Abstract
Background
In 2018, the Infectious Diseases Society of America (IDSA) published guidelines for diagnosis and treatment of Clostridioides (formerly Clostridium) difficile infection (CDI). However, there is little guidance regarding which treatments are cost-effective.
Methods
We used a Markov model to simulate a cohort of patients presenting with an initial CDI diagnosis. We used the model to estimate the costs, effectiveness, and cost-effectiveness of different CDI treatment regimens recommended in the recently published 2018 IDSA guidelines. The model includes stratification by the severity of the initial infection, and subsequent likelihood of cure, recurrence, mortality, and outcomes of subsequent recurrences. Data sources were taken from IDSA guidelines and published literature on treatment outcomes. Outcome measures were discounted quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs).
Results
Use of fidaxomicin for nonsevere initial CDI, vancomycin for severe CDI, fidaxomicin for first recurrence, and fecal microbiota transplantation (FMT) for subsequent recurrence (strategy 44) cost an additional $478 for 0.009 QALYs gained per CDI patient, resulting in an ICER of $31 751 per QALY, below the willingness-to-pay threshold of $100 000/QALY. This is the optimal, cost-effective CDI treatment strategy.
Conclusions
Metronidazole is suboptimal for nonsevere CDI as it is less beneficial than alternative strategies. The preferred treatment regimen is fidaxomicin for nonsevere CDI, vancomycin for severe CDI, fidaxomicin for first recurrence, and FMT for subsequent recurrence. The most effective treatments, with highest cure rates, are also cost-effective due to averted mortality, utility loss, and costs of rehospitalization and/or further treatments for recurrent CDI.
Keywords: Clostridium difficile infection, Clostridioides difficile infection, cost-effectiveness
We estimated the cost-effectiveness of different treatment regimens for Clostridioides difficile infection (CDI), per Infectious Diseases Society of America 2018 guidelines. Optimal treatment is vancomycin for severe CDI, fidaxomicin for first recurrence, and fecal microbiota transplant for subsequent recurrence.
Clostridioides difficile (formerly Clostridium difficile) caused close to half a million incident infections in 2011 and 29 000 deaths, making C. difficile infection (CDI) the most common cause of gastroenteritis-related deaths in the United States [1]. The resulting economic burden of CDI is staggering, with annual CDI-attributable cost ranging from $1.1 billion to $6.3 billion in the United States [2–5]. Cost frequently guides treatment decisions for CDI; metronidazole has been appealing as initial treatment as it is inexpensive. Though fidaxomicin has a lower rate of CDI recurrence compared to the standard treatment course of oral vancomycin [6], the cost of therapy can be prohibitive.
The Infectious Diseases Society of America (IDSA)/Society for Healthcare Epidemiology of America (SHEA) updated the clinical practice guidelines for CDI in 2018, with the notable change of adding vancomycin and fidaxomicin to the list of recommended first-line treatments for initial nonsevere CDI, and fidaxomicin for severe CDI [7]. Metronidazole was demoted from a first-line agent for nonsevere CDI to an alternative agent when first-line drugs are not available. Additionally, the panel agreed that fecal microbiota transplantation (FMT) be reserved for those patients experiencing at least 2 episodes of recurrent CDI. While metronidazole is no longer recommended in most scenarios, the decision for initial treatment remains ambiguous and, in practice, is driven by cost [8]. Multiple therapeutic options are suggested in the guidelines, depending on severity and recurrence, but no guidance exists regarding which treatment is best. The cheaper initial cost of metronidazole to vancomycin or fidaxomicin will likely remain a consideration in healthcare settings regardless of clinical guidelines [8]. Similarly, while the cost-effectiveness analysis of vancomycin compared to fidaxomicin favors fidaxomicin due to decreased rates of recurrence [9–11], fidaxomicin remains prohibitively expensive for patients and insurance providers. The aim of this study is to determine the cost-effectiveness of treatment options for CDI based on the 2018 IDSA/SHEA guidelines.
METHODS
Analytic Overview
We used a Markov model to simulate a cohort of patients presenting with initial CDI diagnosis. We used the model to estimate costs, effectiveness, and cost-effectiveness of all the different CDI treatment regimens that are consistent with the recently published 2018 IDSA/SHEA guidelines. The model includes stratification by the severity of the initial infection, and subsequent likelihood of cure, recurrence, mortality, and outcomes of subsequent recurrences. The cohort was simulated in 2-month time steps, as this was the clinically relevant time frame for capturing treatment outcome and recurrence [12].
We compared a total of 48 treatment strategies, reflecting all permutations of treatment options for initial and recurrent CDIs that are consistent with IDSA/SHEA recommendations. Strategies were compared by direct healthcare costs and quality-adjusted life-years (QALYs). Strategies were evaluated over a 1-year time horizon, with remaining quality-adjusted life expectancy (QALE) and lifetime healthcare costs included for patients who survived to the end of the year. A cost-effectiveness analysis was conducted from a healthcare payer perspective, with costs and health outcomes discounted at 3% per year. We calculated incremental cost-effectiveness ratios (ICERs) of each strategy by dividing the additional cost by the additional QALYs gained compared to the next less expensive strategy. We interpreted ICERs using a willingness-to-pay threshold of US$100 000. Strategies with an ICER <$100 000 were considered cost-effective. The decision analytic model was implemented in TreeAge Pro version 2018 software (TreeAge, Williamstown, Massachusetts).
