in our mediation analysis (eMethods S1; http://links.lww.com/EE/A82).
We chose to evaluate effect modification by population density because prior research had shown varying associations between neighborhood greenness and other health outcomes based on level of population density, including mortality among men with prostate cancer,28 obesity,41 and adolescent mental health.42 We chose to evaluate whether the association between neighborhood greenness and lethal prostate cancer might vary based on address type because prior research on location-based environmental exposures has revealed that the magnitude and direction of association can vary depending on where exposure is assessed,43 providing insights into possible mechanisms.11 We evaluated multiplicative effect modification of the association between continuous NDVI and lethal prostate cancer by census tract-level population density (≥1,000 people/mi2 compared with <1,000 people/mi2) following US government designations of urban areas (US Census 1994) and address type (home compared with work) using likelihood ratio tests. For levels of population density and home and work addresses, we rescaled the quintiles based on the NDVI distribution for participants within each category. We further evaluated multiplicative effect modification by census region (US Census defined North, South, East, West) and PSA screening history and intensity.
Further details regarding mediation analysis as well as sensitivity analysis for cumulative updated average exposure and unmeasured confounding using e-values are provided in eMethods S1; http://links.lww.com/EE/A82.
RESULTS
After exclusions, 47,958 participants (93%) remained in our analytic sample, giving rise to 898 cases of incident lethal prostate cancer accrued over 1,054,743 person-years of follow-up. Age-adjusted characteristics of the study population are described in Table 1 across quintiles of NDVI. Most participants were white (95%) with an average age of 64.4 years over follow-up. Participants in the highest quintile of NDVI reported higher nonvigorous physical activity (NDVI Q5: 17.6 vs. Q1: 15.6 MET-hours/week) and lower vigorous physical activity (NDVI Q5: 8.4 vs. Q1: 9.7 MET hours/week) compared with participants in the lowest quintile. These patterns held in adjusted models (eTable S1; http://links.lww.com/EE/A82). Average census tract population density (NDVI Q5: 1,720 vs. Q1: 8,870 people/mi2) decreased with increasing quintiles of NDVI, while median income increased (NDVI Q5: $58,870 vs. Q1: $52,270). Maps of participant locations (eFigure S1; http://links.lww.com/EE/A82) and NDVI in July 1989 (eFigure S2; http://links.lww.com/EE/A82) display the geographic spread of exposure locations.
Table 1.
Age-standardized characteristics by quintile of NDVI among men in the Health Professionals Follow-up Study from 1986 to 2014a,b
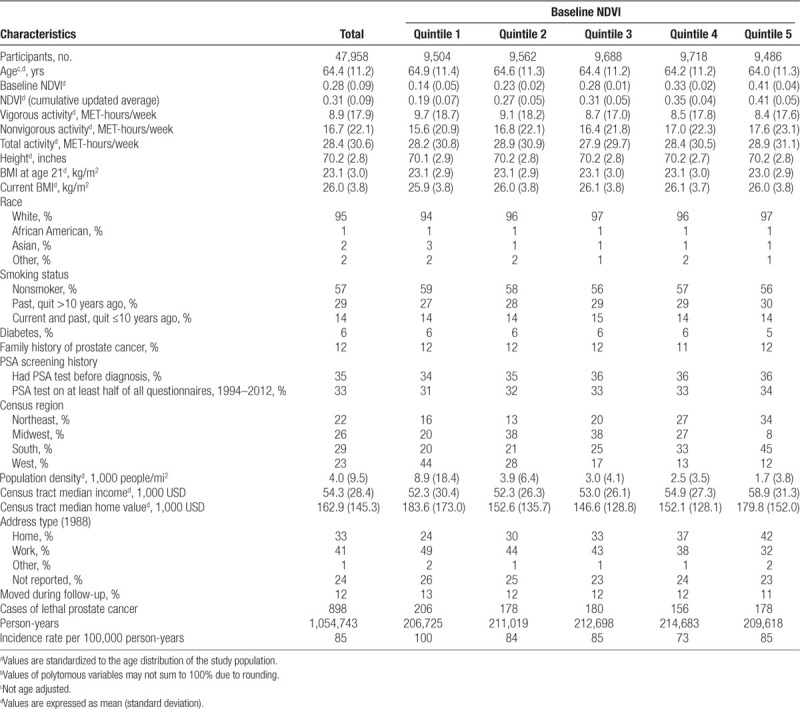
In our analysis of the full cohort (Figure 1, eTable S2; http://links.lww.com/EE/A82), increasing quintiles of baseline NDVI were not significantly associated with lower rates of lethal prostate cancer compared with the lowest quintile (Q1) in age and calendar year- and confounding-adjusted models. Only the estimate for Q4 was statistically significant at the 0.05 level (adjusted hazard ratio [aHR] = 0.78, 95% confidence interval [CI] = 0.63, 0.96, Ptrend = 0.25). Inverse associations were stronger among the 42,492 (89%) participants who did not change addresses during follow-up (813 cases over 930,033 person-years) (Figure 1, eTable S2; http://links.lww.com/EE/A82). Among nonmovers, we observed an 8% lower rate of lethal prostate cancer associated with an IQR increase in NDVI (aHR = 0.92, 95% CI = 0.85, 1.01), with weak evidence of lower rates of lethal prostate cancer associated with increasing NDVI quintiles (Ptrend = 0.068). Results from models further adjusting for vigorous physical activity and BMI were similar to those from confounding models in the total and restricted populations (Figure 1, eTable S2; http://links.lww.com/EE/A82).
