Abstract
This study aimed to investigate the prevalence and clinical significance of lead-related tricuspid regurgitation (TR) in patients with permanent pacemaker (PM). A total of 2,533 patients who underwent permanent PM implantation between January 2008 and December 2017 in a single center were retrospectively reviewed. Among them, 429 patients who underwent transthoracic echocardiography within 90 days before implantation and were followed up at least 3 months after PM implantation were included. Patients who had pre-existing grade 3 or 4 TR, had a single atrial lead, or had undergone tricuspid valve surgery before PM implantation were excluded. Occurrence of PM-related TR (PMTR) was defined as worsening of TR by at least 2 grades on follow-up echocardiography. Cardiovascular outcomes were defined as the composite of cardiovascular death and hospitalization for heart failure. During the median follow-up of 855 days, 42 (9.8%) patients had PMTR and 86 (20.0%) presented with cardiovascular outcomes. In the multivariate logistic regression analysis, the presence of atrial fibrillation (hazard ratio [HR]: 2.07, 95% confidence interval [CI]: 1.27–4.09, p = 0.037]) and history of open-heart surgery (HR: 3.34, 95% CI: 1.68–6.68, p<0.001) were independently associated with PMTR. Patients with PMTR showed significantly higher cardiovascular events than those without (45.2 vs. 17.3%, log-rank p<0.001). Furthermore, PMTR was independently associated with the primary outcome (HR: 2.45, 95% CI: 1.43–4.22, p = 0.001). In conclusion, the occurrence of TR in patients with permanent PM is not uncommon. PMTR is associated with atrial fibrillation, the history of open-heart surgery, and poorer cardiovascular outcomes.
Introduction
Implantation of permanent pacemakers (PM) has gradually increased because of either prolonged life expectancy or increased incidence of cardiac surgery [1, 2]. Tricuspid regurgitation (TR) is recognized as one of the lead-related complications [1, 3, 4]. PM-related TR (PMTR) can occur due to direct leaflet damage, such as leaflet perforation, lead entanglement, or lead adherence from fibrosis [2, 5]. In addition, TR can be aggravated through annular dilatation and chronic right ventricular (RV) dysfunction regardless of the mechanical interference to the valve by the electrode [6].
Several previous studies have demonstrated that moderate or severe TR occurred at a significant higher rate in patients with PM and was associated with higher mortality and heart failure-related hospitalization than that in risk-matched cohorts [7–9]. Although clinical implications of PMTR are largely concordant among previous studies[7–9], diverse predictors of PMTR have been proposed in each study. Alternative strategies to pace the heart without passing through the tricuspid valve, such as the placement of a coronary sinus pacing lead, surgical epicardial placement of leads, and leadless pacing systems, are currently available [10, 11]. Furthermore, echocardiography guidance at the time of PM implantation might be helpful to avoid acute leaflet damage by a transvenous lead in high-risk patients for PMTR. Therefore, we sought to investigate the prevalence, predictors, and prognosis of PMTR in a large, single-center registry involving a wide spectrum of patients who underwent permanent PM implantation in this study.
Materials and methods
Study population
A total of 2,533 patients who underwent de novo permanent PM implantation at a single tertiary center between January 2008 and December 2017 were included in this analysis. Among them, 429 patients who underwent transthoracic echocardiography within 90 days before implantation and underwent follow-up at least 3 months after PM implantation were included. Data on baseline patient characteristics, implantation procedure, device characteristics and setting, and all follow-up visits were retrospectively reviewed. Indications for device implantation, based on international guidelines, were sick sinus syndrome and advanced atrioventricular block in PM recipients. We excluded patients who had grade 3 or 4 TR before PM implantation; were treated with a single-lead atrial PM, implantable cardioverter defibrillator, or cardiac resynchronization therapy; had a history of tricuspid valve repair; had an inadequate quality of echocardiographic data for assessment of RV function and degree of TR; or had undergone a temporary PM implantation (Fig 1). PMTR was defined as TR worsened by at least 2 grades on post-implantation echocardiography during the follow-up period. Patients were divided into two groups according to the occurrence of PMTR: Group 1 (n = 387, no PMTR) and Group 2 (n = 42, PMTR). The study was approved by the Institutional Review Board of Yonsei University Health System (approval number: 4-2020-0032), and it complied with the Declaration of Helsinki. As registry-based retrospective study and the data were analyzed anonymously, it did not require informed consent from the study subjects.
