Abstract
Previous research shows that yawning enhances intracranial circulation and regulates brain temperature. Consistent with these functional outcomes, yawn duration correlates positively with interspecies variation in brain weight across mammals, with robust relationships documented at both the taxonomic rank of class and the more restricted scale of family (e.g., Felidae). This study provides the first investigation into whether differences in brain weight within a single species, domesticated dogs Canis lupus familiaris, can predict intraspecific variation in yawn duration. Measures of yawn duration were obtained from public videos available online and then paired with previously published brain and body weight data of different dog breeds. The final sample consisted of 272 yawns from 198 dogs across 23 breeds. Consistent with recent studies, we find robust correlations between yawn duration and brain weight across breeds. Moreover, these correlations remain significant after controlling for differences in body weight across breeds. These findings replicate and extend upon past work in this area and provide further support that yawns evolved to serve an important and large-scale neurophysiologic function.
Keywords: brain cooling, brain size, cortical arousal, neuroanatomy
Yawning is characterized by a powerful gaping of the jaw with inspiration, followed by a brief period of peak muscle contraction and a passive closure of the jaw with shorter expiration (Barbizet 1958). While yawning appears to be a stereotyped action pattern across vertebrates (Provine 1986), recent research has identified distinct variations in the expression of this behavior. For example, some primate species display different yawn types that vary in intensity and morphology, which may correspond with transitions in daily repertoires, activity patterns, and internal states (Palagi et al. 2009; Vick and Paukner 2010; Leone et al. 2014). This work supports research suggesting yawns might be multifunctional across different contexts (Baenninger 1997; Provine 2005; Gallup 2011).
Studying differences in the expression of yawning across species could also help elucidate the neurophysiological significance of this highly conserved response. Based on hypotheses stating that the motor action pattern of yawns serves to enhance intracranial circulation (Walusinski 2014) and regulate brain temperature (Gallup and Gallup 2007, 2008; Ramirez et al. 2019), the duration or magnitude of this response should correspond to the degree of neurophysiological change. In particular, the brain cooling hypothesis states that the gaping of the jaw and deep inhalation of air during yawning functions to promote thermal homeostasis by altering the rate and temperature of arterial blood traveling to the skull (Gallup and Hack 2011). Support for this hypothesis comes from measuring changes in brain temperature before and after yawns (Shoup-Knox et al. 2010; Shoup-Knox 2011) and naturalistic and experimental studies assessing the relationship between yawning and ambient temperature (e.g., Gallup et al. 2011; Massen et al. 2014; Eldakar et al. 2015). Although the neural structures necessary for yawning appear to be located in the brain stem (Heusner 1946), recent comparative research shows that yawns produce large-scale neurophysiologic effects as evidenced by sizable cooling at the surface of the skull (Eguibar et al. 2017; Gallup et al. 2017). Therefore, according to this hypothesis, animals with larger and more complex brains should display longer and more powerful yawns to achieve these outcomes.
In the first study to test this prediction, Gallup et al. (2016) collected yawn duration data across a diverse sample of mammals and linked these with published data on both brain weight and cortical neuron number. The results of this work revealed average yawn duration to be highly variable across 24 mammalian taxa (range: 0.8–6.5 s). Moreover, consistent with neurophysiologic hypotheses of yawning, interspecific differences in yawn duration were robustly correlated with both brain weight (correlation coefficient = 0.911) and cortical neuron number (correlation coefficient = 0.951) across species. Variation in yawn duration was not tied to particular features of body/jaw size, but was correlated positively with encephalization quotient across species.
As a direct follow-up to this work, Gallup et al. (2017) investigated whether the relationship between yawn duration and brain size could be identified within a single family of mammal species. Examining this link at a restricted taxonomic scale allowed for the determination of whether variability in yawn duration was related specifically to brain size or to other features pertaining to phylogenetic history and morphological diversity between species. In a sample of 58 animals across 7 wild cat species in the family Felidae, a similarly robust association (correlation coefficient = 0.937) was observed between yawn duration and brain weight (Gallup et al. 2017). This study also showed that body size was not a significant predictor of yawn duration. These combined findings support the view that the motor action pattern of yawning serves an important function tied to brain size.
