Abstract
Purpose
Ethiopia is grouped with countries with no national strategy for surveillance of viral hepatitis. Hence, data on hepatitis B virus (HBV) infection in the general population are limited. The aim of this study was to estimate the prevalence and associated factors of HBV infection among adults in Southwest Ethiopia.
Materials and Methods
A community-based cross-sectional study was conducted in Southwest Ethiopia, from November 1, 2017–January 30, 2018. A total of 612 individuals were included in the study using a multistage sampling technique. A structured questionnaire was used to collect data and a whole blood sample was aseptically collected and tested for HBsAg using a commercially available rapid serological test kit. Bivariate and multivariate logistic regression were employed and odds ratio with 95% confidence interval was retrieved. P-value <0.05 was considered as statistically significant.
Results
Among 612 participants, half of them, 310 (50.7%), were in the age range of 25–34 years. The mean age of the respondents was 32.5 [SD ±7.5] years. Seroprevalence of HBsAg among adults was 55/612 (9.0%). Tattooing on gums (AOR=23.9, 95% CI (2.2–26.3)), tattooing on the body (AOR=6.8, 95% CI (1.1–43.1)), and contact with a jaundiced person (AOR=20.7, 95% CI (6.7–63.8)) were significantly associated with seroprevalence of HBsAg.
Conclusion
Hepatitis B virus infection in adults at the community level is highly endemic. Modifiable risk factors such as tattooing on gums, tattooing on body, and contact with a jaundiced person account for the high HBV infection. Hence, behavioral education and communication programs designed to reduce HBV infection need to address these modifiable factors.
Keywords: hepatitis B virus, HBsAg, adult, Ethiopia
Introduction
Chronic viral hepatitis is mainly caused by hepatitis viruses B, C, and D in which the hepatitis B virus (HBV) infection is a major public health problem.1 In 2015 and 2016, the global seroprevalence of the hepatitis B virus surface antigen (HBsAg) was 3.6%2 and 3.9%3 respectively with the highest endemicity in the region of Africa. It is the leading cause of liver disease and the risk of developing hepatocellular carcinoma increased in patients with chronic HBV infection.4 In sub-Saharan Africa, 26% of liver cancer is due to HBV and 19 (14–24) per 1000 deaths attributed due to it.5 The prevalence of chronic hepatitis B virus infection in sub-Saharan Africa ranges from 5% to 25%.6
Hepatitis B is transmitted both sexually and parenterally, most often by percutaneous or mucous membrane exposure to infectious body fluids. Percutaneous exposures leading to the transmission of HBV include needlestick injury, tattooing, piercing, injection drug use with shared needles and sharp materials, transfusion of infected blood or blood products, and hemodialysis. Perinatal transmission is the major route of HBV transmission in highly endemic areas.7,8 Hepatitis B virus surface antigen is a protein on the surface of HBV. It is the hallmark of HBV infection and is the first serological marker to appear in acute HBV infection. It is the most frequently used marker to screen for the presence of HBV infection and persistence of HBsAg in the blood or serum for more than six months suggests chronic HBV infection.9
By the year 2007, universal vaccination of children against HBV was introduced in Ethiopia. The vaccine is available throughout the expanded program on immunization. However, it is not available for the general population including pregnant women.10 The overall full immunization coverage at the national level was 38.3% and 32.7% in the Southern region of the country.11 In addition to universal vaccination of children, screening of pregnant women is included as a part of the prevention and control of HBV in the country.
Ethiopia is grouped among viral hepatitis endemic countries. A recent systematic review and Meta-analysis in Ethiopia showed the pooled prevalence of HBV is 7.4%.12 Community-based seroepidemiological surveys are few in Ethiopia and the available studies indicate the varied distribution of HBV infection across geographical regions of the country.13–15 In addition, previous community-based studies were old and did not show the current burden of the virus.13,14 Knowledge on the prevalence of HBV infection in the general population is significant for health-care workers and policymakers since the risk of health-care workers to occupational exposure with infected body fluids depends upon the prevalence of HBV in the general population.16 However, there is a paucity of data on the prevalence of HBV infection in the general population. A single up-to-date community-based study conducted in the North-west region of Ethiopia showed that 3.1% of adults were positive with the hepatitis B virus surface antigen.15
Ethiopia is grouped with countries with no national strategy for surveillance of viral hepatitis.17 Hence, data on HBV in the general population are limited. Most reports regarding HBV were from institutional-based studies and among the high-risk group of people.18–22 Even though few community-based studies on seroepidemiology of HBV prevalence in Ethiopia have been previously done and indicated that hepatitis B is endemic in Ethiopia with regional variation,15,23 community-based studies are still limited. This study aimed to determine the seroprevalence of HBsAg among adults within the community and identify factors associated with hepatitis B virus infection in Southwest Ethiopia.
