Abstract
Both observational and indirect evidence are widely used to determine the diets of wild animals. Direct observations are often assumed to provide the most comprehensive reflection of diet, but many wild animals are logistically challenging to observe. Despite the regular use of observational and indirect methods for inferring diet in wild animals, they have rarely been compared in detail for the same study population. Over 12 months this study assessed the congruence of methods in estimating the diet of a montane community of eastern chimpanzees Pan troglodytes schweinfurthii in Nyungwe National Park, Rwanda using observational scan samples and macroscopic fecal inspection. The assessment of the number of food species consumed each month was comparable between methods, but the estimation of the composition of items in the diet differed significantly. Most notably, the fecal samples significantly underestimated the consumption of flowers, and certain fruit species, which based on direct behavioral observations were seasonally consumed at very high rates. Conversely, direct observations underestimated the consumption of leaves and pith in comparison to results present in the fecal samples. These results suggest that combining methods where possible is most useful for accurate monitoring of dietary trends, particularly for species that experience significant seasonal shifts in their diet.
Keywords: chimpanzee, comparative methods, dietary analysis, seasonality
Describing the diets of wild animals is a crucial task and central theme in the field of biology, because understanding the dietary ecology of different species provides essential information about habitat requirements, trophic interactions, and food webs (Chapman 1995; Bridgeland et al. 2010; VelezLiendo et al. 2013; Singer et al. 2014). Characterizing the resource base of wild animals can also address questions related to nutritional ecology (Felton et al. 2009; Birnie-Gauvin et al. 2017), resource partitioning (Wrangham et al. 1998; Pardo et al. 2015), and selective pressures that shape morphology and socio-ecology (Marshall and Wrangham 2007; Chapman et al. 2012). Dietary studies also play a central role in assessing the responses of wild populations to anthropogenic habitat alteration (McLennan 2013; Birnie-Gauvin et al. 2017). This kind of information is especially vital for the conservation efforts of rare or endangered species, because it helps to identify habitats or food sources that need to be maintained (Ward et al. 2012; Aryal et al. 2016).
Two principal methods for assessing the dietary ecology of wild animal populations are direct observations of feeding activity and analysis of fecal samples (Moreno-Black 1978; Altmann 1991; Martin and Bateson 2007; Gilby et al. 2010; Koirala et al. 2016). Focal animal sampling and instantaneous scan sampling are typical observational methods used to measure activity budgets, time spent feeding, and intake rates (Altmann 1991, 1998; Zuberbühler and Wittig 2011; Kaplin and William 2013). However, these techniques can be subject to observer error, observer bias, and results may be influenced by data recorded predominantly in parts of the habitat in which a species is most observable (McInnis et al. 1983; Shrestha and Wegge 2006; Schneider et al. 2010; Tuyttens et al. 2014). Additionally, the processing or handling time of different foods may not be proportional to the amount of that food consumed (Hohmann 2009). For example, in howler monkeys Alouatta pigra leaves accounted for 50.6% of time spent feeding, but estimates of dry matter intake of leaves indicated that they accounted for 37% of ingested food (Righini et al. 2017); similarly observations of chimpanzees Pan troglodytes were found to overestimate ingested foods (Hladik 1977). Sampling bias may also be introduced by the length of the sampling period and seasonal variation in diet, time of day, and sex and age of the observed individual (Hladik 1977; Altmann 1991, 1998; Rothman et al. 2012).
Direct observations are not always possible since many wild animals are difficult to locate and reliably observe. Researchers may also make ethical decisions, selecting indirect methods to reduce negative impacts (such as disturbance or disease transmission) on the study population (Gruen et al. 2013; Shutt et al. 2014). Therefore, non-invasive methods, such as macroscopic fecal sampling (Tutin and Fernandez 1993; Basabose 2002), microscopic and molecular fecal analysis (Phillips 2011), DNA barcoding of fecal samples (Quéméré et al. 2013; Mallot et al. 2018), and examination of trail signs and feeding remains (Rogers et al. 1990; Doran et al. 2002), can be important for determining animal diets, particularly of shy or at-risk species. However, the choice of indirect methods and their associated biases will vary depending on the study species and the research questions to be answered.
Macroscopic fecal inspection is used to assess diet in a wide range of species (birds: Inger et al. 2006; elephants and antelope: Kos et al. 2012; bears: Koike et al. 2013), including primates (Julliot and Sabatier 1993; Tutin and Fernandez 1993; Doran et al. 2002; Hanya et al. 2003; Surbeck et al. 2009; Phillips and McGrew 2014; Johnson 2015). However, ingested foods have differential rates of digestion, and this approach may result in bias toward items that show greater digestive resistance due to their biochemical and physiological properties (e.g., seeds, hard leaves, or chitinous invertebrate exoskeletons) (Tutin and Fernandez 1993; Mitra and Flynn 2007; Brett et al. 2017). However, it is worth noting that the detection of hard-bodied invertebrates during macroscopic fecal analysis may be influenced by the presence of a functional chitinase gene that assists with the digestion of chitin (Janiak et al. 2017). Furthermore, food classes that leave no recognizable remains (e.g., some leaves, flowers, and soft-bodied invertebrates) may be absent from estimates—these limitations have been well documented in previous studies (McInnis et al. 1983; Putman 1984; Dellinger and Trillmich 1988; Tutin and Fernandez 1993; Su and Lee 2001). Additionally, some items are difficult to identify (e.g., pith and stems), and this may lead to underestimation of these food types in the diet (Tutin and Fernandez 1993; Su and Lee 2001; Basabose 2002; Parker and Bernard 2006; Phillips and McGrew 2014).
Despite the varying capacities of these approaches to quantify wild animal diets, few studies have made direct intraspecific comparisons of macroscopic fecal sampling and direct feeding observations (reviewed by Nielsen et al. 2018). Research has focused on comparisons of invasive and non-invasive indirect methods (Anthony and Smith 1974; Seefelt and Gillingham 2006; Klare et al. 2011; Weiser and Powell 2011; Bryan 2014) or observational methods (Fragaszy et al. 1992; Margalida et al. 2005; Gilby et al. 2010; Amato et al. 2013). Some studies have directly compared diet composition estimated from observations with macroscopic fecal analysis (McInnis et al. 1983; Mills 1992; Parker and Bernard 2006; Bakaloudis et al. 2012; Phillips and O’Connell 2016), but to date no studies have made direct comparisons using group-level analyses across seasons for chimpanzees (Phillips and McGrew 2014).
Chimpanzees are an ideal species with which to compare the diet profiles generated by these two techniques, since indirect (macroscopic fecal analysis: Tutin and Fernandez 1993; Basabose 2002; McLennan 2013; Phillips and Lancelotti 2014; Carvalho et al. 2015) and direct methods (focal sampling: Wrangham et al. 1996; Potts et al. 2011; scan sampling: Newton-Fisher 1999; Tweheyo et al. 2003) have been employed widely. Determining the breadth and composition of species in the diet using these methods are subject to the above-mentioned biases. Of particular concern is the limited identification and estimation of foods easily digested in fecal samples, and the difficulty observing certain feeding behaviors, which leads to inaccurate estimates. While researchers are often aware of these limitations in general, direct comparisons of methods provide a means for assessing how they might specifically affect results from research on a species or population. Dietary ecology is a particular focus in chimpanzee studies because it can facilitate conservation of this endangered species. Knowledge of potential sources of bias in determining the diet of chimpanzee populations can improve accuracy in identifying important food species and can assist with the protection of those species from anthropogenic influences (Chapman et al. 2000; Felton et al. 2010; Potts 2011).
Comparisons of chimpanzee focal observations and macroscopic fecal inspection in Budongo, Uganda indicated that the proportion of fruits estimated in the diet from both methods were positively correlated, but young leaves were not (Fawcett 2000). The study provided no discussion or explanation as to why this was the case, but it could be argued that the digestibility of young leaves may account for the difference. Research comparing the number of plant or animal items detected from focal observations and macroscopic fecal inspection in Kibale National Park, Uganda concluded that the indirect method was less accurate in describing folivorous and faunivorous diet types (Phillips and McGrew 2013). The difference here was likely due to the difficulties of identifying leaf, pith, or animal fragments in the fecal samples (Tutin and Fernandez 1993; Phillips and McGrew 2013). This research also indicated that when analyzing fecal samples in two subsets, one considering gut passage rates and the other not, the results were similar (Phillips and McGrew 2013). However, the study did not examine the time spent feeding on different food types. An opposite trend was reported in a study on macaques Macaca cyclopis, where more vine/shrub species were detected in fecal samples than through observations (Su and Lee 2001); presumably because feeding on those items was difficult to observe.