Model Structure
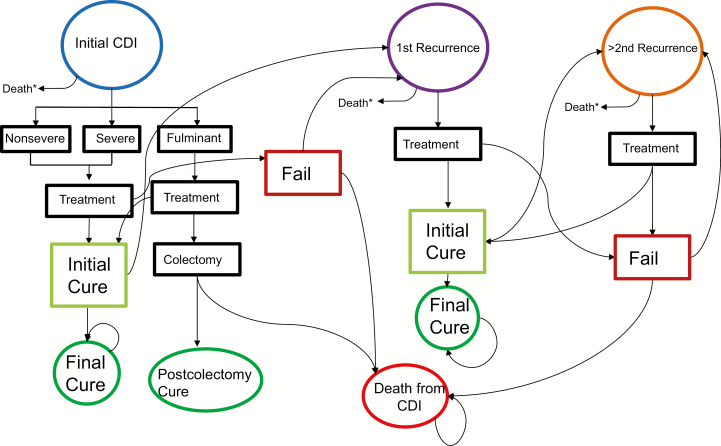
A state-transition diagram of the CDI model is shown in Figure 1. At initial CDI diagnosis, patients were stratified into 3 levels of severity: nonsevere, severe, or fulminant infection. Consistent with IDSA/SHEA guidelines, the severity of CDI dictated possible treatment options. The probability of cure by initial CDI severity and treatment regimen was estimated from the published literature. Patients who failed initial treatment experienced a risk of CDI-related death. Patients who survived following treatment failure progressed to the first recurrence state. Patients who were initially cured by first-line therapy could also experience recurrence within the 2-month simulation cycle; these patients also transitioned to the first recurrence state.
Figure 1.
State transition diagram of patient progression through initial Clostridioides difficile infection (CDI) and potential recurrences in the first 12 months. The states of final cure, postcolectomy cure, and death are absorbing states, as reflected by the self-loop arrows. Any patient who is alive at the end of the 12 months is assumed to have an average life expectancy with no further risk of CDI occurrence or CDI-related death. *Death from other causes.
Patients in the first recurrence state could be treated with regimens recommended by IDSA/SHEA for those with first recurrent CDI. For recurrent CDI, patients could be cured or fail treatment. Those who failed treatment had a probability of CDI-related death. Those who survived treatment failure progressed to second recurrence. Patients who initially responded to therapy also had a risk of recurrence within the 2-month cycle; those who experienced recurrence also progressed to the second recurrent CDI state.
Patients in the second recurrence state would undergo treatment, following IDSA/SHEA guidelines for patients with ≥2 recurrences. Patients who failed treatment were assumed not to have died from CDI-related causes, but would reenter the second recurrence state for treatment of recurrent CDI.
Patients who were initially cured following treatment and did not experience a recurrence within 2 months of treatment were assumed to have achieved long-term cure and no longer had a risk of CDI recurrence. Patients in all health states also experienced a small risk of dying from other, non-CDI causes in each time step, estimated from US life tables for a 67-year-old, the median age of initial CDI patients in this cohort [13].
Treatment Strategies
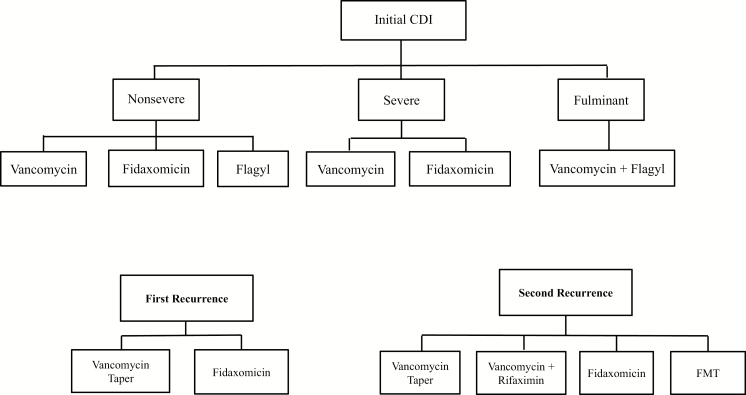
IDSA/SHEA guidelines recommend a number of different treatment regimens depending on the type of CDI (initial vs recurrent) and severity (Figure 2). For initial CDI that is nonsevere, recommended regimens are vancomycin for 10 days, fidaxomicin for 10 days, or metronidazole for 10 days, whereas only vancomycin or fidaxomicin is recommended for severe CDI. For fulminant CDI, there is only a single recommended treatment regimen (vancomycin 500 mg 4 times daily with intravenous metronidazole). Fulminant CDI patients who fail treatment could die from the CDI; those who survived were assumed to undergo a colectomy, with a corresponding probability of surgical death. Patients surviving the colectomy procedure were assumed to be cured of the CDI and no longer at risk of CDI recurrence.
Figure 2.
Available treatment options for Clostridioides difficile infection per Infectious Diseases Society of America guidelines. Abbreviations: CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation.