Materials and Methods
Study Design and Setting
A community-based cross-sectional study was conducted in Bench Maji Zone, Southwest Ethiopia, from November 1, 2017 to January 30, 2018. Bench-Maji is one of the Zones of the Ethiopian southern nations, nationalities, and peoples’ region with a total area of 19,965.90 sq. km and lies between 5.33–7.21 latitude and 34.88 to 36.14 longitudes with an elevation ranging to 2500 m above sea level. It is bordered on the south by the Ilemi Triangle, on the west by South Sudan, on the Northwest by the Gambella region, on the North by Sheka zone, on the Northeast by Keffa zone, and on the East by Debub Omo. According to the unpublished zonal report, the 2017 total projected population of the zone was 858,177. Nearly half (48.27%) of the population was in the age range of 15–59 years old.
This zone has a` total of 11 districts; six of the districts (Debub Bench, Guraferda, Semen Bench, Shewa Bench, Sheko, and Mizan Aman town administration) are agrarian. Whereas, five of the districts (Bero, Maji, Meinit Goldia, Meinit Shasha, and Surma) are semi-pastoral districts. The study was ethically approved by the ethics committee of institute of research, community service, and support, Mizan Tepi University, and written informed consent was obtained from each study participants.
Source and Study Population
The source population was all adult populations age ≥ 18 years in the Bench-Maji zone in the SNNPR region. Adults with age ≥ 18 years from the selected households in the study area during the study period were the study population. Adults who were critically ill and unable to give responses were excluded.
Sample Size Determination and Sampling Procedures
A single population proportion formula, 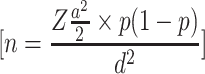 , was used to estimate the sample size. Where;
, was used to estimate the sample size. Where; 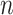 =sample size, Z= standard normal distribution corresponding to significance level at α = 0.05, P=prevalence of HBV from the previous study conducted at Addis Ababa and, d= margin of error. The following assumptions were made while calculating the sample size; the margin of error d = 0.03, Zα/2= 1.96, and the prevalence of HBV = 0.07.23 Since the sampling procedure involved a multistage sampling technique, we multiplied by the design effect of 2. Finally, by adding a 10% non-response rate, the final sample size was 612.
=sample size, Z= standard normal distribution corresponding to significance level at α = 0.05, P=prevalence of HBV from the previous study conducted at Addis Ababa and, d= margin of error. The following assumptions were made while calculating the sample size; the margin of error d = 0.03, Zα/2= 1.96, and the prevalence of HBV = 0.07.23 Since the sampling procedure involved a multistage sampling technique, we multiplied by the design effect of 2. Finally, by adding a 10% non-response rate, the final sample size was 612.
A multistage sampling technique was used to recruit study participants. First, the primary sampling units (districts) were stratified into agrarian and semi-pastoral districts. In this study area, there are six agrarian and five semi-pastoral districts. Among the six agrarian districts, two of them (Mizan-Aman and Shey-Bench) were selected using the random sampling method. Among the five semi-pastoral districts, two of them (Bero and Menit Goldia) were selected using the random sampling method.
Second, the secondary sampling unit (kebeles) in each selected district was stratified into urban and rural. Accordingly, five urban kebeles (which account more than 30% of urban kebeles) from Mizan-Aman, Shey Bench, Bero, and Menit Goldia were selected using the random sampling method. Similarly, 16 rural kebeles (account more than 30% of rural kebeles) from Mizan-Aman, Shey Bench, Bero, and Menit Goldia were selected.
After selecting kebeles, the tertiary sampling units (households) in each selected kebeles that had adults (age ≥18 years old) were identified. A census was conducted to identify adults having age ≥18 years old. A sampling frame was made during the census. Then, the final sample size was proportionally allocated to each selected kebeles using population proportion to size (PPS). Finally, study participants whose age is 18 years old and above were selected using a systematic sampling method. The sampling interval was calculated by dividing the total numbers of adults age ≥18 years in each selected kebeles by the allocated sample size to each kebeles. The overall sampling procedure is found in Figure S1. For households with more than one eligible individual in one household, only one person was selected using the lottery method. In a case where eligible respondents were not available at the time of the survey, revisit was made at least three times.
Data Collection Tools and Procedure
A structured questionnaire adapted from different peer-reviewed literature was used to collect socio-demographic information and data on risk factors for HBV infection among participants.23–25 A face to face interview using a verbal questionnaire was conducted by eight diploma holder laboratory technicians. The questionnaire was pretested among 5% of the sample size in a similar set up before the actual data collection period. The coherence and skipping pattern of the questionnaire were corrected after the pretest.
Operational Definitions
Family Size
The number of individuals in the household including the study participant.