Figure 1.
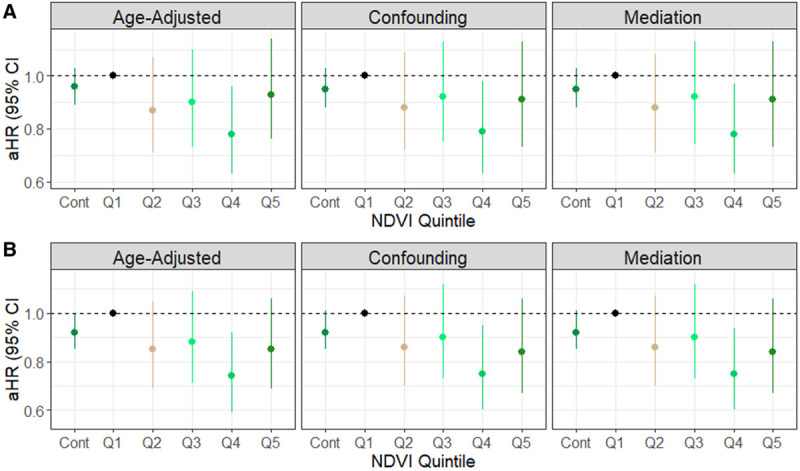
Hazard ratios and confidence intervals for the association between baseline NDVI and lethal prostate cancer incidence in the Health Professionals Follow-up Study, United States, 1986–2014. Sequentially adjusted for age in months and calendar time as strata (Age-adjusted Model), race (categorical), diabetes mellitus (yes or no), height (categorical), family history of prostate cancer (yes or no), BMI at age 21 (categorical), smoking status in 1986 (categorical), 1990 census tract median income (USD), 1990 census tract median home value (USD), population density (binary: high: ≥1,000, low:<1,000 people/mi2), history of prostate-specific antigen testing, intensity of prostate-specific antigen testing (Confounding Model), vigorous physical activity, non-vigorous physical activity, and current BMI (Mediation Model). A, Total population (N = 47,958); (B) participants who did not move over follow-up (N = 42,492). Cont indicates an IQR increase in continuous NDVI of 0.11 units. Q, quintile.
Table 2 presents results from models evaluating the association between NDVI and incidence of lethal prostate cancer within levels of population density and address type. Stronger inverse associations were observed in high (>1,000 people/mi2) compared with low population density neighborhoods (<1,000 people/mi2) though the P value for heterogeneity did not reach statistical significance (Phet = 0.086). In high population density areas, an IQR increase in NDVI was associated with a 10% lower rate of lethal prostate cancer (aHR = 0.90, 95% CI = 0.82, 0.99), while in low population density areas, the direction of this association was reversed (aHR = 1.11, 95% CI = 0.95, 1.29).
Table 2.
