Abstract
The COVID-19 pandemic has put a serious strain on health treatments as well at the economies of many nations. Unfortunately, there is not currently available vaccine for SARS-Cov-2/COVID-19. Various types of patients have delayed treatment or even routine check-ups and we are adapting to a virtual world. In many cases, surgeries are delayed unless they are essential. This is also true with regards to cancer treatments and screening. Interestingly, some existing drugs and nutraceuticals have been screened for their effects on COVID-19. Certain FDA approved drugs, vitamin, natural products and trace minerals may be repurposed to treat or improve the prevention of COVID-19 infections and disease progression. This review article will summarize how the treatments of various cancer patients has changed during the COVID-19 era as well as discuss the promise of some existing drugs and other agents to be repurposed to treat this disease.
Keywords: COVID-19, Cancer, Natural products, Nutraceuticals, Repurposing approved drugs
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, COVID-19) is a highly infectious virus which has caused considerable health problems world-wide. The COVID-19 viral pandemic appears to be unprecedented in the life-spans of most individuals today, both due to its pervasiveness as well as effects on the global economy. Although we constantly have outbreaks of various viruses world-wide, the tolls on human lives and world-wide economies are unprecedented. While some of us have lived during various viral outbreaks (HIV/AIDS. Asian Flu, SARS and others) multiple wars (World War II, Korean Conflict, Vietnam War, Iraq/Afghanistan Wars and others), suicide bombings (9/11 and others), bad political governments, food rationing, the COVID-19 pandemic present new challenges for us to live with as there is currently no proven vaccine or drugs that will effectively prevent COVID-19 infection or treat patients after infection, there has been a reluctance of cancer and other patients to avoid hospitals and clinics and delay treatments or screenings in fear of development of the disease and potentially succumb to the disease. This is especially true with elderly patients as they have higher risk factors (diabetes, heart problems, high blood pressure, hypertension) and immunosuppressed patients.
1.1. Overview, risk factors for cancer patients and COVID-19
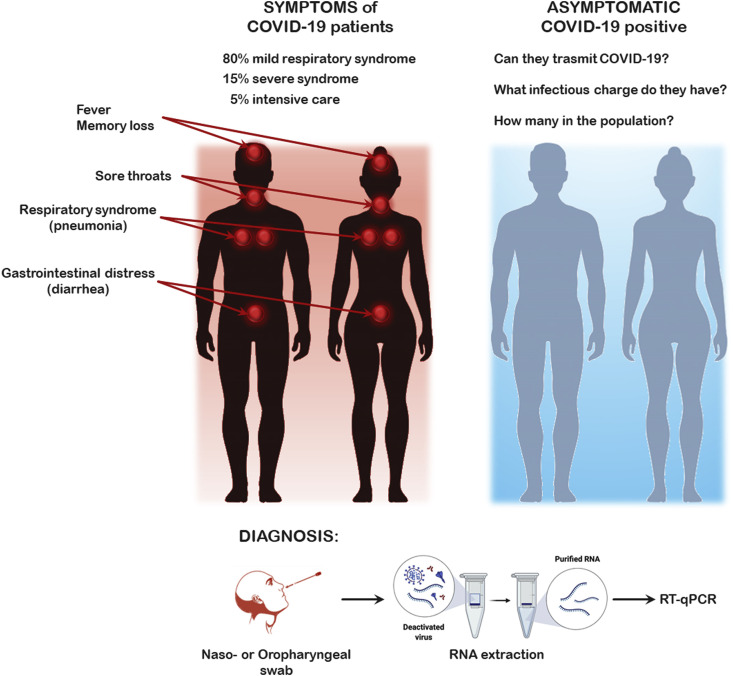
SARS-CoV-2 is a highly pathogenic corona virus also called COVID-19. It is a single-stranded, positive-sense RNA virus, which means the viral RNA can be directly translated in viral proteins in infected cells. Infection with SARS-CoV-2 can cause a wide clinical spectrum of seemingly unrelated symptoms ranging from fever to gastrointestinal distress to memory loss. A large percentage of the infected patients remain asymptomatic. This causes significant problems in terms of transmission as we often do not know who is and who is not infected. Currently approximately 80% of the symptomatic patients exhibit a mild respiratory syndrome, while approximately 15% of the patients develop a severe disease requiring hospitalization and approximately 5% need intensive care (Gordin et al., 2020). Many of the more serious affected patients are elderly and may some have cancer as cancer is predominately a disease of the elderly. An overview of the symptoms and diagnosis of COVID-19 disesase is presented in Fig. 1 .
Fig. 1.
Overview of the symptoms and diagnosis of COVID-19 disesase.
It is a pandemic infectious virus which causes respiratory diseases. Infection with this virus is associated with a high morbidity and mortality. COVID-19 is associated with comorbidities and multi-organ damage. Infection with SARS-CoV-2 is associated with a higher death rate than either SARS-CoV or MERS. SARS-CoV-2 causes damage to alveolar tissues and acute respiratory failure. SARS-CoV-2 causes damage to multiple organs namely central nervous system, eye, heart, gastrointestinal, liver, lung and other body organs (Renu et al., 2020).
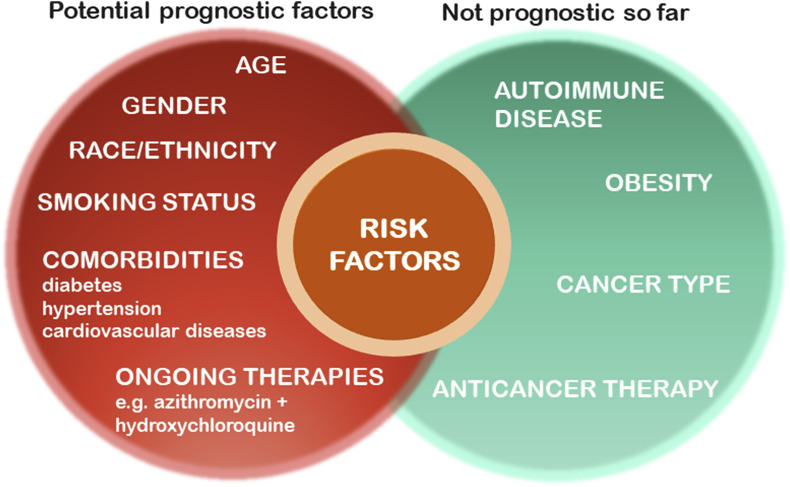
Potential prognostic factors for mortality and severe illness are being evaluated in a cohort study in an ongoing clinical trial with patients with cancer [NCT04354701] from the USA, Canada, and Spain from the COVID-19 and Cancer Consortium (CCC19). Some criteria on the cancer patients evaluated in the survey include: age, sex, smoking status, and obesity. So far, 928 patients met inclusion criteria. The average age was 66 years old and 50% of the patients were male and 50% were females. Breast (21%) and prostate (16%) were the most common malignancies. 39% of the patients were undergoing anticancer therapies. Increased age, cigarette smoking status (former smoker vs never smoked), number of comorbidities, active cancer status (progressing vs remission), and treatment with azithromycin (a macrolide-type antibiotic used to treat a wide variety of bacterial infections) plus hydroxychloroquine (an immunosuppressive and anti-parasite drug used to treat patients with malaria, lupus and arthritis) are being evaluated. So far, it was determined that the 30-day all-cause mortality was high. Interestingly, race, ethnicity, obesity, cancer type, or type of anticancer therapy, were determined not to be associated with mortality in this trial so far. The authors concluded that a longer follow-up time is required to determine the effects of various cancer types and cancer treatments provided to patients infected with COVID-19 (Kuderer et al., 2020). A large study in Israel has documented the role of age and gender in the recovery from COVID-19 infection (Voinky et al., 2020). An overview of some of the potential risk factors associated with COVID-19 disease is presented in Fig. 2 .