Sharing of Sharp Personal Items
Sharing of sharp materials used by an individual that could have a relation with transmission of HBV such as needles, blade, scissors, and also toothbrush.
Contact With a Jaundiced Person
Direct or indirect contact with body fluids excreted from a jaundiced person. It can happen during care of a jaundiced person or sharing of personal items or sleep together.
Unprotected Sexual Behavior
Having sex with inconsistent or no use of condoms with any partner.
Specimen Collection and Laboratory Procedure
Upon completion of the questionnaire, capillary blood was collected through a skin puncture of the middle/ring finger by the sterile blood lancet. The collected whole blood sample was tested for serostatus of HBsAg using advanced qualityTM one step HBsAg test (InTec Products Inc., USA). The kit’s performance ability is; sensitivity 100%, specificity 99.43%, and the predictive value of a positive test 98.57%. The test was done according to the manufacturer’s instructions and interpreted accordingly. First, all reagents and specimens were brought to room temperature. The test card was removed from the foil pouch and placed it on a clean dry space. Then, the test card for each specimen or control was identified. Three drops (100ul) of the specimen were dispensed into the sample well on the card and the test result was interpreted at 15 minutes.
Data Quality Assurance
The quality of socio-demographic data was assured by properly designed and pre-tested of the questionnaire. Furthermore, proper training of the data collectors about the data collection procedures was made. To get appropriate information from respondents, we selected data collectors who are fluent speakers of the respondents’ local language. Furthermore, efforts were made to create a rapport with each participant through respectful approaching, using encouraging gestures and words during the data collection process.
The quality of the laboratory result was guaranteed by applying quality control measures during sample collection and laboratory procedures. A sufficient quantity of capillary blood (Three drops or 100 µl) was collected. Standard operational procedures recommended by the manufacturers of advanced qualityTM one step HBsAg tests were strictly followed. The expiry date of test kits was checked. A positive control (containing 10 ng/mL of HBsAg) and negative control (containing 0 ng/mL of HBsAg) were employed to check the performance of test kits.
Data Processing and Analysis
The data were entered using EPI INFO version 7 and was exported to statistical packages for social science (SPSS) version 21.0 for data cleaning and analysis. The degree of association between independent variables and the dependent variable was assessed using crude and adjusted odds ratios with a 95% confidence interval. Those independent variables with a p-value < 0.25 at the bivariate analysis were included in the multivariate logistic regression model to control for potential confounders. The results were presented in the form of tables, figures, and summary statistics.
Results
Sociodemographic Characteristics of the Respondents
A total of 612 eligible adults comprised 276 (45.1%) males and 336 (54.9%) females participated in the study. The mean age of the participants was 32.5 years [SD ±7.5]. Almost half of the participants, 310 (50.7%) were in the age range of 25–34 years. The majority (71.4%) of the study participants were rural residents. Concerning the educational status, 261 (42.6%) of the study participants were not able to read and write [Table 1].
Table 1.
Sociodemographic Characteristics of the Respondents (n=612) in Southwest Ethiopia
| Characteristics | N | % | |
|---|---|---|---|
| Gender | Male | 276 | 45.1 |
| Female | 336 | 54.9 | |
| Residence | Urban | 175 | 28.6 |
| Rural | 437 | 71.4 | |
| Age | 18–24 | 85 | 13.9 |
| 25–34 | 310 | 50.7 | |
| 35–44 | 188 | 30.7 | |
| ≥45 | 29 | 4.7 | |
| Marital status | Married | 393 | 64.2 |
| Single | 102 | 16.7 | |
| Separated | 74 | 12.1 | |
| Divorced | 30 | 4.9 | |
| Widowed | 13 | 2.1 | |
| Religion | Protestant | 273 | 44.6 |
| Orthodox | 248 | 40.5 | |
| Muslim | 91 | 14.9 | |
| Educational status | Unable to write and read | 261 | 42.6 |
| Able to read and write | 87 | 14.2 | |
| Primary | 95 | 15.5 | |
| Secondary | 136 | 22.2 | |
| Certificate | 13 | 2.1 | |
| Diploma and above | 20 | 3.3 | |
| Occupational status | Farmer | 179 | 29.2 |
| Housewife | 293 | 47.9 | |
| Merchant | 71 | 11.6 | |
| Student | 17 | 2.8 | |
| Daily worker | 20 | 3.3 | |
| Gov’t employee | 32 | 5.2 | |
| Family size | <2 | 31 | 5.1 |
| 2–3 | 30 | 4.9 | |
| 4–5 | 421 | 67.3 | |
| >5 | 139 | 22.7 | |
Prevalence of HBV Infection
The overall prevalence of HBV infection among adults in the community was 9.0% (95% CI: 5.03–12.96). Of the total HBV infected participants, 27 (49.1%) and 28 (50.9%) were males and females, respectively. The majority of HBV seropositive, 36 (65.5%) were rural dwellers. A high proportion of seropositive HBsAg cases were in the age group of 25–34 years, 27 (49.1%), and 35–34 years, 21 (38.2%) [Table 2].