Hazard ratios for the association between baseline NDVIa and lethal prostate cancer incidence in the Health Professionals Follow-up Study, United States, 1986–2014, stratified by population density (high: ≥1,000, low: <1,000 people/mi2) and address type (work, home)

When stratifying by address type, in general, characteristics of participants were similar across address types (eTable S3; http://links.lww.com/EE/A82), though those with work address were more likely to have been screened before diagnosis (42% vs. 35%) and were screened more frequently (40% vs. 31%). We observed stronger inverse associations among participants for whom NDVI was assessed at work (Ptrend = 0.027) compared with home though evidence for effect modification was weak (Phet = 0.10). There was a 13% lower rate of lethal prostate cancer associated with an IQR increase in NDVI (aHR = 0.87, 95% CI = 0.75, 1.01) among men for whom NDVI was assessed at work, compared with a 4% increased rate among those with residential NDVI (aHR = 1.04, 95% CI = 0.91, 1.17). Linear associations for high population density and among those with work addresses were strengthened when restricting to nonmovers (Table 2). Further examination of effect modification by additional factors (PSA screening intensity, prior history of PSA screening, or geographic region) did not reveal any differences (Table 3).
Table 3.
Hazards ratios for the association between baseline NDVIa,b and lethal prostate cancer incidence in the Health Professionals Follow-up Study, United States, 1986–2014, stratified by address, prostate-specific antigen screening, and census region
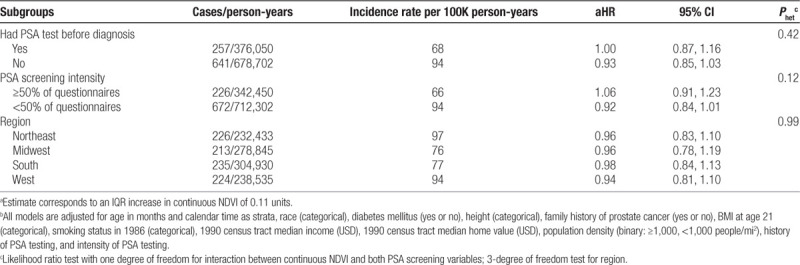
In sensitivity analyses using cumulative updated average NDVI and baseline maximum NDVI, inverse associations were weaker (eTable S4; http://links.lww.com/EE/A82, eTable S5; http://links.lww.com/EE/A82) and failed to reveal evidence of effect modification by population density (eTable S4; http://links.lww.com/EE/A82). Strongest e-values for point estimates (2.12) and confidence intervals (1.29) were observed for NDVI Q5 compared with Q1 among men who did not move with addresses in high population density neighborhoods, suggesting that these estimates are less likely to be completely explained by unmeasured confounding (eTable S6; http://links.lww.com/EE/A82).
DISCUSSION
We observed an inverse association between baseline neighborhood greenness and lethal prostate cancer, though this finding was restricted to those in high population density areas. Contrary to expectation, we found that neighborhood greenness was associated with lower levels of vigorous physical activity in this population of health professionals. We did not observe evidence of a mediating role of vigorous physical activity. Restricting to men who remained at the same address over follow-up strengthened the inverse association between neighborhood greenness and lethal prostate cancer incidence, suggesting that mechanisms are related to environmental context or reduced home or work mobility.
Few studies have assessed the association between neighborhood greenness and prostate cancer.27,44 Our findings corroborate results from a population-based case–control study conducted by Demoury and colleagues27 in Montreal, the second-largest city in Canada. In an urban population, they reported effect estimates of similar magnitude to ours, though they used maximal annual residential NDVI at diagnosis and 10 years before diagnosis. They also found no evidence of physical activity as a mediating pathway. Because we used different exposure and outcome measures, our studies are not directly comparable, but both are consistent with a hypothesis that green spaces and contextual environment could play a role in prostate cancer risk.
There is limited evidence for direct effects of exposure to neighborhood greenness and carcinogenesis. However, physiologic changes that arise from spending time in green environments could serve as a mechanism. Interventional studies conducted in Japan comparing visits to urban areas with forests observed higher parasympathetic activation, lower cardiometabolic response, and lower natural killer cell activity following forest visits.45,46 Cross-sectional studies in the United States reported inverse associations between neighborhood greenness and allostatic load, a composite index derived from biomarkers to capture physiologic adaptation to stress.47 One of these inflammatory biomarkers, interleukin-8, could drive cancer progression by decoupling tumor growth from androgen hormone regulation.48,49 Further studies are needed to clarify biological mechanisms.