Fig. 2.
Overview of Some of the Factors which May Influence the Development of COVID-19 Disease.
An additional cohort study was performed in the United Kingdom (UK) on cancer patients and COVID-19 that were receiving chemotherapy or other cancer treatments. This involved cancer patients enrolled in the UK Coronavirus Cancer Monitoring Project (UKCCMP). Patients were tested for COVID by RT-PCR assay with a specimen prepared from a nose or throat swab. Only COVID-19 RT-PCR positive patients were included in the study. So far, the primary endpoint was either all-cause mortality, or discharge from hospital. 800 patients have been examined from March 18, to April 26, 2020. 52% of the patients had a mild COVID process while 28% of the patients died. The risk of death was associated with increased age, male sex, and the presence of comorbidities including hypertension (high blood pressure) and cardiovascular disease. 35% of the patients had received chemotherapy four weeks before they tested positive for COVID-19. So far, it appears that chemotherapeutic drug treatment did not have a significant effect on mortality form COVID-19. Interestingly, hormonal therapy, immunotherapy, radiotherapy or targeted therapy also did not appear to influence the incidence of death form COVID-19 in this study so far (Lee et al., 2020).
1.2. Breast cancer patients and COVID-19
Treatment of breast cancer patients during the COVID-19 era is challenging as approximately 30% of breast cancer patients diagnosed annually in the USA are greater than seventy years old. In addition, many older breast cancer patients have recurrent and/or metastatic disease. Multidisciplinary care considerations are being proposed to improve their treatment (Freedman et al., 2020; Viale et al., 2020).
One of the consequences of the COVID-19 pandemic has been the need to prevent infections and the delay of breast cancer screenings. The Canadian Association of Breast Imaging/Canadian Association of Radiologist has developed safe guideline for breast imaging and concluded that delaying breast cancer screening should be avoided if possible, during the COVID-19 era (Seely et al., 2020).
1.3. Colorectal cancer patients and COVID-19
Treatment of rectal cancer patients during the COVID-19 era is a challenge as it requires a multidisciplinary approach and considerable resources (Skowron et al., 2020). Guidelines have been established to provide safer treatment of colorectal cancer treatment both in terms of personal protective equipment (PPE) and surgery/operating rooms. Negative-pressure environments may be necessary in the operating rooms to allow the use of aerosol-generating procedures (Wexner et al., 2020).
1.4. Gastrointestinal cancer patients and COVID-19
Treatment of gastrointestinal cancer patients during the COVID-19 era by radiation therapy has also encountered some difficulties. The RADS principle (Remote visits, Avoid radiation, Defer radiation, Shorten radiation) has been proposed to treat gastrointestinal cancer patients with radiation. (Tchelebi et al., 2020).
1.5. Glioblastoma cancer patients and COVID-19
Due to the advanced nature of high-grade glioma, it has been proposed that the treatment of patients with this cancer should not be delayed in the COVID-19 era (Bernhardt et al., 2020).
1.6. Gynecological cancer patients and COVID-19
It has been recommended that patients with gynecological cancer should be managed in hierarchical and individualized manners. In addition, it has been suggested that local conditions related to COVID-19 may influence treatment strategies (Wang et al., 2020).
1.7. Head and neck cancer patients and COVID-19
In Hong Kong adaptive measures were developed for patients with laryngectomy based on the previous experiences developed for the SARs outbreak in 2003. Guidelines were developed to reduce or prevent droplets with physical barriers and sanitation of hands and equipment as well as reducing physical contacts (Yeung et al., 2020).
Hypofractionated radiotherapy (RT-hypo) is an approach to treat certain head and neck squamous cell cancer [HNSCC] patients. RT-hypo consists of 60 Gy in 25 fractions over 5 weeks. RT-hypo was compared with moderately accelerated radiotherapy (RT-acc) alone (70 Gy in 35 fractions over 6 weeks), or concurrent chemoradiotherapy (CCRT). Patients were classified in terms of human papilloma virus (HPV) presence or absence. It was proposed that RT-hypo treatment method be used in place of CCRT for certain stages of HPV+ and HPV- HNSCC patients during the COVID-19 outbreak (Huang et al., 2020).
1.8. Hepatocellular cancer patients and COVID-19
A problem in the treatment of hepatocellular carcinoma patients during the COVID-19 era is that many of them are undergoing liver transplantation and take immunosuppressive drugs (Jeddou and Boudjema, 2020). An additional complication is that some of the treatments for COVID-19 are also associated with liver toxicity. (Ridruejo and Soza, 2020). Some clinicians have proposed that surgical resection for certain high-risk patients should be postponed (Triki et al., 2020).
1.9. Lung cancer patients and COVID-19
Problems with the treatment of lung cancer patients are that progression can be rapid and the risk of life threating COVID-19 infection. It has been suggested that the treatment of lung cancer patients should not be delayed to prevent rapid cancer progression (Singh et al., 2020).
In a different group of lung cancer experts consisting of twenty-four members, including pulmonologists (n = 17), thoracic radiologists (n = 5), and thoracic surgeons (n = 2), a consensus conclusion regarding lung cancer was obtained. They proposed to defer enrollment in lung cancer screening and modify the evaluation of lung nodules, defer surveillance imaging and minimize nonurgent interventions during the evaluation of lung nodules and stage I non-small cell lung cancer. These recommendations were due to the added risks from potential exposure (Mazzone et al., 2020).
In Germany, general strategies to reduce unnecessary patient contact during therapy are recommended. Nivolumab therapy intervals in NSCLC patients may be extended to four (480 mg) instead of two weeks (240 mg) with higher doses according to the German Society of Hematology and Oncology (DGHO) recommendations on COVID-19 in cancer patients. (www.onkopedia.com/de/onkopedia/guidelines/coronavirus-infektion-covid-19-bei-patienten-mit-blut-und-krebserkrankungen).