For a first CDI recurrence, IDSA/SHEA guidelines recommend treatment with either a vancomycin taper or a 10-day course of fidaxomicin, while recommended regimens for second or subsequent recurrences are vancomycin taper, vancomycin plus rifaximin, fidaxomicin, or FMT.
Treatment strategies were constructed by selecting one of the recommended regimens for each CDI type and severity. This resulted in 48 different treatment strategies, representing all possible combinations of recommended regimens.
Post–time Horizon
Any patient alive at the end of the 1-year time horizon was assumed to be cured of their CDI without risk of future recurrence. These patients were assumed to have an average remaining life expectancy and lifetime healthcare costs. Discounted remaining QALE was calculated from US life tables [14] starting from age 68, the age of the cohort at the end of the time horizon, and age-specific utility weights. The discounted QALE after cure was 9.21 years. They also accrued baseline medical expenses not related to CDI. The discounted costs accrued over the remaining lifetime were $54 282, calculated from average age-specific healthcare expenditure estimates in the United States [15].
Model Parameter Estimates
Epidemiology of CDI, probability of cure, failure, and recurrence of each regimen were taken from published medical literature (Table 1). For estimates of cure, failure, and recurrence with each medication regimen, we initially reviewed the literature cited in the IDSA/SHEA CDI guidelines. Thereafter, we searched PubMed using the following Medical Subject Headings (MeSH) search terms: adults, humans, clinical trials, Clostridium difficile, and/or recurrence for additional relevant publications. Medication costs (Table 2) were estimated by applying a 40% discount to the average wholesale price (AWP) published through Micromedex/Redbook [16], which is standard in such cost estimates [17]. Costs for FMT were taken from Centers for Medicare and Medicaid Services and published studies (Table 3, Supplementary Tables 1 and 2). The daily cost of CDI hospitalization was calculated from a published study evaluating the economic burden of CDI [18]. We estimated the probability of hospitalization and length of stay for those hospitalized based on severity of infection [12, 13]. Length of stay was multiplied by the per-day cost of hospitalization to calculate total hospitalization costs by severity of CDI (Supplementary Table 3).
Table 1.
Input Parameters for Epidemiology of Clostridioides difficile Infection, Cure, and Recurrence With Recommended Treatments
| Parameter | Probability | Range or 95% CI | Source(s) |
|---|---|---|---|
| Epidemiology of CDI | Range (±15%) | ||
| Probability of nonsevere infection | 0.33 | 0.26–0.36 | … |
| Probability of severe infection | 0.43 | 0.34–0.46 | [21] |
| Probability of fulminant infection | 0.23 | 0.18–0.24 | [21] |
| Medication outcomes | |||
| Initial infection: nonsevere | 95% CI | ||
| Cure after vancomycin (10 d) | 0.898 | .861–.928 | [6, 26, 27] |
| Cure after fidaxomicin (10 d) | 0.917 | .879–.945 | [6, 27] |
| Cure after metronidazole (10 d) | 0.783 | .709–.846 | [26, 28] |
| Recur after vancomycin (10 d) | 0.215 | .170–.265 | [6, 26, 27] |
| Recur after fidaxomicin (10 d) | 0.134 | .093–.184 | [6, 27] |
| Recur after metronidazole (10 d) | 0.208 | .159–.264 | [26, 28] |
| Initial infection: severe | |||
| Cure after vancomycin (10 d) | 0.846 | .791–.892 | [6, 26, 27] |
| Cure after fidaxomicin (10 d) | 0.800 | .733–.857 | [6, 27] |
| Recur after vancomycin (10 d) | 0.253 | .191–.322 | [6, 26, 27] |
| Recur after fidaxomicin (10 d) | 0.114 | .067–.179 | [6, 27] |
| Initial infection: fulminant | |||
| Cure after vancomycin 500 mg + IV metronidazole (14 d) | 0.515 | .438–.592 | [29, 30] |
| Recur after vancomycin + IV metronidazole (14 d) | 0.253 | .191–.322 | [6, 26, 27] |
| Death after medical treatment for fulminant infection | 0.485 | .397–.551 | [29, 30] |
| Colectomy survival for fulminant infection | 0.660 | .561–.759 | [31] |
| First recurrence | |||
| Cure after vancomycin taper | 0.916 | .834–.965 | [32] |
| Cure after fidaxomicin (10 d) | 0.937 | .858–.979 | [32] |
| Recur after vancomycin taper | 0.355 | .237–.487 | [32] |
| Recur after fidaxomicin | 0.197 | .109–.313 | [32] |
| Second or subsequent recurrence | |||
| Cure after vancomycin taper | 0.690 | .492–.847 | [33] |
| Cure after fidaxomicin (10 d) | 0.816 | .657–.923 | [34] |
| Cure after vancomycin + rifaximin | 0.848 | .681–.949 | [35] |
| Cure after FMT (endoscopic) | 0.896 | .863–.924 | [36–45] |
| Cure after FMT (capsule) | 0.913 | .876–.941 | [42, 45–48] |
| Recur after vancomycin taper | 0.310 | .153–.508 | [33] |
| Recur after fidaxomicin | 0.290 | .246–.333 | [34] |
| Recur after vancomycin + rifaximin | 0.151 | .051–.319 | [35, 49] |
| Recur after FMT (endoscopic) | 0.113 | .085–.147 | [36–45] |
| Recur after FMT (capsule) | 0.087 | .059–.124 | [42, 45–48] |
Abbreviations: CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation; IV, intravenous.