Seasonal differences in diet could lead to seasonal differences in food digestibility because different foods are consumed, and therefore, correlations between macroscopic fecal analysis and behavioral observations may differ across seasons. Research spanning an annual cycle that compares the relative proportions of each main food type in the chimpanzee diet between seasons would demonstrate how reliably the two methods correlate in their predictions of chimpanzee diet over time. This is particularly important for species that experience considerable seasonality in the types of food available to them (van Schaik et al. 1993; Hemingway and Bynum 2005), such as the chimpanzee community in the montane forest of Nyungwe National Park, Rwanda (Matthews et al. 2019).
Little research has undertaken detailed and direct comparisons using group-level analyses of dietary data collected from both direct observations and macroscopic fecal analyses between seasons remain unexplored in chimpanzees. Interpretations of diet for any species from observations, macroscopic fecal sampling, or a combination of methods should (if possible), be made with knowledge of the methodological incongruences. With this in mind, the purpose of this study was to 1) highlight any differences in dietary breadth and diversity estimated using direct group-level scans and macroscopic fecal sampling and 2) determine the comparability of diet composition estimated using those two methods across an annual cycle to include seasonal differences. This study does not aim to promote one method over another, but highlight the key differences of each method in order to promote informed methodological choice, accurate interpretations, and cross-site examinations.
Materials and Methods
Study site and subjects
The study was conducted in Nyungwe National Park, Rwanda (2°17′ −2°50′S and 29°07′ −29°26′E, Figure 1). The 1,019 km2 park is located in southwestern Rwanda and connects with Kibira National Park, Burundi. The park is characterized by steep slopes ranging from 1,600 to 2,950 m ASL and contains a mosaic of primary and secondary forest. During the study period (September 2016 to August 2017) annual rainfall was 1,402 mm and mean daily temperature ranged from 11.4°C to 19.6°C (Meteorological station location: 2°28′43″S, 29°12′00″E, 2465 m ASL Nyirambangutse et al. 2017). September to November and January to March were the rainy seasons (monthly rainfall >100 mm), April to August was the major dry season (monthly rainfall <100 mm), and December was also dry (<100 mm), tree fruit production peaked in the major dry season (Matthews et al. 2019). The park has a rich primate diversity, containing at least 13 species of primates, including one species of great ape: the eastern chimpanzee Pan troglodytes schweinfurthii. The focal subjects for this study were the Mayebe community of chimpanzees, numbering ∼67 free-ranging individuals (18 adult females and 14 adult males individually recognizable, Green et al. 2019), this community receives regular tourists and generally tolerates human presence at >20 m.
Figure 1.
Map of Nyungwe National Park, located in southwestern Rwanda, East Central Africa. Adapted by K. Meisterhans and C. C. Grueter from Nyungwe National Park Management Plan 2012–2021.
Data collection
We collected data from September 2016 to August 2017 for 10 days each month (split into two slots of five consecutive days, with 2 weeks between each sampling period) using 2 methods: instantaneous scan sampling and fecal sampling. Sampling effort occurred on concurrent days throughout the study.
Observational data
We collected observational data through instantaneous scan samples (Altmann 1974) taken at 15-min intervals (n = 1,532 feeding scans over 12 months, 14.2 8.4 scans per day, 2.5 1.7 individuals feeding per scan). While the terrain, dense vegetation, and large home range precluded the possibility of conducting individual focal follows, we took care not to resample the same individuals in the group scans by using distinguishing features (e.g., size, hair color, facial features, physical marks, or disfigurements) as a form of identification. We located the chimpanzees at their nests before dawn (when the previous day’s nesting sites were known) and followed them throughout the day (for full-day focal group follows) to the evening nest site when possible. Alternatively, we used vocalizations and recently used feeding sites or fresh tracks to locate the chimpanzees. During each group-level scan we noted whether each individual was feeding and if so, we recorded the item (e.g., plant, animal, and mushroom), species, and part consumed (fruit, pith/stem, leaf, flower, bark, and wood). We calculated the composition of the diet as the proportion of scans in which individuals fed on a non-plant item or plant part relative to the number of feeding scans (Matthews et al. 2019). When calculating dietary breadth (number of species consumed) from scan sampling data, we also used feeding remains data associated with the scan samples; if for instance, we could not reliably identify a species fed upon due to poor visibility, we entered the area after the chimpanzees had moved away from the food source, and where possible identified the species from remains (one leaf and one mushroom species unidentified).
Fecal sample data
We collected fresh fecal samples (<24 h old) that were undisturbed by insects opportunistically during the same two 5-day sampling periods as behavioral observations (n = 323 samples collected over 12 months of observation). Due to opportunistic collection methods, we were not able to link the fecal samples to a specific age–sex class or known individual. Samples were easily distinguished from other primate feces by size, smell, form, and color. The samples were taken back to camp, stored in plastic zip-lock bags, and analyzed within 5 days of collection. We weighed and then sluiced the entire wet sample in 1 mm mesh sieves (McGrew et al. 2009). We examined each sample macroscopically, and categorized each item (fruit, leaf, pith, flower, animal, or honey), identifying them to species level where possible (two fruit and one leaf species unidentified). We estimated each category visually as a proportion of the total fecal sample volume, to the nearest 5% (Basabose 2002), and counted each fruit seed unless <2 mm in diameter, in which case they were estimated.
All research protocols were reviewed and approved by the University of Western Australia’s Animal Ethics Committee (RA/5/15/1070) as well as the governing body of Nyungwe NP, the Rwanda Development Board—Tourism and Conservation Department.
Analytical methods
We used Pearson’s correlations to assess the relationship between the number of fruit species, non-fruit plant species (of which leaves and pith are consumed), and total plant species that the chimpanzees fed on during the five consecutive observation day periods and the number recorded in fecal samples during the same 5 days (data did not deviate significantly from a normal distribution). We also used Pearson’s correlations to analyze the relationship between dietary diversity and evenness determined from observations and fecal samples. Shannon’s Index [=where is the proportion of feeding time (or proportion of each fecal sample) accounted for by th food type] was used to estimate monthly dietary diversity for observational and fecal data (Shannon and Weaver 1964), and Hill’s index (=H′/x, where x is the total number of species sampled) was used to estimate dietary evenness (Hill 1973; Pielou 1974).
For analyses, we categorized honey and insects together as “non-plant.” During fecal analysis, honey was primarily determined by bee (Apis mellifera or Meliponini) remains, since bees are only consumed when the chimpanzees are consuming honey, so the detection rate of honey is assumed to be comparable to insects: due to low consumption rates, these could not be analyzed separately. We assessed whether method type (observational or fecal) influenced the proportion of each category (fruit, leaf, pith, flower, and non-plant) in the scan or fecal samples using separate generalized linear models (GLMs) with a binomial error distribution and a logit link function. The response was highly skewed for observations (e.g., fruit consumption was observed 100% of the time in 66% of group feeding scans) and fecal samples (e.g., 72% of fecal samples did not contain flowers), precluding the use of a Poisson distribution. The response was partitioned into a binomial denominator (total possible occurrence of a category was 1) and a numerator (the proportion of each food category present in the sample). We included season (wet or dry months) and interactions between season and method as additional predictors were also included to assess whether there was a seasonal or season * method effect on the estimation of diet. The full model was:
To demonstrate trends on a finer scale we also analyzed the data by month using the same method and model as above, but with month as the additional predictor instead of season. Data were analyzed in SPSS (SPSS Inc., Chicago, IL, USA).