The magnitude and direction of the association between neighborhood greenness and incidence rate of lethal prostate cancer varied by levels of high and low population density, though we lacked power to detect statistically significant effect modification. Because neighborhood greenness varies spatially, these different relationships could be related to different geographic patterns of care seeking and treatment for lethal prostate cancer. Geographic patterns of prostate cancer care have been observed in the United States; for example, rural prostate cancer patients are less likely to receive radiotherapy and surgery compared with urban patients.50,51 In rural areas, benefits of greenness could be offset by increased lethal prostate cancer mortality resulting from the absence of these treatment modalities.
Environmental factors could also explain this effect heterogeneity. Ultraviolet light exposure, which has been linked with reduced rates of prostate cancer in prospective studies, could be influenced by neighborhood greenness and vary by population density.52,53 Several reports have documented increased risk of prostate cancer among farmers, hypothesized to arise from long-term use of endocrine disrupting chemicals found in pesticides.54,55 Given that agricultural land accounts for much of the natural green vegetation in rural environments, refining neighborhood greenness exposure to account for source of green vegetation could shed light on possible mechanisms. A recent study reported effect modification of the association between neighborhood greenness and risk of breast cancer, another hormone-dependent cancer, with inverse associations in urban areas, but elevated risk in rural areas with surrounding agricultural land.56 Joint consideration of multiple environmental exposures could improve mechanistic understanding of how neighborhood greenness could influence risk of lethal prostate cancer,57 but these studies would require longitudinal designs with changing trends in these exposures to distinguish confounding from mediation pathways.12 In future studies, refining measurement of exposure to natural green vegetation at different locations, using higher resolution data and detailed information on the type of natural green environment, could reveal underlying mechanisms.17,58
We observed stronger associations among participants for whom greenness was assessed at work compared with home address. A possible explanation could be enhancement of mental and related health benefits from greenness in stressful work environments. A recent prospective study reported lower levels of job-related stress among people living in neighborhoods with higher levels of residential greenness.59 Indirect support for this hypothesis comes from observations that health benefits of greenness appear greater in urban compared with rural settings11,28 and in more deprived neighborhoods.60–64 Many health care professionals engage in shift work or long hours, leading to disruption of circadian rhythm, altered social patterns, and adverse cardiovascular and mental health.65,66 Though the evidence is mixed, there are several reports of increased risk of prostate cancer among shift workers compared with nonshift workers, hypothesized to arise from circadian disruption that could lead to hormonal shifts which promote tumor growth.67,68 In this context, it is possible that for health professionals, greenness exposure at their work place could provide greater benefits than at their residential address.
Interpretation of our results warrants consideration of our study limitations. Unmeasured confounding is a major threat to validity. Though we did not adjust for individual-level socioeconomic status, assuming that area-level socioeconomic status serves as a reliable measure of individual-level socioeconomic status69 and that premove lifestyle factors are not associated with neighborhood selection,70 adjustment for individual lifestyle factors and area-level socioeconomic status would be expected to mitigate confounding bias. Furthermore, this occupational cohort of male health professionals exhibits limited variability in terms of income and education, and therefore, restriction would mitigate confounding from individual-level socioeconomic status. Using e-values, we quantified the magnitude of bias needed to change our inference and found that moderate bias conditional on covariates would be required. Our prospective design allowed us to control for major individual clinical, lifestyle, and socioeconomic contextual factors, making it unlikely for an unmeasured covariate to exhibit associations with neighborhood greenness and lethal prostate cancer as extreme as those presented in our sensitivity analysis.
Nearly a quarter (24%) of participants were missing address type. For the remainder, only home or work address was available. We consider this to be an issue of measurement error, in which we have randomly sampled greenness exposure for some participants at home and others at work within strata of confounding variables. This nondifferential measurement error means that our reported associations are weaker than what one would expect to see with perfect exposure assessment. Our satellite-derived measure captures vegetation exhibiting high levels of photosynthesis and so may not capture green vegetation with low photosynthesis activity. When using NDVI as an exposure, we are limited in the spatial and temporal measures we can use to estimate the full extent of neighborhood greenness exposure that may be etiologically meaningful. Our choice of using seasonal average NDVI as the primary exposure assumes that the etiologically meaningful measure of neighborhood greenness is a weighted average of NDVI exposure measured during each season. Under a classical measurement error structure, this decision could lead to bias if the etiologically relevant measure is better reflected by greenness experienced in a single season. We found stronger associations between seasonal average NDVI and lethal prostate cancer incidence than maximal NDVI from a single season, suggesting that the seasonal measure may better reflect etiologically meaningful exposure. Finally, results obtained from this select population of predominantly white health professionals may not extend to other populations. A different study of residential greenness and mortality among men with prostate cancer reported an inverse association between greenness and cardiovascular mortality among White but not Black men, showing that patterns may vary by race or other characteristics.28 However, restriction based on socioeconomic status and race strengthens internal validity of our study.