Table 2.
Distribution of HBsAg Seroprevalence with Socio-Demographic Characteristics in Southwest Ethiopia
| Variables | HBsAg Seroprevalence | ||
|---|---|---|---|
| Negative N (%) | Positive N (%) | ||
| Gender | Male | 249 (44.7) | 27 (49.1) |
| Female | 308 (55.3) | 28 (50.9) | |
| Residence | Urban | 156 (28.0) | 19 (34.5) |
| Rural | 401 (72.0) | 36 (65.5) | |
| Age | 18–24 | 83 (14.9) | 2 (3.6) |
| 25–34 | 283 (50.8) | 27 (49.1) | |
| 35–44 | 167 (30.0) | 21 (38.2) | |
| ≥45 | 24 (4.3) | 5 (9.1) | |
| Marital status | Married | 373 (67.0) | 20 (36.4) |
| Single | 92 (16.5) | 10 (18.2) | |
| Separated | 71 (12.7) | 3 (5.5) | |
| Divorced | 11 (2.0) | 19 (34.5) | |
| Widowed | 10 (1.8) | 3 (5.5) | |
| Educational status | Unable to write and read | 231 (41.5) | 30 (54.5) |
| Able to read and write | 70 (12.6) | 17 (30.9) | |
| Primary | 90 (16.2) | 5 (9.1) | |
| Secondary | 133 (23.9) | 3 (5.5) | |
| Certificate | 13 (2.3) | 0 (0.0) | |
| Diploma and above | 20 (3.6) | 0 (0.0) | |
| Occupational status | Farmer | 162 (29.1) | 17 (30.9) |
| Housewife | 279 (50.1) | 14 (25.5) | |
| Merchant | 63 (11.3) | 8 (14.5) | |
| Student | 15 (2.7) | 2 (3.6) | |
| Daily worker | 6 (1.1) | 14 (25.5) | |
| Gov’t employee | 32 (5.7) | 0 (0.0) | |
| Family size | <2 | 28 (5.0) | 3 (5.5) |
| 2–3 | 18 (3.2) | 12 (21.8) | |
| 4–5 | 393 (70.6) | 19 (34.5) | |
| >5 | 118 (21.2) | 21 (38.2) | |
| Total | 557 | 55 | |
Risk Factors of Hepatitis B Virus Infection
Based on bivariate logistic regression analysis, tattooing on gums (COR=4.1, 95% CI = (2.3−7.4)), tattooing on body (COR=7.9, 95% CI = (3.8–16.6)), body piercing (COR=8.4, 95% CI = (4.6–15.2)), contact with a jaundiced person (COR=13.6, 95% CI = (7.8–54.1)), history of hospitalization (COR=7.1, 95% CI = (2.9–17.0)), sharing sharp personal items (COR=4.0, 95% CI = (2.0–10.0)), history of circumcision (COR=3.2, 95% CI = (1.8–5.7)), and unprotected sexual behavior (inconsistent or no use of a condom) (COR=3.0, 95% CI = (1.5–5.7)) were the factors found to be significantly associated with the seroprevalence of HBsAg.
After adjusting for the possible confounders with multivariate logistic regression analysis, tattooing on gums (AOR=23.9, 95% CI = (2.2–26.3)), tattooing on body (AOR=6.8, 95% CI = (1.1–43.1)), and contact with a jaundiced person (AOR=20.7, 95% CI = (6.7–63.7)) were significantly associated [Table 3].
Table 3.
