Heart failure accounts for approximately 5% of all hospital admissions, and 30–45% of patients hospitalized with acute decompensated heart failure (ADHF) die within one year.1 Existing risk prediction models for ADHF patients are limited by lack of integration of right-sided filling pressures, as hemodynamic characterization is not routinely performed because of its invasive nature and cost.2,3 Persistently elevated filling pressures or signs and symptoms of congestion at discharge are associated with a poor prognosis in patients with ADHF.4–6 Recent studies have suggested the use of liver stiffness as a non-invasive surrogate marker of central venous pressure to offer additional prognostic information in patients hospitalized with ADHF.2,4,7,8 However, the available data are limited by small studies. Therefore, the primary objective of this systematic review and meta-analysis was to evaluate the association of liver stiffness and cardiovascular outcomes in hospitalized heart failure patients.
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines.9 Two reviewers (TJS and MSK) independently searched MEDLINE and Scopus databases in February 2018 for all published articles on human studies that included measurement of liver stiffness and assessment of all-cause mortality and/or adverse cardiac events. The search was conducted combining the following words and Medical Subject Headings terms: liver stiffness, liver cirrhosis, liver fibrosis, non-alcoholic fatty liver disease, elasticity imaging techniques, elastography and heart failure. Detailed search strategy for each database is provided in Table S1 in the Supplementary Material online. Only studies that included patients with established heart failure without prior history of chronic liver disease were included. Other data sources including bibliographies of relevant articles and proceedings of scientific meetings were also searched. No language or time restrictions were set. Abstracts and studies published from the same institute in overlapping time frames were excluded. Data were abstracted on a standardized collection sheet from the short-listed articles, and verified by two reviewers (TJS and MSK). In the case of any discrepancy, a third reviewer (SUK) was consulted. Endpoints were defined as reported in the individual studies. Adverse cardiac events were defined as either cardiac death or repeat heart failure hospitalization. The refined Quality In Prognosis Studies tool was used to assess the risk of bias in the included studies.10 When evaluating bias and validity in studies of prognostic factors, six important domains are considered: study participation, study attrition, prognostic factor measurement, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. Egger regression test was performed to evaluate publication bias. Review Manager (Version 5.5; Cochrane Collaboration, Oxford, UK) was used to perform the statistical analyses. Hazard ratios and their 95% confidence intervals (CIs) from individual studies were converted to log hazard ratios and corresponding standard errors, which were then pooled using a generic invariance weighted random effects model. An outcome needed a minimum of two studies to be analyzed and reported in our quantitative analysis.
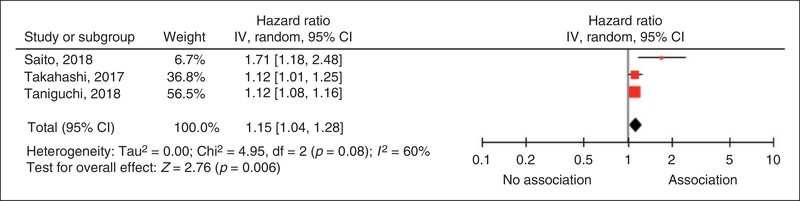
Four studies met criteria and were included in our systematic review.2,4,7,8 The PRISMA flowchart (Supplementary Figure S1) summarizes the literature search. Baseline characteristics of the included trials are summarized in Table 1. Three studies with available data on adverse cardiac outcomes comprising 792 participants hospitalized with ADHF (37% female, mean age 71 years) were included in our meta-analysis.2,4,7 The participants had a high prevalence of comorbidities: 30% had coronary artery disease, 31% had diabetes mellitus, and 49% had hypertension. The median follow-up time was 273 days (range: 153–464 days). Liver stiffness was assessed by transient elastography in two studies2,4 and by liver fibrosis scores (LFSs) (non-alcoholic fatty liver disease fibrosis score) in one study.7 Transient elastography utilizes ultrasonography to estimate liver stiffness whereas LFS utilizes a combination of liver function tests to quantify liver stiffness.2,7 The studies overall had low risk of bias (Supplementary Table S2). No evidence of publication bias was found (p=0.07). Our pooled analysis shows that increased liver stiffness was associated with higher risk of adverse cardiac events (hazard ratio=1.15, 95% CI=1.04–1.28, p=0.006, I2=60%) (Figure 1).
Table 1.