Table 2.
Medication Costs
| Medication | Unit, mg | AWP for 1 Unit | Estimated Purchasing Price for 1 Unit | Course Duration, d | Total Price for Course (0.4 of AWP) | Range (0.2–0.6 of AWP) | Source |
|---|---|---|---|---|---|---|---|
| Vancomycin | 125 | $0.88 | $0.35 | 10 | $14.08 | $7.04–$21.12 | [16, 17] |
| Metronidazole | 500 | $0.73 | $0.29 | 10 | $8.76 | $4.38–$13.14 | [16, 17] |
| Rifaximin | 1000 | $9.20 | $3.68 | 20 | $88.32 | $44.16–$132.48 | [16, 17] |
| Fidaxomicin | 200 | $220.90 | $88.36 | 10 | $1767.20 | $883.60–$2650.80 | [16, 17] |
| Metronidazole (IV) | 500 | $2.34 | $0.94 | 14 | $39.12 | $19.56–$58.68 | [16, 17] |
All costs are given as US dollars.
Abbreviations: AWP, average wholesale price; IV, intravenous.
Table 3.
Fecal Microbiota Transplantation Costs
| Product | HCPC | Total Charges | Range | Source |
|---|---|---|---|---|
| FMT prep instillation | G0455 | $226.54 | $226.54–$485.00 | [50], Open Biome website |
| Diagnostic colonoscopy | 45378 | $568.70 | … | [50] |
| Vancomycin (10 d) | … | $14.08 | $7.04–$21.12 | [16] |
| Donor testing | … | $488.41 | $488.41–$697.07 | Supplementary Table 1 |
| Outpatient visit | 99203 | $205.31 | … | [50] |
| Total FMT cost | … | $1503.04 | $1495.00–$1977.20 | … |
All costs are given as US dollars.
Abbreviations: FMT, fecal microbiota transplantation; HCPC, Healthcare Common Procedure Coding.
All costs from published literature were converted to 2018 US dollars. Utility weights were derived from 4 published sources (Supplementary Table 4). For our model, the lower and upper limits of the weights reflect the range of utilities reported in the literature.
Sensitivity Analysis
Due to the potentially prohibitive cost of fidaxomicin, we varied this cost in a one-way sensitivity analysis to determine the threshold for which fidaxomicin would be a cost-effective choice for nonsevere, severe, or recurrent CDI. We additionally performed a one-way sensitivity analysis varying the cost of FMT, as costs of FMT are variable depending on the source of stool and where it is being performed. We explored at what threshold FMT would no longer be cost-effective for recurrent CDI.
Finally, we performed a probabilistic sensitivity analysis (PSA) varying input parameters of interest within a defined distribution. For each point estimate, a 95% confidence interval was calculated to determine the distribution (Table 1). For medication costs, we varied costs from 20% of AWP to 60% of AWP (Table 2). Probabilities were sampled from β-distributions. For other values (cost, utility), we used normal distributions. We sampled 10 000 parameter sets from these distributions and evaluated the model for each parameter set. We constructed a cost-effectiveness acceptability curve by varying the willingness-to-pay threshold from $0 to $300 000 per QALY gained and calculating the proportion of PSA samples for which each strategy was optimal (achieving the greatest benefit while falling below the willingness-to-pay threshold per QALY gained).
RESULTS
Model Validation
We validated our simulation model by comparing the short-term model–predicted mortality to that observed in large CDI patient cohorts [12, 19–21]. Our average 60-day mortality for all 48 strategies was 8%, whereas many studies report 30-day mortality of 9%–10%. It should be noted that the literature likely overestimates current CDI-related mortality, because many of these studies used data that are several years old (between 2005 and 2014), when metronidazole use was more common, and FMT was not yet common practice. In our model, 1-year mortality was 9.2%, consistent with prior published studies (approximately 11%) [19].
Model Output
The expected cost and effectiveness of all 48 treatment strategies were evaluated (Supplementary Table 5). After eliminating dominated strategies, only 6 treatment strategies remained (Table 4). Note that the treatment regimen for fulminant initial CDI is not listed in Table 4 as it is the same across all 48 treatment strategies, consistent with a single treatment option recommended in the IDSA/SHEA guidelines.
Table 4.