Here, we extend upon this line of research by testing whether intraspecific variation in brain weight predicts differences in yawn duration within a single species. Domesticated dogs Canis lupus familiaris were chosen due to large differences in brain size across breeds and the availability of open access yawning data on the Internet. A report by Bronson (1979) that documented the average adult brain and body weights from over 2 dozen dog breeds was used as a basis for the inclusion of breeds within this study. This report also included a scaling function for estimating brain weight based on the average body weight for a given breed, which was recently validated and applied to a much larger sample of domesticated dogs (Horschler et al. 2019). The same methods used in Gallup et al. (2016, 2017) were applied to acquire representative yawn durations from each breed using public videos available online. We hypothesized that dog breeds with larger brains would display longer yawns, and that this relationship would remain significant after controlling for body size.
Materials and Methods
Using the list of 26 dog breeds compared in Bronson (1979), a researcher was instructed to find up to 12 adult dogs yawning from each breed by searching videos posted on YouTube and related websites. For each video identified, the researcher noted the time(s) at which the yawn(s) on the clip occurred and the duration of all yawning events using the operational definition provided by Barbizet (1958). Durations were recorded to the nearest 0.01 s using the stopwatch feature of a smartphone. Data collection occurred during Fall 2016, and yielded a total of 299 yawns from 213 dogs across the 26 breeds.
In Fall 2017, an independent rater was provided with the complete list of uniform resource locators (URLs) identified from the original search and instructed to score the duration of each yawn across the sample. In doing so, 4 videos were no longer available and 8 yawns were excluded because they were noted as either being too difficult to distinguish or there was no clear way to determine their beginning or end. In addition, links for 3 dogs were excluded because they were identified by both raters as juveniles. The inter-rater reliability for yawn duration was high for the remaining sample: intraclass correlation = 0.87.
The average yawn duration for each breed was then calculated by summing all the respective durations from members of that group and then dividing by the number of yawners. To avoid pseudo-replication in cases where the same individual displayed multiple yawns in a given video, the average duration for this animal was used as a single data point when generating the overall average for that breed. The maximum yawn duration was also recorded from each breed. Following the same criterion set within Gallup et al. (2016, 2017), analyses were restricted to breeds with yawns from at least 3 individuals.
The final sample included a total of 272 yawns from 198 dogs across 23 breeds. Individual yawning data from the following breeds were included in the sample: Beagle (11), Boston Terrier (12), Boxer (12), Bulldog (11), Chihuahua (9), Cocker Spaniel (3), Collie (5), Dachshund (12), Doberman Pinscher (9), German Shepherd (11), Golden Retriever (12), Great Dane (12), Labrador Retriever (12), Miniature Poodle (8), Miniature Schnauzer (6), Old English Sheepdog (3), Pekingese (6), Pug (11), Standard Poodle (4), Standard Schnauzer (4), Toy Fox Terrier (3), Toy Poodle (12), and Weimaraner (10). The Supplemental Material includes URLs to all videos used in the study. For the average brain and body weights across breeds, Bronson (1979) provided data based on an average sample of 31.69 dogs per breed (range: N = 5–110). This report also included a scaling function for estimating brain weight based on the average body weight for a given breed. Horschler et al. (2019) recently validated this scaling function and applied it to a much larger sample of domesticated dogs (N = 7,397 across 74 breeds). Therefore, where applicable in this study (19/23 breeds), these recent and more representative body weight and estimated brain weight data were used (see Table 1).
Table 1.
