Abstract
Background
The Early Pediatric Initiation Canada Child Cure Cohort (EPIC4) study is a prospective, multicenter, Canadian cohort study investigating human immunodeficiency virus–1 (HIV-1) reservoirs, chronic inflammation, and immune responses in children with perinatally acquired HIV-1 infection. The focus of this report is HIV-1 reservoirs and correlates in the peripheral blood of children who achieved sustained virologic suppression (SVS) for ≥5 years.
Methods
HIV-1 reservoirs were determined by measuring HIV-1 DNA in peripheral blood mononuclear cells and inducible cell-free HIV-1 RNA in CD4+ T-cells by a prostratin analogue stimulation assay. HIV serology was quantified by signal-to-cutoff ratio (S/CO).
Results
Of 228 enrolled participants, 69 achieved SVS for ≥5 years. HIV-1 DNA, inducible cell-free HIV-1 RNA, and S/COs correlated directly with the age of effective combination antiretroviral therapy (cART) initiation (P < .001, P = .036, and P < .001, respectively) and age when SVS was achieved (P = .002, P = .038, and P < .001, respectively) and inversely with the proportion of life spent on effective cART (P < .001, P = .01, and P < .001, respectively) and proportion of life spent with SVS (P < .001, P = .079, and P < .001, respectively). Inducible cell-free HIV-1 RNA correlated with HIV-1 DNA, most particularly in children with SVS, without virologic blips, that was achieved with the first cART regimen initiated prior to 6 months of age (rho = 0.74; P = .037) or later (rho = 0.87; P < .001). S/COs correlated with HIV-1 DNA (P = .003), but less so with inducible cell-free HIV-1 RNA (P = .09).
Conclusions
The prostratin analogue stimulation assay, with its lower blood volume requirement, could be a valuable method for evaluating inducible HIV-1 reservoirs in children. Standard commercial HIV serology may be a practical initial indirect measure of reservoir size in the peripheral blood of children with perinatally acquired HIV-1 infection.
Keywords: HIV-1 DNA, inducible RNA, child, reservoir, prostratin analogue
Human immunodeficiency virus–1 (HIV-1) reservoir sizes in peripheral blood—measured by HIV-1 DNA, inducible cell-free RNA, and quantitative serology—correlate with earlier initiation of and proportions of life spent on effective treatment and with virologic suppression.
The initiation of combination antiretroviral therapy (cART) early in human immunodeficiency virus–1 (HIV-1) infection has been associated with lower HIV-1 reservoirs in peripheral blood in both adults and children [1–9]. Furthermore, posttreatment control, whereby the HIV-1 viral load (VL) remains undetectable for a prolonged period after cessation of cART, has been observed in up to 15% of adults started on cART during early infection, despite unfavorable human leukocyte antigen (HLA) markers [10–12]. While there are no equivalent cohort data for children, several case reports support the possibility of prolonged virologic remission after treatment cessation. In the “Mississippi baby,” cART was started at 30 hours of life, virologic suppression (<48 copies/mL) confirmed at 29 days of life, and treatment stopped at 18 months of life, after which the VL remained undetectable for 21.9 months [13, 14]. In another case from France, virologic remission lasting more than 12 years was observed after treatment cessation in a child who earlier had received 5.5 to 6.5 years of cART, beginning at 3 months of age [15].
Measuring the size of the HIV-1 reservoir in a pediatric population is a challenge, because the volume of blood available for sampling is limited. Therefore, HIV-1 DNA, a sensitive and reproducible marker of HIV-1 persistence, is often used [2–9]. However, it is well established that HIV-1 DNA measures largely overestimate the size of the replication-competent HIV-1 reservoir, since the majority of HIV-1 genomes are defective [16, 17]. More recently, the robust correlation and close approximation of reservoir size by inducible cell-free HIV-1 RNA in CD4+ T-cells and the quantitative viral outgrowth assay (QVOA) was demonstrated [18]. We used a novel assay, based on prostratin analog (SUW013 [Stanford University Wender lab compound 013]) stimulation of CD4+ T-cells, to measure the inducible HIV-1 reservoir in the CD4+ T-cells of children with perinatally acquired HIV-1 infections [19].
The main objectives of the present study were to investigate the cross-sectional correlates of HIV-1 reservoir size in the peripheral blood of children with perinatally acquired HIV-1 infection who had a sustained virologic suppression (SVS) of at least 5 years duration, and to evaluate the utility of the prostratin analogue stimulation assay [19] as a measure of the reservoir size in children.
METHODS
Study Design
The Early Pediatric Initiation Canada Child Cure Cohort (EPIC4) study is a prospective, multicenter, Canadian cohort study investigating HIV-1 reservoirs, chronic inflammation, and immune responses in children with perinatally acquired HIV-1 infection. Children and young adults were recruited from the 8 major pediatric HIV care centers across the country. The current report is a cross-sectional analysis of predictors of HIV-1 reservoir size in peripheral blood in the subset of children who had achieved SVS for at least 5 years. Children who fulfilled this criterion were categorized a priori into 3 groups: Group 1 consisted of those initiated on effective cART during the first 6 months of life and who achieved SVS on their first cART regimen; Group 2 were those initiated on effective cART at or after 6 months of life and who achieved SVS on their first cART regimen; and Group 3 consisted of those who had failed at least 1 cART regimen, but who achieved SVS on a subsequent regimen. The study was approved by the Research Ethics Boards of all participating institutions. Voluntary informed consent was provided by the participant or their legal guardian, as appropriate.