Results
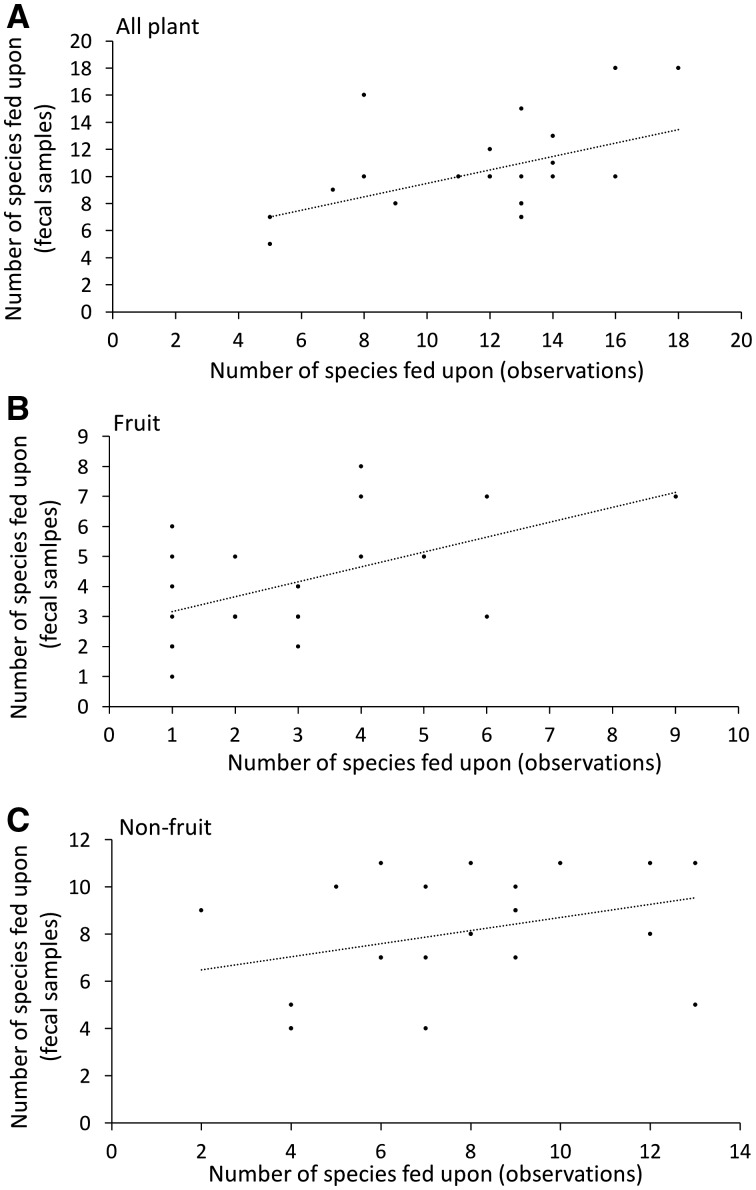
A comparison of the plant foods consumed for each method type revealed that the number of plant species fed upon during direct foraging observations positively predicted the number of plant species detected through macroscopic fecal inspection (Pearson correlation, n = 21 5-day comparisons, r = 0.48, P < 0.05; Figure 2A). When considering fruit and non-fruit (e.g., herbaceous) species separately, the number of fruit species consumed was highly correlated between methods, but non-fruits were not, specifically feeding observations detected fewer non-fruit species (fruit: n = 21, r = 0.54, P = 0.007; non-fruit: n = 21, r = 0.34, P = 0.11; Figure 2B,C). Dietary diversity (H′) and evenness (J′) were not significantly correlated between data collection methods during the 5-day field trips (H′: n = 10, r = 0.16, P = 0.61; J′: n = 10, r = 0.07, P = 0.81).
Figure 2.
The relationship between the number of A) all plant species B) fruit species, and C) non-fruiting (i.e., herbaceous) species detected in the chimpanzee diet through direct feeding observations and macroscopic fecal samples during bi-monthly field trips from August 2016 to September 2017 in Nyungwe National Park, Rwanda.
There was a significant effect of the interaction between season and method on the proportion of fruit, leaves, pith, flowers, and non-plant items detected in the observational scans and fecal samples, with greater similarities in dietary information between method collection types in one season than the other (Table 1). While the model in Table 1 was assessed at a seasonal level, Figures 3–7 and model in Table 2 are presented at the month level to demonstrate precisely where the differences and similarities lie. The proportion of fruits consumed by chimpanzees using the two sampling methods was comparable only during September (wet season), December, and May (dry season, Figure 3 and Table 2). The most substantial differences were apparent during November, January, February, and August (wet season and end of the dry season, Figure 3). In these months, direct foraging observations predicted much higher proportions of fruit in the diet than fecal samples; the reverse trend was apparent during March, April, and June (dry season and end of the wet season, Figure 3).
Table 1.
GLM analysis of the effect of method (observational scans and macroscopic fecal sampling) and season on the proportion of fruit, pith, leaf, flower, and non-plant items (honey and insects) in the chimpanzee diet over a 12-month period (2016–2017)
| Food type | Predictors | χ2 | P-value | Effect size±SE | 95% CI |
|---|---|---|---|---|---|
| Fruit | Method | 35 | <0.001 | Fec: −0.36 ± 0.06 (Obs: 0a) | −0.48, −0.24 |
| Season | 23 | <0.001 | Dry: 0.29 ± 0.06 (Wet: 0a) | 0.17, 0.41 | |
| Method * Season | 83 | <0.001 | Fec*Dry: −0.08 ± 0.08 | −0.26, 0.08 | |
| Obs*Dry: −0.02 ± 0.09 | −0.19, 0.15 | ||||
| Fec*Wet: −0.67 ± 0.09 (Obs*Wet: 0a) | −0.84, −0.50 | ||||
| Pith | Method | 241 | <0.001 | Fec: 2.11 ± 0.13 (Obs: 0a) | 1.84, 2.37 |
| Season | 18 | <0.001 | Dry: 0.41 ± 0.09 (Wet: 0a) | −0.61, −0.22 | |
| Method * Season | 272 | <0.001 | Fec*Dry: 2.19 ± 0.23 | 1.73, 2.65 | |
| Obs*Dry: 0.69 ± 0.26 | 0.17, 1.21 | ||||
| Fec*Wet: 2.80 ± 0.23 (Obs*Wet: 0a) | 2.35, 3.26 | ||||
| Leaf | Method | 53 | <0.001 | Fec: 0.65 ± 0.08 (Obs: 0a) | 0.47, 0.82 |
| Season | 8 | <0.05 | Dry: −0.16 ± 0.08 (Wet: 0a) | 0.04, 0.26 | |
| Method * Season | 56 | <0.001 | Fec*Dry: 0.50 ± 0.12 | 0.25, 0.75 | |
| Obs*Dry: −0.12 ± 0.14 | −0.39, 0.16 | ||||
| Fec*Wet: 0.68 ± 0.12 (Obs*Wet: 0a) | 0.43, 0.93 | ||||
| Flower | Method | 235 | <0.001 | Fec: −2.92 ± 0.19 (Obs: 0a) | −3.29, −2.55 |
| Season | 10 | 0.002 | Dry: 0.34 ± 0.10 (Wet: 0a) | 0.55, 0.12 | |
| Method * Season | 245 | <0.001 | Fec*Dry: −2.98 ± 0.22 | −3.43, −2.54 | |
| Obs*Dry: −0.42 ± 0.11 | −0.64, −0.19 | ||||
| Fec*Wet: −3.54 ± 0.36 (Obs*Wet: 0a) | −4.26, −3.34 | ||||
| Non-plant | Method | 8 | 0.002 | Fec: −0.87 ± 0.25 (Obs: 0a) | −0.93, −0.53 |
| Season | 29 | <0.001 | Dry: −1.79 ± 0.33 (Wet: 0a) | −2.45, −1.15 | |
| Method * Season | 30 | <0.001 | Fec*Dry: −2.54±0.60 | −3.73, −2.35 | |
| <0.001 | Obs*Dry: −1.56 ± 0.39 | −2.33, −1.77 | |||
| Fec*Wet: −0.30 ± 0.27 (Obs*Wet: 0a) | −1.34, −0.24 |
0a is set to zero because this category level is the reference category. Fec, fecal sampling; Obs, observational sampling.
Figure 3.
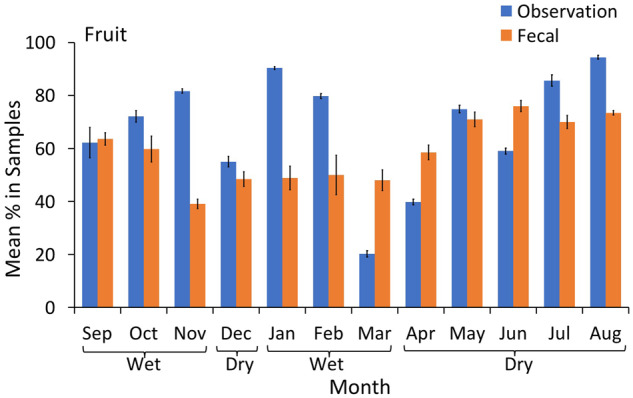
Mean percentage of fruit (with 95% CI) in the chimpanzee diet over 12 months of continuous sampling (2016–2017), using observational scans and macroscopic fecal sampling.