In a 28-year prospective study of 47,958 health professionals, we observed an inverse association between neighborhood greenness and rate of lethal prostate cancer in high population density areas. These findings suggest that health benefits of neighborhood greenness could include reduced incidence of lethal prostate cancer. Future studies should apply more precise measurements of exposure to greenness, clarify mechanisms, and assess transportability of these findings.
Conflicts of interest statement
M.D.H. declares relationships with Bayer AG (provides aspirin/placebo for trial NCG02927249, consulting for Arla Foods), Cambridge Savings Bank (advisory board member), and United States Social Securing Administration, VISIONS Inc. The other authors have no conflicts to report.
ACKNOWLEDGMENTS
We thank the participants of the Health Professionals Follow-up Study for providing the detailed data used in this study, as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. The authors assume full responsibility for analyses and interpretation of these data. We are grateful for administrative support from the Channing Division of Network Medicine, Department of Medicine, Brigham & Women’s Hospital and Harvard Medical School, Boston, MA. We are also grateful for technical GIS support from Jeffrey Blossom at the Harvard Center for Geographic Analysis.
The Health Professionals Follow-up Study is supported by National Cancer Institute grant UM1 CA167552. H.S.I. was supported by National Cancer Institute grant T32 CA009001 and National Cancer Institute grant P20-CA233255. J.E.H. and F.L. were supported by National Institute of Environmental Health Sciences grant P30 ES000002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Footnotes
Published online 27 March 2020
Sharing of Data and Code: Please contact the corresponding author to request code used for this analysis. Further information including the procedures to obtain and access data from the Health Professionals Follow-up Study is described at https://sites.sph.harvard.edu/hpfs/for-collaborators/.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammarsten J, Damber JE, Haghsheno MA, Mellström D, Peeker R. A stage-dependent link between metabolic syndrome components and incident prostate cancer. Nat Rev Urol. 2018;15:321–333. doi: 10.1038/nrurol.2018.8. [DOI] [PubMed] [Google Scholar]
- 4.Pernar CH, Ebot EM, Wilson KM, et al. The epidemiology of prostate cancer. Cold Spring Harb Perspect Med. 2018;8:a030361. doi: 10.1101/cshperspect.a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookman-May SD, Campi R, Henríquez JDS, et al. Latest evidence on the impact of smoking, sports, and sexual activity as modifiable lifestyle risk factors for prostate cancer incidence, recurrence, and progression: a systematic review of the literature by the European Association of Urology Section of Oncological Urology (ESOU). Eur Urol Focus. 2019;5:756–787. doi: 10.1016/j.euf.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Wekesa A, Harrison M, Watson RW. Physical activity and its mechanistic effects on prostate cancer. Prostate Cancer Prostatic Dis. 2015;18:197–207. doi: 10.1038/pcan.2015.9. [DOI] [PubMed] [Google Scholar]
- 7.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;39:887–903. doi: 10.1016/0277-9536(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 9.Lynch SM, Rebbeck TR. Bridging the gap between biologic, individual, and macroenvironmental factors in cancer: a multilevel approach. Cancer Epidemiol Biomarkers Prev. 2013;22:485–495. doi: 10.1158/1055-9965.EPI-13-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebbeck TR. Precision prevention of cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2713–2715. doi: 10.1158/1055-9965.EPI-14-1058. [DOI] [PubMed] [Google Scholar]
- 11.James P, Banay RF, Hart JE, Laden F. A review of the health benefits of greenness. Curr Epidemiol Rep. 2015;2:131–142. doi: 10.1007/s40471-015-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markevych I, Schoierer J, Hartig T, et al. Exploring pathways linking greenspace to health: theoretical and methodological guidance. Environ Res. 2017;158:301–317. doi: 10.1016/j.envres.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Frumkin H, Bratman GN, Breslow SJ, et al. Nature contact and human health: a research agenda. Environ Health Perspect. 2017;125:075001. doi: 10.1289/EHP1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong KC, Hart JE, James P. A review of epidemiologic studies on greenness and health: updated literature through 2017. Curr Environ Health Rep. 2018;5:77–87. doi: 10.1007/s40572-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twohig-Bennett C, Jones A. The health benefits of the great outdoors: a systematic review and meta-analysis of greenspace exposure and health outcomes. Environ Res. 2018;166:628–637. doi: 10.1016/j.envres.