Bivariate and Multivariate Analysis of Risk Factors of HBV Infection in Southwest Ethiopia
| Variables | Seroprevalence of HBsAg | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| Negative N (%) | Positive N (%) | |||
| Tattooing of gums | ||||
| Yes | 78 (78) | 22 (22) | 4.1 (2.3–7.4)* | 23.9 (2.2–26.3)* |
| No | 479 (93.6) | 33 (6.4) | 1.00+ | 1.00+ |
| Tattooing on body | ||||
| Yes | 218 (82.6) | 46 (17.4) | 7.9 (3.8–16.6)* | 6.8 (1.1–43.1)* |
| No | 339 (97.4) | 9 (2.6) | 1.00+ | 1.00+ |
| Body piercing | ||||
| Yes | 96 (73.3) | 35 (26.7) | 8.4 (4.6–15.2)* | 5.2 (0.2–109.3) |
| No | 461 (95.8) | 20 (4.2) | 1.00+ | 1.00+ |
| Uvulectomy | ||||
| Yes | 73 (76.8) | 22 (23.2) | 4.4 (0.4–7.9) | 4.3 (0.4–46.1) |
| No | 484 (93.6) | 33 (6.4) | 1.00+ | 1.00+ |
| Ever shaved at barbershop | ||||
| Yes | 173 (86.1) | 28 (13.9) | 2.3 (0.3–4.0) | 2.9 (0.1–61.4) |
| No | 384 (93.4) | 27 (6.6) | 1.00+ | 1.00+ |
| Contact with a jaundiced person | ||||
| Yes | 4(10.8) | 33 (89.2) | 13.6 (7.8–54.1)* | 20.7 (6.7–63.7)* |
| No | 553 (96.2) | 22 (3.8) | 1.00+ | 1.00+ |
| History of circumcision | ||||
| Yes | 188 (84.7) | 34 (15.3) | 3.2 (1.8–5.7)* | 1.5 (0.05–38.9) |
| No | 369 (94.6) | 21 (5.4) | 1.00+ | 1.00+ |
| Dental extraction at home | ||||
| Yes | 6 (13) | 40 (87) | 1.00+ | 1.00+ |
| No | 551 (97.3) | 15 (2.7) | 2.0 (1.0–4.0) | 2.0 (1.0–4.1) |
| History of hospitalization | ||||
| Yes | 15 (62.5) | 9 (37.5) | 7.1 (2.9–17.0)* | 1.1 (0.1–10.8) |
| No | 542 (92.2) | 46 (7.8) | 1.00+ | 1.00+ |
| Dental extraction at health institutions | ||||
| Yes | 10 (71.4) | 4 (28.6) | 1.00+ | 1.00+ |
| No | 547 (91.5) | 51 (8.5) | 1.2 (1.1–3.8) | 3.2 (0.1–160.8) |
| Catheterization | ||||
| Yes | 4 (30.8) | 9 (69.2) | 1.00+ | 1.00+ |
| No | 553 (62.3) | 46 (7.7) | 1.0 (1.0–3.1) | 0.3 (0.01–6.5) |
| Alcohol drinking or drugs/substances utilization | ||||
| Yes | 293 (88.8) | 37 (11.2) | 1.8 (0.01–3.3) | 6.48 (0.1–38.6) |
| No | 264 (93.6) | 18 (6.4) | 1.00+ | 1.00+ |
| Unprotected sexual behavior (inconsistent or no use of a condom) | ||||
| Yes | 305 (87.6) | 43 (12.4) | 3.0 (1.5–5.8)* | 0.1 (0.01–1.3) |
| No | 252 (95.5) | 12 (4.5) | 1.00+ | 1.00+ |
| Ever smoke cigarette | ||||
| Yes | 8 (26.7) | 22 (73.3) | 1.00+ | 1.00+ |
| No | 549 (64.3) | 33 (5.7) | 1.0 (0.05–13.0) | 0.1 (0.01–1.8) |
| Sharing of sharp personal items | ||||
| Yes | 9 (26.5) | 25 (73.5) | 4.0 (2.0–10.0)* | 1.0 (0.04–2.5) |
| No | 548 (94.8) | 30 (5.2) | 1.00+ | 1.00+ |
Notes: *Significant, +Reference category.
Abbreviations: COR, crude odds ratio; AOR, adjusted odds ratio; CI, confidence interval.