Fig 1. Flowchart of the study.
Echocardiography
Standard two-dimensional and Doppler measurements were performed following the American Society of Echocardiography guidelines [12]. TR severity was graded semi-quantitatively using colored and continuous wave Doppler data using a multi-parametric approach [13, 14]. RV systolic dysfunction was evaluated by two-dimensional echocardiography. RV area measurements were obtained from the apical four-chamber focused RV view at end-diastole and end-systole, and RV fractional area change was calculated as the ratio between the difference at the end-diastolic and end-systolic RV areas and the end-diastolic area [12]. The TV annulus dimension (TVAD) was measured as the end-diastole in the apical four-chamber view and then indexed by the body surface area (BSA) [13]. The RV systolic pressure was calculated from the maximum velocity of the TR jet according to the modified Bernoulli equation [12]. Echocardiographic data were carefully reviewed by two experienced cardiologists who were blinded to the clinical data.
Follow-up and outcomes
Patients were regularly scheduled to visit the PM clinic every 6 months after PM implantation. Follow-up data were obtained by reviewing medical records. If patients did not visit on the scheduled day, outcome data were assessed via telephone interview. The primary outcome was defined as a composite of cardiovascular death and hospitalization due to heart failure during the follow-up period.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as number (percentage). Between-group comparisons were performed using standard χ2 tests for categorical variables and paired t-tests for continuous variables. Univariate and multivariate logistic regression analyses were performed. Comparison of worsening of TR, mitral regurgitation (MR) and RV systolic pressure (RVSP) in tertile of ventricular pacing percentage were performed using the standard χ2 tests and analysis of variance (ANOVA) test. Pearson's correlation coefficient was used to test the linear relation between the percentage of ventricular pacing and RVSP. Survival curves were constructed using the Kaplan–Meier method, and comparisons were made using the log-rank test. All statistical analyses were performed using SPSS Statistics software version 25.0 (IBM, Armonk, NY, USA); p-values < 0.05 were considered statistically significant.
Results
Baseline characteristics
Among 429 patients, 42 (9.8%) had PMTR. Clinical and echocardiographic characteristics of the study population, patients without PMTR, and those with PMTR are summarized in Table 1. Patients with PMTR tended to be older and of the female sex (without statistical significance). Patients with PMTR had a significantly higher incidence of previous open-heart surgery than those without PMTR. Regarding PM data, patients with PMTR showed a higher incidence of VVI mode although the V pacing percentage and indications for PM implantation were comparable. In terms of echocardiographic characteristics, patients with PMTR showed smaller RV end-diastole area and RV end-systole area than those without PMTR, although other echocardiographic variables were comparable, including left heart size and function, TVAD, RV systolic pressure, and initial TR grades. The smaller RV area in patients with PMTR seems to be associated with fewer cases of grade 1 or 2 TR in that group than in the group without PMTR, owing to the criteria for dividing the two groups.
Table 1. Baseline characteristics.
