Abstract
Background
Studies have demonstrated that the roots of Averrhoa carambola L. (Oxalidaceae), a traditional Chinese medicine, can be used to treat diabetes and diabetes-related diseases. Nevertheless, the potential beneficial effects and mechanism of benzoquinone isolated from the roots of Averrhoa carambola L. (BACR) on diabetes remain unclear.
Methods
Diabetic Kunming mice were injected with STZ (120 mgkg−1) in the tail vein. Fasting blood glucose (FBG) and the change of body weight were measured after oral administration of BACR (120, 60, 30 mg/kg/d) every week. The levels of the total cholesterol (TC), triglyceride (TG), free fatty acids (FFA), glucosylated hemoglobin (GHb), fasting insulin (FINS), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were measured. The histological examination of pancreatic tissues and the TLR4/NF-κB pathway was analyzed by RT-PCR, immunohistochemistry and Western blot.
Results
The study found that clearly the BACR obviously reduced the blood glucose, serum lipids, GHb and FINS. In addition, BACR treatment markedly reduced the release of inflammatory factors, including IL-6 and TNF-α, and down-regulated the expression of the TLR4/NF-κB pathway.
Conclusion
BACR has potential benefits for the treatment of diabetes by ameliorating metabolic functions and attenuating the inflammatory response via inhibition of the activation of theTLR4/NF-κB pathway.
Keywords: benzoquinone of Averrhoa carambola L. root, diabetes, inflammation, TLR4/NF-κB pathway
Introduction
Diabetes mellitus is a multifactorial, chronic metabolic disorder, characterized by hyperglycemia due to deficient insulin secretion and/or action. At the same time, it causes abnormal lipid metabolism and protein metabolism, which lead to the disorder of the internal environment of the organism.1,2 The clinical treatment of diabetes mellitus is difficult, and there is still a lack of specific and targeted treatment drugs. Therefore, the research and development of effective drugs for diabetes mellitus is the direction of joint efforts of medical workers at present.
Toll-like receptors (TLRs) play a major role in the development of the innate immunity, and can activate innate immunity by regulating immune and inflammatory responses. Among all TLRs, TLR4 especially can activate a myeloid differentiation factor 88 (MyD88)-dependent pathway, resulting in an increased release of NF-κB from IκBα, subsequently intensifying the generation of inflammation responses.3,4 Increasing evidence has indicated that the TLR4/NF-κB pathway is related to the development of diabetes.5
Many natural compounds have an important role in antidiabetic effects. Averrhoa carambola L. is an ancient herbal medicine with local characteristics in oriental countries, which has been used to treat hyperglycemia, headaches and alleviate stomach diseases.6 Previous studies have reported that extract and compounds of Averrhoa carambola L. roots decreased the fasting blood glucose level, improved blood lipid metabolism and enhanced antioxidant capacity in diabetic mice.7–10. However, the potential beneficial effects and mechanism on diabetes of benzoquinone extracted from the roots of Averrhoa Carambola L. (which mainly contains DMDD and MNDD) remain unclear. Consequently, the main purpose of the current study was to reveal the protective efficacy and mechanism of BACR in STZ-induced diabetic mice.
Materials and Methods
Preparation of BACR
The roots of Averrhoa carambola L. in this study were obtained from Lingshan County, Guangxi Autonomous Region, China. BACR was prepared by a method described in our previous study.11 Briefly, Averrhoa carambola L. roots were pulverized and extracted three times with 60% aq. EtOH, and then using silica gel column chromatography.
Animals
Male Kunming mice weighing 18–22g were supplied by the Laboratory Animal Centre, Guangxi Medical University, China. All mice were housed in standard conditions, with ten mice per cage under twelve-hour, light–dark cycles and given a normal chow diet and allowed free access to water. All the experimental protocols involving animals were followed and the research was conducted with approval from the Ethical Committee of the Experimental Use of Animals at Guangxi Medical University. Animal utilization protocols were designed in accordance with the Guideline of the Treatment of Laboratory Animals issued by the Ministry of Science and Technology of the People’s Republic of China and the Laboratory Animal-Guideline for Ethical Review of Animal Welfare issued by the National Standard GB/T35892-2018 of the People’s Republic of China.
Experimental Design
The mice were injected once with 120 mg/kg of streptozotocin (STZ) after 12 hours fasting. After 72 hours, blood glucose was withdrawn from the tail veins of the mice, and fasting blood glucose (FBG) levels of 11.1mmol/L were to indicate diabetic mice. Subsequently, the diabetic mice were randomized into six groups (the mice per group) as follows: normal control group; model control group; metformin group (320mg/kg); high concentration of BACR group (120mg/kg); middle concentration of BACR (60mg/kg); low concentration of BACR (30mg/kg), respectively. Each group was treated for 21 days, and the body weight and FBG levels of all mice were monitored each week.