Discussion
Hepatitis B virus infection is highly endemic in the current study area, nearly one in ten adults (9.0%) were positive with HBsAg. The prevalence of HBsAg observed in the current study is generally categorized as high endemic according to the world health organization criteria (≥8.0%)26 and close to the subgroup meta-analysis of community-based studies in Ethiopia (8.0%). However, the significant heterogeneity among these community-based studies was reported.12 It is also consistent with the national HBsAg prevalence estimation in Uganda in 2007 (10.3%).27 Nevertheless, a recent community-based study in Northern Uganda found a relatively high prevalence of HBsAg (17.6%)28 which clearly shows regional variation within one country. A relatively high proportion of HBsAg positive adults in Northern Uganda may indicate early childhood exposure. This is most likely true because the majority of participants (23.9%) within the age range of 15–24 years old were positive with HBsAg in Northern Uganda.28
On the contrary, the prevalence of HBsAg in the current study is higher than community-based study findings in other East-African countries including Madagascar (6.9%),29 Kenya (2.1%),30 and Northwest Ethiopia (3.1%).15 This variation could be due to differences in study population and exclusion criteria. For instance, study populations in Kenya were exclusively HIV negative adults23 and thus the prevalence of HBsAg may be underestimated. Due to the common/shared way of transmission, it is believed that HBV is higher among HIV positive individuals. However, this needs to be cautiously interpreted and needs further confirmation as studies in the literature to suggest that HBV infections occur during early childhood via horizontal transmission, and iatrogenic exposure accounts for the majority of HBV infections in Africa.31
We assessed risk factors associated with HBsAg seropositivity to identify the subgroup of adult populations with the risk of hepatitis B infection. We found that the prevalence did not vary significantly according to socio-demographic variables including age, gender, marital status, educational status, and occupation. Studies also documented that those socio-demographic factors could not significantly influence the prevalence of HBsAg as far as other confounding variables such as traditional practices and risky sexual behaviors are controlled. For instance, Andriamandimby et al found that gender and age do not significantly vary between HBsAg positive and negative individuals in Madagascar.29 Similarly, no significant difference was observed between age, gender, resident, marital status, and HBsAg status in Northern Ethiopia.15
After controlling potential confounders, tattooing on gum and tattooing on the body were significantly and positively associated with HBV infection which is in line with study findings from Ethiopia.32,33 This indicates that the sharing of unsafe materials for traditional practices still contributes to the transmission of HBV in rural Ethiopia. It has been documented that community awareness of the transmission of hepatitis B is low in Ethiopia.34 In this study setup, body tattooing, piercing of ear, and lips are common traditional practices and the communities maintain them as their cultural identity. Hepatitis B virus can survive and retain its infectivity for more than a week on the surfaces of non-sterile materials. So, the sharing of non-sterile materials during traditional practices might contribute to the transmission of HBV in this study setup.
In addition to traditional practices, contact with a jaundiced person was found to be an independent risk factor. This finding is consistent with the study conducted in Northwest Ethiopia which showed that study subjects who had a previous history of contact were five times more likely to have HBV infection compared to those who have not.35 In addition, a recent study in Kenya reported 50.6% prevalence of HBsAg among patients presenting with jaundice.36 The prevalence of HBV infection among patients with jaundice is high and the presence of close contact with patients presenting with jaundice increases the chance of HBV transmission.
To prevent future hepatitis B virus infection in this study area, universal vaccination of children should be promoted and the hepatitis B birth dose vaccine needs to be implemented in the country. However, HBV vaccination alone is insufficient to reduce HBV related deaths because adults who may establish chronic infection before the introduction of the hepatitis vaccine will continue to carry a high risk of dying from hepatitis associated liver diseases. Thus, alternative solutions proposed by a recent modeling study like population screening and treatment of HBV may reduce the transmission of HBV.37 In addition to vaccination, other HBV prevention and control measures have to be promoted to reduce the transmission of hepatitis B like; safe sexual practice, avoid direct contact with blood and body fluids, avoid sharing sharp items such as razors, nail clippers, toothbrushes, and earrings or body rings, and use sterile needles for ear or body piercing, and tattoos.
Strengths and Limitations
One of the strengths of the current study is the study populations were representatives of the wider population in the Bench-Maji zone covering all rural and urban adults as well as semi-pastoralists. Furthermore, to ensure the validity of our findings, we strictly followed quality control methods for both the collection of socio-demographic and laboratory-based data. However, this study has some limitations. First, occult HBV infections were not identified since polymerase chain reaction test was not carried out in this study. Second, the small number of persons with HBsAg in each row by column table may create concerns about wider confidence interval around the HBsAg estimates that were stratified by demographic and behavioral characteristics. Third, there may be a possibility of exposure misclassification bias related to willingness to disclose risky behaviors that may be stigmatized such as alcohol drinking and circumcision. However, we believe that the exposure misclassification is non-differential. Fourth, selection bias is expected as males tend to work outside the home and may not be available at the time of data collection. However, our findings indicate that the number of male and female participates is nearly equivalent. Thus, the introduction of selection bias is minimal. Finally, we only studied people aged 18 years or above and did not assess the prevalence in children. Therefore, the interpretation of results from this study and future studies need to consider these limitations. Despite these limitations, we believe that the use of population-based data provides valuable information for determining the regional burden of infection in countries where surveillance for these diseases is limited.
Conclusions
The current study showed that the burden of HBV infection in adults at the community level is highly endemic. Modifiable risk factors such as tattooing on gums, tattooing on the body, and contact with a jaundiced person account for the high prevalence of HBV infection in the study area. Behavioral education and communication need to address these modifiable risk factors to reduce HBV infection and transmission. In addition, population-based screening and treatment of HBV at national level recommended.
Acknowledgments
We are grateful to Mizan Tepi University for its financial and technical support. We are also very indebted to extend our gratitude to the study participants. Special thanks goes to the research assistants who participated in data collection.
Funding Statement
Funding for this study was made possible through grants offered by Mizan Tepi University. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
HBV, hepatitis B virus; HBsAg, hepatitis B virus surface antigen.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the primary author on reasonable request (Alex.sayihalem2012@gmail.com).