Table 2.
GLM analysis of the effect of method (observational scans and macroscopic fecal sampling) and month on the proportion of fruit, pith, leaves, and flowers in the chimpanzee diet over a 12-month period (2016–2017)
| Fruit: |
Pith: |
Leaf: |
Flower: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | χ2 | Effect±SE | 95% CI | χ2 | Effect±SE | 95% CI | χ2 | Effect±SE | 95% CI | χ2 | Effect±SE | 95% CI | ||
| Method | 38 | 247 | 54 | 266 | ||||||||||
| Fec | −0.39±0.06 | −0.52, −0.27 | 2.15±0.13 | 1.89, 2.42 | −0.67±0.09 | 0.49, 0.85 | −3.24±0.19 | −3.63, −2.85 | ||||||
| Obs | 0a | 0a | 0a | 0a | ||||||||||
| Met*Mon | 509 | See Figure 3 | 244 | See Figure 4 | 243 | See Figure 5 | 547 | See Figure 6 | ||||||
| Month | 329 | 0a | 121 | 0a | 129 | 0a | 287 | 0a | ||||||
| Sep | 0.45±0.18 | −0.03, 0.95 | −0.58±0.24 | −1.06, −0.10 | −0.71±0.23 | −1.16, −0.26 | – | – | ||||||
| Oct | 0.53±0.15 | 0.24, 0.81 | −1.08±0.21 | −1.51, −0.66 | −0.43±0.17 | −0.76, −0.09 | – | – | ||||||
| Nov | 0.42±0.14 | 0.14, 0.70 | −0.74±0.20 | −1.13, −0.35 | −0.90±0.18 | −1.26, −0.83 | 0a | |||||||
| Dec | 0a | 0a | 0a | – | – | |||||||||
| Jan | 0.81±0.15 | 0.52, 1.10 | −0.52±0.19 | −0.89, −0.14 | −1.14±0.20 | −1.50, −0.73 | – | – | ||||||
| Feb | 0.68±0.15 | 0.39, 0.97 | −0.84±0.20 | −1.24, −0.43 | −1.19±0.20 | −1.58, −0.79 | 1.68±0.36 | 0.97, 2.01 | ||||||
| Mar | −0.69±0.15 | −0.97, −0.40 | −0.71±0.20 | −1.10, −0.32 | −0.58±0.18 | −0.93, −0.24 | 3.61±0.35 | 2.92, 3.94 | ||||||
| Apr | 0.96±0.15 | 0.66, 1.25 | −0.91±0.20 | −1.32, −0.51 | −0.71±0.18 | −1.06, −0.36 | 2.99±0.35 | 2.31, 3.28 | ||||||
| May | −0.18±0.14 | −0.46, 0.09 | −1.87±0.26 | −2.39, −1.34 | −0.90±0.19 | −1.27, −0.53 | 1.48±0.36 | 0.75, 1.82 | ||||||
| Jun | 0.79±0.15 | 0.51, 1.09 | −2.04±0.28 | −2.59, −1.50 | −1.69±0.23 | −2.15, −1.23 | 2.28±0.35 | 1.58, 2.67 | ||||||
| Jul | 1.12±0.15 | 0.81, 1.41 | −1.61±0.23 | −2.11, −1.13 | −1.40±0.21 | −1.82, −0.98 | 0.84±0.38 | 0.76, 1.62 | ||||||
| Aug | 1.63±0.16 | 1.30, 1.96 | −1.73±0.25 | −1.99, −0.98 | −2.02±0.26 | −2.54, −1.51 | 0.74±0.38 | −0.04, 1.52 | ||||||
0a is set to zero because this category level is the reference category.
Pith was consistently estimated at significantly higher proportions in the fecal samples than direct observations (Figure 4 and Table 2); similarly, leaves were estimated at higher rates in the fecal samples during 9 out of the 12 months (Figure 5 and Table 2). The opposite trend was observed for flowers: they were estimated at higher proportions during direct observations than fecal samples in 6 out of 12 months (Figure 6). Meat was present in fecal samples during 4 months of the year (wet and dry season), but no hunting or meat-eating was detected during observational sampling. Honey and insects were detected at low rates using both methods, and the proportions of these items in the chimpanzee diet appeared to be similar only during October (wet season, Figure 7). The data indicated that in 5 months (mostly wet season) these items were more prevalent in the fecal samples, while in 4 months (mostly dry season) they were more prevalent in observations. Overall low detection rates precluded a month by month statistical comparison and made a clear trend difficult to identify (Figure 7).
Figure 4.
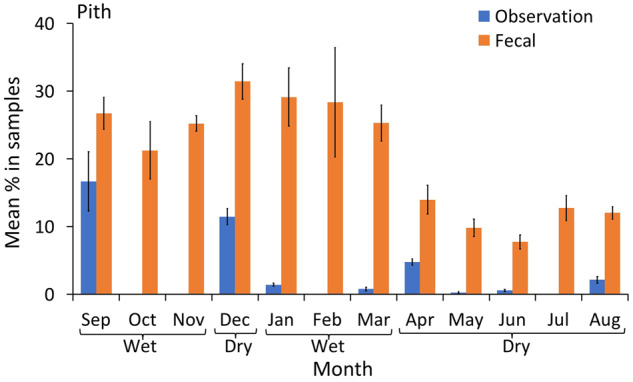
Mean percentage of pith (with 95% CI) in the chimpanzee diet over 12 months of continuous sampling (2016–2017), using observational scans and macroscopic fecal sampling.
Figure 5.
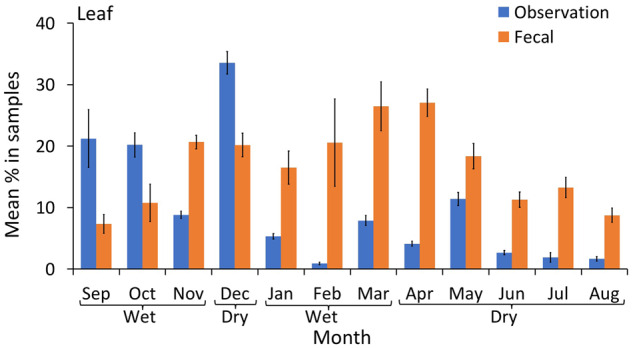
Mean percentage of leaves (with 95% CI) in the chimpanzee diet over 12 months of continuous sampling (2016–2017), using observational scans and macroscopic fecal sampling.
Figure 6.
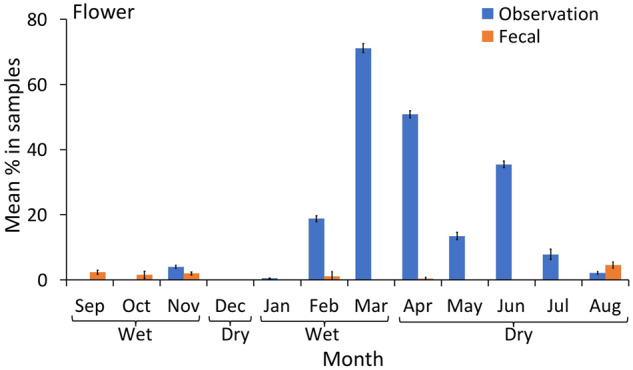
Mean percentage of flowers (with 95% CI) in the chimpanzee diet over 12 months of continuous sampling (2016–2017), using observational scans and macroscopic fecal sampling.
Figure 7.
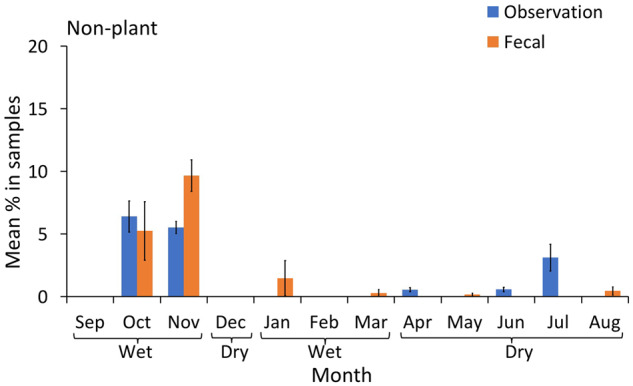
Mean percentage of insects and honey (with 95% CI) in the chimpanzee diet over 12 months of continuous sampling (2016–2017), using observational scans and macroscopic fecal sampling.