Descriptive statistics for yawn duration and brain and body weights
| Breed (alphabetical order) | Dog N | Mean ± SD yawn duration (s) | Maximum yawn duration (s) | Mean brain weight (g) | Mean body weight (kg) |
|---|---|---|---|---|---|
| Beaglea | 11 | 2.35 ± 0.49 | 3.28 | 79.66 | 12.79 |
| Boston Terriera | 12 | 1.67 ± 0.28 | 2.09 | 73.22 | 9.41 |
| Boxera | 12 | 2.20 ± 0.39 | 3.00 | 98.95 | 28.16 |
| Bulldoga | 11 | 1.84 ± 0.32 | 2.45 | 94.78 | 24.08 |
| Chihuahuaa | 9 | 1.58 ± 0.44 | 2.17 | 55.10 | 3.35 |
| Cocker Spaniela | 3 | 1.88 ± 0.34 | 2.25 | 77.76 | 11.72 |
| Colliea | 5 | 2.03 ± 0.43 | 2.56 | 98.91 | 28.12 |
| Dachshunda | 12 | 1.78 ± 0.30 | 2.24 | 68.03 | 7.20 |
| Doberman Pinschera | 9 | 2.14 ± 0.74 | 3.27 | 104.01 | 33.77 |
| German Shepherda | 11 | 2.59 ± 0.73 | 3.97 | 104.63 | 34.50 |
| Golden Retrievera | 12 | 2.25 ± 0.65 | 3.72 | 101.63 | 31.04 |
| Great Danea | 12 | 2.31 ± 0.43 | 2.94 | 120.54 | 57.75 |
| Labrador Retrievera | 12 | 2.21 ± 0.46 | 3.00 | 102.12 | 31.59 |
| Miniature Poodlea | 8 | 1.70 ± 0.46 | 2.72 | 67.54 | 7.02 |
| Miniature Schnauzera | 6 | 1.88 ± 0.33 | 2.48 | 69.76 | 7.89 |
| Old English Sheepdogb | 3 | 2.47 ± 0.86 | 3.40 | 104.40 | 38.60 |
| Pekingeseb | 6 | 1.43 ± 0.39 | 2.11 | 53.40 | 4.90 |
| Puga | 11 | 1.72 ± 0.30 | 2.20 | 71.85 | 8.79 |
| Standard Poodlea | 4 | 2.29 ± 1.09 | 3.90 | 92.53 | 22.06 |
| Standard Schnauzera | 4 | 2.20 ± 0.45 | 2.66 | 84.09 | 15.58 |
| Toy Fox Terrierb | 3 | 1.52 ± 0.72 | 2.34 | 52.30 | 3.40 |
| Toy Poodleb | 12 | 1.76 ± 0.31 | 2.40 | 59.10 | 3.20 |
| Weimaranera | 10 | 2.83 ± 0.64 | 3.64 | 101.33 | 30.71 |
Based on output from Kolmogorov–Smirnov normality tests, Pearson correlations were used to assess the relationships between measures of yawn duration and brain and body weights across the 23 breeds. Separate partial correlations were then run controlling for body and brain weight, respectively. Despite our a priori hypotheses, all analyses included more conservative 2-tailed tests with the alpha set at 0.05. Moreover, Benjamini and Hochberg corrections were applied for multiple comparisons, and thus adjusted P-values are provided.