Definitions
SVS was defined as continuous VL measurements below the level of detection (target not detected, <20, <40, and <50 HIV-1 RNA copies/mL plasma, by different assays over time and at different centers), allowing for virologic blips. Effective cART was defined as cART that led to SVS. A virologic blip was defined as a detectable VL <500 HIV-1 RNA copies/mL of plasma, if the VL measurements immediately preceding and following the blip were below the limit of detection. As previously described, the proportion of life on effective cART refers to the sum of all time periods during which the patient received effective cART associated with SVS, in days (numerator), divided by the patient age, in days (denominator) [20]. The proportion of life with SVS was defined as the sum of all time periods during which SVS was achieved, in days (numerator), divided by the patient age, in days (denominator).
Laboratory Methods
Total HIV-1 DNA was quantified as previously described [21, 22]. Briefly, total peripheral blood mononuclear cells (PBMCs) were digested with proteinase K. Then, a first round of polymerase chain reaction (PCR) preamplification was performed directly on cell lysates using long terminal repeats (LTR)-gag amplification. A nested real-time PCR was then carried out on a Rotor-Gene Q instrument (Qiagen, Mississauga, Canada) using TaqMan probes. The number of copies of the CD3 gene was determined, to accurately quantify the number of cells in each reaction. Results were expressed as HIV-1 DNA copies per 106 PBMCs.
The level of inducible cell-free HIV-1 RNA in CD4+ T-cells was measured using the prostratin analogue stimulation assay [19]. Briefly, CD4+ T-cells were negatively purified from PBMCs using the EasySep Human CD4+ T-cell Isolation Kit (STEMCELL Technologies, Vancouver, Canada) and resuspended (107 cells/mL) in Roswell Park Memorial Institute (RPMI) medium, supplemented with 10% fetal bovine serum, 1000 IU/mL interleukin-2 (IL-2), and 50 nM of the SUW013 prostratin analogue (compound 11c in reference 19) [19]. After 48 h of incubation at 37°C, viral RNA were measured in supernatants using the droplet digital PCR method [23]. Results of the prostratin analogue stimulation assay were expressed as number of copies of HIV-1 RNA produced by 106 CD4+ T-cells.
Ultrasensitive VL testing was performed on plasma samples using the droplet digital PCR method. Briefly, HIV-1 RNA was extracted from 1000 µl of a plasma sample, or from 1000 µl of supernatant from prostratin analogue stimulation, with the Nuclisens easyMAG (BioMerieux, Marcy-l’Étoile, France). First-strand cDNA synthesis was performed using SuperScript VILO Master Mix (ThermoFisher Scientific, Waltham, MA). The 1-step droplet digital PCR reaction mixtures were done in triplicates for negative and positive controls, while tested samples were amplified across 6 reactions. Droplets were generated from the PCR reaction mixtures in DG32 Automated Droplet Generator Cartridges, using the Automated Droplet Generator (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. PCR was performed using a C1000 Touch Thermal Cycler (Bio-Rad Laboratories, Hercules, CA). The fluorescence intensities of each droplet from the samples were measured using the QX200 Droplet Reader (Bio-Rad Laboratories, Hercules, CA) and analyzed in the QuantaSoft software.
HIV serology was performed using the Architect HIV-1/2 Ag/Ab Combo Test (Abbott Diagnostics, Mississauga, Canada). The strength of the serologic response was quantified as the signal-to-cutoff value (S/CO) of the assay.
Statistical Analysis
Statistical analysis was performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC). Demographic characteristics were summarized using proportions for categorical variables and medians with interquartile ranges (IQR) for continuous variables. The comparisons of baseline characteristics between groups were done using the chi-square test, Fisher’s exact test, or Wilcoxon rank sum test, as appropriate. For the analyses of reservoir size according to group, the Wilcoxon rank sum test or Fisher’s exact test was performed. Regression analyses (linear or logistic, as appropriate) were also performed to adjust for potential confounders (reservoir sizes on log10 scale for linear regression). Variables were selected for inclusion in the model on the basis of potential clinical relevance or a P value of <0.1 in the univariate analysis. Baseline characteristics that were highly dependent on the group definition were not included, to avoid overadjustment. For the secondary analysis, correlates of reservoir size (HIV-1 DNA, inducible cell free HIV-1) and quantitative HIV-1 serology were evaluated. Correlates of reservoir size (HIV-1 DNA, inducible cell free HIV-1) and quantitative HIV-1 serology were investigated using the partial Spearman’s rank correlation coefficient (rho), while adjusting for potential confounders.