1.10. Melanoma cancer patients and COVID-19
Due to the COVID-19 outbreak, the majority of dermatological visits were postponed in some regions of certain countries This could result in a delay in early diagnosis which is essential for improved patient survival. Delayed diagnosis of melanoma could result in increased morbidity, mortality and health care costs. Alternative models of skin cancer screening have been proposed for use during the COVID-19 era (Villani et al., 2020).
In another study with melanoma and other skin cancer patients, the authors recommended delaying the treatment of patients with T0 to T1 stages of the disease for three months providing there was no macroscopic residual disease. Treatment of patients with tumors ≥ T2 was proposed to be delayed for 3 months if the biopsy margins are negative. Thus, the treatment of melanoma and other skin cancer patients at certain cancer centers is dependent on the tumor stage during the COVID-19 era (Baumann et al., 2020).
The programmed death-1 (PD-1)/programmed death ligand-1 (PDL-1) pathway is an important immunotherapeutic target in melanoma and other cancers. In a study with a single elderly woman, who had additional co-morbidities and was taking nivolumab (a PD-1 inhibitor) for metastatic malignant melanoma, a potential anti-viral effect was observed. Inhibition of PD-1 has been proposed to have effects on viral infections. Additional research needs to be performed on the effects of inhibition of the PD-1/PD-L1 pathway on COVID-19 replication (Yekeduz et al., 2020).
1.11. Multiple myeloma cancer patients and COVID-19
It has been postulated that patients with multiple myeloma are at increased risk for more severe COVID-19 infection. This may be due to in the older age, immunocompromised state and other comorbidities of the MM patients. The European Myeloma Network has provided a set of guidelines with regards to management of MM patients in the COVID-19 era. They suggested that in countries or regions where COVID-19 is high, MM patients should have a PCR test to determine the presence of SARS-Cov-2 before hospital admission on a nasal swab. This is to avoid infection of other patients and should be performed on the patient before starting a new treatment line, cell apheresis or autologous stem cell transplant (ASCT). The authors proposed that autologous and allogeneic transplantation should be delayed, and watchful waiting should be considered for standard risk patients. MM patients with COVID-19 disease should interrupt MM treatment (Terpos et al., 2020).
1.12. Ovarian cancer patients and COVID-19
Ovarian cancer patients tend to be diagnosed later in life; thus they are susceptible to the devastating effects of COVID-19 infection (Bogani et al., 2020).
1.13. Prostate cancer patients and COVID-19
The treatment of prostate cancer (PC) patients in the COVID-19 era has been difficult as prostate cancer is detected in older men. In Italy, the presence of physicians and staff in urological departments and other patient services were reduced during the peak of the COVID-19 pandemic in that country. Interestingly, the only group of PC patients did not receive a significant reduction were medical therapies for advanced hormone sensitive (HS) or castration resistant (CR) PC (Sciarra et al., 2020).
1.14. Thyroid cancer patients and COVID-19
In general, thyroid cancer patients are not considered at high risk for COVID-19 infection and mortality in comparison to the general population (Vrachimis et al., 2020).
1.15. Complications of treating cancer patients in the COVID-19 era
As previously mentioned, there are problems treating certain HCC patients with certain drugs directed against COVID-19 infections due to hepatotoxicity. Interactions with chemotherapeutic drugs and drug used to treat COVID-19 may also be a problem (Jafari et al., 2020).
The surgical operating room also remains a problem during the COVID-19 era for cancer patients. Many hospitals have limited elective surgeries in order to prevent COVID-19 infections and only allow surgeries necessary for survival of the cancer patient. Guidelines have been proposed such as the “Covid-minimal surgery pathway” to minimize the risk of nosocomial infections (Boffa et al., 2020).
An additional consideration with surgery of cancer patients is they are often older than the general population and many may be immunosuppressed due to their treatments. Thus, frequently only emergencies are preformed, and elective surgeries delayed (Chen et al., 2020).
Blood transfusions during chemotherapy should not be restricted as the risk of SARS-Cov-2 transmission is estimated to be very low. Neutropenia and hypoglobulinemia e.g. during anti-CD20 directed therapy in chronic lymphocytic leukemia patients (CLL) should be treated consistently. (German Society of Hematology and Oncology (DGHO) recommendations on COVID-19 in cancer patients (www.onkopedia.com/de/onkopedia/guidelines/coronavirus-infektion-covid-19-bei-patienten-mit-blut-und-krebserkrankungen)
2. Identification of proteins/drugs binding SARS-CoV-2 virally encoding proteins
26 of the 29 proteins encoded by SARS-CoV-2 were cloned, tagged with a marker and expressed in human cells in an attempt to identify potential drug targets. Human proteins interacting with these proteins were identified by affinity-purification mass spectrometry (AP-MS). 332 high-confidence SARS-CoV-2-human protein-protein interactions (PPIs) were discovered. 66 druggable human proteins or host factors were determined to be targeted by 69 compounds (29 FDA-approved drugs, 12 drugs in clinical trials, and 28 preclinical compounds). Two sets of pharmacological agents were determined to have anti-viral activity in viral assays. These drugs are inhibitors of mRNA translation. Specifically, the inhibitors regulate the Sigma1 and Sigma2 receptors (Gordon et al., 2020).
Various signaling pathways have been identified by the mapping the proteins that interact with SARS-CoV-2 proteins. Some of these proteins are present in oncogenic pathways. Some existing drugs, originally developed to inhibit the oncogenes may be repurposed and prove effective in treating COVID-19 (Tutuncuoglu et al., 2020).
3. Repurposing of existing drugs/natural products and minerals to treat cancer patients with COVID-19
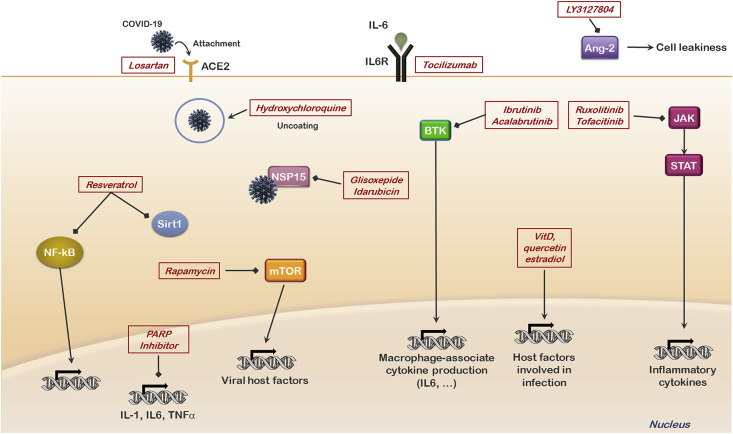
Remdesivir (RDV) is an antiviral drug. It has shown some effects on Middle East Respiratory Syndrome (MERS-CoV) infected rhesus macaque model (de Wit et al., 2020). The effects of RDV and combination of lopinavir (LPV), ritonavir (RTV) and interferon beta against MERS-CoV have been examined in mouse models (Sheahan et al., 2020). In vitro studies indicated that combining RDV and IFNβ had better antiviral activity in comparison to LPV and RTV. In studies with mice, treatment with RDV improved pulmonary function, and reduced viral loads in the lung and the severe lung pathology normally observed after infection with MERS-CoV. There are thirty-one clinical trials with RDV and various aspects of COVID-19 listed on the ClinicalTrials.gov website. An overview of some approaches being examined to inhibit development of COVID-19 disease is presented in Fig. 3 .