| Total (n = 429) | No PMTR (n = 387) | PMTR (n = 42) | P-value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age, years | 67.0 ± 12.8 | 66.7 ± 13.0 | 70.3 ± 10.4 | 0.078 |
| Male sex, n (%) | 176 (41.0) | 164 (42.4) | 12 (28.6) | 0.118 |
| Body mass index, kg/m2 | 24.2 ± 3.5 | 24.2 ± 3.5 | 23.9 ± 3.5 | 0.580 |
| Hypertension, n (%) | 256 (59.7) | 226 (58.4) | 30 (71.4) | 0.142 |
| Diabetes mellitus, n (%) | 104 (24.2) | 91 (23.5) | 13 (31.0) | 0.380 |
| Atrial fibrillation, n (%) | 131 (30.5) | 114 (29.5) | 17 (40.5) | 0.195 |
| CAD, n (%) | 53 (12.4) | 47 (12.1) | 6 (14.3) | 0.878 |
| Open-heart surgery, n (%) | 71 (16.6) | 57 (14.7) | 14 (33.3) | 0.004 |
| MV surgery | 21 (4.9) | 16 (4.1) | 5 (11.9) | 0.066 |
| AV surgery | 43 (10.0) | 35 (9.0) | 8 (19.0) | 0.075 |
| CABG | 8 (1.9) | 6 (1.6) | 2 (4.8) | 0.389 |
| Others | 11 (2.6) | 10 (2.6) | 1 (2.4) | >.999 |
| Pacemaker data | ||||
| V pacing percentage, % | 61.1 ± 42.5 | 61.5 ± 42.8 | 57.5 ± 40.3 | 0.571 |
| Indication | ||||
| AV node disease, n (%) | 264 (61.5) | 238 (61.5) | 26 (61.9) | >.999 |
| Sinus node disease, n (%) | 165 (38.5) | 149 (38.5) | 16 (38.1) | >.999 |
| Mode | ||||
| DDD, n (%) | 348 (81.1) | 322 (83.2) | 26 (61.9) | 0.002 |
| VDD, n (%) | 7 (1.6) | 7 (1.8) | 0 (0.0) | 0.812 |
| VVI, n (%) | 25 (5.8) | 17 (4.4) | 8 (19.0) | < .001 |
| Echocardiographic data | ||||
| LVEF, % | 65.6 ± 9.6 | 65.7 ± 9.5 | 65.2 ± 10.2 | 0.770 |
| LA volume index, ml/m2 | 43.3 ± 17.8 | 43.3 ± 17.6 | 43.7 ± 19.9 | 0.902 |
| RVEDA, cm | 18.8 ± 7.3 | 19.0 ± 7.6 | 17.0 ± 4.2 | 0.008 |
| RVESA, cm | 10.2 ± 3.7 | 10.3 ± 3.8 | 9.3 ± 2.8 | 0.048 |
| RVFAC, % | 45.8 ± 9.0 | 45.8 ± 9.1 | 45.0 ± 8.9 | 0.568 |
| TVAD, mm | 29.5 ± 4.6 | 29.5 ± 4.6 | 29.5 ± 4.0 | 0.951 |
| TVAD/BSA, mm/m2 | 18.1 ± 3.1 | 18.0 ± 3.1 | 18.3 ± 2.9 | 0.531 |
| RVSP, mmHg | 33.2 ± 12.6 | 33.4 ± 13.0 | 31.5 ± 9.1 | 0.224 |
| Tricuspid regurgitation | 0.398 | |||
| No, n (%) | 287 (66.9) | 255 (65.9) | 32 (76.2) | |
| Grade 1, n (%) | 110 (25.6) | 102 (26.4) | 8 (19.0) | |
| Grade 2, n (%) | 32 (7.5) | 30 (7.8) | 2 (4.8) |
PMTR, pacemaker-related tricuspid regurgitation; CAD, coronary artery disease; MV, mitral valve; AV, aortic valve; CABG, coronary artery bypass graft; AVB, Atrioventricular block; LVEF, left ventricular ejection fraction; LA, left atrium; RVEDA, right ventricular end-diastole area; RVESA, right ventricular end-systole area; TVAD, tricuspid valve annular diameter; BSA, body surface area; RVSP, right ventricular systolic pressure.
Predictors for PMTR
Table 2 shows the factors associated with the development of PMTR. In the multivariate logistic regression analysis, the presence of atrial fibrillation (hazard ratio [HR] 2.07, 95% confidence interval [CI] 1.27–4.09, p = 0.037) and history of open-heart surgery (HR 3.34. 95% CI 1.68–6.68, p < 0.001) were independently associated with PMTR. Among echocardiographic variables, TVAD/BSA tended to be associated with PMTR, but the statistical significance was marginal (HR 1.12, 95% CI 0.99–1.26, p = 0.054). The presence of grade 1 or 2 TR on pre-implantation echocardiography did not increase the risk of PMTR compared to the absence of TR. The results of subgroup analysis of multivariate Cox proportional hazard ratios for PMTR according to the history of open-heart surgery are shown in Table 3. Comparison of worsening of TR, MR and RVSP in tertile of ventricular pacing percentage were presented in Table 4. There were no significant relationships between increase of ventricular pacing percentage and worsening of TR or MR. On the contrary, RVSP was correlated with percentage of ventricular pacing in Pearson’s correlation coefficient (R = 0.154, p = 0.001).
