To the Editor:
In 2019 the United States experienced an outbreak of respiratory illnesses that were associated with e-cigarette use. e-Cigarette, or vaping, product use-associated lung injury (EVALI) resulted in >2800 hospitalizations and 68 deaths, but numbers have dwindled recently.1 This decline may relate to a decrease in high-risk products, predominantly illicit tetrahydrocannabinol products that contain vitamin E acetate, which an adulterant that is added to improve dealers’ profit margins.2 Reports of EVALI continue despite changes in usage patterns, and the diagnosis has been complicated by the emergence of coronavirus disease 2019 (COVID-19). Like EVALI, COVID-19 may cause acute respiratory failure, constitutional complaints, and ground glass opacities on chest imaging.3,4 Herein we report a case series of patients who have been diagnosed with EVALI since the start of the COVID-19 pandemic and provide strategies for the diagnosis and treatment of patients with EVALI in the time of COVID-19.
All patients with EVALI at the University of Utah and Intermountain Healthcare have been tabulated since June 2019. We included all patients who were diagnosed with EVALI who had negative influenza testing and at least one negative COVID-19 test from March 1, 2020, when testing began in Utah, to May 15, 2020. Clinical course, treatment, outcomes, and severe acute respiratory syndrome coronavirus 2 (SARS-coV-2) tests are reported with the use of descriptive statistics. This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. It was approved by the Institutional Review Board at Intermountain Healthcare (1051214) and University of Utah Health (00128817).
Twelve patients met inclusion criteria, 11 of whom met Centers for Disease Control criteria for “confirmed” EVALI and one “probable” (Table 1). Our findings align with previous reports5,6: patients were predominantly young (mean age, 30.8 years; range, 18 to 53 years), white (100%; of whom 2 of 12 [17%] were Hispanic), and male (67%) and had used tetrahydrocannabinol products (67%). Severity of illness varied: nine patients were admitted to ICUs; two patients were admitted to wards, and one patient was treated in the ED. One-half of the patients had psychiatric comorbidities that included anxiety and depression. Clinicians ruled out influenza in all patients; expanded respiratory virus polymerase chain reactions (PCRs) were negative in ten patients (83%), and respiratory syncytial virus test results were negative in another one. Two patients underwent bronchoscopy, and the SARS-coV-2 PCR was negative in both, which shows macrophage and neutrophil-predominant cell counts with negative infectious workup. All patients received antibiotics for pneumonia, and eight patients (67%) received corticosteroids. Patients were hospitalized an average of 6.0 days.
Table 1.
Characteristics of Patients With EVALI
| Variable | Total (N = 12) |
|---|---|
| Age, mean ± SD; median; range (minimum-maximum) | 30.8 ± 12.1; 27; (18-53) |
| Sex: Male, No. (%) | 8 (67) |
| Ethnicity, No. (%) | |
| Hispanic | 2 (17) |
| Non-Hispanic | 10 (83) |
| Race: white, No. (%) | 12 (100) |
| Comorbidity, No. (%) | 8 (67) |
| Reported or confirmed tetrahydrocannabinol use, No. (%) | 9 (75) |
| WBC count, mean ± SD, k/uL | 15.3 ± 3.5 |
| Absolute lymphocyte count | 1.3 ± 0.5 |
| Eosinophils total | 0.2 ± 0.5 |
| C-reactive protein, mean ± SD, mg/dL | 69.9 ± 93.8 |
| SARS-coV-2 tests, mean ± SD; median; (range minimum maximum) | 2.5 ± 1.0; 2; (1-4) |
| 1 Test, No. (%) | 1 (8) |
| 2 Tests, No. (%) | 7 (58) |
| 3 Tests, No. (%) | 2 (17) |
| 4 Tests, No. (%) | 2 (17) |
| SARS-coV-2 test, No. (%) | |
| PCR | 30 (97) |
| Nasopharyngeal | 28 |
| BAL | 2 |
| IgG antibody | 1 (3) |
| Chest radiographs, No. (%) | 12 (100) |
| Chest CT scan, No. (%) | 12 (100) |
| ICU admission, No. (%) | 9 (75) |
| Ward admission, No. (%) | 2 (17) |
| ED treatment, No. (%) | 1 (8) |
| Hospital length of stay, mean ± SD | 6.0 ± 3.7 |
| Bronchoscopy, No. (%) | 2 (16.7) |
| Treatment with corticosteroids, No. (%) | 8 (67) |
| Initial corticosteroid dose in prednisone milligram equivalents, median (interquartile range); (minimum, maximum) | 56 (15); (50, 88) |
| Treatment with antibiotics, No. (%) | 12 (100) |
| Highest oxygen support, No. (%) | |
| Invasive mechanical ventilation | 3 (25) |
| Noninvasive positive pressure ventilation | 1 (8) |
| High-flow nasal cannula | 3 (25) |
| Nasal cannula | 5 (42) |
EVALI = e-cigarette, or vaping, product use-associated lung injury; PCR = polymerase chain reaction; SARS-coV-2 = severe acute respiratory syndrome coronavirus 2.