RESULTS
Of 228 children and young adults with perinatally acquired HIV-1 infection enrolled in EPIC4, 69 (30.3%) had achieved SVS for at least 5 years. This subgroup of children had a median current age of 15.5 years (IQR 12.9, 17.6), median age of effective cART initiation of 3.7 years (IQR 1.5, 8.0), and median duration of SVS of 9.1 years (IQR 6.9, 12.5). The baseline characteristics of the participants, according to group, are described in Table 1. The Group 1 individuals were younger, more likely to be female, more likely to be born in Canada, and tended to have less advanced HIV-1 disease, as defined by less severe worst Centers for Disease Control and Prevention (CDC) clinical category, less severe worst CDC immunologic category, and higher CD4% nadir, and to have had fewer virologic blips. As would be expected by the nature of the group definitions, the early treated group was significantly more likely to have achieved SVS at a younger age and exhibited a higher proportion of life on effective cART and higher proportion of life with SVS.
Table 1.
Baseline Characteristics According to Age of Effective Combination Antiretroviral Therapy Initiation
| Parameter | Group 1 (n = 10) | Group 2 (n = 30) | Group 3 (n = 29) | P Value |
|---|---|---|---|---|
| Current age, in years | 10.1 (8.4, 13.2) | 15.7 (13.1, 17.6) | 17.0 (14.4, 18.1) | <.001 |
| Biological sex, % male | 30.0% | 50.0% | 72.4% | .04 |
| Ethnicity/race | … | … | … | .50 |
| African/Caribbean/Black | 55.6% | 62.1% | 44.8% | … |
| White | 22.2% | 17.2% | 13.8% | … |
| Indigenous | 11.1% | 6.9% | 27.6% | … |
| Other | 11.1% | 13.7% | 13.7% | … |
| Canadian born, % | 100% | 40% | 72.4% | .001 |
| CDC clinical category, worsta | … | … | … | .19 |
| None/mild, N/A | 71.5% | 58.3% | 24.0% | … |
| Moderate, B | 14.3% | 16.7% | 36.0% | … |
| AIDS defining conditions, C | 14.3% | 25.0% | 40.0% | … |
| CDC immunological category, worsta | … | … | … | .18 |
| No suppression | 57.1% | 24.0% | 34.6% | … |
| Moderate suppression | 42.9% | 48.0% | 26.9% | … |
| Severe suppression | … | 28.0% | 38.5% | … |
| Current ART | … | … | … | .80 |
| NNRTI-based cART | 30.0% | 48.3% | 40.7% | … |
| PI-based cART | 50.0% | 34.5% | 33.3% | … |
| INSTI-based cARTb | 20.0% | 17.2% | 25.9% | … |
| Current CD4 count, cells/µL | 841 (533, 1145) | 670 (510, 839) | 640 (510, 773) | .29 |
| Current CD4% | 38.0 (36.0, 42.0) | 36.5 (32.0, 41.0) | 36.0 (32.0, 40.0) | .55 |
| CD4% nadir | 24.5 (18.0, 32.0) | 21.5 (12.0, 29.0) | 19.0 (12.0, 25.0) | .15 |
| Viral load peak, log | 5.02 (4.83, 5.70) | 4.95 (4.12, 5.63) | 5.67 (4.99, 5.90) | .03 |
| Virologic blips | 20.0% | 56.7% | 34.5% | .07 |
| Age at start of effective cART, years | 0.2 (0.1, 0.4) | 3.2 (1.5, 5.4) | 6.8 (3.2, 8.7) | <.001 |
| Age at SVS, years | 0.8 (0.4, 1.4) | 4.3 (2.2, 6.4) | 8.5 (3.7, 9.5) | <.001 |
| Duration of SVS, years | 8.7 (7.2, 10.3) | 10.5 (7.5, 13.8) | 8.0 (6.3, 10.1) | .04 |
| Proportion of life on effective cART | 0.98 (0.95, 0.99) | 0.82 (0.62, 0.89) | 0.65 (0.53, 0.84) | <.001 |
| Proportion of life with SVS | 0.94 (0.83, 0.97) | 0.74 (0.53, 0.83) | 0.57 (0.47, 0.77) | <.001 |
Group 1 had effective cART initiated at <6 months of life, with SVS on the first cART regimen; Group 2 had effective cART initiated at ≥6 months of life with SVS on the initial regimen; Group 3 failed at least 1 cART regimen, but later achieved SVS on another regimen. Results are shown as medians with IQRs for continuous variables and as proportions for categorical variables.
Abbreviations: AIDS, acquired immunodeficiency syndrome; cART, combination antiretroviral therapy; CDC, Centers for Disease Control and Prevention; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SVS, sustained virologic suppression.
aThe worst CDC clinical category was unknown for 13 cases and the worst immunologic category was unknown for 11 cases.
bIncludes children on INSTI + NRTI and INSTI + PI + NRTI.