Table 2. Multivariate Cox proportional hazard ratios for pacemaker-related tricuspid regurgitation.
| HR (95% CI) | P-value | |
|---|---|---|
| Age | 1.03 (0.99–1.06) | 0.072 |
| Male sex | 0.67 (0.32–1.37) | 0.271 |
| Atrial fibrillation | 2.07 (1.27–4.09) | 0.037 |
| Open-heart surgery | 3.34 (1.68–6.68) | <0.001 |
| V pacing percentage | 1.00 (0.99–1.01) | 0.901 |
| LVEF | 1.01 (0.97–1.04) | 0.789 |
| LA volume index | 1.00 (0.98–1.02) | 0.893 |
| RVEDA | 0.93 (0.86–1.02) | 0.114 |
| RVFAC | 0.99 (0.95–1.02) | 0.444 |
| TVAD/BSA | 1.12 (0.99–1.26) | 0.054 |
| RVSP | 1.00 (0.97–1.03) | 0.893 |
| Tricuspid regurgitation | ||
| No | Ref | Ref |
| Grade 1 | 0.47 (0.20–1.11) | 0.086 |
| Grade 2 | 0.43 (0.08–2.24) | 0.314 |
HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; LA, left atrium; RVEDA, right ventricular end-diastole area; RVESA, right ventricular end-systole area; TVAD, tricuspid valve annular diameter; BSA, body surface area; RVSP, right ventricular systolic pressure.
Table 3. Subgroup analysis of multivariate Cox proportional hazard ratios for PMTR according to the history of open-heart surgery.
| No open-heart surgery (n = 358) | Open-heart surgery (n = 71) | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.03 (0.98–1.05) | 0.395 | 1.07 (0.98–1.05) | 0.045 |
| Male sex | 0.84 (0.34–2.07) | 0.698 | 0.61 (0.34–2.07) | 0.529 |
| Atrial fibrillation | 1.91 (0.79–4.62) | 0.151 | 3.24 (0.87–12.06) | 0.080 |
| V pacing percentage | 1.00 (0.99–1.01) | 0.885 | 1.00 (0.98–1.02) | 0.948 |
| LVEF | 0.99 (0.95–1.04) | 0.827 | 1.03 (0.95–1.04) | 0.349 |
| LA volume index | 0.99 (0.96–1.01) | 0.313 | 0.99 (0.96–1.01) | 0.617 |
| RVEDA | 0.92 (0.83–1.02) | 0.101 | 1.02 (0.84–1.24) | 0.832 |
| RVFAC | 0.99 (0.95–1.04) | 0.717 | 1.01 (0.94–1.09) | 0.840 |
| TVAD/BSA | 1.12 (0.96–1.30) | 0.158 | 1.28 (0.98–1.65) | 0.065 |
| RVSP | 1.01 (0.97–1.06) | 0.554 | 1.02 (0.95–1.10) | 0.557 |
| TR | ||||
| No | Ref | Ref | Ref | |
| Grade 1 | 0.16 (0.03–0.77) | 0.022 | 1.60 (0.33–7.71) | 0.559 |
| Grade 2 | 0.01 (0.08-inf) | 0.997 | 1.54 (0.16–15.12) | 0.712 |
HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; LA, left atrium; RVEDA, right ventricular end-diastole area; RVESA, right ventricular end-systole area; TVAD, tricuspid valve annular diameter; BSA, body surface area’ RVSP, right ventricular systolic pressure.
Table 4. Comparison of worsening of TR, MR and RVSP in tertile of ventricular pacing percentage.
| V pacing <30% (n = 147) | V pacing 30 to 99.5% (n = 133) | V pacing >99.5% (n = 149) | P-value | |
|---|---|---|---|---|
| PMTR, n (%) | 14 (9.5) | 17 (12.8) | 11 (7.4) | 0.311 |
| Worsening of MR, n (%) | 23 (15.6) | 24 (18.0) | 17 (11.4) | 0.282 |
| RV systolic pressure, mmHg ± SD | 30.7 ± 9.8 | 34.8 ± 14.1 | 34.4 ± 13.4 | 0.011 |
V pacing, Ventricular pacing percentage; PMTR, pacemaker-related tricuspid regurgitation; MR, mitral regurgitation; RV, right ventricle.