Discussion
Our comparison of the two techniques of dietary data collection over the time period in the same study population demonstrated that there was a highly significant difference in the estimation of dietary diversity and seasonal dietary composition of fruit, leaf, pith, flowers, and non-plant items using observational scans and macroscopic fecal inspection. However, differences were not consistent throughout the study, and during some seasons, and months, the two methods generated comparable results, while others differed markedly. Highly obvious disparities were evident in the low estimation of leaves and pith in the observational data, and low estimation of flowers in the fecal samples. The study also indicated that each method detected comparable numbers of fruit, but not non-fruit plant items such as leaves and pith in the monthly diet.
Studies comparing dietary analysis methods on a range of species have also highlighted disparities among techniques in determining dietary breadth (Klare et al. 2011; Bakaloudis et al. 2012) and composition (McInnis et al. 1983; Seefelt and Gillingham 2006; van Lieverloo et al. 2009; Wam and Hjeljord 2010). For example, pellet analysis of double-crested cormorant Phalacrocorax auritus diets undervalued the importance of the prey species alewife Alosa pseudoharengus compared with other methods (Seefelt and Gillingham 2006). Seefelt and Gillingham (2006) highlighted the significance of this oversight, since alewife is an important prey species for the cormorant diet, with an important link between cormorant reproductive success and alewife population dynamics (Weseloh and Ewins 1994; Wires et al. 2001). During November (wet season), observations of Nyungwe chimpanzees indicated that they fed almost exclusively on the fruit of Syzygium guineense (Matthews et al. 2019). However, seed handling behavior (wadging in this instance, i.e., extracting juice and nutrients from fruits while spitting the skin, fiber, and seeds out in a ball) influenced what remained of S. guineense in the fecal samples, underestimating the importance of this fruit species in the diet over the same period considerably.
The chimpanzees are an important seed disperser of S. guineense (Gross-Camp et al. 2009), and analyzing fecal samples alone would not reveal this relationship. Likewise, in January, February, and August, the dominant species consumed (Ficus sur and Podocarpus latifolius) were both wadged, and the fecal samples yielded lower proportions of fruits than the observations (Matthews et al. 2019). We explored the alternative explanation that fruit estimates in November, January, February, and August were influenced by a lack of correlation between seed size and fruit size of the dominant plant species consumed. While this does not appear to have had a substantial impact on our results in these months, detailed data on seed and fruit size are not available for all species consumed, and therefore this may have been a source of bias in other months. Future studies estimating fruit consumption from fecal samples should consider the relationship between seed and fruit size when making their estimations.
We observed very high rates of flower consumption toward the end of the wet season and into the dry season (March, April, and June), yet flowers were detected at meager rates or not at all in the fecal samples. At the end of the wet season (March) this difference was so significant that >70% of the observed diet was comprised of flowers, while 0% of flower remains were detected in fecal samples. If we had relied on fecal samples alone we would be unaware of the large disparity in the two methods, and would not have noticed this interesting dietary behavior given that flowers are not typically an important food item in chimpanzee diets (reviewed in Watts et al. 2012). This result supports previous suggestions that macroscopic fecal sampling results in the underrepresentation of foods that are easily broken down during digestion (Tutin and Fernandez 1993), compared with foods such as mature leaves that are high in cellulose and dietary fiber and are more difficult to digest.
On the other hand, during some months macroscopic fecal inspection detected honey and insects in higher quantities than observations. Underrepresentation of rarer items such as insects by observations compared with other methods of dietary estimation has also been noted in previous studies of primates (Moreno-Black 1978; Tutin and Fernandez 1993; Su and Lee 2001; Radespiel et al. 2006), and is evidently a methodological shortfall of studies relying solely on observations. It is also possible that the 15-min scan interval used in this study could have omitted shorter feeding bouts that included insect feeding. However, there were also months in which observations detected honey and insect consumption while fecal samples did not, suggesting that utilizing either method alone would result in biased detection rates.
The other obvious disparity between methods was the high proportion of leaves and pith estimated in the fecal samples during a majority of months compared with direct feeding observations. This overestimation is likely due to recognizable fibrous strands of pith and stems remaining in the samples while flowers, fruit pulp, and leaves were digested more easily, further indicating that the biochemical and physical properties of food items, as well as their digestibility, can influence estimates of dietary composition from macroscopic fecal sampling. Another source of bias highlighted by Bakaloudis et al. (2012) suggested that the bias in diet determined by observations of the long-legged buzzard Buteo rufinus compared with other methods may in part have stemmed from their selection of easily accessible nests for viewing and the resultant environmental homogeneity. Although we did not select specific areas for viewing the chimpanzees (as in the above study), certain areas and behaviors were more easily visible than others (e.g., tree-feeding versus ground-feeding); this may have similarly biased our observational results as non-fruit plants such as herbaceous vegetation were typically at ground-level. Additionally, estimates of leaves and pith in the fecal samples from February to June (end of wet season/beginning of the dry season) may be inflated due to underestimation of flowers or particular fruiting species during the same period.
Passage rates of foods consumed by the chimpanzees in the analysis of fecal samples may explain some of the discrepancies between sampling methods in this study. Mean gut retention time for chimpanzees in captivity is estimated to be 31–48 h (Milton and Demment 1988; Lambert 2002); estimating passage rates for chimpanzees in the wild is much more difficult due to varied diets and repeated consumption of the same foods over the course of a day, as well as consecutive fecal samples containing the same species (Phillips and McGrew 2013). In this study, the fecal samples collected on day 1 of each 5-day field trip would have contained foods that were consumed before observations conducted on day 1. This could have contributed to the divergence in diet estimations when averaging across each sampling period. However, Phillips and McGrew (2013) demonstrated that the results were similar when considering gut passage rates during comparisons of fecal sample analysis and direct observations. Future comparative studies may account for this potential bias by offsetting observations with fecal sampling to account for the lag between observations of food consumption and when food would likely appear in the fecal samples. In addition, the lack of correlation between methods used in this study demonstrates that the proportion of time that chimpanzees were observed feeding on particular species does not translate to the amount of food consumed, and thus the inclusion of information such as food intake from bite/unit consumption rates in future comparisons may produce a better correlation with fecal samples (Rothman et al. 2012).
When monthly dietary breadth was compared, the number of fruit species recorded in the diet using both methods was correlated, but the number of non-fruit (e.g., herbaceous) plant species was not. In contrast to our findings, there was no correlation between the mean number of species observed to be fed upon by chimpanzees and those detected in fecal samples in Budongo Forest, Uganda (Fawcett 2000). The reason for this difference is unclear, but it can be argued that in our study the correlation of fruiting species detected by both methods is likely due to chimpanzees being more observable while feeding in fruiting trees, and fruit seeds being easily identified in fecal samples (Tutin and Fernandez 1993). The effect of visibility on observations is likely also the reason that dietary diversity (H′) and evenness (J′) were both significantly higher in fecal samples compared with direct observation. This reveals that the choice of method can significantly influence the outcome of diet analyses, and this may lead to different conclusions regarding the ecology and habitat requirements of a species (Klare et al. 2011).
Data analyzed in this paper confirms that the two data collection types we used differed significantly in their estimation of dietary composition. In the present study on the Nyungwe chimpanzees, direct observations underestimated dietary diversity and the amount of leaf and pith plant items in the diet; while reliance on fecal sampling significantly underestimated the consumption of flowers. Both techniques have significant biases and flaws and without direct comparisons such as the one undertaken in this study, predicting the extent of the biases is challenging, particularly for species with a broad diet of items that vary widely in digestibility. Our results suggest that when addressing questions related to dietary breadth and diversity, a combination of both observational and fecal sampling methods will provide the most comprehensive description of the diet. While our results indicate that both methods used in this study could not provide a knowingly accurate account of diet composition, this is a common methodological issue with field studies that is not often addressed. Using multiple methods (e.g., macroscopic and microscopic analysis of fecal samples as well as observations, camera traps, and if possible, bite/unit consumption counts) to collect data concurrently can demonstrate where bias may be present, thus improving the interpretability of results as different methods can result in different conclusions about a species’ ecological and habitat needs. Other studies have similarly concluded that a combination of methods is the most effective approach (Parker and Bernard 2006; Bakaloudis et al. 2012). A study comparing methods of dietary analysis of giraffes Giraffa camelopardalis found congruency in their results from direct observations and fecal analysis in identifying seasonal changes in the top food species, yet the authors still recommended that a combination of methods should be used (Parker and Bernard 2006). These findings suggest that features of the animal’s foraging behavior and its environment (e.g., selective feeder, diurnal, and open habitat) influence the suitability and accuracy of different methods. However, we also highlight that when selecting appropriate methodological approaches, it is important to consider how the chosen methods relate to the research questions and goals, the feasibility of applying within the system of interest, and any ethical and conservation-related issues.