HIV-1 reservoir size, as measured by HIV-1 DNA in PBMC, was significantly lower in Group 1, compared to Groups 2 and 3 (Figure 1A; Table 2). A similar but nonsignificant trend was observed when the size of the inducible HIV-1 reservoir (cell-free HIV-1 RNA in CD4+ T-cells) was estimated using the prostratin analogue stimulation assay (Figure 1B; Table 2). A higher proportion of children in Group 1 had no detectable HIV-1 DNA in PBMC (<1 copy per 106 PBMC), compared to Group 2 or 3 (70%, 13.3%, and 14.3%, respectively; P < .001). In the regression analysis that adjusted for biological sex, CD4% nadir, VL peak, and virologic blips, the significant difference in HIV-1 DNA level between groups was retained (P = .001), as was the nonsignificant trend for inducible cell-free HIV-1 RNA level (P = .17).
Figure 1.
Impact of age of initiation of effective cART on the size of the viral reservoir, measured by quantitative assay of HIV-1 DNA in PBMCs (A), copies of HIV-1 RNA produced by prostratin analogue stimulated CD4+ T-cells (B), and quantitative HIV-1–specific serologic response in children with perinatally acquired HIV-1 infection (C). The horizontal lines correspond to the median and IQR. The significant and nonsignificant differences between groups were retained in the analysis adjusted for biological sex, CD4% nadir, VL peak, and virologic blips (data not shown). Abbreviations: cART, combination antiretroviral therapy; HIV-1, human immunodeficiency virus–1; IQR, interquartile range; PBMCs, peripheral blood mononuclear cells; VL, viral load.
Table 2.
Reservoir Size According to Age of Effective Combination Antiretroviral Therapy Initiation
| Parameter | Group 1 (n = 10) | Group 2 (n = 30) | Group 3 (n = 29) | P Valuea | |
|---|---|---|---|---|---|
| HIV-1 DNAb | Median (IQR) | 10.0 (10.0, 18.0) | 117.2 (57.5, 247.5) | 77.3 (29.8, 170.1) | <.001 |
| % undetectable | 70.0% | 13.3% | 14.3% | <.001 | |
| Cell-free HIV-1 RNAc | Median (IQR) | 3.0 (1.2, 12.0) | 5.5 (2.4, 21.7) | 14.7 (3.4, 63.6) | .17 |
| % undetectable | 20.0% | 13.3% | 13.8% | .81 | |
| Ultrasensitive VLd | Median (IQR) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | .67 |
| % negative | 90.0% | 81.5% | 79.2% | .91 | |
| Quantitative serology (S/CO) | Median (IQR) | 3.4 (0.2, 22.4) | 145.9 (12.4, 348.6) | 141.8 (45.9, 303.9) | .002 |
| % negative | 30.0% | 0% | 0% | .002 |
Abbreviations: HIV-1, human immunodeficiency virus–1; IQR, interquartile range; PBMC, peripheral blood mononuclear cell; S/CO, signal-to-cutoff ratio; VL, viral load
aThe unadjusted P values are presented. In the regression analysis adjusting for biological sex, CD4% nadir, VL peak, and virologic blips, no significant changes to the results were observed. Minor adjusted P value changes were in the mean log10 HIV-1 DNA (P = .001), percent undetectable HIV-1 DNA (P = .018), and mean log10 S/CO (P < .001).
bExpressed as copies per 106 PBMC; <10 copies per 106 PBMC are considered undetectable.
cExpressed as copies of HIV RNA produced per 106 stimulated CD4+ T-cells; <1 copy per 106 CD4+ T-cells is considered undetectable.
dLevel of detection set at 5 copies/mL.
In a separate analysis of the cohort as a whole, those who had no detectable HIV-1 DNA in PBMC were shown to have achieved SVS at a significantly younger age than those with detectable HIV-1 DNA in PBMC (1.4 years [IQR 0.5, 3.5] versus 5.9 years [IQR 3.5, 8.9], respectively; P < .001). Inducible cell-free HIV-1 RNA was detected in similar proportions from all 3 groups (80% in Group 1, 86.7% in Group 2, and 86.2% in Group 3), but there was a significant association between the absence of inducible cell-free HIV-1 RNA and younger age at SVS in the full cohort (1.9 years [IQR 1.2, 3.9] with no inducible cell-free HIV-1 RNA versus 5.5 years [IQR 2.8, 8.8] with inducible cell-free HIV-1 RNA; P = .03).
The levels of HIV-1 DNA in PBMC, adjusted for biological sex, correlated directly with the age of initiation of effective cART (rho = 0.52; P < .001) and age when SVS was achieved (rho = 0.44; P = .002) and correlated inversely with the proportion of life spent on effective cART (rho = −0.51; P < .001) and proportion of life with SVS (rho = −0.41; P < .001; Table 3). Similar, though less robust correlations, were noted for inducible cell-free HIV-1 RNA level in peripheral blood CD4+ T-cells. In an analysis restricted to children with no virologic blips (n = 40), adjusted for biological sex, significant correlations of similar magnitudes were retained for all 4 variables in relation to HIV-1 DNA in PBMC (age of initiation of effective cART, rho = 0.49 [P = .003]; age when SVS was achieved, rho = 0.55 [P < .001]; proportion of life spent on effective cART, rho = −0.55 [P = .001]; proportion of life with SVS, rho = −0.44 [P = .006]); only age when SVS was achieved and proportion of life on effective cART retained significance for inducible cell-free HIV-1 RNA (rho = 0.37 [P = .021] and rho = −0.41, [P = .013], respectively).