At the end of the experiment, all mice were put down under anesthesia. Blood was collected from eyeballs and the mice were dissected to collect the pancreases. Pancreas tissues were fixed in 10% formalin, and the blood and tissues were kept frozen at −80°C for further determination.
Oral Glucose Tolerance Test (OGTT)
An OGTT was performed to determine the glucose tolerance after21days of treatment. Afterfastingfor12 hours, all groups were orally administrated with glucose (2g/kg). The blood glucose levels at 0, 15, 30, 60, and 120 minutes were determined using Accu-Chek glucometers.
Biochemical Estimations
The levels of TC, TG and FFAs in the serum were estimated using radioimmunoassay kits (Nanjing Institute of Bioengineering, Nanjing, China) following the instructions. The levels of insulin, GHb, TNF-α, and IL-6 in the serum were measured according to the protocols for ELISA kits (Shanghai Yuanye Bio-Technology Co, Shanghai, China). The formula computation of insulin sensitivity index (ISI) was: ISI=ln (FBG×FINS)−1.
Pancreas Histopathological Examinations
For microscopic analysis, the pancreatic samples were fixed in 10% formalin for about 24 hours and then embed in paraffin. The pancreatic tissue was stained with hematoxylin and eosin (H&E) staining for the pathologic structures analysis, and then the slides were observed under light microscopy at 400×.
Immunohistochemical Analysis
The expression levels of TLR4 (1:2000), MyD88 (1:500), TNF-a (1:1000), IL-6 (1:500), and p-NF-κB (1:1000) were analyzed following the manufacturer’s recommended protocols of immunohistochemistry. To calculate the positive expression of the immunohistochemical sections of the IOD values, Image-Pro Plus 6.0 software was used.
Relative Quantitative Real-Time PCR (RT- PCR)
The total RNA was extracted from the frozen pancreas by using the Trizol reagent following the manufacturer’s instructions, and cDNA was extracted from RNA with a HiScript® II Reverse Transcriptase reagent Kit (Takara Biotechnology Co. Ltd., Dalian, China). The TransScript™SYBR® Green Master Mix kit was assayed to the RT-PCR analysis by ABI Prism 7300 real-time thermocycler (Applied Biosystems, Foster City, CA, USA). The primer sequences for the PCR are exhibited in Table 1. Relative gene expression values were calculated by the 2−ΔΔCT method, with an internal control of GAPDH.
Table 1.
Primers Information for RT-PCR
| Gene | Forward Primer (5ʹ-3ʹ) | Reverse Primer (5ʹ-3ʹ) |
|---|---|---|
| TLR4 | GCCATCATTATGAGTGCCAATT | AGGGATAAGAACGCTGAGAATT |
| MyD88 | CGGAACTTTTCGATGCCTTTAT | CACACACAACTTAAGCCGATAG |
| NF-κBp65 | TCCAGGCTCCTGTTCGAGTCTC | CGGTGGCGATCATCTGTGTCTG |
| TNF-α | GCGACGTGGAACTGGCAGAAG | GCCACAAGCAGGAATGAGAAGAGG |
| IL-6 | CTCCCAACAGACCTGTCTATAC | CCATTGCACAACTCTTTTCTCA |
| GAPDH | GGTTGTCTCCTGCGACTTCA | TGGTCCAGGGTTTCTTACTCC |
Western Blotting Analysis
Proteins were extracted from the pancreas tissues from each group by homogenizing in a RIPA buffer and with a BCA protein quantification kit (Fude Biotechnology Co. Ltd., Hangzhou China) to calculate the concentration. The proteins were subjected to reducing 10%SDS-PAGE, transferred onto PVDF membranes which performed with different antibodies: TLR4 (1:1000), MyD88 (1:500), NF-κB (1:1000), IκBα (1:1000), p-IκBα (1:1000), p-NF-κB (1:1000), and GAPDH (1:1000) overnight at 4°C, and then incubated the secondary antibody was incubated (1:5000, goat anti-rabbit IgG) at RT 1h. The protein bands were revealed using the ECL method and quantified by Image J software.
Statistical Analysis
The results were analyzed using the SPSS 19.0 and Graph Pad Prism. The data are presented as means ± standard deviations (S.D.). A one-way analysis of variance (ANOVA) was employed for statistical analysis. Multiple comparisons between the groups were performed using the S-N-K method. Statistical significance was at P < 0.05 or P < 0.01.