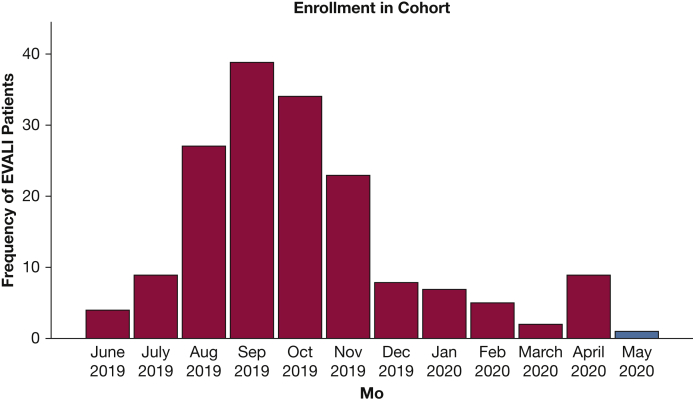
Our case series highlights several important features. Whereas the nationwide total of EVALI cases has faded in recent months, we have observed an uptick in Utah (Fig 1). Several patients reported substantial anxiety, which is a frequent comorbidity in EVALI,5 which led them to vape more to cope with pandemic stressors. Their agent of choice, tetrahydrocannabinol oil, remains outlawed in many states including Utah, which indicates that patients may obtain it through unregulated sources. Ongoing investigations by federal authorities shed doubt on one distinct causal agent,1,7 which suggests that the causes may be heterogenous and that cases may persist despite efforts to curtail vitamin E acetate. We definitively linked only two patients to vitamin E acetate through state laboratory testing. This convergence of social and medical factors suggests that we will continue to see patients with EVALI for the foreseeable future.
Figure 1.
Frequency of reported patients with EVALI by month of diagnosis. Data presented through May 15, 2020. The first case of EVALI in our cohorts was reported June 24, 2019. The first case in this case series with negative COVID testing was diagnosed March 17, 2020. The first case of COVID-19 diagnosed in Utah was March 6, 2020. May 2020 is highlighted because data are incomplete and current as of May 15, 2020. COVID-19 = coronavirus disease 2019; EVALI = e-cigarette, or vaping, product use-associated lung injury.