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James P, Hart JE, Banay RF, Laden F. Exposure to greenness and mortality in a Nationwide Prospective Cohort Study of women. Environ Health Perspect. 2016;124:1344–1352. doi: 10.1289/ehp.1510363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.South EC, Hohl BC, Kondo MC, MacDonald JM, Branas CC. Effect of greening vacant land on mental health of community-dwelling adults: a Cluster Randomized Trial. JAMA Netw Open. 2018;1:e180298. doi: 10.1001/jamanetworkopen.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James P, Hart JE, Hipp JA, et al. GPS-based exposure to greenness and walkability and accelerometry-based physical activity. Cancer Epidemiol Biomarkers Prev. 2017;26:525–532. doi: 10.1158/1055-9965.EPI-16-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villeneuve PJ, Jerrett M, Su JG, Weichenthal S, Sandler DP. Association of residential greenness with obesity and physical activity in a US cohort of women. Environ Res. 2018;160:372–384. doi: 10.1016/j.envres.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson Å, Pyko A, Lind T, et al. Urban residential greenness and adiposity: a cohort study in Stockholm County. Environ Int. 2018;121(pt 1):832–841. doi: 10.1016/j.envint.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Azevedo Da Silva M, Singh-Manoux A, et al. Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. Eur J Epidemiol. 2012;27:537–546. doi: 10.1007/s10654-012-9692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Win S, Parakh K, Eze-Nliam CM, Gottdiener JS, Kop WJ, Ziegelstein RC. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the Cardiovascular Health Study. Heart. 2011;97:500–505. doi: 10.1136/hrt.2010.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter RF, Christian H, Veitch J, Astell-Burt T, Hipp JA, Schipperijn J. The impact of interventions to promote physical activity in urban green space: a systematic review and recommendations for future research. Soc Sci Med. 2015;124:246–256. doi: 10.1016/j.socscimed.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Leslie E, Giles-Corti B, Owen N. Associations of neighbourhood greenness with physical and mental health: do walking, social coherence and local social interaction explain the relationships? J Epidemiol Community Health. 2008;62:e9. doi: 10.1136/jech.2007.064287. [DOI] [PubMed] [Google Scholar]
- 25.de Vries S, van Dillen SM, Groenewegen PP, Spreeuwenberg P. Streetscape greenery and health: stress, social cohesion and physical activity as mediators. Soc Sci Med. 2013;94:26–33. doi: 10.1016/j.socscimed.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Kim ES, Kawachi I. Perceived neighborhood social cohesion and preventive healthcare use. Am J Prev Med. 2017;53:e35–e40. doi: 10.1016/j.amepre.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demoury C, Thierry B, Richard H, Sigler B, Kestens Y, Parent ME. Residential greenness and risk of prostate cancer: a case-control study in Montreal, Canada. Environ Int. 2017;98:129–136. doi: 10.1016/j.envint.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Iyer HS, Valeri L, James P, et al. The contribution of residential greenness to mortality among men with prostate cancer: a registry-based cohort study of Black and White men. Environ Epidemiol. 2020;4:e087. doi: 10.1097/EE9.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165:1005–1010. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- 30.Pernar CH, Ebot EM, Pettersson A, et al. A prospective study of the association between physical activity and risk of prostate cancer defined by clinical features and TMPRSS2:ERG. Eur Urol. 2019;76:33–40. doi: 10.1016/j.eururo.2018.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and equifax nationwide death search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 32.Wilson KM, Giovannucci EL, Mucci LA. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J Androl. 2012;14:365–374. doi: 10.1038/aja.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen-era. Int J Cancer. 2015;137:2795–2802. doi: 10.1002/ijc.29408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kriegler FJ, Malila WA, Nalepka RF, et al. Proceedings of the Sixth International Symposium on Remote Sensing of Environment. Ann Arbor, MI: Institute of Science and Technology of the University of Michigan; 1969. Preprocessing transformations and their effects on multispectral recognition. pp. 97–131. [Google Scholar]
- 35.Weier J, Herring D. Measuring Vegetation (NDVI & EVI). 2000 Available at: http://earthobervatory.nasa.gov/Features/MeasuringVegetation/. Accessed 28 May 2019.