Results
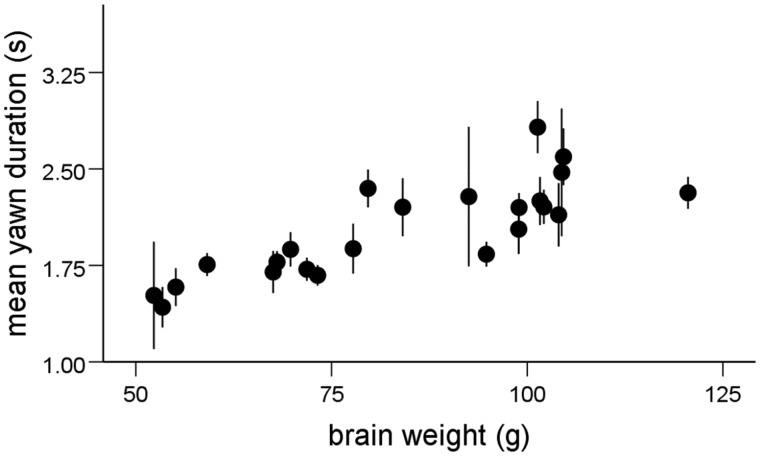
The average yawn duration for all dogs in the sample was 2.04 ± 0.59 s, with the breed average ranging from 1.43–2.83 s (Table 1). Consistent with previous research, there were strong positive correlations between measures of yawn duration and brain weight across breeds (average duration: r23 = 0.819, P < 0.001; maximum duration: r23 = 0.703, P < 0.001; Figure 1). Breed brain and body weights were highly correlated across the sample (r23 = 0.957, P < 0.001), and thus yawn duration was also positively correlated with body weight (average duration: r23 = 0.749, P < 0.001; maximum duration: r23 = 0.649, P = 0.001). Partial correlations showed that average yawn duration remained significantly correlated with brain weight after controlling for body weight (average duration: r20 = 0.535, P = 0.016; maximum duration: r20 = 0.374, P = 0.111). However, yawn duration was not correlated with body weight when controlling for brain weight (average duration: r20 = −0.222, P = 0.361; maximum duration: r20 = −0.125, P = 0.579).
Figure 1.
Scatterplot depicting the linear relationship between average yawn duration (Mean ± SEM) and average brain weight across dog breeds. Breeds in order of mean yawn duration: Pekingese, Toy Fox Terrier, Chihuahua, Boston Terrier, Miniature Poodle, Pug, Toy Poodle, Dachshund, Bulldog, Cocker Spaniel, Miniature Schnauzer, Collie, Doberman Pinscher, Boxer, Standard Schnauzer, Labrador Retriever, Golden Retriever, Standard Poodle, Great Dane, Beagle, Old English Sheepdog, German Shepherd, and Weimaraner.
Discussion
Recent research has shown that mammalian brain weight is a robust predictor of yawn duration across different taxonomic scales (Gallup et al. 2016, 2017), supporting past research suggesting that this ubiquitous motor action pattern serves an important neurological function. This study reveals that yawn duration is also tied to intraspecific variation in brain size among domesticated dogs. In particular, we show a wide range in the duration of this action pattern across breeds, with larger brained dogs tending to have longer yawns. Notably, over two-thirds of the variability in the average yawn duration across breeds can be explained by brain weight differences between these populations. Partial correlations show that this association is not due to differences in body size across breeds, but rather differences in yawning appear to be specifically associated with variation in the brain size of these animals. It remains unknown exactly which neurological features are driving this association, but past research suggests that cortical neuron number is the strongest predictor of interspecies differences in yawn duration (Gallup et al. 2016). Although research on neuron numbers in dogs is currently limited, in a comparison of 2 animals Jardim-Messeder et al. (2017) provide some evidence that larger breeds have more neurons (i.e., Golden Retriever: 627 million; unspecified smaller breed: 429 million).
While we show here that intraspecific variation in brain size appears to be a robust predictor of yawn duration in domesticated dogs, it is unknown how these measures would correlate within a given breed or in a different wild type species. Due to artificial selection, the variation in brain and body weights across dog breeds exceeds that of any other species, making this study a distinctive and atypical within-species comparison. Based on the findings of recent work uncovering breed differences in neuroanatomical organization (Hecht et al. 2019), one could argue that assessing neurological differences across dog breeds becomes similar to interspecies comparisons. Thus, until further research is conducted, we are hesitant to conclude that yawn duration would be predictive of intraspecific variation in brain size in other animals. One intriguing species to investigate would be humans, since there is sizable variation in the overall brain volume among healthy adults (1.25–1.88 dm3; Lüders et al. 2002). Research on humans or another species could also provide a more precise analysis of this relationship, by linking brain volumes acquired through magnetic resonance imaging to electromyography from the masseter and submental muscles to measure yawn duration and intensity (see Ertekin et al. 2015).