Fig. 3.
Overview of potential sites of therapeutic intervention in COVID-19 disease.
Additional drugs have been reported to have some effects in the treatment of COVID-19 patients. One that previously was mentioned is hydroxychloroquine (HDQ), the immunosuppressive and anti-parasite drug used to treat patients with malaria, lupus and arthritis (Quiros Roldan et al., 2020). From a historical point of view, it is worth recalling that during the Spanish flu pandemic in 1918, a Chicago physician (H.K. Klein) reported he had been quite successful in treating patients in the early stage of the disease (i.e. within 3 days after the appearance of the first symptoms) with i.v. quinine hydrochloride and oral quinine bisulphate (Klein, 1918). Quinine was used for many years as first-line treatment for malaria and remained the antimalarial drug of choice until after World War II. Since then, however, other drugs displaying fewer adverse effects, such as chloroquine and hydroxychloroquine, have largely replaced it (Tripathy et al., 2020).
The Eudra clinical (trial number: 2020-001704-42) is a double-blind, randomized, prospective, controlled clinical trial with hydroxychloroquine in Spain with health care professionals. Approximately 12–15% of health care professionals are infected with SARS-CoV-2. HDQ inhibits the growth of the coronavirus in vitro. This study will attempt to determine if oral administration of HDQ reduces SARS-CoV2 infection. (Cuadrado-Lavin et al., 2020). However, a recent study where hydroxychloroquine was tested as postexposure prophylaxis after moderate-risk or high-risk exposure to SARS-CoV2, demonstrated that the drug did not prevent illness when used within 4 days after exposure (Boulware et al., 2020).
In a study in a hospital in Madrid Spain, COVID-19 cancer patients who were treated with hydroxychloroquine and azithromycin resulted in 3/18 deaths. Interestingly age, staging, histology, cancer treatment and comorbidities were not determined to be linked with mortality. The authors concluded that hydroxychloroquine and azithromycin combined treatment may a good treatment option. (Rogado et al., 2020a, Rogado et al., 2020b). Nevertheless, in a large non-randomized study performed on hospitalized patients in New York City, treatment with hydroxychloroquine was not associated with either a greatly lowered or an increased risk of the composite end point of intubation or death (Geleris et al., 2020). There are 215 clinical trials with hydroxychloroquine and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
Artemisinin is a sesquiterpene lactone that is derived from the herb Artemisia annua. It has been used in traditional medicine for the treatment of fever, chills and malaria. Artemisinin also has some antiviral and anti-tumor activities. It has also been investigated in respiratory diseases and lung cancer. It has been proposed that artemisinin may have some effects against SARS-CoV-2 infection.
(Cheong et al., 2020). There are two clinical trials with artemisinin and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
The combination of vitamin D, quercetin, and estradiol has been proposed to have effects against COVID-19. The angiotensin-converting enzyme 2 (ACE2) and FURIN paired basic amino acid residue-cleaving enzyme (FURIN) proteins are required for SARS-CoV-2 entry into cells. They were used as baits in molecular traps to determine potential drugs and natural products that would affects SARS2-CoV-2 entry into cells. Vitamin D, quercetin and estradiol were identified molecules which would affect SARS2 the most. Quercetin is a natural product (nutraceutical) present in many common plants and fruits. Quercetin may alter the expression 30% of human genes encoding potential protein targets of SARS-CoV-2. Vitamin D may also alter the expression of 25% of potential human genes encoding protein targets of SARS-CoV-2. Estradiol may also affect expression of 61% of proteins which are targets of SARS-CoV-2. In contrast, testosterone did not demonstrate these effects which was speculated to have an influence on the higher male mortality rate in COVID-19-infected patients (Glinsky, 2020). There are sixteen clinical trials with vitamin D and various aspects of COVID-19 listed on the ClinicalTrials.gov website. There is one clinical trial with quercetin and various aspects of COVID-19 listed on the ClinicalTrials.gov website. There is one clinical trial with patients wearing an estrogen patch and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
Angiotensin-converting enzyme 2 is a potential drug target in the treatment of COVID-19 disease. SARS-CoV-2 binds angiotensin-converting enzyme (ACE)2. ACE2 is present on the membranes of many cell type including alveolar monocytes/macrophages. Losartan is a commonly-prescribed drug to treat high blood pressure (hypertension). Down-regulation of ACE2 results in production of angiotensin (ANG) II by the related enzyme ACE and simulation of the ANG type 1a receptor (AT1R). Losartan is an AT1R blocker (Gurwitz, 2020a; Magrone et al., 2020). Such analysis on drug repurposing document the important of clinical records and prescription records mining (Gurwitz, 2020b). There are four clinical trials with patients and losartan and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
LY3127804 is a MoAb that was developed by Eli Lily to treat patients with acute respiratory distress syndrome (ARDS), cancer and malaria. ARDS is a characteristic of severe COVID-19 patients. LY3127804 is directed to Angiopoietin-2 (Ang2). Ang2 causes endothelial cells in the lungs to become leaky. Increased levels of Ang2 are present in ARDS patients. The effects of suppressing Ang2 in COVID-19 patients will be examined with this MoAb to determine whether it is possible to reduce the progression to ARDS and the need for ventilators in the treatment of COVID-19 patients. There is one clinical trial with LY3127804 and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
Bruton's tyrosine kinase (BTK) is a non-receptor kinase that plays a crucial role in aberrant signaling pathways that are critical for proliferation and survival of cancer cells in several B cell malignancies (Pal Singh et al., 2019). Ibrutinib is an FDA-approved BTK inhibitor that has shown significant clinical activity in chronic lymphocytic leukemia and small lymphocytic lymphoma patients (Pula et al., 2019). There is one clinical trial with Ibrutinib with patients and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
It should be considered that patients with severe COVID-19 display a hyperinflammatory immune response (also referred to as “cytokine storm”) which suggests macrophage activation syndrome due to the release of multiple inflammatory cytokines/chemokines (IL-1β, IL-6, IL-7, IL-8, IL-9, IL-10, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, and MIP-1α) (Soy et al., 2020; Vabret et al., 2020). It had been previously speculated that BTK is involved in controlling macrophage functions (Lionakis et al., 2017). Interestingly, it was demonstrated that Acalabrutinib, a selective BTK inhibitor, when administered off-label over 10–14 days to 19 patients hospitalized with severe COVID-19 (eleven on supplemental oxygen; eight on mechanical ventilation), improved oxygenation in most of patients, often within 1–3 days, and had no discernible adverse effects (Roschewski et al., 2020). An ex-vivo analysis demonstrated elevated BTK activity, as shown by autophosphorylation, and increased IL-6 production in blood monocytes from patients with severe COVID-19 compared with blood monocytes from healthy donors (Roschewski et al., 2020). Therefore, these findings might indicate that targeting excessive inflammation with a BTK inhibitor is a potential therapeutic strategy in severe COVID-19 patients. There are two clinical trials with Acalabrutinib with patients and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
It is interesting that a study based on network proximity analysis have revealed how yet another drug used for targeted therapy of cancer, rapamycin (an mTOR inhibitor), may hold the potential to be effective in COVID-19 patients (Zhou et al., 2000). Nevertheless, problems with the use of rapamycin in COVID-19 patients include an increased risk of infections and development of interstitial pneumonitis, alveolar hemorrhage and acute respiratory distress syndrome that could superimpose to and aggravate those caused by the SARS-Cov2 (Moujaess et al., 2020). There are three clinical trials with rapamycin with patients and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
Moreover, PARP inhibitors, a class of drugs that are emerging as promising therapeutics in different cancer types (Gonçalves et al., 2020), have the potential to be used for treating COVID-19 patients, as they could decrease IL-1, IL-6, and TNFα levels and could alleviate subsequent lung fibrosis, as demonstrated in murine models and clinical trials (Kim et al., 2008; Lucarini et al., 2017; Sahu et al., 2020).