- 36.James P, Berrigan D, Hart JE, et al. Effects of buffer size and shape on associations between the built environment and energy balance. Health Place. 2014;27:162–170. doi: 10.1016/j.healthplace.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker P, Gilliland J. The effect of season and weather on physical activity: a systematic review. Public Health. 2007;121:909–922. doi: 10.1016/j.puhe.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Shephard RJ, Aoyagi Y. Seasonal variations in physical activity and implications for human health. Eur J Appl Physiol. 2009;107:251–271. doi: 10.1007/s00421-009-1127-1. [DOI] [PubMed] [Google Scholar]
- 39.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 40.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Liu GC, Wilson JS, Qi R, Ying J. Green neighborhoods, food retail and childhood overweight: differences by population density. Am J Health Promot. 2007;21(4 suppl):317–325. doi: 10.4278/0890-1171-21.4s.317. [DOI] [PubMed] [Google Scholar]
- 42.Bezold CP, Banay RF, Coull BA, et al. The association between natural environments and depressive symptoms in adolescents living in the United States. J Adolesc Health. 2018;62:488–495. doi: 10.1016/j.jadohealth.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez-Pedrerol M, Rivas I, López-Vicente M, et al. Impact of commuting exposure to traffic-related air pollution on cognitive development in children walking to school. Environ Pollut. 2017;231(pt 1):837–844. doi: 10.1016/j.envpol.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 44.Datzmann T, Markevych I, Trautmann F, Heinrich J, Schmitt J, Tesch F. Outdoor air pollution, green space, and cancer incidence in Saxony: a semi-individual cohort study. BMC Public Health. 2018;18:715. doi: 10.1186/s12889-018-5615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Morimoto K, Kobayashi M, et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int J Immunopathol Pharmacol. 2008;21:117–127. doi: 10.1177/039463200802100113. [DOI] [PubMed] [Google Scholar]
- 46.Park BJ, Tsunetsugu Y, Kasetani T, Kagawa T, Miyazaki Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): evidence from field experiments in 24 forests across Japan. Environ Health Prev Med. 2010;15:18–26. doi: 10.1007/s12199-009-0086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egorov AI, Griffin SM, Converse RR, et al. Vegetated land cover near residence is associated with reduced allostatic load and improved biomarkers of neuroendocrine, metabolic and immune functions. Environ Res. 2017;158:508–521. doi: 10.1016/j.envres.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 49.Michaud DS, Daugherty SE, Berndt SI, et al. Genetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8, and IL-10 and risk of prostate cancer. Cancer Res. 2006;66:4525–4530. doi: 10.1158/0008-5472.CAN-05-3987. [DOI] [PubMed] [Google Scholar]
- 50.Muralidhar V, Rose BS, Chen YW, Nezolosky MD, Nguyen PL. Association between travel distance and choice of treatment for prostate cancer: does geography reduce patient choice? Int J Radiat Oncol Biol Phys. 2016;96:313–317. doi: 10.1016/j.ijrobp.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 51.Cary C, Odisho AY, Cooperberg MR. Variation in prostate cancer treatment associated with population density of the county of residence. Prostate Cancer Prostatic Dis. 2016;19:174–179. doi: 10.1038/pcan.2015.65. [DOI] [PubMed] [Google Scholar]
- 52.Gilbert R, Metcalfe C, Oliver SE, et al. Life course sun exposure and risk of prostate cancer: population-based nested case-control study and meta-analysis. Int J Cancer. 2009;125:1414–1423. doi: 10.1002/ijc.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin SW, Wheeler DC, Park Y, et al. Prospective study of ultraviolet radiation exposure and risk of cancer in the United States. Int J Cancer. 2012;131:E1015–E1023. doi: 10.1002/ijc.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koutros S, Beane Freeman LE, Lubin JH, et al. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am J Epidemiol. 2013;177:59–74. doi: 10.1093/aje/kws225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis-Mikhael AM, Bueno-Cavanillas A, Ofir Giron T, Olmedo-Requena R, Delgado-Rodríguez M, Jiménez-Moleón JJ. Occupational exposure to pesticides and prostate cancer: a systematic review and meta-analysis. Occup Environ Med. 2016;73:134–144. doi: 10.1136/oemed-2014-102692. [DOI] [PubMed] [Google Scholar]