Baseline characteristics of included studies.
| Reference | N | Method for liver stiffness assessment | Study location | Country | Variables adjusted for in the primary outcome | Primary outcome | Outcome follow-up, median, days | Average age, years | BMI, kg/m2 | Male, % | NYHA Glass III or IV, % | Mean LVEF, % (±SD) | Prevalence of comorbidities, n (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HL | CAD | AF | HTN | DM | |||||||||||||
| Saito ec al., 20184 | 105 | Transient elastography | Single centre | Japan | Age, sex and NT-proBNP | Adverse cardiac event | 153 | 78 | 20.6 | 72 | 61 | 42 (202) | NA | NA | 31 (30) | 4 (4) | NA |
| Takahashi et al., 20187 | 516 | NFSa | Single centre | Japan | Age, BMI, NY HA, albumin, AST/ALT, Hb, eGFR and BNP | Adverse cardiac event | 464 | 71 | 214 | 59 | 82 | 49 (173) | 150 (29) | 154 (30) | NA | 304 (59) | 164 (32) |
| Taniguchi et al., 20182 | 171 | Transient elastography | Single centre | Japan | Age, sex and systemic organ functions (estimated glomerular filtration ratio, total bilirubin), right ventricular functions by echo | Adverse cardiac event | 203 | 65 | 20.5 | 68 | 15 | 40 (24.6) | 105 (61) | 49 (29) | 74 (44) | 77 (45) | 46 (27) |
| Sato et a1., 20178 | 1058 | Fibrosis-4 Indexb | Single centre | Japan | Sex, BMI, NYHA, CAD, HTN, DM, HL, AF, Hb, eGFR, B-type natriuretic peptide, LVEF, renin-angiotensin-aldosterone system inhibitors, (β-blockers, diuretics, inotropic agents | All-cause mortality | 1047 | 67 | 23.3 | 61 | 3 | 50 (163) | 828 (79) | 277 (26) | 410 (39) | 801 (76) | 432 (41) |
NFS was calculated using the following formula: – 1.675 + 0.037 Ẳ~ age (years) + 0.094 Ẳ~ BMI (kg/m2) +1.13 Ẳ~ impaired fasting glucose or diabetes (yes = 1, no = 0) + 0.099 Ẳ~ AST/ALT ratio − 0.013 (Ẳ~ 0.109/1) − 0.66 Ẳ~ albumin (g/dl).7
Fibrosis-4 Index was calculated using the following formula: age (years) x AST (IU/1)/platelet count (109/1) x square root of ALT (IU/1).8
N: number of patients with heart failure; NFS: non-alcoholic fatty liver disease fibrosis score; NYHA: New York Heart Association classification; LVEF: left ventricular ejection fraction; SD: standard deviation; HL: hyperlipidemia; CAD: coronary artery disease; AF: atrial fibrillation; HTN: hypertension; DM: diabetes mellitus; NT-proBNP: N-terminal pro-B type natriuretic peptide; BMI: body mass index; NA: not available; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Hb: hemoglobin; eGFR: estimated glomerular filtration rate
Figure 1.
Forest plot assessing the association between liver stiffness and adverse cardiac events.
CI: confidence interval; IV: inverse variance.
This meta-analysis suggests that liver stiffness may be a novel, independent prognostic marker of cardiovascular outcomes in patients hospitalized with ADHF when assessed in the absence of liver disease. Increased liver stiffness may reflect residual congestion secondary to volume and pressure overload and/or inadequate liver perfusion with low cardiac output in patients hospitalized with ADHF.4 The use of liver stiffness may be especially helpful in situations when hemodynamic status is unable to be readily assessed at the bedside on physical examination, as the assessment of liver stiffness by transient elastography or LFS is rapid, simple, and objective. Further study is required to determine whether serial measurements to detect change in liver stiffness may be useful to guide treatment of volume status in ADHF and thereby improve outcomes.2
This meta-analysis has several limitations. First, the systematic search yielded mostly small observational studies that are unable to fully account for confounding factors. Second, our findings were subject to considerable heterogeneity. Third, all the studies were from Japan in a homogenous population and thus generalizability may be limited. In conclusion, increased liver stiffness is associated with poor cardiovascular outcomes in hospitalized heart failure patients and may serve as a promising tool in ADHF patients to identify those at high-risk for adverse outcomes.
Supplementary Material
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant number KL2TR001424 (SSK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi T, Ohtani T, Kioka H, et al. Liver stiffness reflecting right-sided filling pressure can predict adverse outcomes in patients with heart failure. JACC Cardiovasc Imaging, Epub ahead of print 12 January 2018. DOI: 10.1016/j.jcmg.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Arzilli C, Aimo A, Vergaro G, et al. N-terminal fraction of pro-B-type natriuretic peptide vs. clinical risk scores for prognostic stratification in chronic systolic heart failure. Eur J Prev Cardiol 2018; 25: 889–895. [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Kato M, Nagashima K, et al. Prognostic relevance of liver stiffness assessed by transient elastography in patients with acute decompensated heart failure. Circ J 2018; 82: 1822–1829. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen ME, Andersson C, Vasan RS, et al. Characteristics and prognosis of heart failure with improved compared with persistently reduced ejection fraction: A systematic review and meta-analyses. Eur J Prev Cardiol 2018; 25: 366–376. [DOI] [PubMed] [Google Scholar]
- 6.Baert A, Pardaens S and de Smedt D. Factors associated with health-related quality of life in stable ambulatory congestive heart failure patients: Systematic review. Eur J Prev Cardiol 2018; 25: 472–481. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Watanabe T, Shishido T, et al. The impact of non-alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels 2018; 33: 733–739. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y, Yoshihisa A, Kanno Y, et al. Liver stiffness assessed by Fibrosis-4 Index predicts mortality in patients with heart failure. Open Heart 2017; 4: e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.