High levels of IL-6 are associated with poor outcome in COVID-19 patients (Del Valle et al., 2020). Tocilizumab is MoAb which inhibits IL-6. Some encouraging results in Tocilizumab-treated severe COVID-19 patients have been reported (Campins et al., 2020). There are 54 clinical trials with Tocilizumab and patients with various aspects of COVID-19 listed on the ClinicalTrials.gov website.
The JAK protein kinase is frequently activated by multiple interleukins, cytokines, and growth factors. Some encouraging results have been observed in a cohort of severe COVID-19 patients treated with the JAK inhibitor, Ruxolitinib (Cao et al., 2020). There are seventeen clinical trials with Ruxolitinib with patients and various aspects of COVID-19 listed on the ClinicalTrials.gov website. Tofacitinib is a small molecule inhibitor which inhibits JAK signaling. There are four clinical trials with tofacitinib and patients with various aspects of COVID-19 listed on the ClinicalTrials.gov website.
Resveratrol (RES) is a polyphenol present in many fruits and berries and is consumed by many people as a nutraceutical. RES can activate sirtuin (Sirt)1 [McCubrey et al., 2017; McCubrey et al., 2018 reviews. Sirt1 can decrease AT1R expression by increasing ACE2 expression. RES has anti-viral effects and may be evaluated in treatment of COVID-19 patients (Marinella, 2020). There is one clinical trial with RES with patients and various aspects of COVID-19 listed on the ClinicalTrials.gov website.
SARS-CoV-2 encodes nonstructural protein-15 (NSP15) which is an uridylate-specific endoribonuclease (EndoU) enzyme. NSP15 is essential for the SARS-CoV-2 life cycle and could be a target for drug development. By performing an in silico based virtual screening of FDA approved drugs, glisoxepide and idarubicin were selected as strong binders of Endo U. They are used for treatment for diabetes and leukemia respectively (Chandra et al., 2020).
Selenium is a trace element and also a dietary supplement contained in multivitamin tablets it has anti-viral properties. It is postulated that selenium may have potential effects on COVID-19 infections (Kieliszek and Lipinski, 2020).
Zinc is a trace element and also a dietary supplement contained in multivitamin tablets, it has anti-viral properties and may have effects on COVID-19 (Kumar et al., 2000).
4. Summary
In our review we have reviewed the impact of COVID-19 on cancer patients and treatments. While we currently lack an approved, effective vaccine for SAR2-Cov-2 virus, there are approaches which we can employ to lower the potential of infection. We have discussed the altered conditions in hospitals which have resulted from the COVID-19 outbreak. In addition, we have discussed the potential repurposing of approved drugs that may show some effects on COVID-19 patients. Much more research needs to be performed to determine whether potential drugs/natural products/trace elements which have been identified to affect SARS-2-Cov-2 can improve treatment of patients suffering from COVID-19.
Last but not least, given that the cytokine storm activates major signaling pathways implicated in aberrant cell growth and might weaken the immune system response to tumors, survivors of severe COVID-19 are potentially at risk of developing cancer. Future investigations are required to support this hypothesis, both in in vitro models and animal models.
Author contributions
Conceptualization: SMA, SLA, LSS, SC, ML, KL, LC, GR, SR, MYF, AMM, WLB, MP, GM, MC, MN, JB and JAM researched the various topic areas and wrote multiple sections. Funding acquisition; JAM and LC were involved with funding acquisition. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no conflicts of interest with publication of this manuscript.
Acknowledgements
JAM, SLA, and LSS were supported in part by grants from East Carolina University Grants (#111104 and #111110-668715-0000). LC, GR, SR and MYF were supported in part by grants from: Intesa San Paolo Foundation. WLB and MP were supported in part by the Italian Association for Research on Cancer (AIRC).