It is important to acknowledge limitations to this study with regard to the methods of data collection. Although visual media can provide a powerful tool for investigating and developing insights into animal behavior (Nelson and Fijn 2013), and research utilizing YouTube for this purpose has increased within the past decade (see Measey et al. 2019), one potential issue with human posted videos of this nature is nonrandom sampling (Burn 2014). While we do not view this as a major concern given the strength of the correlations both for average and maximum yawn duration, naturalistic observations could be conducted to confirm the true variability in spontaneous yawn duration for a given breed. The inability to determine the contextual triggers of yawning from online videos also presents a limitation. While there is consistency in that most videos occurred within the context of close dog–human interaction, other potentially important factors known to alter the morphology of yawning in other species (i.e., Leone et al. 2014; Palagi et al. 2009; Vick and Paukner 2010) could not be evaluated. To date, however, no study has reported differential yawn types for dogs.
Overall, this study replicates and extends upon previous results showing a robust link between yawn duration and brain weight, providing further support for predictions derived from the brain cooling hypothesis. However, it is important to acknowledge that the current findings do not necessarily exclude other neurophysiological hypotheses of yawning. We propose that, in addition to examining the connection between yawn duration and brain size in other classes of vertebrates (e.g., avian species), future research could investigate how differences in the duration of yawning events correlate with immediate changes in neurophysiological measures and cognitive processing. Work of this nature could help elucidate the evolutionary significance of this ubiquitous response.
Supplementary Material
Acknowledgments
We are thankful to Jeff Clemishaw for helping to score video data and the College of Arts and Sciences at SUNY Polytechnic Institute for providing support for this research. We are also grateful to Anne Clark for carefully reviewing some of the data presented in Table 1 prior to publication.
Authors’ contributions
A.C.G. conceived of the study and analyzed the data; L.M. collected the data; and A.C.G., L.M., and J.J.M.M. wrote the article.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
References
- Baenninger R, 1997. On yawning and its functions. Psychon Bull Rev 4:198–207. [DOI] [PubMed] [Google Scholar]
- Barbizet J, 1958. Yawning. J Neurol Neurosurg Psychiatry 21:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson RT, 1979. Brain weight-body weight scaling in breeds of dogs and cats. Brain Behav Evol 16:227–236. [DOI] [PubMed] [Google Scholar]
- Burn CC, 2014. Social media offers new insights into human and animal behavior: how to harness them scientifically In: Spink AJ, van den Broek EL, Loijens L, Woloszynowska-Fraser M, Noldus L, editors. Proceedings of Measuring Behavior 2014. Wageningen: Noldus Information Technology; 254–257. [Google Scholar]
- Eguibar JR, Uribe CA, Cortes C, Bautista A, Gallup AC, 2017. Yawning reduces facial temperature in the high-yawning subline of Sprague-Dawley rats. BMC Neurosci 18:3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldakar OT, Dauzonne M, Prilutzkaya Y, Garcia D, Thadal C. et al. , 2015. Temperature-dependent variation in self-reported contagious yawning. Adapt Hum Behav Physiol 1:460–466. [Google Scholar]
- Ertekin C, Bulbul NG, Uludag IF, Tiftikcioglu BI, Arici S. et al. , 2015. Electrophysiological association of spontaneous yawning and swallowing. Exp Brain Res 233:2073–2080. [DOI] [PubMed] [Google Scholar]
- Gallup AC, 2011. Why do we yawn? Primitive versus derived features. Neurosci Biobehav Rev 35:765–769. [DOI] [PubMed] [Google Scholar]
- Gallup AC, Gallup GG, 2007. Yawning as a brain cooling mechanism: nasal breathing and forehead cooling diminish the incidence of contagious yawning. Evol Psychol 5:92–101. [Google Scholar]