Table 3.
Correlates of Human Immunodeficiency Virus–1 (HIV-1) Reservoir Size in Peripheral Blood and Quantitative HIV-1 Serology
| Variable | HIV-1 DNA | Cell-free HIV-1 RNA | HIV-1 Serology | |||
|---|---|---|---|---|---|---|
| Rho | P Value | Rho | P Value | Rho | P Value | |
| Age at cART initiation, years | 0.52 | <.001 | 0.26 | .036 | 0.63 | <.001 |
| Age at SVS, years | 0.44 | .002 | 0.25 | .038 | 0.65 | <.001 |
| Duration of SVS, years | −0.12 | .35 | −0.08 | .54 | −0.32 | .008 |
| Proportion of life on effective cART | −0.51 | <.001 | −0.32 | .01 | −0.66 | <.001 |
| Proportion of life with SVS | −0.41 | <.001 | −0.21 | .079 | −0.68 | <.001 |
| HIV serology (S/CO) | 0.34 | .005 | 0.21 | .089 | … | … |
The values shown are adjusted for biological sex. All other variables considered for the adjusted analysis—including country of birth (Canada vs elsewhere), CD4 nadir, peak VL, and virologic blips—were highly correlated with other predictor variables depicted in the table and, therefore, not included in the model. In the univariate analysis, “current CD4 count” was significantly associated with HIV-1 serology (rho = 0.28; P = .022); in the adjusted analysis this significance was lost (rho = 0.21; P = .088).
Abbreviations: cART, combination antiretroviral therapy; HIV-1, human immunodeficiency virus–1; rho, Spearman partial correlation coefficient; S/CO, signal-to-cutoff ratio; SVS, sustained virologic suppression.
Quantitative HIV-1 serology, as measured by S/CO, was significantly lower for children in Group 1 than for those in Groups 2 or 3 in both the unadjusted and adjusted analyses (Figure 1C; Table 2). Furthermore, all 3 children with negative HIV-1 serology were from Group 1: 2 were initiated on effective cART within 48 hours of birth and 1 at 1.7 months of life. As was the case for HIV-1 DNA in PBMC and inducible cell-free HIV-1 RNA level in CD4+ T-cells, quantitative HIV-1 serology, adjusted for biological sex, correlated directly with the age of initiation of effective cART and the age when SVS was achieved and correlated inversely with the proportion of life spent on effective cART and the proportion of life with SVS (Table 3). In addition, the duration of SVS correlated inversely with quantitative serology. Quantitative HIV-1 serology correlated directly with HIV-1 DNA in PBMC, but less so with inducible cell-free HIV-1 RNA level (Table 3).
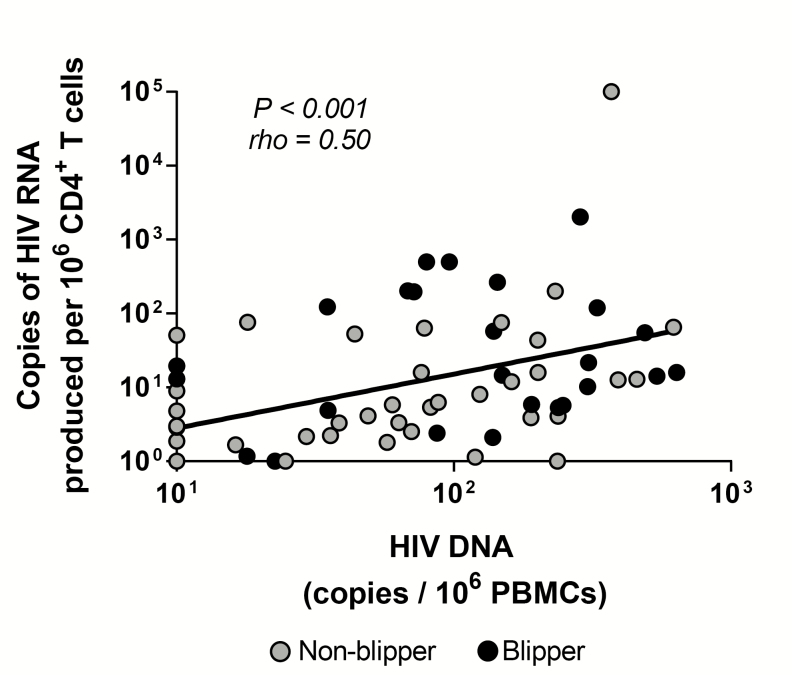
A positive correlation was noted between HIV-1 DNA in PBMC and inducible cell-free HIV-1 RNA level in peripheral blood CD4+ T-cells (rho = 0.50; P < .001; Figure 2). This correlation was particularly robust for those children in Groups 1 and 2 who had not had any blips subsequent to achieving SVS (rho = 0.74 [P = .037] for Group 1, n = 8; rho = 0.87 [P < .001] for Group 2, n = 13). There was no significant correlation between HIV-1 DNA in PBMC and inducible cell-free HIV-1 RNA level in peripheral blood CD4+ T-cells in Group 3 patients (rho = 0.34; P = .17).