References
- Baumann B.C., MacArthur K.M., Brewer J.D., Mendenhall W.M., Barker C.A., Etzkorn J.R., Jellinek N.J., Scott J.F., Gay H.A., Baumann J.C., Manian F.A., Devlin P.M., Michalski J.M., Lee N Y., Thorstad W.L., Wilson L.D., Perez C.A., Miller C.J. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020 doi: 10.1002/cncr.32969. 2020 Jun 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt D., Wick W., Weiss S.E., Sahgal A., Lo S.S., Suh J.H., Chang E.L., Foote M., Perry J., Meyer B., Vajkoczy P., Wen P.Y., Straube C., Pigorsch S., Wilkens J.J., Combs S.E. Neuro-oncology management during the COVID-19 pandemic with a focus on WHO grade III and IV gliomas. Neuro Oncol. 2020 doi: 10.1093/neuonc/noaa113. 2020 May 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffa D.J., Judson B.L., Billingsley K.G., Galetta D., Fontanezl P., Odermatt C., Lindner K., Mitchell M.R., Henderson C.H., Carafeno T., Pinto J., Wagner J., Ancuta M., Beley P., Turner A.L., Banack T., Laurans M.S., Johnson D.C., Yoo P., Morton J.M., Zurich H., Davis K., Ahuja N. Pandemic recovery using a Covid-minimal cancer surgery pathway. Ann. Thorac. Surg. 2020 doi: 10.1016/j.athoracsur.2020.05.003. 10.1016/j.athoracsur.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogani G., Casarin J., Pinelli C., Di Donato V., Bosio S., Ruisi S., Brusadelli C., Guerrisi R., Sarpietro G., Ditto A., Ghezzi F., Raspagliesi F. Management of patients with ovarian cancer in the COVID-19 era. J. Surg. Oncol. 2020 doi: 10.1002/jso.26057. May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W., Cheng M.P., LaBar D., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Zarychanski R., Kelly L.E., Schwartz I.S., McDonald E.G., Rajasingham R., Lee T.C., Hullsiek K.H. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2016638. 2020 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campins L., Boixeda R., Perez-Cordon L., Aranega R., Lopera C., Force L. Early tocilizumab treatment could improve survival among COVID-19 patients. Clin. Exp. Rheumatol. 2020;38(3):578. May-Jun. [PubMed] [Google Scholar]
- Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., Huang L., Meng F., Huang L., Wang N., Zhou X., Luo H., Mao Z., Chen X., Xie J., Liu J., Cheng H., Zhao J., Huang G., Wang W., Zhou J. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.05.019. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A., Gurjar V., Qamar I., Singh N. Identification of potential inhibitors of SARS-COV-2 endoribonuclease (EndoU) from FDA approved drugs: a drug repurposing approach to find therapeutics for COID19. J. Biomol. Struct. Dyn. 2020;28:1–16. doi: 10.1080/07391102.2020.1775127. 2020 May. Online ahead of print. PMID: 32462970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Wu X., Wang W., Wang Q. When cancer encounters COVID-19 in China: what have we suffered, experienced and learned. Jpn. J. Clin. Oncol. 2020 doi: 10.1093/jjco/hyaa077. 2020 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong D.H.J., Tan D.W.S., Wong F.W.S., Tran T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol. Res. 2020;158:104901. doi: 10.1016/j.phrs.2020.104901. 10.1016/j.phrs.2020.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Lavin A., Olmos J.M., Cifrian J.M., Gimenez T., Gandarillas M.A., Garcia-Saiz M., Rebollo M.H., Martinez-Taboada V., Lopez-Hoyos M., Farinas M.C., Crespo J. Controlled, double-blind, randomized trial to assess the efficacy and safety of hydroxychloroquine chemoprophylaxis in SARS CoV2 infection in healthcare personnel in the hospital setting: a structured summary of a study protocol for a randomised controlled trial. Trials [Electronic Resource] 2020;21(1):472. doi: 10.1186/s13063-020-04400-4. 2020 Jun 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U. S. A. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. 2020 Mar 24. Epub 2020 Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle D.M., Kim-Schulze S., Hsin-Hui H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T., Madduri D., Stock A., Marron T., Xie H., Patel M.K., van Oekelen O., Rahman A., Kovatch P., Aberg J., Schadt E., Jagannath S., Mazumdar M., Charney A., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature helps predict COVID-19 severity and death. medRxiv. 2020 doi: 10.1101/2020.05.28.20115758. May 30:2020.05.28.20115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R.A., Sedrak M.S., Bellon J.R., Block C.C., Lin N.U., King T.A., Minami C., VanderWalde N., Jolly T.A., Muss H.B., Winer E.P. Weathering the storm: managing older adults with breast cancer amid COVID-19 and beyond. J. Nat. Canc.Inst. 2020 doi: 10.1093/jnci/djaa079. 10.1093/jnci/djaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. 2020. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. 2020 May 7:NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsky G.V. Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines. 2020 May 21;8(5):E129. doi: 10.3390/biomedicines8050129. PMID: 32455629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A., Bertucci A., Bertucci F. PARP inhibitors in the treatment of early breast cancer: the step beyond? Cancers. 2020;12(6):E1378. doi: 10.3390/cancers12061378. 2020 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin E.A., Day A., Stankova L., Heitman E., Sadler J. Care in the time of coronavirus: ethical considerations in head and neck oncology. Head Neck. 2020 doi: 10.1002/hed.26272. 10.1002/hed.26272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Huettenhain R.K., aake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meye B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Si Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verb a K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., García-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. 2020 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020 doi: 10.1002/ddr.21656. Mar 4:10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Repurposing current therapeutics for treating COVID-19: a vital role of prescription records data mining. Drug Dev. Res. 2020 doi: 10.1002/ddr.21689. 2020 May 18:10.1002/ddr.21689. Online ahead of print. PMID: 32420637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.H., O'Sullivan B., Su J., Ringash J., Bratman S.V., Kim J., Hosni A., Bayley A., Cho J., Giuliani M., Hope A., Spreafico A., Hansen A.R., Siu L.L., Gilbert R., Irish J.C., Goldstein D., de Almeida J., Tong L., Xu W., Waldron J. Hypofractionated radiotherapy alone with 2.4 Gy per fraction for head and neck cancer during the COVID-19 pandemic: the Princess Margaret experience and proposal. Cancer. 2020 doi: 10.1002/cncr.32968. 2020 Jun 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari A., Dadkhahfar S., Perseh S. Considerations for interactions of drugs used for the treatment of COVID-19 with anti-cancer treatments. [Review] Crit. Rev. Oncol. Hematol. 2020;151:102982. doi: 10.1016/j.critrevonc.2020.102982. 2020 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddou H., Boudjema K. Surgical resection for liver cancer during the COVID-19 outbreak. Updates Surg. 2020 doi: 10.1007/s13304-020-00799-2. 