While our discussion relates mainly to macroscopic fecal sampling we also highlight the contribution that microscopic sampling techniques such as DNA barcoding or pollen analysis provide in studies assessing the dietary diversity, composition, and seasonal shifts of a range of species (Valentini et al. 2009; Scanlon and Petit 2013; Garnick et al. 2018; Komura et al. 2018; Robeson et al. 2018), particularly as these methods can deliver precision in species identification (Quéméré et al. 2013; Hamad et al. 2014). It is important to note that the DNA barcoding marker used to analyze plant diet can impact which species are identified (Bradley et al. 2007; Mallot et al. 2018), and when identifying vertebrates in the diet through DNA analysis there is the possibility that vertebrates in the local environment contaminated the feces (Hofreiter et al. 2010). Evidently, an integrative approach that utilizes macroscopic fecal analyses and/or behavioral observations to confirm DNA results would be the most effective (Ortmann et al. 2006; Bradley et al. 2007).
The differences in diet composition and diversity estimated by the two sampling methods used in this study highlight some of the key considerations to be made when using one single method to calculate wild animal diets. Future studies should empirically assess whether differences exist among methods used to determine diet in a range of species. Of particular interest are animals in highly seasonal environments where differences may change over an annual cycle, and rare or endangered species, since practical conservation efforts often rely on accurate knowledge of a species’ ecology.
Acknowledgments
We thank the government of Rwanda for allowing us to work in Nyungwe National Park and the Rwanda Development Board, specifically Innocent Ndikubwimana and Kambogo Ildephonse for facilitating the research. Field work was made possible through assistance and dedication from Fidele Muhayayezu and the chimpanzee trackers, and Samantha Green and Bradley Smith for their collaboration in the field. We also thank Göran Wallin for providing climate data. We are grateful to the anonymous reviewers who provided feedback on this manuscript, which has improved the clarity and quality of this work.
Funding
Funding was provided by the University of Western Australia, UWA Postgraduate Student Association and Basler Stiftung für Biologische Forschung (Switzerland).
References
- Altmann J, 1974. Observational study of behavior: sampling methods. Behaviour 49:227–266. [DOI] [PubMed] [Google Scholar]
- Altmann SA, 1991. Diets of yearling female primates Papio cynocephalus predict lifetime fitness. Proc Natl Acad Sci U S A 88:420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann SA, 1998. Foraging for Survival: Yearling Baboons in Africa. Chicago (IL: ): The University of Chicago Press. [Google Scholar]
- Amato KR, Van Belle S, Wilkinson B, 2013. A comparison of scan and focal sampling for the description of wild primate activity, diet and intragroup spatial relationships. Folia Primatol 84: 87–101. [DOI] [PubMed] [Google Scholar]
- Anthony RG, Smith NS, 1974. Comparison of rumen and fecal analysis to describe deer diets. J Wildl Manag 38:535–540. [Google Scholar]
- Aryal A, Lamsal RP, Ji W, Raubenheimer D, 2016. Are there sufficient prey and protected areas in Nepal to sustain an increasing tiger population?. Ethol Ecol Evol 28:1–4. [Google Scholar]
- Bakaloudis DE, Iezekiel S, Vlachos CG, Bontzorlos VA, Papakosta M. et al. , 2012. Assessing bias in diet methods for the long-legged buzzard Buteo rufinus. J Arid Environ 77:59–65. [Google Scholar]
- Basabose AK, 2002. Diet composition of chimpanzees inhabiting the montane forest of Kahuzi, Democratic Republic of Congo. Am J Primatol 58:1–21. [DOI] [PubMed] [Google Scholar]
- Birnie-Gauvin K, Peiman KS, Raubenheimer D, Cooke SJ, 2017. Nutritional physiology and ecology of wildlife in a changing world. Conserv Physiol 5:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BJ, Stiller M, Doran-Sheehy DM, Harris T, Chapman CA. et al. , 2007. Plant DNA sequences from feces: potential means for assessing diets of wild primates. Am J Primatol 69: 699−705. [DOI] [PubMed] [Google Scholar]
- Brett MT, Bunn SE, Chandra S, Galloway AWE, Guo F. et al. , 2017. How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshw Biol 62:833–853. [Google Scholar]
- Bridgeland WT, Beier P, Kolb T, Whitham TG, 2010. A conditional trophic cascade: birds benefit faster growing trees with strong links between predators and plants. Ecology 91:73–84. [DOI] [PubMed] [Google Scholar]
- Bryan A, 2014. Identifying bearded and ringed seal diet: a comparison of stomach contents, stable isotopes, fatty acids, and fecal DNA [masters thesis]. [Fairbanks (USA)]: University of Alaska Fairbanks.
- Carvalho JS, Vicente L, Marques TA, 2015. Chimpanzee Pan troglodytes verus diet composition and food availability in a human-modified landscape at Lagoas de Cufada Natural Park, Guinea-Bissau. Int J Primatol 36:802–822. [Google Scholar]
- Chapman CA, 1995. Primate seed dispersal: coevolution and conservation implications. Evol Anthropol Issues News Rev 4:74–82. [Google Scholar]
- Chapman CA, Balcomb SR, Gillespie TR, Skorupa JP, Struhsaker TT, 2000. Long-term effects of logging on African primate communities: a 28-year comparison from Kibale National Park, Uganda. Conserv Biol 14:207–217. [Google Scholar]
- Chapman CA, Rothman JM, Lambert JE, 2012. Food as a selective force in primates In: Mitani JC, Call J, Kappeler PM, Palmobit J, Silk JB, editors. The Evolution of Primate Societies. Chicago (IL: ): The University of Chicago Press, 149–168. [Google Scholar]
- Dellinger T, Trillmich F, 1988. Estimating diet composition from scat analysis in otariid seals (Otariidae): is it reliable? Can J Zool 66:1865–1870. [Google Scholar]
- Doran DM, McNeilage A, Greer D, Bocian C, Mehlman P. et al. , 2002. Western lowland gorilla diet and resource availability: new evidence, cross‐site comparisons, and reflections on indirect sampling methods. Am J Primatol 58: 910−116. [DOI] [PubMed] [Google Scholar]
- Fawcett K, 2000. Female relationships and food availability in a forest community of chimpanzees [PhD thesis]. [Edinburgh (UK)]: University of Edinburgh.