- 56.O’Callaghan-Gordo C, Kogevinas M, Cirach M, et al. Residential proximity to green spaces and breast cancer risk: the multicase-control study in Spain (MCC-Spain). Int J Hyg Environ Health. 2018;221:1097–1106. doi: 10.1016/j.ijheh.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Klompmaker JO, Janssen NAH, Bloemsma LD, et al. Associations of combined exposures to surrounding green, air pollution, and road traffic noise with cardiometabolic diseases. Environ Health Perspect. 2019;127:87003. doi: 10.1289/EHP3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Labib SM, Lindley S, Huck JJ. Spatial dimensions of the influence of urban green-blue spaces on human health: a systematic review. Environ Res. 2020;180:108869. doi: 10.1016/j.envres.2019.108869. [DOI] [PubMed] [Google Scholar]
- 59.Herrera R, Markevych I, Berger U, et al. Greenness and job-related chronic stress in young adults: a prospective cohort study in Germany. BMJ Open. 2018;8:e021599. doi: 10.1136/bmjopen-2018-021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell R, Popham F. Effect of exposure to natural environment on health inequalities: an observational population study. Lancet. 2008;372:1655–1660. doi: 10.1016/S0140-6736(08)61689-X. [DOI] [PubMed] [Google Scholar]
- 61.Brown SC, Lombard J, Wang K, et al. Neighborhood greenness and chronic health conditions in medicare beneficiaries. Am J Prev Med. 2016;51:78–89. doi: 10.1016/j.amepre.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 62.de Keijzer C, Tonne C, Basagaña X, et al. Residential surrounding greenness and cognitive decline: a 10-year follow-up of the Whitehall II Cohort. Environ Health Perspect. 2018;126:077003. doi: 10.1289/EHP2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Keijzer C, Basagaña X, Tonne C, et al. Long-term exposure to greenspace and metabolic syndrome: a Whitehall II study. Environ Pollut. 2019;255(pt 2):113231. doi: 10.1016/j.envpol.2019.113231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yitshak-Sade M, James P, Kloog I, et al. Neighborhood greenness attenuates the adverse effect of PM2.5 on cardiovascular mortality in neighborhoods of lower socioeconomic status. Int J Environ Res Public Health. 2019;16:E814. doi: 10.3390/ijerph16050814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson JL. The impact of shift patterns on healthcare professionals. J Nurs Manag. 2002;10:211–219. doi: 10.1046/j.1365-2834.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 66.Matheson A, O’Brien L, Reid JA. The impact of shiftwork on health: a literature review. J Clin Nurs. 2014;23:3309–3320. doi: 10.1111/jocn.12524. [DOI] [PubMed] [Google Scholar]
- 67.Wendeu-Foyet MG, Menegaux F. Circadian disruption and prostate cancer risk: an updated review of epidemiological evidences. Cancer Epidemiol Biomarkers Prev. 2017;26:985–991. doi: 10.1158/1055-9965.EPI-16-1030. [DOI] [PubMed] [Google Scholar]
- 68.Dickerman BA, Markt SC, Koskenvuo M, et al. Sleep disruption, chronotype, shift work, and prostate cancer risk and mortality: a 30-year prospective cohort study of Finnish twins. Cancer Causes Control. 2016;27:1361–1370. doi: 10.1007/s10552-016-0815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.James P, Hart JE, Arcaya MC, Feskanich D, Laden F, Subramanian SV. Neighborhood self-selection: the role of pre-move health factors on the built and socioeconomic environment. Int J Environ Res Public Health. 2015;12:12489–12504. doi: 10.3390/ijerph121012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