Figure 2.
Correlation of HIV-1 reservoir size in PBMC measured using the HIV-1 DNA assay and cell-free HIV-1 RNA in CD4+ T-cells using the prostratin analogue stimulation assay. Abbreviations: HIV-1, human immunodeficiency virus–1; PBMC, peripheral blood mononuclear cell.
DISCUSSION
This study shows direct correlation of reservoir sizes in peripheral blood, as measured by both HIV-1 DNA in PBMC and inducible cell-free HIV-1 RNA in CD4+ T-cells, with both the age at initiation of effective cART and the age at which SVS was achieved [4, 6, 8]. In addition, as recently reported from the EPIICAL (Early-treated Perinatally HIV-infected Individuals: Improving Children's Actual Life with Novel Immunotherapeutic Strategies) Consortium, we observed a strong inverse correlation between the size of the viral reservoir measured with both assays and the proportion of life spent on effective cART or proportion of life with SVS [7]. Taken together, these findings indicate that smaller reservoir size in peripheral blood is a function of earlier treatment initiation, as well as higher proportion of life on effective treatment and with virologic suppression. The findings of this study extend those of other pediatric studies demonstrating lower reservoir size in peripheral blood following the early initiation of effective cART [4–9].
Most pediatric studies have relied on measurement of HIV-1 DNA in PBMC to assess reservoir size [4–9]. While this methodology has the advantages of being easier to perform and requiring smaller blood volumes, it cannot distinguish replication-competent virus, as measured by the QVOA, from defective virus and tends to overestimate the size of the replication-competent reservoir by as much as 100-fold [16, 17, 24]. Poor correlation between the QVOA and HIV-1 DNA measurement have been observed in some [16], but not all, studies [25]. In this study, we demonstrate for the first time that the size of the inducible HIV-1 reservoir in the peripheral blood CD4+ T-cells of children, as measured by the prostratin analogue stimulation assay, correlates with that measured by HIV-1 DNA. Our results further show that this correlation is most robust among those children who were treated very early, achieved SVS, have had no virologic blips, and have very low levels of the virus in their peripheral blood. The strong correlation between the 2 assays in this subgroup may reflect a circumstance where less cumulative viral replication has taken place, thereby limiting both the reservoir size and the accumulation of defective genomes. This suggests that in children treated very early, virologic blips may be associated with sustained, low-level viral replication, with gradual increase in the proportion of defective genomes.
Previous studies have demonstrated that HIV-1 serology can be negative in perinatally infected children with SVS after initiating cART during the first 3–6 months of life [4, 9, 26]. In the present study, we demonstrate that standard, commercial HIV serology, measuring total antibodies to gp41, p24, and gp120, quantified by S/CO, correlates with HIV-1 reservoir size in peripheral blood. This contrasts with findings in a cohort of 97 children with perinatally acquired HIV-1 infection in Mali, where anti-gp41 antibody activity, quantified by S/CO, did not correlate with HIV-1 DNA level in PBMC, despite a significant correlation with the age of cART initiation [27]. A more recent publication demonstrated significant association of anti-gp160 and anti-gp41 antibody levels with HIV-1 DNA level in PBMCs [28]. In an analysis of 69 children from the EPIICAL cohort, a Western blot band intensity score correlated directly with the time of cART initiation (P < .001) and with HIV-1 DNA (P = .032) [29]. In our study, antibody level correlated more robustly with HIV-1 DNA level than with inducible virus level. Our data suggest that the Architect HIV-1/2 Ag/Ab Combo Assay could be used as a relatively accessible and inexpensive first-step measure of reservoir size in the peripheral blood of children with perinatally acquired HIV-1 infection.
A limitation of this study was its cross-sectional design, though this was mitigated by the high recruitment rate of subjects with SVS from all participating centers and, hence, the good sample size. The limited volume of blood that could be drawn from younger children precluded the quantification of very low levels of the virus in peripheral blood (<10 copies/106 PBMC for HIV-1 DNA; <1 copy/106 CD4+ T-cells for inducible virus) or evaluation of replication-competent virus using the QVOA, which is considered the current gold standard in terms of reservoir measurement. However, the prostratin analogue stimulation assay correlated well with HIV-1 DNA level and could represent a useful and cost-effective tool for assessment of the inducible HIV-1 reservoir in the peripheral blood of children.