This experience highlights the difficulty of diagnosing EVALI during the COVID-19 pandemic. Presentations may appear remarkably similar, and we risk premature closure diagnosing COVID-19 in patients presenting with acute respiratory failure. Most patients were treated initially as having COVID-19, and EVALI was not considered in several cases because patients did not volunteer their vaping history until well into their illnesses. The diagnosis of EVALI is still considered one of exclusion,1 which has proven problematic. Physicians struggle diagnosing EVALI, given variable SARS-coV-2 testing availability and imperfect sensitivities and specificities and barriers to bronchoscopy due to aerosolization concerns. In addition to excluding “usual” alternative diagnoses like alveolar hemorrhage,8 when do clinicians feel that they have satisfactorily “ruled out” COVID-19 and the probability of EVALI supersedes it? Our experience illustrates this self-doubt, because we sent a collective 31 tests to ensure these 12 patients did not have COVID-19, despite first PCR positivity in >95% of cases (internal data). Even then, clinicians still experienced significant apprehension in diagnosing EVALI and administering systemic corticosteroids. Assessing patients with a broad differential diagnosis and obtaining key relevant history and risk factors, in addition to understanding local SARS-coV-2 test characteristics, are key in making the right diagnosis in patients who present with respiratory symptoms.
Though no pathognomonic findings can distinguish EVALI from COVID-19, we noted some differentiating features. First, COVID-19 often leads to normal or low WBC counts often with lymphopenia.3,9 In contrast, most patients with EVALI present with leukocytosis,5,6 as did 11 of 12 of patients (92%) here (mean WBC, 15.3 k/uL); thus, the patient with leukopenia appears more likely to have COVID-19. Second, a wide age of discrepancy exists between “typical” EVALI and patients with COVID-19. Youths who become severely ill from COVID-19 are relatively uncommon, and EVALI suspicions should rise in the young patient who vapes and presents with respiratory failure. One cannot, however, use age as the sole discriminator between diseases because EVALI can afflict older patients and be morbid.1 Repeated efforts at obtaining a vaping history remain key in making the diagnosis. Third, the administration of systemic corticosteroids appears helpful because both are a therapeutic and diagnostic endeavor. Whereas experts debate the utility of corticosteroids in COVID-19 pneumonia,10 case series suggest their utility in treating EVALI, and we recognize that some patients have a clinical phenotype comparable with eosinophilic pneumonia.2,5 In patients with indistinguishable clinical features, we found improvement within 1 to 3 days with corticosteroids to be highly suggestive of EVALI. We recommend a short course of moderate-dose corticosteroids (prednisone 40-60 mg for 5-10 days) when the diagnosis of EVALI seems probable.
In summary, EVALI still exists in 2020. A single, unifying causal agent for EVALI is not apparent, and it remains a diagnosis of exclusion and clinical probability. The psychologic ramifications of the COVID-19 pandemic may promote more cases of EVALI. COVID-19 complicates the care of patients with EVALI by confounding the diagnosis and seeding doubt in the physician’s mind when considering treatment with corticosteroids. In the time of COVID-19, it remains essential to consider differential diagnoses that include EVALI and to obtain careful vaping histories in patients presenting with respiratory complaints.
Acknowledgments
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: None declared.
FUNDING/SUPPORT: S. J. C., M. J. L., and D. P. B. were supported in part by a grant from the National Institutes of Health [grant U01HL123018-06S1].
References
- 1.Werner A.K., Koumans E.H., Chatham-Stephens K. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589–1598. doi: 10.1056/NEJMoa1915314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonas AM, Raj R. Vaping-related acute parenchymal lung injury: a systematic review. Chest. 2020;158(4):155-1565. [DOI] [PubMed]
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blagev D.P., Harris D., Dunn A.C., Guidry D.W., Grissom C.K., Lanspa M.J. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet. 2019;394(10214):2073–2083. doi: 10.1016/S0140-6736(19)32679-0. [DOI] [PubMed] [Google Scholar]
- 6.Layden J.E., Ghinai I., Pray I. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin: final report. N Engl J Med. 2020;382(10):903–916. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration Lung Injuries Associated with Use of Vaping Products. 2020. https://www.fda.gov/news-events/public-health-focus/lung-injuries-associated-use-vaping-products
- 8.Aberegg SK, Maddock SD, Blagev DP, Callahan SJ. Diagnosis of EVALI: general approach and the role of bronchoscopy. Chest. 2020;158(2):820-827. [DOI] [PubMed]
- 9.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020;ciaa478. [DOI] [PMC free article] [PubMed]