Impact of PMTR on clinical outcomes
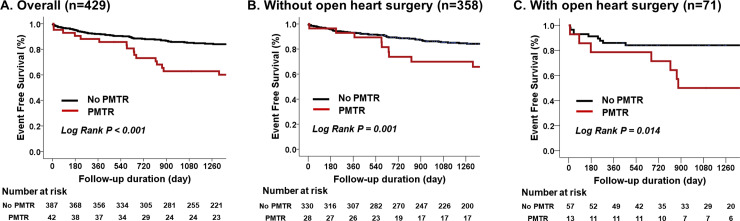
During the median follow-up of 855 days, patients with PMTR showed significantly higher cardiovascular events than those without (45.2% vs. 17.3%, log-rank p<0.001) (Fig 2A). PMTR was independently associated with primary outcomes (HR: 2.45, 95% CI: 1.43–4.22, p = 0.001) even after controlling for age; sex; comorbidities including coronary artery disease, atrial fibrillation, and history of open-heart surgery; and echocardiographic variables including LV ejection fraction and LA volume index (Table 5). In the subgroup analysis according to the history of open-heart surgery, the clinical impact of PMTR was consistent in patients without a history of open-heart surgery (Fig 2B, log-rank p = 0.002, HR 2.44, 95% CI 1.28–4.62, p = 0.007, Table 6) and in those with a history of open-heart surgery (Fig 2C, log-rank p = 0.014, HR 1.43, 95% CI 1.48–4.33, p = 0.012, Table 6).
Fig 2.
Kaplan–Meier curves for the primary outcome-free survival according to the presence of pacemaker-related tricuspid regurgitation A. overall patients, B. patients without previous open-heart surgery, and C. patients with previous open-heart surgery.
Table 5. Multivariate Cox proportional hazard ratios for primary outcomes.
| HR (95% CI) | P-value | |
|---|---|---|
| Age | 1.05 (1.03–1.08) | <0.001 |
| Male sex | 1.19 (0.77–1.85) | 0.437 |
| CAD | 1.14 (0.64–2.05) | 0.656 |
| Atrial fibrillation | 1.23 (0.78–1.96) | 0.377 |
| Open-heart surgery | 0.86 (0.48–1.53) | 0.602 |
| LVEF | 0.96 (0.94–0.98) | <0.001 |
| LA volume index | 1.00 (0.99–1.01) | 0.707 |
| PMTR | 2.45 (1.43–4.22) | 0.001 |
CAD, coronary artery disease; LVEF, left ventricular ejection fraction; LA, left atrium; PMTR, pacemaker-related tricuspid regurgitation.
Table 6. Subgroup analysis of the multivariate Cox proportional hazard ratios for primary outcomes according to the history of open-heart surgery.
| No open-heart surgery (n = 358) | Open-heart surgery (n = 71) | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.06 (1.03–1.09) | < .001 | 1.07 (1.03–1.11) | 0.623 |
| Male sex | 1.39 (0.84–2.28) | 0.199 | 2.85 (1.25–6.50) | 0.614 |
| CAD | 1.10 (0.57–2.15) | 0.770 | 0.47 (0.13–1.65) | 0.717 |
| Atrial fibrillation | 1.52 (0.92–2.50) | 0.101 | 1.28 (0.56–2.96) | 0.258 |
| LVEF | 0.97 (0.94–0.99) | 0.005 | 0.96 (0.93–0.99) | 0.028 |
| LA volume index | 1.01 (0.99–1.02) | 0.155 | 1.00 (0.97–1.02) | 0.433 |
| PMTR | 2.44 (1.28–4.62) | 0.007 | 1.43 (1.48–4.33) | 0.012 |
CAD, coronary artery disease; LVEF, left ventricular ejection fraction; LA, left atrium; PMTR, pacemaker-related tricuspid regurgitation.
Discussion
The primary findings of this study were as follows: 1) the prevalence of PMTR was 9.7% in this study population during a median follow-up of 855 days; therefore, it was not uncommon; 2) the occurrence of PMTR was associated with atrial fibrillation and a history of open-heart surgery; and 3) PMTR was associated with poorer cardiovascular outcome. Therefore, we would suggest that echocardiographic surveillance of PMTR should be performed in patients who underwent PM implantation, especially in those with atrial fibrillation or a history of open-heart surgery.