In conclusion, we have demonstrated that the size of the HIV-1 reservoir in peripheral blood correlates directly with the age of effective cART initiation and age of SVS and correlates inversely with the proportion of life spent on effective cART and proportion of life with SVS. We have also shown that the size of the inducible HIV-1 reservoir in peripheral blood CD4+ T-cells can be estimated in children using the prostratin analogue stimulation assay and that the results of this assay correlate well with HIV-1 DNA levels, particularly in children who were treated very early, have achieved SVS, and have had no virologic blips. As such, the prostratin analogue stimulation assay, with its lower blood volume requirement, may become a valuable method for estimating replication-competent HIV-1 reservoirs in children. Finally, we demonstrated that quantified HIV-1 serologic responses, using a standard, commercially available HIV-1 serologic assay, may serve as a practical initial indirect measure of reservoir size in the peripheral blood of children with perinatally acquired HIV-1 infection.
Notes
The Early Pediatric Initiation Canada Child Cure Cohort (EPIC 4 ) research group. Ariane Alimenti, British Columbia (BC) Women’s Hospital & Health Centre, Vancouver; Petronela Ancuta, Centre de Recherche du Centre Hospitalier de l’Université de Montréal; Ari Bitnun, Hospital for Sick Children, Department of Pediatrics, University of Toronto; Jason Brophy, Children’s Hospital of Eastern Ontario, Department of Pediatrics, University of Ottawa; Jared Bullard, Children’s Hospital of Winnipeg, University of Manitoba; Tae-Wook Chun, National Institute of Allergy and Infectious Diseases, Bethesda; Hélène C. F. Côté, University of British Columbia, Vancouver; Joanne Embree, Children’s Hospital of Winnipeg, University of Manitoba; Michael T. Hawkes, Department of Pediatrics, Stollery Children’s Hospital, Department of Pediatrics, University of Alberta, Edmonton; Fatima Kakkar, Centre Hospitalier Universitaire (CHU) Sainte-Justine, Department of Pediatrics, Université de Montréal; Christos Karatzios, Montreal Children’s Hospital, Department of Pediatrics, McGill University; Rupert Kaul, University Health Network, Department of Medicine, University of Toronto; John Kim, National Human Immunodeficiency Virus (HIV) and Retrovirology Laboratory (NHRL), Public Health Agency of Canada (PHAC), Winnipeg; Valérie Lamarre, CHU Sainte-Justine, Department of Pediatrics, Université de Montréal; Normand Lapointe, CHU Sainte-Justine, Department of Pediatrics, Université de Montréal; Pascal Lavoie, BC Women’s & Children’s Hospital, Vancouver; Terry Lee, Canadian Institutes of Health Research (CIHR) Canadian HIV Trials Network (CTN), Vancouver; Deborah M. Money, BC Women’s Hospital & Health Centre, University of British Columbia, Vancouver; Dorothy Moore, Montreal Children’s Hospital, Department of Pediatrics, McGill University; Stanley Read, Hospital for Sick Children, Department of Pediatrics, University of Toronto; Robert Reinhard, Public/Global Health Consultant, San Francisco; Lindy Samson, Children’s Hospital of Eastern Ontario, Department of Pediatrics, University of Ottawa; Paul Sandstorm, NHRL, PHAC, Winnipeg; Laura Sauve, BC Women’s Hospital & Health Centre, Department of Pediatrics, University of British Columbia, Vancouver; Sandra Seigel, McMaster Children’s Hospital, Department of Pediatrics, McMaster University, Hamilton; Joel Singer, CIHR CTN, Vancouver; Hugo Soudeyns, Centre de Recherche du CHU Sainte-Justine, and Department of Microbiology, Infectiology & Immunology and Department of Pediatrics, Université de Montréal; Ben Tan, Department of Pediatrics, University of Saskatchewan, Saskatoon; and Wendy Vaudry, Stollery Children’s Hospital, Department of Pediatrics, University of Alberta, Edmonton.
Acknowledgments. The authors thank all the study participants, as well as Cheryl Arneson, Christine Bon, Lise Bourrier, Jennifer Bowes, Martine Caty, Cathy den Hollander, Chantal Dessureault, Jodi Gallant, Shanlea Gordon, Hanh Dao, Evelyn Marquis, Zoe Hassall, Audrée Janelle-Montcalm, Danny Dong Hyun Kim, Nicole Kimball, Matthew Kocal, Ye-Von Lee, Evelyn Mann, Dorothy McKelvey, Karen Mochoruk, Mbaye Ndiaye, Barb Neufeld, Laura Puri, Annie Qiu, Suzanne Taillefer, and Silvie Valois for expert and technical assistance. They thank Dr Carole Lavigne and Ms Xuefen Yang, who contributed to the validation of some of the technical methods in the early phase of this study.
Financial support. This work was supported by the Canadian Institutes of Health Research, the Canadian Foundation for AIDS Research, and the International AIDS Society (grant number HIG-133051 ), and by an infrastructure grant from Réseau SIDA-Maladies Infectieuses of the Fonds de la recherche du Québec-Santé.