Results
Effects of BACR on the Bodyweight of Diabetic Mice
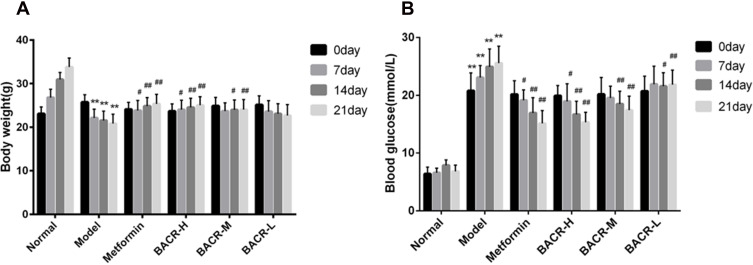
The body weights of the mice were determined every week. The body weight of the mice in the normal control group increased in a stable manner. The mice in the STZ control group had significantly more weight loss compared with the mice in the normal control group. After the 21 days of treatment, the mice administered metformin and BACR showed increased body weight compared with the model group (Figure 1A).
Figure 1.
Effect of BACR on body weight (A) and on the blood glucose (B). Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR-M group: 60mg/kg; BACR-L group: 30mg/kg. Data are presented as the mean ± SD (n = 10). **P< 0.01 vs Normal control group. #P < 0.05 or ##P<0.01 vs Model control group.
Effects of BACR on the Blood Glucose Levels of Diabetic Mice
Figure 1B shows that the blood glucose levels of diabetic mice were significantly higher than those of normal mice. However, metformin and BACR treatment could obviously reduce the blood glucose levels after administration for 21 days compared with the model control group.
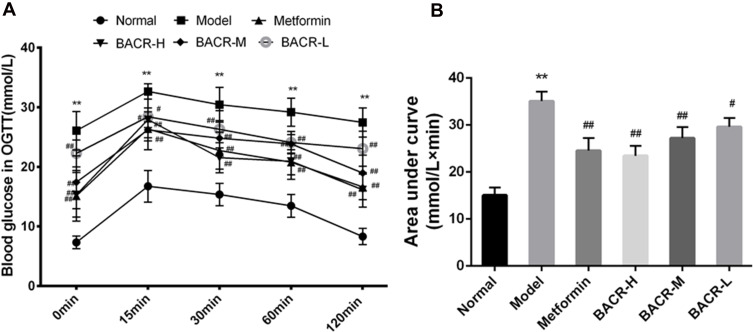
Effect of BACR on OGTT
The OGTT results are shown in Figure 2. The blood glucose levels of the STZ control group had a notable elevation compared with the normal control group. However, the blood glucose levels after three weeks of treatment with metformin and BACR had a significant reduction compared with the model control group.
Figure 2.
Effect of BACR on OGTT (A) and AUC (B). Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR-M group: 60mg/kg; BACR-L group: 30mg/kg. Data are presented as the mean ± SD (n = 10). **P< 0.01 vs Normal control group. #P < 0.05 or ##P<0.01 vs Model control group.
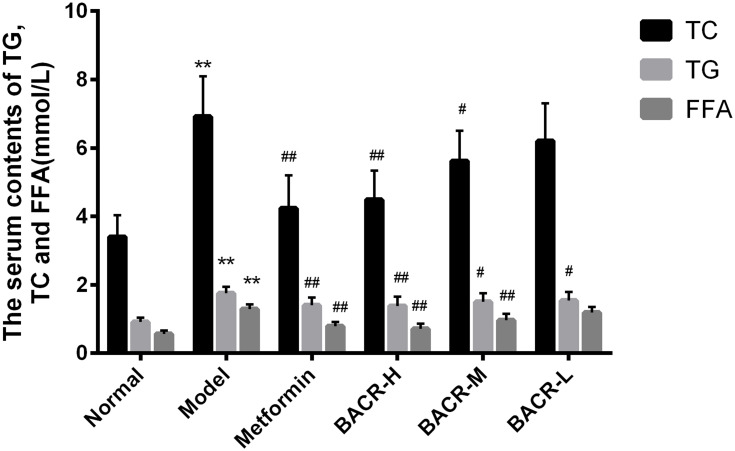
Effect of BACR on the Levels of TC, TG and FFA in Serum
The data presented in Figure 3 shows the effect of BACR on TC, TG and FFAs in the serum of STZ diabetic mice. The levels of TC, TG and FFA in the serum of the model control group had a more prominent enhancement than in normal mice. Notably, the levels of TC, TG and FFA of BACR-treated mice had a significant reduction in comparison with the model control group. The results above indicated that BACR has a lipid-lowering activity which the same therapeutic effect as metformin.
Figure 3.
Effect of BACR on the levels of TC, TG and FFA in the serum. Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR–M group: 60mg/kg; BACR-Lgroup: 30mg/kg. Data are presented as the mean ± SD (n = 10), **P< 0.01 vs Normal control group. #P < 0.05 or ##P<0.01 vs Model control group.