- Gallup AC, Gallup GG, 2008. Yawning and thermoregulation. Physiol Behav 95:10–16. [DOI] [PubMed] [Google Scholar]
- Gallup AC, Hack GD, 2011. Human paranasal sinuses and selective brain cooling: a ventilation system activated by yawning? Med Hypoth 77:970–973. [DOI] [PubMed] [Google Scholar]
- Gallup AC, Church AM, Pelegrino AJ, 2016. Yawn duration predicts brain weight and cortical neuron number in mammals. Biol Lett 12:20160545.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup AC, Crowe B, Yanchus M, 2017. Yawn duration predicts brain size in wild cats (Felidae). Int J Comp Psychol 30: 1–5. [Google Scholar]
- Gallup AC, Herron E, Militello J, Swartwood L, Cortes C. et al. , 2017. Thermal imaging reveals sizable shifts in facial temperature surrounding yawning in budgerigars Melopsittacus undulatus. Temperature 4:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup AC, Miller RR, Clark AB, 2011. Changes in ambient temperature trigger yawning but not stretching in rats. Ethology 117:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht EE, Smaers JB, Dunn WJ, Kent M, Preuss TM. et al. , 2019. Significant neuroanatomical variation among domestic dog breeds. J Neurosci 39:7748–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusner AP, 1946. Yawning and associated phenomena. Physiol Rev 26:156–168. [DOI] [PubMed] [Google Scholar]
- Horschler DJ, Hare B, Call J, Kaminski J, Miklósi Á. et al. , 2019. Absolute brain size predicts dog breed differences in executive function. Anim Cogn 22:187–198. [DOI] [PubMed] [Google Scholar]
- Jardim-Messeder D, Lambert K, Noctor S, Pestana FM, de Castro Leal ME. et al. , 2017. Dogs have the most neurons, though not the largest brain: trade-off between body mass and number of neurons in the cerebral cortex of large carnivoran species. Front Neuroanat 11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone A, Ferrari PF, Palagi E, 2014. Different yawns, different functions? Testing social hypotheses on spontaneous yawning in Theropithecus gelada. Sci Rep 4: 4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders E, Steinmetz H, Jäncke L, 2002. Brain size and grey matter volume in the healthy human brain. Neuroreport 13:2371–2374. [DOI] [PubMed] [Google Scholar]
- Massen JJ, Dusch K, Eldakar OT, Gallup AC, 2014. A thermal window for yawning in humans: yawning as a brain cooling mechanism. Physiol Behav 130:145–148. [DOI] [PubMed] [Google Scholar]
- Measey J, Basson A, Rebelo A, Nunes A, Vimercati G. et al. , 2019. Why have a pet amphibian? Insights from YouTube. Front Ecol Evol 52: 1–8. [Google Scholar]
- Nelson XJ, Fijn N, 2013. The use of visual media as a tool for investigating animal behaviour. Anim Behav 85:525–536. [Google Scholar]
- Palagi E, Leone A, Mancini G, Ferrari PF, 2009. Contagious yawning in gelada baboons as a possible expression of empathy. Proc Natl Acad Sci USA 106:19262–19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provine RR, 1986. Yawning as a stereotyped action pattern and releasing stimulus. Ethology 72:109–122. [Google Scholar]
- Provine RR, 2005. Yawning: the yawn is primal, unstoppable and contagious, revealing the evolutionary and neural basis of empathy and unconscious behavior. Am Sci 93:532–539. [Google Scholar]
- Ramirez V, Ryan CP, Eldakar OT, Gallup AC, 2019. Manipulating neck temperature alters contagious yawning in humans. Physiol Behav 207:86–89. [DOI] [PubMed] [Google Scholar]
- Shoup-Knox ML, 2011. Physiology of Yawning: Proximate Mechanisms Supporting an Ultimate Function. PhD dissertation. State University of New York at; Albany. [Google Scholar]
- Shoup-Knox ML, Gallup AC, Gallup GG, McNay EC, 2010. Yawning and stretching predict brain temperature changes in rats: support for the thermoregulatory hypothesis. Front Evol Neurosci 2:108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick SJ, Paukner A, 2010. Variation and context of yawns in captive chimpanzees Pan troglodytes. Am J Primatol 72:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walusinski O, 2014. How yawning switches the default‐mode network to the attentional network by activating the cerebrospinal fluid flow. Clin Anat 27:201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.