The results of this study are generally concordant with those of previous studies on the poor prognostic impact of PMTR [7–9]. However, the study population of each study is notably different. Höke et al. demonstrated that significant lead-induced TR is associated with poor long-term prognosis in 287 patients who underwent Implantable cardioverter defibrillator (ICD) or pacemaker implantation. They included 191 patients with ICD and excluded patients who underwent cardiac valve surgery. Therefore, the baseline LV ejection fraction of the study was 39 ± 14% because of a large proportion of patients with ICD implantation for primary or secondary prevention [9]. In contrast, the baseline LV ejection fraction in this study was 66 ± 10%, as we excluded patients who underwent ICD implantation or cardiac resynchronization therapy. Moreover, 16.6% of patients had a history of open-heart surgery, and of these, the majority were cardiac valve surgeries in the mitral or aortic position. Delling et al. also revealed that permanent PM-related significant TR was not a benign phenomenon but was associated with an increased mortality risk compared to that in patients with permanent PM without significant TR [9]. The study did not exclude patients with pre-existing grade 3 or 4 TR before PM implantation, and information regarding the presence of atrial fibrillation at baseline was lacking. Another study by Al-Bawardy et al. also did not exclude patients with pre-existing grade 3 or 4 TR from their study, and 62% of their study population had ICDs [7]. Therefore, this study provides further information regarding the prevalence, predictors, and prognosis of PMTR by including only patients with permanent PM in a wide spectrum of underlying disease including patients with history of open-heart surgery.
The etiology and mechanism of PMTR are not fully elucidated. Earlier studies mainly showed direct damage to TV leaflet or subvalvular apparatus during pacemaker lead implantation can lead to PMTR by perforation of TV leaflet, laceration, or injury of chordae tendineae [15–17]. However, more recent studies have demonstrated that mechanical interference of TV leaflet or subvalvular structures which resulted in incomplete coaptation of TV [3, 5]. Another mechanism of lead-related PMTR is fibrotic response and adhesion of lead and TV leaflet. One study reported that lead adherence to the TV leaflet occurred in 14 out of 41 patients with severe TR due to a permanent pacemaker or ICD [2]. Pacing-related TR also one of the possible mechanism of PMTR. Since LV dyssynchrony and left bundle branch block is an established cause of mitral regurgitation [18], RV dyssynchrony due to permanent pacing is considered can lead to a worsening of TR [6]. In this study, atrial fibrillation and a history of open-heart surgery were found to be independent predictors of PMTR. This finding suggested that the mechanism of PMTR in this population is likely to involve secondary TR, including interference of TV leaflet coaptation, lead adherence, or pacing-related TR rather than primary TR related with direct leaflet damage by the PM lead. Moreover, the larger TVAD/BSA on baseline echocardiogram before PM implantation tends to be associated with PMTR after adjusting for the RV area and presence of atrial fibrillation, which also supports the suggested mechanism.
With the increase in the aging population and recent advances in surgical and transcatheter intervention, many patients who underwent permanent PM implantation had atrial fibrillation and a history of valve procedure. Conduction abnormality requiring permanent PM implantation are frequent and associated with an increased risk of heart failure-related hospitalization and lack of functional improvement and reverse remodeling after either transcatheter or surgical aortic valve implantation [19–21]. Data regarding the prognostic impact of PM implantation after valve intervention are somewhat controversial. A few studies demonstrated no prognostic impact of PM implantation after valve implantation [19, 22]; however, some studies showed worse outcome in patients who received permanent PM implantation [21, 23]. These discrepancies may possibly be related to the involvement of the occurrence of PMTR between the PM implantation and clinical outcome. In this study, the clinical implication of PMTR was also significant in the subgroup of patients with a history of open-heart surgery.
This study has several limitations. First, this study was retrospectively designed, resulting in an inherent potential limitation. Patients who did not undergo follow-up echocardiography were excluded, and the interval after PM implantation in each patient was not preset in real clinical practice. Therefore, the prevalence of PMTR may possibly be underestimated. Second, a few studies have demonstrated that three-dimensional echocardiography might be better for the assessment of lead-TV interaction [24, 25], but we did not perform an analysis using three-dimensional echocardiography findings. Therefore, the mechanism of PMTR could not be determined in detail. Third, although one of the possible mechanism of pacing related TR is RV dyssynchrony, we did not include RV dyssynchrony analysis due to unavailable echocardiographic data for the analysis. However, we assumed that the main findings of the study are not substantially affected by RV dyssynchrony because percentage of ventricular pacing was not significantly associated with increasing of TR.