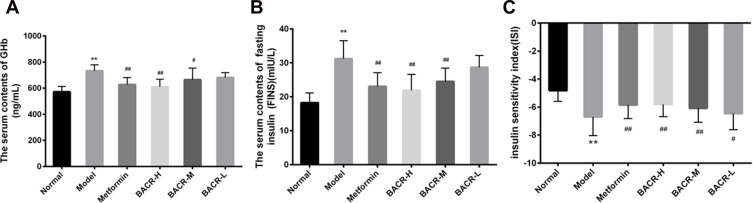
Effect of BACR on GHb, FINS and ISI
To investigate the use of BACR in the control of glucose homeostasis and insulin sensitivity, the levels of GHb and FINS were measured. As demonstrated in Figure 4A, the GHb of STZ control mice were distinctly increased compared with the normal mice, while the mice administered BACR and metformin clearly had an elevated GHb content. At the same time, FINS levels and ISI in the STZ control group increased markedly compared with those of the normal group (Figure 4B and C). In contrast, after the administration of BACR (120 and 60mg/kg), the FINS content and ISI were obviously reduced. Therefore, BACR treatment effectively improves insulin sensitivity in diabetic mice which the same therapeutic effect as metformin.
Figure 4.
Effect of BACR on GHb (A), FINS (B) and ISI (C) in the serum. Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR-M group: 60mg/kg; BACR-L group: 30mg/kg. Data are presented as the mean ± SD (n = 10). **P< 0.01 vs Normal control group. #P < 0.05 or ##P<0.01 vs Model control group.
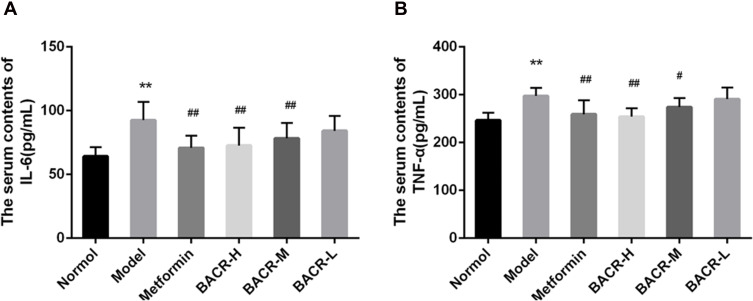
Effect of BACR on the Levels of TNF-α and IL-6 in the Serum
It has been reported that hyperglycemia causes an inflammatory reaction. In our study, serum levels of TNF-α and IL-6 were markedly higher in STZ control mice than in normal mice (Figure 5). However, compared with the STZ control group, serum levels of TNF-α and IL-6 were clearly reduced after treatment with BACR (120 and 60mg/kg). The results above indicated that EACR effectively inhibited inflammatory reaction.
Figure 5.
Effect of BACR on the levels of TNF-α (A) and IL-6 (B) in the serum. Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR-M group: 60mg/kg; BACR-L group: 30mg/kg. Data are presented as the mean ± SD (n = 10). **P< 0.01 vs Normal control group. #P < 0.05 or ##P<0.01 vs Model control group.
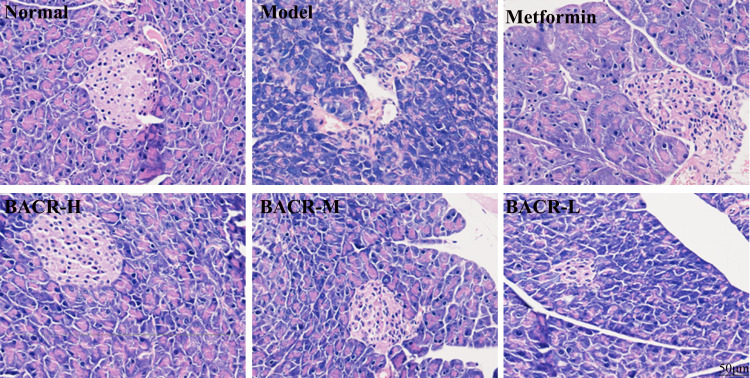
Effect of BACR on STZ-Induced Pathological Changes in Pancreas Tissue
In order to investigate whether EACR has a potential impact on pancreatic tissue damage in diabetic mice, histopathology analysis was performed on pancreatic tissues. As shown in Figure 6, the pancreases of normal mice had a normal histological appearance, an intact cellular structure of pancreatic β cells with abundant and regular distribution. Conversely, the STZ control group showed significant pathological changes, such β cell decrease or shrinkage, the signs of islet β cell apoptosis, and inflammatory infiltration. Interestingly, the function of pancreatic islets was markedly alleviated by metformin or BACR treatment.
Figure 6.
Pancreatic histological changes by HE staining (400×) (n=10). Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR-M group: 60mg/kg; BACR-L group: 30mg/kg.