- Felton AM, Felton A, Foley WJ, Lindenmayer DB, 2010. The role of timber tree species in the nutritional ecology of spider monkeys in a certified logging concession, Bolivia. Forest Ecol Manag 259:1642–1649. [Google Scholar]
- Felton AM, Felton A, Lindenmayer DB, Foley WJ, 2009. Nutritional goals of wild primates. Funct Ecol 23:70–78. [Google Scholar]
- Fragaszy DM, Boinski S, Whipple J, 1992. Behavioral sampling in the field: comparison of individual and group sampling methods. Am J Primatol 26:259–275. [DOI] [PubMed] [Google Scholar]
- Garnick S, Barboza PS, Walker JW, 2018. Assessment of animal-based methods used for estimating and monitoring rangeland herbivore diet composition. Rangeland Ecol Manag 71: 449–457. [Google Scholar]
- Gilby IC, Pokempner AA, Wrangham RW, 2010. A direct comparison of scan and focal sampling methods for measuring wild chimpanzee feeding behaviour. Folia Primatol 81:254–264. [DOI] [PubMed] [Google Scholar]
- Green SJ, Boruff BJ, Grueter CC, 2019. From ridge tops to ravines: landscape drivers of chimpanzee ranging patterns. bioRxiv 10.1101/795393. [DOI]
- Gross-Camp ND, Masozera M, Kaplin BA, 2009. Chimpanzee seed dispersal quantity in a tropical montane forest of Rwanda. Am J Primatol 71:901–911. [DOI] [PubMed] [Google Scholar]
- Gruen L, Fultz A, Pruetz J, 2013. Ethical issues in African great ape field studies. ILAR J 54:24–32. [DOI] [PubMed] [Google Scholar]
- Hamad I, Delaporte E, Raoult D, Bittar F, 2014. Detection of termites and other insects consumed by African great apes using molecular fecal analysis. Sci Rep 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanya G, Noma N, Agetsuma N, 2003. Altitudinal and seasonal variations in the diet of Japanese macaques in Yakushima. Primates 44:51–59. [DOI] [PubMed] [Google Scholar]
- Hemingway CA, Bynum N, 2005. The influence of seasonality on primate diet and ranging In: Brockman DK, van Schaik CP, editors. Seasonality in Primates: Studies of Living and Extinct Human and Non-Human Primates. Cambridge (UK: ): Cambridge University Press, 57–104. [Google Scholar]
- Hill MO, 1973. Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432. [Google Scholar]
- Hladik CM, 1977. Chimpanzees of Gabon and chimpanzees of Gombe: some comparative data on diet In: Clutton−Brock TH, editor. Primate Ecology: Studies of Feeding and Ranging Behavior in Lemurs, Monkeys and Apes. London (UK: ): Academic Press, 481–501. [Google Scholar]
- Hofreiter M, Kreuz E, Eriksson J, Schubert G, Hohmann G, 2010. Vertebrate DNA in fecal samples from bonobos and gorillas: evidence for meat consumption or artefact? PLoS ONE 5:e9419.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann G, 2009. The diets of non-human primates: frugivory, food processing, and food sharing In: Hublin JJ, Richards MP, editors. The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence. Dordrecht (Netherlands: ): Springer, 1–14. [Google Scholar]
- Inger R, Ruxton GD, Newton J, Colhoun K, Robinson JA. et al. , 2006. Temporal and intrapopulation variation in prey choice of wintering geese determined by stable isotope analysis. J Anim Ecol 75:1190–1200. [DOI] [PubMed] [Google Scholar]
- Janiak MC, Chaney ME, Tosi AJ, 2017. Evolution of acidic mammalian chitinase genes (CHIA) is related to body mass and insectivory in primates. Mol Biol Evol 35:607–622. [DOI] [PubMed] [Google Scholar]
- Johnson C, 2015. The feeding and movement ecology of yellow baboons Papio cynocephalus in a primate rich habitat: The Issa Valley of Western Tanzania [PhD thesis]. [Wales (UK)]: Swansea University.
- Julliot C, Sabatier D, 1993. Diet of the red howler monkey Alouatta seniculus in French Guiana. Int J Primatol 14:527–550. [Google Scholar]
- Kaplin BA, William A, 2013. Behaviour within groups In: Sterling EJ, Bynum N, Blair ME, editors. Primate Ecology and Conservation: A Handbook of Techniques. Oxford (UK: ): Oxford University Press, 58–78. [Google Scholar]
- Klare U, Kamler JF, Macdonald DW, 2011. A comparison and critique of different scat-analysis methods for determining carnivore diet. Mammal Rev 41:294–312. [Google Scholar]
- Koike S, Nakashita R, Naganawa K, Koyama M, Tamura A, 2013. Changes in diet of a small, isolated bear population over time. J Mammal 94:361–368. [Google Scholar]
- Koirala RK, Raubenheimer D, Aryal A, Pathak ML, Ji W, 2016. Feeding preferences of the Asian elephant Elephas maximus in Nepal. BMC Ecol 16:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura T, Ando H, Horikoshi K, Suzuki H, Isagi Y, 2018. DNA barcoding reveals seasonal shifts in diet and consumption of deep-sea fishes in wedge-tailed shearwaters. PLoS ONE 13:e0195385.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos M, Hoetmer AJ, Pretorius Y, de Boer WF, de Knegt H. et al. , 2012. Seasonal diet changes in elephant and impala in mopane woodland. Eur J Wildl Res 58:279–287. [Google Scholar]
- Lambert JE, 2002. Digestive retention times in forest guenons (Cercopithecus spp.) with reference to chimpanzees Pan troglodytes. Int J Primatol 23:1169–1185. [Google Scholar]
- Mallot EK, Garber PA, Malhi RS, 2018. trnL outperforms rbcL as a DNA metabarcoding marker when compared with the observed plant component of the diet of wild white-faced capuchins (Cebus capucinus, Primates). PLoS ONE 13:e0199556.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalida A, Bertran J, Boudet J, 2005. Assessing the diet of nestling bearded vultures: a comparison between direct observation methods. J Field Ornithol 76:40–45. [Google Scholar]
- Marshall AJ, Wrangham RW, 2007. Evolutionary consequences of fallback foods. Int J Primatol 28:1219–1235. [Google Scholar]
- Martin P, Bateson PPG, 2007. Measuring Behaviour: An Introductory Guide. Cambridge (UK: ): Cambridge University Press. [Google Scholar]
- Matthews JK, Ridley A, Niyigaba P, Kaplin BA, Grueter CC, 2019. Chimpanzee feeding ecology and fallback food use in the montane forest of Nyungwe National Park, Rwanda. Am J Primatol 81:e22971.. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF, Phillips CA, 2009. Standardised protocol for primate faecal analysis. Primates 50:363–366. [DOI] [PubMed] [Google Scholar]
- McInnis ML, Vavra M, Krueger WC, 1983. A comparison of four methods used to determine the diets of large herbivores. J Range Manag 36:302–306. [Google Scholar]
- McLennan MR, 2013. Diet and feeding ecology of chimpanzees Pan troglodytes in Bulindi, Uganda: foraging strategies at the forest-farm interface. Int J Primatol 34:585–614. [Google Scholar]
- Mills MGL, 1992. A comparison of methods used to study food habits of large African carnivores In: McCullough DR, Barrett RH, editors. Wildlife 2001: Populations. London (UK: ): Elsevier, 1112–1124. [Google Scholar]
- Milton K, Demment MW, 1988. Digestion and passage kinetics of chimpanzees fed high and low fiber diets and comparison with human data. J Nutr 118:1082–1088. [DOI] [PubMed] [Google Scholar]
- Mitra A, Flynn K, 2007. Importance of interactions between food quality, quantity, and gut transit time on consumer feeding, growth, and trophic dynamics. Am Nat 169:632–646. [DOI] [PubMed] [Google Scholar]
- Moreno-Black G, 1978. The use of scat samples in primate diet analysis. Primates 19:215–221. [Google Scholar]
- Newton-Fisher NE, 1999. The diet of chimpanzees in the Budongo Forest Reserve, Uganda. Afr J Ecol 37:344–354. [Google Scholar]
- Nielsen JM, Clare EL, Hayden B, Brett MT, Kratina P, 2018. Diet tracing in ecology: method comparison and selection. Methods Ecol Evol 9:278–291. [Google Scholar]
- Nyirambangutse B, Zibera E, Uwizeye FK, Nsabimana D, Bizuru E. et al. , 2017. Carbon stocks and dynamics at different successional stages in an Afromontane tropical forest. Biogeosciences 14:1285–1303. [Google Scholar]
- Ortmann S, Bradley BJ, Stolter C, Ganzhorn JU, 2006. Estimating the quality and composition of wild animal diets: a critical survey of methods In: Hohmann G, Robbins MM, Boesch C, editors. Feeding Ecology in Apes and Other Primates: Ecological, Physical and Behavioral Aspects. Cambridge (UK: ): Cambridge University Press, 397–420. [Google Scholar]
- Pardo SA, Burgess KB, Teixeira D, Bennett MB, 2015. Local-scale resource partitioning by stingrays on an intertidal flat. Mar Ecol Prog Ser 533:205–218. [Google Scholar]
- Parker DM, Bernard RTF, 2006. A comparison of two diet analysis techniques for a browsing megaherbivore. J Wildl Manag 70:1477–1480. [Google Scholar]
- Phillips CA, 2011. Chimpanzee diet: analyses at macroscopic, microscopic and molecular [PhD thesis]. [Cambridge (UK)]: University of Cambridge.