Cost-effectiveness of Clostridioides difficile Infection Treatment Strategies
| Strategy No. | Nonsevere | Severe | First Recurrence | Second or Later Recurrence | Cost | Incremental Cost | Effectiveness, QALYs | Incremental Effectiveness, QALYs | ICER, $/QALY |
|---|---|---|---|---|---|---|---|---|---|
| 3 | Metronidazole | Vancomycin | Vancomycin | Vancomycin | $64 509 | … | 12.280 | … | … |
| 1 | Vancomycin | Vancomycin | Vancomycin | Vancomycin | $64 692 | $183 | 12.352 | 0.072 | 2537 |
| 25 | Vancomycin | Vancomycin | Vancomycin | Vancomycin + rifaximin | $64 917 | $225 | 12.406 | 0.054 | 4208 |
| 37 | Vancomycin | Vancomycin | Vancomycin | FMT | $65 125 | $208 | 12.426 | 0.020 | 10 383 |
| 43 | Vancomycin | Vancomycin | Fidaxomicin | FMT | $65 411 | $286 | 12.442 | 0.016 | 27 428 |
| 44 | Fidaxomicin | Vancomycin | Fidaxomicin | FMT | $65 889 | $478 | 12.451 | 0.009 | 31 751 |
All costs are given as US dollars.
Abbreviations: FMT, fecal microbiota transplantation; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
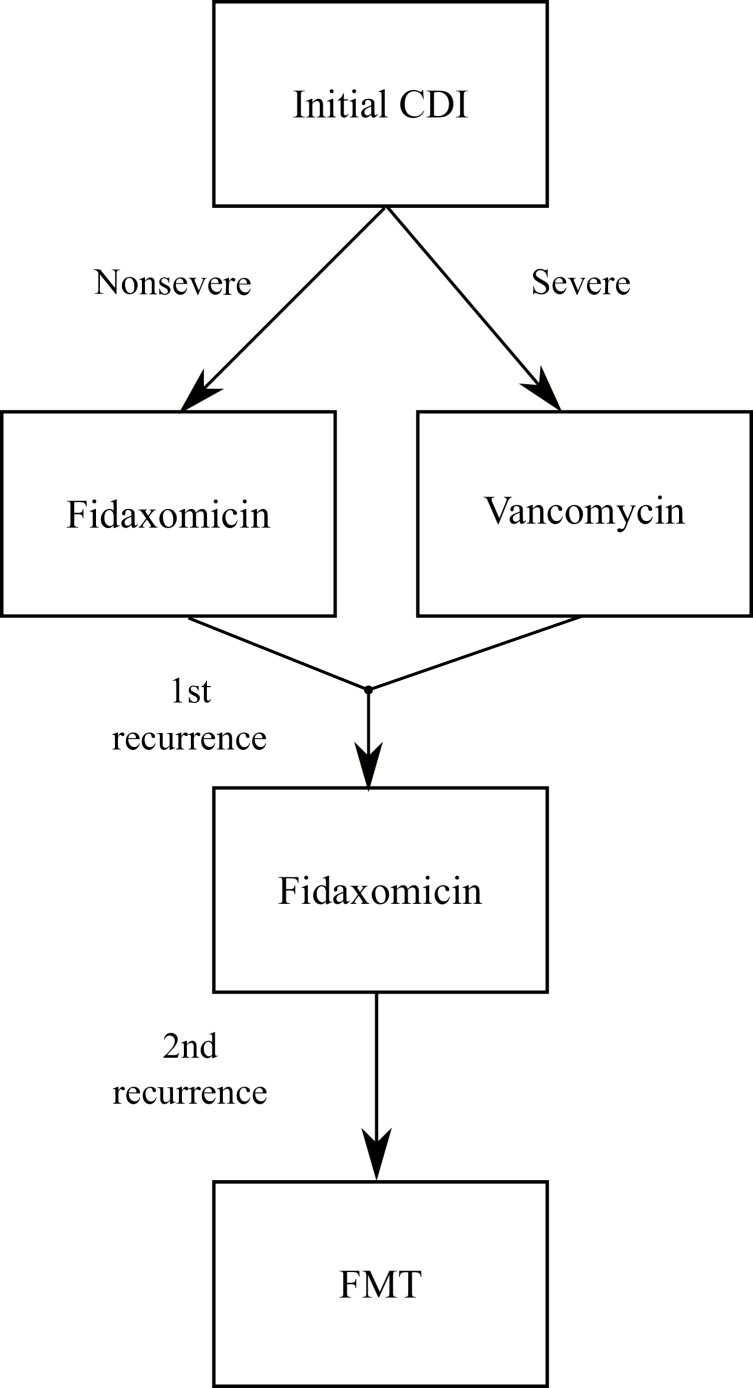
The least expensive, least beneficial strategy was the use of metronidazole for initial nonsevere CDI and vancomycin for severe initial CDI and all recurrences of CDI (strategy 3). The most effective strategy was the use of fidaxomicin for nonsevere initial CDI, vancomycin for severe CDI, fidaxomicin for first recurrence, and FMT for subsequent recurrence (strategy 44). This strategy had an ICER of $31 751 per QALY, which falls below the willingness-to-pay threshold of $100 000 per QALY gained. Thus, from a cost-effectiveness standpoint, strategy 44 is the optimal CDI treatment strategy (Figure 3).
Figure 3.
Optimal strategy for treatment of Clostridioides difficile infection (CDI): fidaxomicin for nonsevere CDI, vancomycin for severe CDI, fidaxomicin for first recurrence, and FMT for second and subsequent recurrence. Abbreviations: CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation.