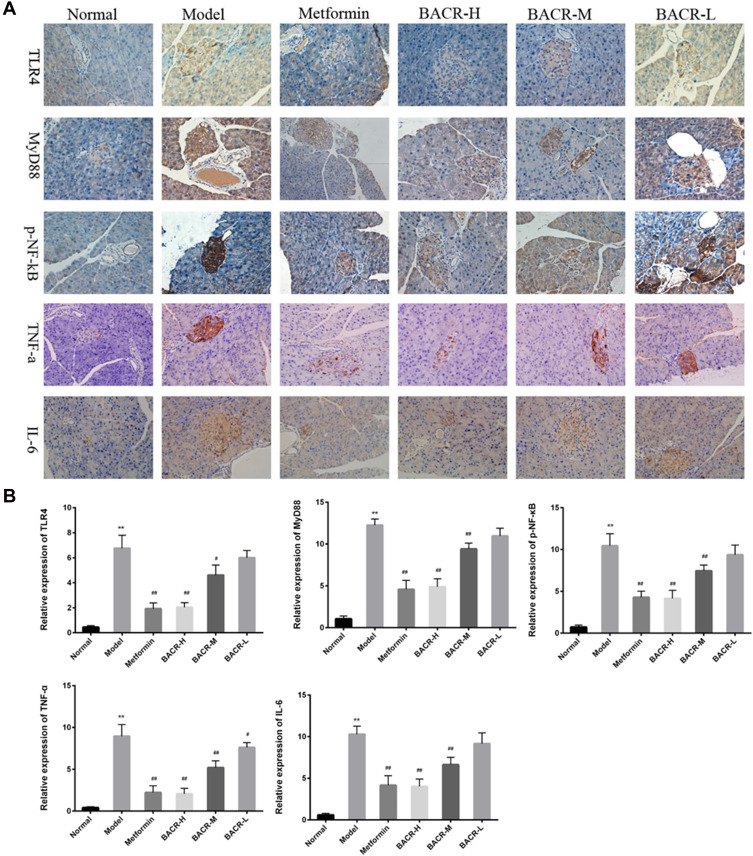
Immunohistochemistry Analysis for the Expression Levels of TLR4, MyD88, p-NF-κB, TNF-α, and IL-6 in Pancreas Tissue
As shown in Figure 7A and B, as compared with the normal mice, the expression levels of TLR4, MyD88, p-NF-κB, TNF-α and IL-6 markedly increased in the model group. On the other hand, the abnormal expression levels of these proteins significantly reduced after treatment with BACR, which resulted in the reduction of positive cells with pancreas tissues.
Figure 7.
Immunohistochemical (IHC) staining (400×) showed expression of TLR4, MyD88, p-NF-κB, TNF-α, and IL-6 in pancreas tissues. The positive rate was analyzed with Image-Pro Plus. Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR–M group: 60mg/kg; BACR-Lgroup: 30mg/kg. Data are presented as the mean ± SD (n = 10). **P< 0.01 vs Normal control group. #P < 0.05 or ##P<0.01 vs Model control group.
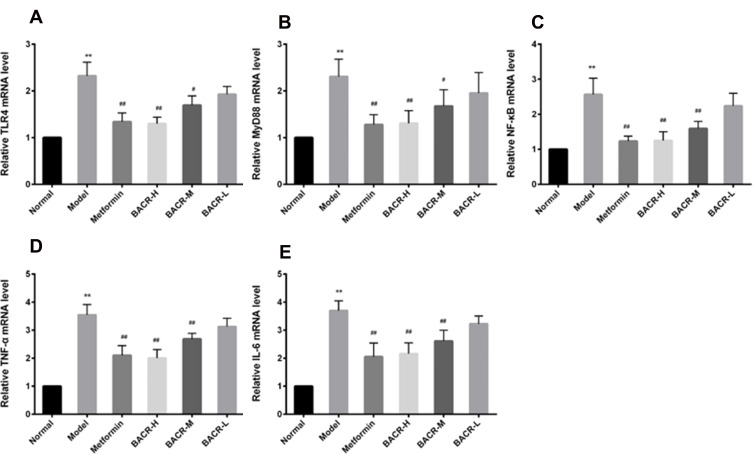
Effects of BACR on the Levels of TLR4, MyD88, NF-Кb, TNF-α, and IL-6 mRNA
In this study, in the STZ control group, RT-PCR assay displayed a notable increase in the mRNA expression levels of TLR4, MyD88, NF-кB, TNF-α and IL-6 mRNA in pancreatic tissues compared to the normal group. As expected, treatment with BACR down-regulated the mRNA expressions of these genes as compared with model group, and obviously inhibited theTLR4/NF-κB pathway (Figure 8).
Figure 8.