- Phillips CA, Lancelotti C, 2014. Chimpanzee diet: phytolithic analysis of feces. Am J Primatol 76:757–773. [DOI] [PubMed] [Google Scholar]
- Phillips CA, McGrew WC, 2013. Identifying species in chimpanzee Pan troglodytes feces: a methodological lost cause? Int J Primatol 34:792–807. [Google Scholar]
- Phillips CA, McGrew WC, 2014. Macroscopic inspection of ape feces: what’s in a quantification method? Am J Primatol 76:539–550. [DOI] [PubMed] [Google Scholar]
- Phillips CA, O’Connell TC, 2016. Fecal carbon and nitrogen isotopic analysis as an indicator of diet in Kanyawara chimpanzees, Kibale National Park, Uganda. Am J Phys Anthropol 161:685.. [DOI] [PubMed] [Google Scholar]
- Pielou EC, 1974. Population and Community Ecology: Principles and Methods. New York (NY: ): Gordon and Breach. [Google Scholar]
- Potts KB, 2011. The long-term impact of timber harvesting on the resource base of chimpanzees in Kibale National Park, Uganda. Biotropica 43:256–264. [Google Scholar]
- Potts KB, Watts DP, Wrangham RW, 2011. Comparative feeding ecology of two communities of chimpanzees Pan troglodytes in Kibale National Park, Uganda. Int J Primatol 32:669–690. [Google Scholar]
- Putman RJ, 1984. Facts from faeces. Mammal Rev 14:79–97. [Google Scholar]
- Quéméré E, Hibert F, Miquel C, Lhuillier E, Rasolondraibe E. et al. , 2013. A DNA metabarcoding study of a primate dietary diversity and plasticity across its entire fragmented range. PLoS ONE 8:e58971.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radespiel U, Reimann W, Rahelinirina M, Zimmermann E, 2006. Feeding ecology of sympatric mouse lemur species in northwestern Madagascar. Int J Primatol 27:311–321. [Google Scholar]
- Righini N, Garber PA, Rothman JM, 2017. The effects of plant nutritional chemistry on food selection of Mexican black howler monkeys Alouatta pigra: the role of lipids. Am J Primatol 79:1–15. [DOI] [PubMed] [Google Scholar]
- Robeson MS, Khanipov K, Golovko G, Wisely SM, White MD. et al. , 2018. Assessing the utility of metabarcoding for diet analyses of the omnivorous wild pig Sus scrofa. Ecol Evol 8:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Maisels F, Williamson EA, Fernandez M, Tutin CEG, 1990. Gorilla diet in the Lopé Reserve, Gabon: a nutritional analysis. Oecologia 84:326–339. [DOI] [PubMed] [Google Scholar]
- Rothman JM, Chapman CA, Soest PJ, 2012. Methods in primate nutritional ecology: a user’s guide. Int J Primatol 33:542–566. [Google Scholar]
- Scanlon AT, Petit S, 2013. How fecal subsampling methods affect the accuracy of dietary pollen detection. J Mammal 94:1321–1330. [Google Scholar]
- Schneider I, Tielen IHM, Rode J, Levelink P, Schrudde D, 2010. Behavioral observations and notes on the vertical ranging pattern of the critically endangered Cat Ba langur Trachypithecus poliocephalus poliocephalus in Vietnam. Primate Conserv 25:111–117. [Google Scholar]
- Seefelt N, Gillingham J, 2006. A comparison of three methods to investigate the diet of breeding double-crested cormorants Phalacrocorax auritus in the Beaver Archipelago, northern Lake Michigan. Hydrobiologia 567:57–67. [Google Scholar]
- Shannon CE, Weaver W, 1964. The Mathematical Theory of Communication. Urbana (IL: ): The University of Illinois Press. [Google Scholar]
- Shrestha R, Wegge P, 2006. Determining the composition of herbivore diets in the Trans-Himalayan rangelands: a comparison of field methods. Rangeland Ecol Manag 59:512–518. [Google Scholar]
- Shutt K, Heistermann M, Kasim A, Todd A, Kalousova B. et al. , 2014. Effects of habituation, research and ecotourism on faecal glucocorticoid metabolites in wild western lowland gorillas: implications for conservation management. Biol Conserv 172:72–79. [Google Scholar]
- Singer MS, Lichter-Marck IH, Farkas TE, Aaron E, Whitney KD. et al. , 2014. Herbivore diet breadth mediates the cascading effects of carnivores in food webs. Proc Natl Acad Sci U S A 111:9521–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Lee L, 2001. Food habits of formosan rock macaques Macaca cyclopis in Jentse, northeastern Taiwan, assessed by fecal analysis and behavioral observation. Int J Primatol 22:359–377. [Google Scholar]
- Surbeck M, Fowler A, Deimel C, Hohmann G, 2009. Evidence for the consumption of arboreal, diurnal primates by bonobos Pan paniscus. Am J Primat 71:171–174. [DOI] [PubMed] [Google Scholar]
- Tutin CEG, Fernandez M, 1993. Faecal analysis as a method of describing diets of apes: examples from sympatric gorillas and chimpanzees at Lope, Gabon. Tropics 2:189–197. [Google Scholar]
- Tuyttens FAM, de Graaf S, Heerkens JLT, Jacobs L, Nalon E. et al. , 2014. Observer bias in animal behaviour research: can we believe what we score, if we score what we believe? Anim Behav 90:273–280. [Google Scholar]
- Tweheyo M, Lye KA, Weladji RB, 2003. Chimpanzee diet and habitat selection in the Budongo Forest Reserve, Uganda. Forest Ecol Manag 188:267–278. [Google Scholar]
- Valentini A, Miquel C, Nawaz M, Bellemain E, Coissac E. et al. , 2009. New perspectives in diet analysis based on DNA barcoding and parallel pyrosequencing: the trnL approach. Mol Ecol Resourc 9:51–60. [DOI] [PubMed] [Google Scholar]
- van Lieverloo RJ, Schuiling BF, de Boer WF, Lent PC, de Jong CB. et al. , 2009. A comparison of faecal analysis with backtracking to determine the diet composition and species preference of the black rhinoceros Diceros bicornis minor. Eur J Wildl Res 55:1–11. [Google Scholar]
- van Schaik CP, Terborgh JW, Wright SJ, 1993. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annu Rev Ecol Evol Syst 24:353–377. [Google Scholar]
- VelezLiendo X, Strubbe D, Matthysen E, 2013. Effects of variable selection on modelling habitat and potential distribution of the Andean bear in Bolivia. Ursus 24:127–138. [Google Scholar]
- Wam HK, Hjeljord O, 2010. Moose summer diet from feces and field surveys: a comparative study. Rangeland Ecol Manag 63:387–395. [Google Scholar]
- Ward EJ, Levin PS, Lance MM, Jeffries SJ, Acevedo-Gutiérrez A, 2012. Integrating diet and movement data to identify hot spots of predation risk and areas of conservation concern for endangered species. Conserv Lett 5:37–47. [Google Scholar]
- Watts DP, Potts KB, Lwanga JS, Mitani JC, 2012. Diet of chimpanzees Pan troglodytes schweinfurthii at Ngogo, Kibale National Park, Uganda, 1. Diet composition and diversity. Am J Primatol 74:114–129. [DOI] [PubMed] [Google Scholar]
- Weiser EL, Powell AN, 2011. Evaluating gull diets: a comparison of conventional methods and stable isotope analysis. J Field Ornithol 82:297–310. [Google Scholar]
- Weseloh DV, Ewins PJ, 1994. Characteristics of a rapidly increasing colony of double-crested cormorants Phalacrocorax auritus in Lake Ontario: population size, reproductive parameters and band recoveries. J Great Lakes Res 20:443–456. [Google Scholar]
- Wires LR, Cuthbert FJ, Trexel DR, Joshi AR, 2001. Status of the double-crested cormorant (Phalacrocorax auritus) in North America. Final Report to USFWS.
- Wrangham RW, Chapman CA, Clark AP, Isabirye-Basuta G, 1996. Social ecology of Kanyawara chimpanzees: implications for understanding the costs of great ape groups In: McGrew WC, Marchant LF, Nishida T, editors. Great Ape Societies. Cambridge (UK: ): Cambridge University Press, 45–57. [Google Scholar]
- Wrangham RW, Conklin-Brittain NL, Hunt KD, 1998. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants. Int J Primatol 19:949–970. [Google Scholar]
- Zuberbühler K, Wittig M, 2011. Field experiments with non-human primates: a tutorial In: Setchell JM, Curtis DJ, editors. Field and Laboratory Methods in Primatology: A Practical Guide. Cambridge (UK: ): Cambridge University Press. [Google Scholar]