Sensitivity Analysis
In one-way sensitivity analysis of fidaxomicin pricing, fidaxomicin remained the optimal treatment regimen for nonsevere CDI up to a price of $5100 (115% AWP) per 10-day course. Above this price, vancomycin was preferred for nonsevere CDI. Above a cost of $4590 (104% of AWP) per 10-day course of fidaxomicin, vancomycin was also preferred for first recurrent CDI, and fidaxomicin was not cost-effective for any severity or recurrence of CDI.
When varying FMT pricing, only at a cost greater than $14 806 (>9 times the estimated base case cost) was FMT no longer cost-effective, with vancomycin plus rifaximin preferred for treating second or subsequent CDI recurrence.
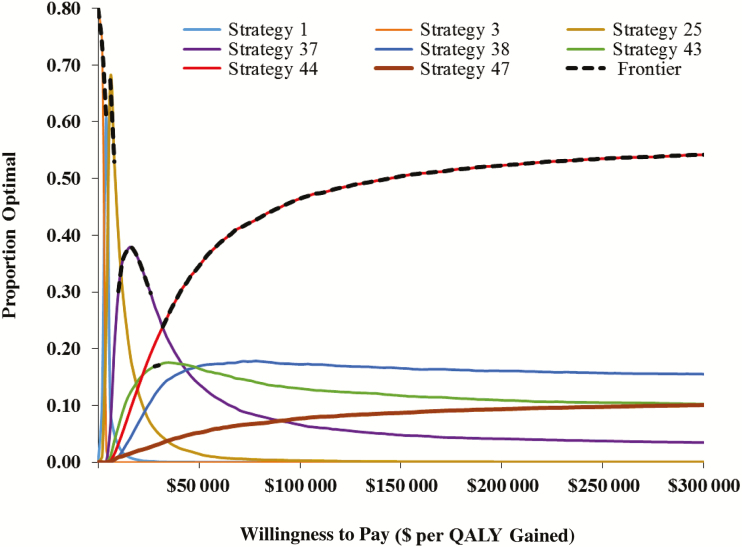
In PSA, assuming a willingness-to-pay threshold of $100 000 per QALY gained, strategy 44 was optimal in 47% of simulations (Figure 4). As long as the willingness-to-pay threshold was above $30 000/QALY, this was the optimal strategy on average as well. The next best option was vancomycin for nonsevere and severe initial CDI, vancomycin for first recurrence, and FMT for subsequent recurrence (strategy 37); this strategy was optimal in 17% of simulations. Vancomycin for nonsevere and severe initial CDI, fidaxomicin for first recurrence, and FMT for subsequent recurrence (strategy 43) was optimal in 13% of simulations.
Figure 4.
Cost-effectiveness acceptability curve for Clostridioides difficile infection (CDI) treatment strategies. Strategy 3 (orange) represents metronidazole for nonsevere initial CDI, and vancomycin for severe CDI and recurrent CDI. Strategy 37 (purple) represents vancomycin for all initial CDI and first recurrence, and fecal microbiota transplantation (FMT) for subsequent recurrence. Strategy 43 (green) represents vancomycin for initial CDI (nonsevere and severe), fidaxomicin for first recurrence, and FMT for subsequent recurrence. Strategy 44 (red) represents fidaxomicin for initial nonsevere CDI and first recurrence, vancomycin for initial severe CDI, and FMT for subsequent recurrence. Strategy 47 (brown) represents fidaxomicin for all initial CDI and first recurrence, and FMT for subsequent recurrence. The black dashed curve highlights the cost-effectiveness frontier, which is the optimal strategy on average across all probabilistic sensitivity analysis samples. Strategy 44 is on the cost-effectiveness frontier above a willingness-to-pay threshold of $30 000 per quality-adjusted life-year (QALY).
DISCUSSION
Based on possible treatment algorithms from the 2018 IDSA/SHEA guidelines, we found that initial treatment for nonsevere CDI with fidaxomicin, followed by fidaxomicin for first recurrence and FMT for any subsequent recurrence, to be cost-effective. For severe CDI, initial treatment with vancomycin, followed by fidaxomicin for first recurrence and FMT for any subsequent recurrence, was the cost-effective treatment strategy. Strategies using metronidazole for nonsevere CDI, while less expensive, were also less beneficial than strategies involving more expensive therapies and would not be optimal at the commonly accepted willingness-to-pay thresholds in the United States. Our results demonstrate that the most effective treatments with highest cure rates are also cost-effective due to averted mortality, utility loss, and costs of rehospitalization and/or further treatments for recurrent CDI.
Our results are consistent with prior literature that supports earlier use of fidaxomicin (either for initial treatment of CDI or first recurrence) [9–11]. For initial severe CDI, vancomycin was favored over fidaxomicin, due to the combined subgroup analysis from 3 randomized controlled trials (RCTs) for CDI treatments. The primary study that drove our estimate for vancomycin cure in severe CDI was an RCT of fidaxomicin to vancomycin for CDI, which found initial cure rates for vancomycin to be superior to fidaxomicin (88.6% vs 82.1%, respectively) [6]. Although this was not statistically significant, and confidence intervals for each estimate overlap, if cure rates for vancomycin and fidaxomicin are essentially equal in severe CDI, vancomycin will be favored due to its lower cost. This was a high-quality RCT, and the best data to date of clinical cure with severe CDI. Furthermore, we performed a PSA, where the ranges for cure in severe CDI with vancomycin vs fidaxomicin overlapped, and our results were consistent.