Effects of BACR on the levels of TLR4 (A), MyD88 (B), NF-кB (C), TNF-α (D), and IL-6 (E) mRNA: Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR-M group: 60mg/kg; BACR-L group: 30mg/kg. Data are presented as the mean ± SD (n = 3). **P< 0.01 vs Normal control group. #P < 0.05 or ##P<0.01 vs Model control group.
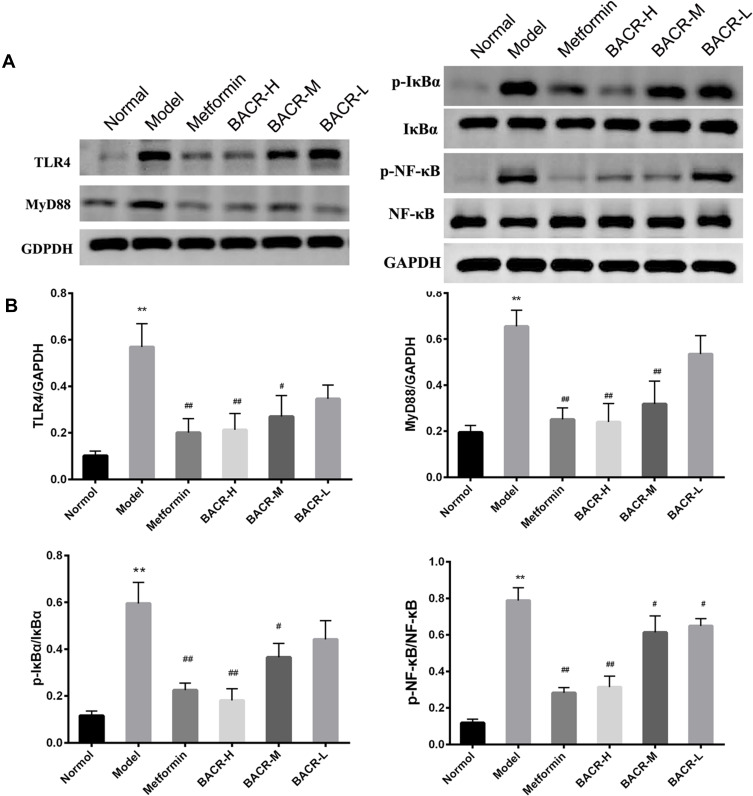
BACR Inhibited the Activation of theTLR4/NF-κB Pathway
To investigate the potential mechanism of BACR on the TLR4/NF-κB pathway, the TLR4/NF-κB pathway-related key proteins were detected. The Western blot assay showed a significant increase in the protein expressions of TLR4, MyD88, p-IκBα and p-NF-κB in the pancreatic tissues of the model group. However, BACR treatment markedly reduced these protein expressions. Therefore, these results indicated that BACR reduced the activation of the TLR4/NF-κB pathway (Figure 9A and B).
Figure 9.
BACR inhibited the activation of theTLR4/NF-κB pathway: Metformin group: 320mg/kg; BACR-H group: 120mg/kg; BACR-M group: 60mg/kg; BACR-L group: 30mg/kg. Data are presented as the mean ± SD (n = 3). **P< 0.01 vs Normal control group. #P < 0.05 or ##P<0.01 vs Model control group.
Discussion
Diabetes mellitus is characterized by persistent hyperglycemia, which resulting in lipid metabolism and other metabolic disorders, and then leading to diabetic complications. With the improvement of living standards, diabetes mellitus has become one of the most harmful chronic diseases in the world, and the mortality rate of diabetes mellitus patients is only exceeded by those with cancer, coronary heart disease and hypertension. It has become one of the major public health problems facing the world, and one of the main causes of human health issues and death.
Traditional Chinese medicine has long been used in folk medicine for treating various ailments, and might evolve into being accepted by traditional medicine for effective treatment. In recent years, a large number of herbal medications have increasingly been used as an important source of medicine to treat several ailments, including diabetes mellitus.12 Our previous works have revealed that the extract of Averrhoa carambola L. root had a high hypoglycemic activity on streptozotocin-induced diabetic mice.13,14 The present research was projected to increase the hypoglycemic activity of BACR in diabetic animals.
STZ is a kind of glucosamine-nitrosourea, which is recognized as a classic chemical inducer for the preparation of the diabetic mice model. It has specific toxicity for the pancreatic β-cells, and then leads to their necrosis, which results in disorders in lipid and protein metabolism, deficient insulin secretion and hyperglycemia.15,16 In this experiment, STZ was used to destroy pancreatic β-cells, increase blood sugar and cause a disorder of the blood lipid metabolism to establish the diabetic mice model.