10.1007/s13304-020-00799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med. Hypotheses. 2020;143:109878. doi: 10.1016/j.mehy.2020.109878. 2020 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Suk M.H., Yoon D.W., Kim H.Y., Jung K.H., Kang E.H., Lee S.Y., Suh I.B., Shin C., Shim J.J., In K.H., Yoo S.H., Kang K.H. Inflammatory and transcriptional roles of poly (ADP-ribose) polymerase in ventilator-induced lung injury. Crit. Care. 2008;12(4):R108. doi: 10.1186/cc6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H.A. The treatment of “Spanish influenza”. J. Am. Med. Assoc. 1918;71:1510. [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.Y., Desai A., de Lima Lopes G., Jr., Grivas P., Painter C.A., Peters S., Thompson M.A., Bakouny Z., Batist G., Bekaii-Saab T., Bilen M.A., Bouganim N., Larroya M.B., Castellano D., Del Prete S.A., Doroshow D.B., Egan P.C., Elkrief A., Farmakiotis D., Flora D., Galsky M.D., Glover M.J., Griffiths E.A., Gulati A.P., Gupta S., Hafez N., Halfdanarson T.R., Hawley J.E., Hsu E., Kasi A., Khaki A.R., Lemmon C.A., Lewis C., Logan B., Masters T., McKay R.R., Mesa R.A., Morgans A.K., Mulcahy M.F., Panagiotou O.A., Peddi P., Pennell N.A., Reynolds K., Rosen L.R., Rosovsky R., Salazar M., Schmidt A Shah, Shaya J.A., Steinharter J., Stockerl-Goldstein K.E., Subbiah S., Vinh D.C., Wehbe F.H., Weissmann L.B., Wu J.T., Wulff-Burchfield E., Xie Z., Yeh A., Yu P.P., Zhou A.Y., Zubiri L., Mishra S., Lyman G.H., Rini B.I., Warner J.L., COVID-19 and Cancer Consortium Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020 May 28 doi: 10.1016/S0140-6736(20)31187-9. SA, S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19 [published online ahead of print, 2020 May 25] Med. Hypotheses. 2000:109848. doi: 10.1016/j.mehy.2020.109848. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y.W., Cazier J.B., Starkey T., Turnbull C.D., UK Coronavirus Cancer Monitoring Project Team, Kerr R., Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31173-9. 2020. 2020 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis M.S., Dunleavy K., Roschewski M., Widemann B.C., Butman J.A., Schmitz R., Yang Y., Cole D.E., Melani C., Higham C.S., Desai J.V., Ceribelli M., Chen L., Thomas C.J., Little R.F., Gea-Banacloche J., Bhaumik S., Stetler-Stevenson M., Pittaluga S., Jaffe E.S., Heiss J., Lucas N., Steinberg S.M., Staudt L.M., Wilson W.H. Inhibition of B cell receptor signaling by Ibrutinib in primary CNS lymphoma. Canc. Cell. 2017;31:833–843. doi: 10.1016/j.ccell.2017.04.012. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarini L., Durante M., Lanzi C., Pini A., Boccalini G., Calosi L., Moroni F., Masini E., Mannaioni G. HYDAMTIQ, a selective PARP-1 inhibitor, improves bleomycin-induced lung fibrosis by dampening the TGF-beta/SMAD signalling pathway. J. Cell Mol. Med. 2017;21(2):324–335. doi: 10.1111/jcmm.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrone T., Magrone M., Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - a perspective. Endocr. Metab. Immune Disord. - Drug Targets. 2020 doi: 10.2174/1871530320666200427112902. 2020 Apr 27. Online ahead of print. PMID: 32338224. [DOI] [PubMed] [Google Scholar]
- Marinella M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19 [published online ahead of print, 2020 May 15] Int. J. Clin. Pract. 2020 doi: 10.1111/ijcp.13535. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P.J., Gould M.K., Arenberg D.A., Chen A.C., Choi H.K., Detterbeck F.C., Fong K.M., Iaccarino J.M., Janes S.M., Kanne J.P., Kazerooni E.A., MacMahon H., Naidich D.P., Powell C.A., Raoof S., Rivera M.P., Tanner N.T., Tanoue L.K., Tremblay A., Vachani A., White C.S., Wiener R.S., Silvestri G.A. Management of lung nodules and lung cancer screening during the COVID-19 Pandemic: CHEST expert panel report. Chest. 2020 doi: 10.1016/j.chest.2020.04.020. ., F. 10.1016/j.chest.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J.A., Lertpiriyapong K., Steelman L.S., Abrams S.L., Yang L.V., Murata R.M., Rosalen P.L., Scalisi A., Neri L.M., Cocco L., Ratti S., Martelli A.M., Laidler P., Dulińska-Litewka J., Rakus D., Gizak A., Lombardi P., Nicoletti F., Candido S., Libra M., Montalto G., Cervello M. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany NY) 2017;9:1477–1536. doi: 10.18632/aging.101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J.A., Abrams S.L., Lertpiriyapong K., Cocco L., Ratti S., Martelli A.M., Candido S., Libra M., Murata R.M., Rosalen P.L., Lombardi P., Montalto G., Cervello M., Gizak A., Rakus D., Steelman L.S. Effects of berberine, curcumin, resveratrol alone and in combination with chemotherapeutic drugs and signal transduction inhibitors on cancer cells-Power of nutraceuticals. Adv. Biol. Regul. 2018;67:190–211. doi: 10.1016/j.jbior.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Moujaess E., Kourie H.R., Kattan J. Targeted therapies for cancer during the COVID-19 pandemic: a threat or a blessing? Pharmacogenomics. 2020 Jun 10 doi: 10.2217/pgs-2020-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal Singh S., Dammeijer F., Hendriks R.W. Role of Bruton's tyrosine kinase in B cells and malignancies. Mol. Canc. 2019;17(1):57. doi: 10.1186/s12943-018-0779-z. 2018 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puła B., Gołos A., Górniak P., Jamroziak K. Overcoming Ibrutinib resistance in chronic lymphocytic leukemia. Cancers. 2019;11(12):1834. doi: 10.3390/cancers11121834. Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros Roldan E., Biasiotto, Magro P., Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol. Res. 2020;158:104904. doi: 10.1016/j.phrs.2020.104904. 2020 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renu K., Prasanna P.L., Valsala Gopalakrishnan A. Coronaviruses pathogenesis, comorbidities and multi-organ damage - a review. [Review] Life Sci. 2020;255:117839. doi: 10.1016/j.lfs.2020.117839. 2020 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridruejo E., Soza A. The liver in times of COVID-19: what hepatologists should know. Ann. Hepatol. 2020 doi: 10.1016/j.aohep.2020.05.001. 10.1016/j.aohep.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogado J., Obispo B., Pangua C., Serrano-Montero G., Martin Marino A., Perez-Perez M., López-Alfonso A., Gullón P., Lara M.Á. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clinical & Translational Oncology: Official Publication of the Federation of Spanish Oncology Societes & of the National Cancer Institute of Mexico. 2020 doi: 10.1007/s12094-020-02381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogado J., Pangua C., Serrano-Montero G., Obispo B., Marino A.M., Pérez-Pérez M., López-Alfonso A., Gullón P., Lara M.Á. Covid-19 and lung cancer: A greater fatality rate? Lung Cancer. 2020;146:19–22. doi: 10.1016/j.lungcan.2020.05.034. Online ahead of print.PMID: 32505076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A., Roshon M., Wrzesinski S.H., Desai J.V., Zarakas M.A., Collen J., Rose K., Hamdy A., Izumi R., Wright G.W., Chung K.K., Baselga J., Staud L.M., Wilson W.H. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abd0110. 2020 Jun 5. Epub 2020 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu B., Narota A., Naura A.S. Pharmacological inhibition of poly (ADP-ribose) polymerase by olaparib, prevents acute lung injury associated cognitive deficits potentially through suppression of inflammatory response. Eur. J. Pharmacol. 2020;877:173091. doi: 10.1016/j.ejphar.2020.173091. [DOI] [PubMed] [Google Scholar]