Despite the higher upfront cost of fidaxomicin, multiple cost-effectiveness studies have found fidaxomicin to be cost-effective over vancomycin for initial CDI therapy [9–11, 22, 23], due to reduced risk of recurrence and subsequent hospitalizations. However, a number of these studies were sponsored by the manufacturer, or supported by Astellas, which has non-US rights to fidaxomicin, potentially introducing bias in the conclusions. Additionally, unlike prior studies, we evaluated the entire treatment algorithm from initial CDI to first and subsequent recurrence. Accounting for the risk of recurrence favors fidaxomicin due to averted recurrence and its associated mortality and healthcare costs. Two recent systematic reviews of economic evaluations support our findings that fidaxomicin is a cost-effective option in initial and recurrent CDI [23] and FMT is cost-effective in recurrent CDI [24].
Our model has a few notable limitations. First, we did not stratify individuals into risk categories for recurrence, such as those with inflammatory bowel disease, transplant patients, or malignancy. These subgroups may have higher risk of recurrence and additional factors related to their underlying disease that would certainly affect treatment outcomes and costs. Additionally, we did not incorporate prior antibiotic exposure into our model, nor did we model prophylactic vancomycin with systemic antibiotics [25]. FMT was limited to second or subsequent recurrence per IDSA/SHEA guidelines. Moreover, input parameters for CDI proportion hospitalized and hospital length of stay were taken from a single study, and in reality are likely highly variable. Furthermore, there are no direct comparisons of all therapies for second and subsequent recurrence. Many of the input parameters come from small studies, including some populations with inflammatory bowel disease, which may not represent the general population. We attempted to account for this variability using the PSA and still found our results to be robust.
In conclusion, based on the 2018 update from IDSA/SHEA clinical practice guidelines, fidaxomicin is the cost-effective initial therapy for nonsevere CDI, followed by fidaxomicin for the first recurrence. Oral vancomycin is cost-effective for severe CDI, followed by fidaxomicin for first recurrence. Regardless of initial severity, for any second or subsequent recurrence, FMT is optimal therapy. Though the use of metronidazole for initial, nonsevere CDI was a less costly strategy, it was also less effective; more expensive strategies provided additional QALY gains at costs far below the commonly accepted willingness-to-pay threshold in US healthcare systems, and hospital pharmacy formularies should consider the overall cost-effectiveness of these therapies when creating institutional guidelines, not just cost. Fidaxomicin and FMT should take a more prominent role in the management of recurrent CDI due to the cost-effective nature of these therapies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH) (grant numbers K23AI13885 to R. R. and K25AI118476 to E. A. E.).
Potential conflicts of interest. E. A. E. has received grants from the NIH, and personal fees from ViiV Healthcare. B. P. V. has received personal fees from AbbVie and Janssen, and grants from Takeda, Roche, Celgene, and Diasorin. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis 2016; 16:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55(Suppl 2):S88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect 2010; 74:309–18. [DOI] [PubMed] [Google Scholar]
- 5. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013; 173:2039–46. [DOI] [PubMed] [Google Scholar]
- 6. Louie TJ, Miller MA, Mullane KM, et al. ; OPT-80-003 Clinical Study Group Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 7. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 8. Fabre V, Dzintars K, Avdic E, Cosgrove SE. Role of metronidazole in mild Clostridium difficile infections. Clin Infect Dis 2018; 67:1956–8. [DOI] [PubMed] [Google Scholar]
- 9. Marković V, Kostić M, Iličković I, Janković SM. Cost-effectiveness comparison of fidaxomicin and vancomycin for treatment of Clostridium difficile infection: a Markov model based on data from a south west Balkan country in socioeconomic transition. Value Health Reg Issues 2014; 4:87–94. [DOI] [PubMed] [Google Scholar]
- 10. Watt M, McCrea C, Johal S, Posnett J, Nazir J. A cost-effectiveness and budget impact analysis of first-line fidaxomicin for patients with Clostridium difficile infection (CDI) in Germany. Infection 2016; 44:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health 2013; 16:297–304. [DOI] [PubMed] [Google Scholar]
- 12. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med 2017; 177:546–53. [DOI] [PubMed] [Google Scholar]
- 13. Reveles KR, Lawson KA, Mortensen EM, et al. National epidemiology of initial and recurrent Clostridium difficile infection in the Veterans Health Administration from 2003 to 2014. PLoS One 2017; 12:e0189227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arias E, Heron M, Xu J. National vital statistics reports. United States life tables, 2014 Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_04.pdf. Accessed 15 August 2018. [PubMed]
- 15. Stagnitti MN. Medical Expenditure Panel Survey. National health care expenses per person in the US civilian noninstitutionalized population, 2014 Available at: https://meps.ahrq.gov/data_files/publications/st493/stat493.shtml. Accessed 15 August 2018.
- 16. Truven Health Analytics. IBM Micromedex Redbook (electronic version) 2018. Available at: http://www.micromedexsolutions.com.ezp1.lib.umn.edu/. Accessed 15 June 2018.