Pancreases possessing islet β cells which secrete insulin are the key organ determining the glucose level in vivo.17 The secretion and sensitivity of insulin have been considered to be essential for maintaining glucose homeostasis.18 The over-concentration of FFAs levels cause glucotoxicity and lipotoxicity in the pancreas, and glucotoxicity and lipotoxicity in beta cells might result in decreased insulin secretion.19–21 In the present experiment, the results indicated that weight loss and glucose gain, enhanced insulin sensitivity, and significant relief of renal pathological damage can effectively be controlled after administration of BACR from HE observations induced by diabetic mice. Furthermore, the serum concentrations of FFAs, TC and TG of BACR-treated mice had a significant reduction which is the same role of metformin. This shows that BACR can ameliorate dysglycemia and has a strong relation to mitigating lipotoxicity and reducing lipogenesis.
More and more attention has been focused on investigating the mechanisms related to the development of diabetes mellitus, and many studies demonstrated the TLR4 signaling pathway is one of the principal mechanisms may contribute to mediating its development.22–24 Furthermore, TLR4 seems to play a critical role in the pathogenesis of diabetes mellitus in the activation of the innate immune system via activating an inflammatory signaling pathway. MyD88 is the major adaptor protein of TLR4 and the central transduction protein of NF-κB. The activation of TLR4 and MyD88 leads to upregulation of the transcription factor of NF-κB, which activates the release of several inflammatory factors such as TNF-α and IL-6.25–27 In the present study, to further investigate the underlying mechanism of BACR on TLR4 signaling, we analyzed the expressions of mRNA and protein of TLR4, MyD88,NF-кB and levels of TNF-α and IL-6. Interestingly, from the results, we found not only that the expression levels of TLR4, MyD88, and NF-κB, but also TNF-α and IL-6 levels, were notably reduced in mice treated with BACR, and increased in diabetic mice.
Although previous studies have investigated the effects of total extracts and DMDD of Averrhoa carambola L. root on diabetes, compared with the total extract of Averrhoa Carambola L. root, the benzoquinone (mainly contains DMDD and MNDD) further clarified the Averrhoa Carambola L. root active components. On the other hand, the results show that benzoquinone has the same efficacy as DMDD, but the benzoquinone extraction is much larger than DMDD, it is about 10 times. A Averrhoa Carambola L. root can only be picked after several decades, and because the extraction amount of DMDD is very small (about 5kg carambola root can extract 1g), so the BACR is more suitable for long-term research.
In summary, our study demonstrated that BACR has protective effect on STZ induced hyperglycemia, and the possible mechanism might be that BACR has an aggressive suppression of inflammatory effect, resulting from inhibiting the TLR4/NF-κB signaling pathway.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81760665, 81460205, 81360129), Innovation Project of Guangxi Graduate Education (YCBZ2018043, YCBZ2017043), and the Postdoctoral Science Foundation of China (No. 2017M613271XB).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Taneeraa J, Dhaibana S, Mohammeda AK, et al. GNAS gene is an important regulator of insulin secretory capacity in pancreatic β-cells. Gene. 2019;715:144028. doi: 10.1016/j.gene.2019.144028 [DOI] [PubMed] [Google Scholar]
- 2.Bin-Jumah MN. Antidiabetic effect of monolluma quadrangula is mediated via modulation of glucose metabolizing enzymes, antioxidant defenses, and adiponectin in type 2 diabetic rats. Oxid Med Cell Longev. 2019;11:6290143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi X, Wang S, Luan H, et al. Clinopodium chinense attenuates palmitic acid-induced vascular endothelial inflammation and insulin resistance through TLR4-mediated NF-κB and MAPK pathways. Am J Chin Med. 2019;47(1):97–117. doi: 10.1142/S0192415X19500058 [DOI] [PubMed] [Google Scholar]
- 4.Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20(5):379–385. doi: 10.1097/MOL.0b013e32832fa5c4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang H, Wang H, Luo L, et al. Toll-like receptor 4 promotes high glucose-induced catabolic and inflammatory responses in chondrocytes in an NF-κB-dependent manner. Life Sci. 2019;228:258–265. doi: 10.1016/j.lfs.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 6.Cabrini DA, Moresco HH, Imazu P, et al. Analysis of the potential topical anti-inflammatory activity of averrhoa carambola L. in mice. Evid Based Complement Alternat Med. 2011;2011:1–7. doi: 10.1093/ecam/neq026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Liang T, Lin X, et al. Effect of the total extract of Averrhoacarambola (Oxalidaceae) root on the expression levels of TLR4 and NF-κB in streptozotocin-induced diabetic mice. Cell Physiol Biochem. 2015;36(6):2307–2316. doi: 10.1159/000430194 [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Liang T, Wen Q, et al. Protective effects of total extracts of Averrhoa carambola L. (Oxalidaceae) roots on streptozotocin-induced diabetic mice. Cell Physiol Biochem. 2014;33(5):1272–1282. doi: 10.1159/000358695 [DOI] [PubMed] [Google Scholar]