- Sciarra A., Salciccia S., Maggi M., Del Giudice F., Busetto G.M., Musio D., Ciardi A., Catalano C., Cortesi E., Panebianco V. Elective procedures for prostate cancer in the time of Covid-19: a multidisciplinary team experience. Prostate Cancer Prostatic Dis. 2020:1–3. doi: 10.1038/s41391-020-0240-4. 10.1038/s41391-020-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J.M., Scaranelo A.M., Yong-Hing C., Appavoo S., Flegg C., Kulkarni S., Kornecki A., Wadden N., Loisel Y., Schofield S., Leslie S., Gordon P. COVID-19: safe guidelines for breast imaging during the pandemic. Can. Assoc. Radiol. J. 2020 doi: 10.1177/0846537120928864. 846537120928864. 10.1177/0846537120928864. [DOI] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. 2020 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.P., Berman A.T., Marmarelis M.E., Haas A.R., Feigenberg S.J., Braun J., Ciunci C.A., Bauml J.M., Cohen R.B., Kucharczuk J.C., Shulman L.N., Langer C.J., Aggarwal C. Management of lung cancer during the COVID-19 pandemic. JCO Oncol. Pract. 2020 doi: 10.1200/OP.20.00286. OP2000286. 10.1200/OP.20.00286. [DOI] [PubMed] [Google Scholar]
- Skowron K.B., Hurst R.D., Umanskiy K., Hyman N.H., Shogan B. Caring for patients with rectal cancer during the COVID-19 pandemic. J. Gastrointest. Surg. 2020 doi: 10.1007/s11605-020-04645-z. 10.1007/s11605-020-04645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy M., Kese G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020:1–10. doi: 10.1007/s10067-020-05190-5. 2020 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchelebi L.T., Haustermans K., Scorsetti M., Hosni A., Huguet F., Hawkins M.A., Dawson L.A., Goodman K.A. Recommendations for the use of radiation therapy in managing patients with gastrointestinal malignancies in the era of COVID-19. Radiother. Oncol. 2020;148:194–200. doi: 10.1016/j.radonc.2020.04.010. 10.1016/j.radonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Engelhardt M., Cook G., Gay F., Mateos M.V., Ntanasis-Stathopoulos I., van de Donk N.W.C.J., Avet-Loiseau H., Hajek R., Vangsted A.J., Ludwig H., Zweegman S., Moreau P., Einsele H., Boccadoro M., San Miguel J., Dimopoulos M.A., Sonneveld P. Management of patients with multiple myeloma in the era of COVID-19 pandemic: a consensus paper from the European Myeloma Network (EMN) Leukemia. 2020 doi: 10.1038/s41375-020-0876-z. 10.1038/s41375-020-0876-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triki H., Rogado J., Obispo B., Pangua C., Serrano-Montero G., Martin Marino A., Perez-Perez M., López-Alfonso A., Gullón P., Lara M.Á. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin. Transl. Oncol.: Official Publication of the Federation of Spanish Oncology Societes & of the National Cancer Institute of Mexico. 2020 doi: 10.1007/s12094-020-02381-z. 10.1007/s12094-020-02381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S., Dassarma B., Roy S., Chabalala H., Matsabisa M.G. A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic. Int. J. Antimicrob. Agents. 2020:106028. doi: 10.1016/j.ijantimicag.2020.106028. May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutuncuoglu B., Cakir M., Batra J., Bouhaddou M., Eckhardt M., Gordon D.E., Krogan N.J. The landscape of human cancer proteins targeted by SARS-CoV-2. Canc. Discov. 2020 doi: 10.1158/2159-8290.CD-20-0559. 10.1158/2159-8290.CD-20-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber G., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., Pia L., Risson E., Saffern M., Salomé B., Selvan M.E., Spindler M.P., Tan J., van der Heide V., Gregory J.K., Alexandropoulos K., Bhardwaj N., Brown B.D., Greenbaum B., Gumus Z.H., Homann D., Horowitz A., Kamphorst A.O., Curotto de Lafaille M.A., Mehandru S., Merad M., Samstein R.M. Immunology of COVID-19: current state of the science. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale G., Licata L., Sica L., Zambelli S., Zucchinelli P., Rognone A., Aldrighetti D., Di Micco R., Zuber V., Pasetti M., Di Muzio N., Rodighiero M., Panizza P., Sassi I., Petrella G., Cascinu S., Gentilini O.D., Bianchini G. Personalized risk-benefit ratio adaptation of breast cancer care at the epicenter of COVID-19 outbreak. Oncol. 2020 doi: 10.1634/theoncologist.2020-0316. 10.1634/theoncologist.2020-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A., Fabbrocini G., Costa C., Scalvenzi M. Melanoma Screening Days during the coronavirus disease 2019 (COVID-19) pandemic: strategies to adopt. Dermatol. Ther. 2020 doi: 10.1007/s13555-020-00402-x. 2020 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinsky I., Baristaite G., Gurwitz D. Effects of age and sex on recovery from COVID-19: analysis of 5769 Israeli patients. J. Infect. 2020 doi: 10.1016/j.jinf.2020.05.026. 2020 May 16:S0163-4453(20)30303-0. Online ahead of print. PMID: 32425274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrachimis A., Iacovou I., Giannoula E., Giovanella L. Endocrinology in the time of COVID-19: management of thyroid nodules and cancer. Eur. J. Endocrinol. 2020 doi: 10.1530/EJE-20-0269. 10.1530/EJE-20-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang S., Wei L., Lin Z., Wang X., Wang J., Hua K., Cui M., Wang J., Wang S., Di W., Wang Y., An R., Xill M., Guo R., Zhou Q., Xie X., Xue F. Recommendations on management of gynecological malignancies during the COVID-19 pandemic: perspectives from Chinese gynecological oncologists. J. Gynecol. Oncol. 2020 doi: 10.3802/jgo.2020.31.e68. 10.3802/jgo.2020.31.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexner S.D., Cortes-Guiral D., Gilshtein H., Kent I., Reymond M.A. COVID-19: impact on colorectal surgery. Colorectal Dis. 2020 doi: 10.1111/codi.15112. 10.1111/codi.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekeduz E., Dursun B., Aydin G.C., Yazgan S.C., Ozturk H.H., Azap A., Utkan G., Ürün Y. Clinical course of COVID-19 infection in elderly patient with melanoma on nivolumab. J. Oncol. Pharm. Pract. 2020 doi: 10.1177/1078155220924084. 1078155220924084. 10.1177/1078155220924084. [DOI] [PubMed] [Google Scholar]
- Yeung D.C.M., Lai R., Wong E.W.Y., Chan J.Y.K. Care of patients with a laryngectomy during the COVID-19 pandemic. Otolaryngol. Head Neck Surg. 2020 doi: 10.1177/0194599820933185. 194599820933185, 2020 Jun 02. UI: 32482154. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., Hou, Y., Shen, J.,Huang, Y., Martin, W., Cheng, F. 2000. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 6:14. Published 2020 Mar 16. doi:10.1038/s41421-020-0153. [DOI] [PMC free article] [PubMed]