In conclusion, worsening of TR is not uncommon in patients with permanent PM and is associated with the presence of atrial fibrillation and a history of open-heart surgery. PMTR is independently associated with poor cardiovascular outcome. Thus, baseline and regular follow-up echocardiographic surveillance should be performed after PM implantation, especially in patients with atrial fibrillation and a history of open-heart surgery.
Data Availability
Minimal data set files are available from the figshare database (URL: https://figshare.com/articles/Minimal_Dataset_xls/12357869).
Funding Statement
This study was supported in part by internal funding. C.Y.S. is funded by the Yonsei University College of Medicine (Faculty research grant, Award Number: 6-2014-0153, URL: https://www.yuhs.or.kr). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Al-Mohaissen MA, Chan KL. Prevalence and mechanism of tricuspid regurgitation following implantation of endocardial leads for pacemaker or cardioverter-defibrillator. J Am Soc Echocardiogr. 2012;25(3):245–52. 10.1016/j.echo.2011.11.020 . [DOI] [PubMed] [Google Scholar]
- 2.Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM, 3rd, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45(10):1672–5. 10.1016/j.jacc.2005.02.037 . [DOI] [PubMed] [Google Scholar]
- 3.Kim JB, Spevack DM, Tunick PA, Bullinga JR, Kronzon I, Chinitz LA, et al. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: an observational study. J Am Soc Echocardiogr. 2008;21(3):284–7. 10.1016/j.echo.2007.05.022 . [DOI] [PubMed] [Google Scholar]
- 4.Paniagua D, Aldrich HR, Lieberman EH, Lamas GA, Agatston AS. Increased prevalence of significant tricuspid regurgitation in patients with transvenous pacemakers leads. Am J Cardiol. 1998;82(9):1130–2, A9. 10.1016/s0002-9149(98)00567-0 . [DOI] [PubMed] [Google Scholar]
- 5.Lee RC, Friedman SE, Kono AT, Greenberg ML, Palac RT. Tricuspid Regurgitation Following Implantation of Endocardial Leads: Incidence and Predictors. Pacing Clin Electrophysiol. 2015;38(11):1267–74. 10.1111/pace.12701 . [DOI] [PubMed] [Google Scholar]
- 6.Vaturi M, Kusniec J, Shapira Y, Nevzorov R, Yedidya I, Weisenberg D, et al. Right ventricular pacing increases tricuspid regurgitation grade regardless of the mechanical interference to the valve by the electrode. Eur J Echocardiogr. 2010;11(6):550–3. 10.1093/ejechocard/jeq018 . [DOI] [PubMed] [Google Scholar]
- 7.Al-Bawardy R, Krishnaswamy A, Rajeswaran J, Bhargava M, Wazni O, Wilkoff B, et al. Tricuspid regurgitation and implantable devices. Pacing Clin Electrophysiol. 2015;38(2):259–66. 10.1111/pace.12530 . [DOI] [PubMed] [Google Scholar]
- 8.Delling FN, Hassan ZK, Piatkowski G, Tsao CW, Rajabali A, Markson LJ, et al. Tricuspid Regurgitation and Mortality in Patients With Transvenous Permanent Pacemaker Leads. Am J Cardiol. 2016;117(6):988–92. 10.1016/j.amjcard.2015.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoke U, Auger D, Thijssen J, Wolterbeek R, van der Velde ET, Holman ER, et al. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart. 2014;100(12):960–8. 10.1136/heartjnl-2013-304673 . [DOI] [PubMed] [Google Scholar]
- 10.Tjong FV, Reddy VY. Permanent Leadless Cardiac Pacemaker Therapy: A Comprehensive Review. Circulation. 2017;135(15):1458–70. 10.1161/CIRCULATIONAHA.116.025037 . [DOI] [PubMed] [Google Scholar]