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki and after ethical clearance was obtained from the institute of research, community development and support (IRCDS) office of the Mizan Tepi University (Ref No: MTU/CHS/56/785/61/10). After explaining the objectives of the study, written informed consent was obtained from each study participant. For the participants who were not able to read and write, finger-print was used as a signature. All the data and samples obtained from them were kept confidential by using codes instead of any personal identifiers and meant only for the purpose of the study. For those study participants who resulted positive based on our findings, all necessary information was informed of the individual and finally linked to the nearby health center. Further follow-up was performed by health extension workers.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
We declare that we have no competing interests.
References
- 1.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X [DOI] [PubMed] [Google Scholar]
- 3.Razavi-Shearer D, Gamkrelidze I, Nguyen MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6 [DOI] [PubMed] [Google Scholar]
- 4.Chien Y-C, Jan C-F, Kuo H-S, Chen C-J. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev. 2006;28(1):126–135. doi: 10.1093/epirev/mxj010 [DOI] [PubMed] [Google Scholar]
- 5.Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA oncol. 2017;3(12):1683–1691. doi: 10.1001/jamaoncol.2017.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allain JP, Owusu‐Ofori S, Bates I. Blood transfusion in sub‐Saharan Africa. Transfusion Altern Transfusion Med. 2004;6(1):16–23. doi: 10.1111/j.1778-428X.2004.tb00108.x [DOI] [Google Scholar]
- 7.WHO. Hepatitis B; 2019. Available from: https://www.who.int/news-room/fact-sheet/detail/hepatitis-b. Accessed on 29May 2020.
- 8.CDC. Viral Hepatitis; 2020. Available from: https://www.cdc.gov/hepatitis/abc/index.htm. Accessed on 29May 2020..
- 9.Kao J-H. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev Gastroenterol Hepatol. 2014;8(4):553–562. [DOI] [PubMed] [Google Scholar]
- 10.François G, Dochez C, Jeffrey Mphahlele M, Burnett R, Van Hal G, Meheus A. Hepatitis B vaccination in Africa: mission accomplished? South Afr J Epidemiol Infect. 2008;23(1):24–28. doi: 10.1080/10158782.2008.11441296 [DOI] [Google Scholar]
- 11.Tamirat KS, Sisay MM. Full immunization coverage and its associated factors among children aged 12–23 months in Ethiopia: further analysis from the 2016 Ethiopia demographic and health survey. BMC Public Health. 2019;19(1):1019. doi: 10.1186/s12889-019-7356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16(1):761. doi: 10.1186/s12879-016-2090-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapicetta M, Hailu K, Morace G, et al. Prevalence of HBeAg, anti-HBe serological markers and HBV-DNA in asymptomatic carriers in Ethiopia. Eur J Epidemiol. 1989;5(4):481–485. doi: 10.1007/BF00140145 [DOI] [PubMed] [Google Scholar]
- 14.Gebreselassie L, Loche M, Dayal R, Perrin LH. Prevalence of specific markers of viral hepatitis A and B among an Ethiopian population. Bull World Health Organ. 1983;61(6):991. [PMC free article] [PubMed] [Google Scholar]
- 15.Abera B, Adem Y, Yimer M, Mulu W, Zenebe Y, Mekonnen Z. Community seroprevalence of hepatitis B, C and human immunodeficiency virus in adult population in gojjam zones, northwest Ethiopia. Virol J. 2017;14(1):21. doi: 10.1186/s12985-017-0696-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosgeroglu N, Ayranci U, Vardareli E, Dincer S. Occupational exposure to hepatitis infection among Turkish nurses: frequency of needle exposure, sharps injuries and vaccination. Epidemiol Infect. 2004;132(1):27–33. doi: 10.1017/S0950268803001407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarus J. Global policy report on the prevention and control of viral hepatitis in WHO Member States. 2014. [Google Scholar]
- 18.Tessema B, Yismaw G, Kassu A, et al. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at gondar university teaching hospital, Northwest Ethiopia: declining trends over a period of five years. BMC Infect Dis. 2010;10(1):111. doi: 10.1186/1471-2334-10-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulu W, Zenebe Y, Abera B, Yimer M, Hailu T. Prevalence of human immunodeficiency virus and hepatitis B virus infections in young women seeking abortion care in Ethiopia: a cross-sectional study. BMC Public Health. 2016;16(1):996. doi: 10.1186/s12889-016-3658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deressa T, Damtie D, Fonseca K, et al. The burden of hepatitis B virus (HBV) infection, genotypes and drug resistance mutations in human immunodeficiency virus-positive patients in Northwest Ethiopia. PLoS One. 2017;12(12):e0190149. doi: 10.1371/journal.pone.0190149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abera B, Zenebe Y, Mulu W, Kibret M, Kahsu G. Seroprevalence of hepatitis B and C viruses and risk factors in HIV infected children at the felgehiwot referral hospital, Ethiopia. BMC Res Notes. 2014;7(1):838. doi: 10.1186/1756-0500-7-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hundie GB, Raj VS, GebreMichael D, Haagmans BL. Seroepidemiology of hepatitis B and C virus infections among blood donors in Ethiopia. J Med Virol. 2017;89(7):1300–1303. doi: 10.1002/jmv.24770 [DOI] [PubMed] [Google Scholar]