Potential conflicts of interest. F. K. is a Junior 1 Fellow of the Fonds de la recherche du Québec-Santé. P. A. W. is supported by the National Institutes of Health (grant number R01 CA031845). N. C. reports grants from Merck & Co, Emanuel Merck, Darmstadt Serono, and Innavirvax and personal fees from Theravectys, all outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Early Pediatric Initiation Canada Child Cure Cohort (EPIC4) Research Group:
Alimenti Ariane, Ancuta Petronela, Bitnun Ari, Brophy Jason, Bullard Jared, Chun Tae-Wook, C F Côté Hélène, Embree Joanne, T Hawkes Michael, Kakkar Fatima, Karatzios Christos, Kaul Rupert, Kim John, Lamarre Valérie, Lapointe Normand, Lavoie Pascal, Lee Terry, M Money Deborah, Moore Dorothy, Read Stanley, Reinhard Robert, Samson Lindy, Sandstorm Paul, Sauve Laura, Seigel Sandra, Singer Joel, Soudeyns Hugo, Tan Ben, and Vaudry Wendy
References
- 1. Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 2005; 191:1410–8. [DOI] [PubMed] [Google Scholar]
- 2. Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013; 208:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laanani M, Ghosn J, Essat A, et al. ; Agence Nationale de Recherche sur le Sida PRIMO Cohort Study Group Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 2015; 60:1715–21. [DOI] [PubMed] [Google Scholar]
- 4. Persaud D, Patel K, Karalius B, et al. ; Pediatric Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome Cohort Study Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 2014; 168:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foster C, Pace M, Kaye S, et al. ; CHERUB Investigators Early antiretroviral therapy reduces HIV DNA following perinatal HIV infection. AIDS 2017; 31:1847–51. [DOI] [PubMed] [Google Scholar]
- 6. Martínez-Bonet M, Puertas MC, Fortuny C, et al. Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis 2015; 61:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tagarro A, Chan M, Zangari P, et al. Early and highly suppressive antiretroviral therapy are main factors associated with low viral reservoir in European perinatally HIV-infected children. J Acquir Immune Defic Syndr 2018; 79:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhn L, Paximadis M, Da Costa Dias B, et al. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PLOS One 2018; 13:e0195514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ananworanich J, Puthanakit T, Suntarattiwong P, et al. ; Human Immunodeficiency Virus-NAT 194 Study Group Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014; 28:1015–20. [DOI] [PubMed] [Google Scholar]
- 10. Namazi G, Fajnzylber JM, Aga E, et al. The control of HIV after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis 2018; 218:1954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin GE, Frater J. Post-treatment and spontaneous HIV control. Curr Opin HIV AIDS 2018; 13:402–7. [DOI] [PubMed] [Google Scholar]
- 12. Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. ; ANRS VISCONTI Study Group Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLOS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luzuriaga K, Gay H, Ziemniak C, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 2015; 372:786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frange P, Faye A, Avettand-Fenoël V, et al. ; ANRS EPF-CO10 Pediatric Cohort and the ANRS EP47 VISCONTI study group HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 3:e49–54. [DOI] [PubMed] [Google Scholar]
- 16. Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLOS Pathog 2013; 9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Massanella M, Yek C, Lada SM, et al. Improved assays to measure and characterize the inducible HIV reservoir. EBioMedicine 2018; 36:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beans EJ, Fournogerakis D, Gauntlett C, et al. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci USA 2013; 110:11698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gulhati V, Soo J, Ransey DG, et al. Higher levels of Angiopoietin-1 are associated with early and sustained viral suppression in children living with vertically acquired HIV. J Acquir Immune Defic Syndr 2019; 80:590–5. [DOI] [PubMed] [Google Scholar]
- 21. Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandergeeten C, Fromentin R, Merlini E, et al. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol 2014; 88:12385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strain MC, Lada SM, Luong T, et al. Highly precise measurement of HIV DNA by droplet digital PCR. PLOS One 2013; 8:e55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol 2015; 23:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiselinova M, De Spiegelaere W, Buzon MJ, Malatinkova E, Lichterfeld M, Vandekerckhove L. Integrated and total HIV-1 DNA predict ex vivo viral outgrowth. PLoS Pathogens 2016; 12:e1005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis 2014; 59:1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brice J, Sylla M, Sayon S, et al. Qualitative and quantitative HIV antibodies andviral reservoir size characterization in vertically infected children with virological suppression. J Antimicrob Chemother 2017; 72:1147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McManus M, Henderson J, Gautam A, et al. Quantitative human immunodeficiency virus (HIV)-1 antibodies correlate with plasma HIV-1 RNA and cell-associated DNA levels in children on antiretroviral therapy. Clin Infect Dis 2019; 68:1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rocca S, Zangari P, Cotugno N, et al. Human immunodeficiency virus (HIV)-antibody repertoire estimates reservoir size and time of antiretroviral therapy initiation in virally suppressed perinatally HIV-Infected Children. J Pediatric Infect Dis Soc 2018. Epub ahead of print. doi:10.1093/jpids/piy080 [DOI] [PMC free article] [PubMed] [Google Scholar]