- 11.Chang JD, Manning WJ, Ebrille E, Zimetbaum PJ. Tricuspid Valve Dysfunction Following Pacemaker or Cardioverter-Defibrillator Implantation. J Am Coll Cardiol. 2017;69(18):2331–41. 10.1016/j.jacc.2017.02.055 . [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39 e14. 10.1016/j.echo.2014.10.003 . [DOI] [PubMed] [Google Scholar]
- 13.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010;11(4):307–32. 10.1093/ejechocard/jeq031 . [DOI] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–71. 10.1016/j.echo.2017.01.007 . [DOI] [PubMed] [Google Scholar]
- 15.Wilner BR, Coffey JO, Mitrani R, Carrillo RG. Perforated tricuspid valve leaflet resulting from defibrillator leads: a review of the literature. J Card Surg. 2014;29(4):470–2. Epub 2014/05/08. 10.1111/jocs.12352 . [DOI] [PubMed] [Google Scholar]
- 16.Schilling RJ. Pacing lead entanglement in the tricuspid valve apparatus during implantation. Europace. 1999;1(3):201 Epub 2001/02/28. 10.1053/eupc.1999.0040 . [DOI] [PubMed] [Google Scholar]
- 17.Gould L, Reddy CV, Yacob U, Teich M, DeMartino A, DePalma D, et al. Perforation of the tricuspid valve by a transvenous pacemaker. JAMA. 1974;230(1):86–7. Epub 1974/10/07. . [PubMed] [Google Scholar]
- 18.Fanari Z, Hammami S, Hammami MB, Hammami S, Shuraih M. The effects of right ventricular apical pacing with transvenous pacemaker and implantable cardioverter defibrillator on mitral and tricuspid regurgitation. J Electrocardiol. 2015;48(5):791–7. Epub 2015/07/29. 10.1016/j.jelectrocard.2015.07.002 . [DOI] [PubMed] [Google Scholar]
- 19.Chamandi C, Barbanti M, Munoz-Garcia A, Latib A, Nombela-Franco L, Gutierrez-Ibanez E, et al. Long-Term Outcomes in Patients With New Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11(3):301–10. 10.1016/j.jcin.2017.10.032 . [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, Shim CY, Hong GR, Cho IJ, You SC, Chang HJ, et al. Adverse effects of left ventricular electrical dyssynchrony on cardiac reverse remodeling and prognosis after aortic valve surgery. J Cardiol. 2018;72(5):385–92. 10.1016/j.jjcc.2018.03.013 . [DOI] [PubMed] [Google Scholar]
- 21.Mehaffey JH, Haywood NS, Hawkins RB, Kern JA, Teman NR, Kron IL, et al. Need for Permanent Pacemaker After Surgical Aortic Valve Replacement Reduces Long-Term Survival. Ann Thorac Surg. 2018;106(2):460–5. 10.1016/j.athoracsur.2018.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohananey D, Jobanputra Y, Kumar A, Krishnaswamy A, Mick S, White JM, et al. Clinical and Echocardiographic Outcomes Following Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement: Meta-Analysis and Meta-Regression. Circ Cardiovasc Interv. 2017;10(7). 10.1161/CIRCINTERVENTIONS.117.005046 . [DOI] [PubMed] [Google Scholar]
- 23.Greason KL, Lahr BD, Stulak JM, Cha YM, Rea RF, Schaff HV, et al. Long-Term Mortality Effect of Early Pacemaker Implantation After Surgical Aortic Valve Replacement. Ann Thorac Surg. 2017;104(4):1259–64. 10.1016/j.athoracsur.2017.01.083 . [DOI] [PubMed] [Google Scholar]
- 24.Mediratta A, Addetia K, Yamat M, Moss JD, Nayak HM, Burke MC, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7(4):337–47. 10.1016/j.jcmg.2013.11.007 . [DOI] [PubMed] [Google Scholar]
- 25.Seo Y, Ishizu T, Nakajima H, Sekiguchi Y, Watanabe S, Aonuma K. Clinical utility of 3-dimensional echocardiography in the evaluation of tricuspid regurgitation caused by pacemaker leads. Circ J. 2008;72(9):1465–70. 10.1253/circj.cj-08-0227 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Minimal data set files are available from the figshare database (URL: https://figshare.com/articles/Minimal_Dataset_xls/12357869).