- 9.Zheng N, Lin X, Wen Q, et al. Effect of DMDD on renal injury in type 2 diabetic KKAy mice. Toxicol Lett. 2013;219(1):77–84. doi: 10.1016/j.toxlet.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 10.Li J, Wei X, Xie Q, et al. Protective effects of 2-Dodecyl-6-Methoxycyclohexa −2,5 -Diene-1,4-Dione Isolated from Averrhoa Carambola L. (Oxalidaceae) roots on high-fat diet-induced obesity and insulin resistance in mice. Cell Physiol Biochem. 2016;40(5):993–1004. doi: 10.1159/000453156 [DOI] [PubMed] [Google Scholar]
- 11.Wen Q, Lin X, Liu Y, et al. Phenolic and lignan glycosides from the butanol extract of averrhoa carambola L. root. Molecules. 2012;17(10):12330–12340. doi: 10.3390/molecules171012330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Peng C, Xie X, et al. Antidiabetic effect of flavonoids from Malus toringoides (Rehd.) Hughes leaves in diabetic mice and rats. J Ethnopharmacol. 2014;153(3):561–567. doi: 10.1016/j.jep.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 13.Lu SY, Zhang HL, Wei XJ, et al. 2-dodecyl-6-methoxycyclohexa-2,5-diene −1,4-dione isolated from Averrhoa carambola L. root ameliorates diabetic nephropathy by inhibiting the TLR4/MyD88/NF-κB pathway[J]. Diabetes MeTable Syndr Obes. 2019;12:1355–1363. doi: 10.2147/DMSO.S209436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HL, Wei XJ, Lu SY, et al. Protective effect of DMDD, isolated from the root of Averrhoa CarambolaL., for high glucose induced EMT in HK-2 cells by inhibiting the TLR4-BAMBI Smad2/3 signaling pathway[J]. Biomed Pharmacother. 2019;113:108705. doi: 10.1016/j.biopha.2019.108705 [DOI] [PubMed] [Google Scholar]
- 15.Gai W, Schott-Ohly P, Schulte Im Walde S, Gleichmann H. Differential target molecules for toxicity induced by streptozotocin and alloxan in pancreatic islets of mice in vitro. Exp Clin Endocrinol Diabetes. 2004;112(1):29–37. doi: 10.1055/s-2004-815724 [DOI] [PubMed] [Google Scholar]
- 16.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 17.Zhang F, Yuan W, Wei Y, et al. The alterations of bile acids in rats with high-fat diet/streptozotocin-induced type 2 diabetes and their negative effects on glucose metabolism. Life Sci. 2019;229:80–92. doi: 10.1016/j.lfs.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Qian L, Wang B, et al. Synergistic hypoglycemic effects of pumpkin polysaccharides and puerarin on type II diabetes mellitus mice. Molecules. 2019;24(5):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleason CE, Gonzalez M, Harmon JS, Robertson RP. Determinants of glucose toxicity and its reversibility in the pancreatic islet beta-cell line, HIT-T15. Am J Physiol Endocrinol Metab. 2000;279(5):997–1002. doi: 10.1152/ajpendo.2000.279.5.E997 [DOI] [PubMed] [Google Scholar]
- 20.Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–1285. doi: 10.1038/nm.2851 [DOI] [PubMed] [Google Scholar]
- 21.Itoh Y, Hinuma S. GPR40, a free fatty acid receptor on pancreatic β cells, regulates insulin secretion. Hepatol Res. 2005;33(2):171–173. doi: 10.1016/j.hepres.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 22.Jia L, Vianna CR, Fukuda M, et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:3878. doi: 10.1038/ncomms4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaraj S, Tobias P, Jialal I. Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine. 2011;55(3):441–445. doi: 10.1016/j.cyto.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 24.Santoni M, Andrikou K, Sotte V, et al. Toll like receptors and pancreatic diseases: from a pathogenetic mechanism to a therapeutic target. Cancer Treat Rev. 2015;41(7):569–576. doi: 10.1016/j.ctrv.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 25.Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100(11):1589–1596. doi: 10.1161/CIRCRESAHA.106.142851 [DOI] [PubMed] [Google Scholar]
- 26.Ding Y, Yang H, Xiang W, et al. CD200R1 agonist attenuates LPS-induced inflammatory response in human renal proximal tubular epithelial cells by regulating TLR4-MyD88-TAK1-mediated NF-κB and MAPK pathway.Biochem. Biophys Res Commun. 2015;460(2):287–294. doi: 10.1016/j.bbrc.2015.03.026 [DOI] [PubMed] [Google Scholar]
- 27.Zeng K, Zhang T, Fu H, et al. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur J Pharmacol. 2012;692(1–3):29–37. doi: 10.1016/j.ejphar.2012.05.030 [DOI] [PubMed] [Google Scholar]