- 23.Abebe A, Nokes D, Dejene A, Enquselassie F, Messele T, Cutts F. Seroepidemiology of hepatitis B virus in Addis Ababa, Ethiopia: transmission patterns and vaccine control. Epidemiol Infect. 2003;131(1):757–770. doi: 10.1017/S0950268803008574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayele AG, Gebre-Selassie S. Prevalence and risk factors of hepatitis B and hepatitis C virus infections among patients with chronic liver diseases in public hospitals in Addis Ababa, Ethiopia. ISRN Trop Med. 2013;2013:1–7. doi: 10.1155/2013/56382125285308 [DOI] [Google Scholar]
- 25.Awole M, Gebre-Selassie S. Seroprevalence of HBsAg and its risk factors among pregnant women in Jimma, Southwest Ethiopia. Ethiopian J Health Dev. 2005;19(1):45–50. doi: 10.4314/ejhd.v19i1.9970 [DOI] [Google Scholar]
- 26.Spearman CW, Afihene M, Ally R, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterology Hepatol. 2017;2(12):900–909. doi: 10.1016/S2468-1253(17)30295-9 [DOI] [PubMed] [Google Scholar]
- 27.Bwogi J, Braka F, Makumbi I, et al. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 2009;9(2). [PMC free article] [PubMed] [Google Scholar]
- 28.Ochola E, Ocama P, Orach CG, et al. High burden of hepatitis B infection in Northern Uganda: results of a population-based survey. BMC Public Health. 2013;13(1):727. doi: 10.1186/1471-2458-13-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andriamandimby SF, Olive -M-M, Shimakawa Y, et al. Prevalence of chronic hepatitis B virus infection and infrastructure for its diagnosis in Madagascar: implication for the WHO’s elimination strategy. BMC Public Health. 2017;17(1):636. doi: 10.1186/s12889-017-4630-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ly KN, Kim AA, Umuro M, et al. Prevalence of hepatitis B virus infection in Kenya, 2007. Am J Trop Med Hyg. 2016;95(2):348–353. doi: 10.4269/ajtmh.16-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews PC, Geretti AM, Goulder PJ, Klenerman P. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in Sub-Saharan Africa. J Clin Virol. 2014;61(1):20–33. doi: 10.1016/j.jcv.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 32.Umare A, Seyoum B, Gobena T, Mariyam TH. Hepatitis B virus infections and associated factors among pregnant women attending antenatal care clinic at Deder Hospital, Eastern Ethiopia. PLoS One. 2016;11(11):e0166936. doi: 10.1371/journal.pone.0166936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tegegne D, Desta K, Tegbaru B, Tilahun T. Seroprevalence and transmission of Hepatitis B virus among delivering women and their new born in selected health facilities, Addis Ababa, Ethiopia: a cross sectional study. BMC Res Notes. 2014;7(1):239. doi: 10.1186/1756-0500-7-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiferaw F, Letebo M, Bane A. Chronic viral hepatitis: policy, regulation, and strategies for its control and elimination in Ethiopia. BMC Public Health. 2016;16(1):769. doi: 10.1186/s12889-016-3459-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molla S, Munshea A, Nibret E. Seroprevalence of hepatitis B surface antigen and anti HCV antibody and its associated risk factors among pregnant women attending maternity ward of Felege Hiwot Referral Hospital, northwest Ethiopia: a cross-sectional study. Virol J. 2015;12(1):204. doi: 10.1186/s12985-015-0437-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochwoto M, Kimotho JH, Oyugi J, et al. Hepatitis B infection is highly prevalent among patients presenting with jaundice in Kenya. BMC Infect Dis. 2016;16(1):101. doi: 10.1186/s12879-016-1409-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayagam S, Thursz M, Sicuri E, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16(12):1399–1408. doi: 10.1016/S1473-3099(16)30204-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO. Hepatitis B; 2019. Available from: https://www.who.int/news-room/fact-sheet/detail/hepatitis-b. Accessed on 29May 2020.
- CDC. Viral Hepatitis; 2020. Available from: https://www.cdc.gov/hepatitis/abc/index.htm. Accessed on 29May 2020..