- 17. Hay JW, Smeeding J, Carroll NV, et al. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report—part I. Value Health 2010; 13:3–7. [DOI] [PubMed] [Google Scholar]
- 18. McGlone SM, Bailey RR, Zimmer SM, et al. The economic burden of Clostridium difficile. Clin Microbiol Infect 2012; 18:282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olsen MA, Stwalley D, Demont C, Dubberke ER. Clostridium difficile infection increases acute and chronic morbidity and mortality. Infect Control Hosp Epidemiol 2019; 40:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Appaneal HJ, Caffrey AR, Beganovic M, Avramovic S, LaPlante KL. Predictors of mortality among a national cohort of veterans with recurrent Clostridium difficile infection. Open Forum Infect Dis 2018; 5:ofy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mora Pinzon MC, Buie R, Liou JI, et al. Outcomes of community and healthcare-onset Clostridium difficile infections. Clin Infect Dis 2019; 68:1343–50. [DOI] [PubMed] [Google Scholar]
- 22. Watt M, Dinh A, Le Monnier A, Tilleul P. Cost-effectiveness analysis on the use of fidaxomicin and vancomycin to treat Clostridium difficile infection in France. J Med Econ 2017; 20:678–86. [DOI] [PubMed] [Google Scholar]
- 23. Burton HE, Mitchell SA, Watt M. A systematic literature review of economic evaluations of antibiotic treatments for Clostridium difficile infection. Pharmacoeconomics 2017; 35:1123–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le P, Nghiem VT, Mullen PD, Deshpande A. cost-effectiveness of competing treatment strategies for Clostridium difficile infection: a systematic review. Infect Control Hosp Epidemiol 2018; 39:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Hise NW, Bryant AM, Hennessey EK, Crannage AJ, Khoury JA, Manian FA. Efficacy of oral vancomycin in preventing recurrent Clostridium difficile infection in patients treated with systemic antimicrobial agents. Clin Infect Dis 2016; 63:651–3. [DOI] [PubMed] [Google Scholar]
- 26. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 27. Cornely OA, Crook DW, Esposito R, et al. ; OPT-80-004 Clinical Study Group Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12:281–9. [DOI] [PubMed] [Google Scholar]
- 28. Johnson S, Louie TJ, Gerding DN, et al. ; Polymer Alternative for CDI Treatment (PACT) Investigators Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59:345–54. [DOI] [PubMed] [Google Scholar]
- 29. Rokas KE, Johnson JW, Beardsley JR, Ohl CA, Luther VP, Williamson JC. The addition of intravenous metronidazole to oral vancomycin is associated with improved mortality in critically ill patients with Clostridium difficile infection. Clin Infect Dis 2015; 61:934–41. [DOI] [PubMed] [Google Scholar]
- 30. Lamontagne F, Labbé AC, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg 2007; 245:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peprah D, Chiu AS, Jean RA, Pei KY. Comparison of outcomes between total abdominal and partial colectomy for the management of severe, complicated Clostridium difficile infection. J Am Coll Surg 2018. doi: 10.1016/j.jamcollsurg.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012; 55(Suppl 2):S154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769–75. [DOI] [PubMed] [Google Scholar]
- 34. Spiceland CM, Khanna S, Pardi DS. Outcomes with fidaxomicin therapy in Clostridium difficile infection. J Clin Gastroenterol 2018; 52:151–4. [DOI] [PubMed] [Google Scholar]
- 35. Garey KW, Ghantoji SS, Shah DN, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J Antimicrob Chemother 2011; 66:2850–5. [DOI] [PubMed] [Google Scholar]
- 36. Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002. [DOI] [PubMed] [Google Scholar]
- 37. Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–87. [DOI] [PubMed] [Google Scholar]
- 38. Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:761–7. [DOI] [PubMed] [Google Scholar]
- 39. Jorup-Rönström C, Håkanson A, Sandell S, et al. Fecal transplant against relapsing Clostridium difficile-associated diarrhea in 32 patients. Scand J Gastroenterol 2012; 47:548–52. [DOI] [PubMed] [Google Scholar]
- 40. Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–6. [DOI] [PubMed] [Google Scholar]
- 41. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015; 41:835–43. [DOI] [PubMed] [Google Scholar]
- 42. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis 2014; 58:1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med 2016; 165:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischer M, Sipe BW, Rogers NA, et al. Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: description of a protocol with high success rate. Aliment Pharmacol Ther 2015; 42:470–6. [DOI] [PubMed] [Google Scholar]
- 45. Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2017; 318:1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Staley C, Hamilton MJ, Vaughn BP, et al. Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated fecal microbiota: pragmatic cohort study. Am J Gastroenterol 2017; 112:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Youngster I, Mahabamunuge J, Systrom HK, et al. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med 2016; 14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis 2015; 15:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Major G, Bradshaw L, Boota N, et al. Follow-on rifaximin for the prevention of recurrence following standard treatment of infection with Clostridium difficile (RAPID): a randomised placebo controlled trial. Gut 2018. doi: 10.1136/gutjnl-2018-316794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kipfer RE, Moertel CG, Dahlin DC. Mesenteric lipodystrophy. Ann Intern Med 1974; 80:582–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.