Abstract
We report a case of severe COVID-19 pneumonia complicated by fatal co-infection with a multi-triazole resistant Aspergillus fumigatus and highlight the importance of recognising the significance of Aspergillus sp. isolation from respiratory samples. Early diagnosis and detection of triazole resistance are essential for appropriate antifungal therapy to improve outcome in patients with coronavirus associated invasive aspergillosis.
Keywords: COVID-19 pneumonia, Invasive pulmonary aspergillosis, Multi-triazole resistance
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing COVID-19 infection, is a newly recognised pathogen that has been traced to Wuhan (Hubei province) in China [1]. The clinical spectrum of COVID-19 varies from asymptomatic infection to severe pneumonia requiring mechanical ventilation. The overall case fatality rate is estimated to be 2.7%, which increases to 4.5% in those ≥ 60 years old [2]. Secondary infections with bacterial and fungal pathogens have been reported in patients with COVID-19 pneumonia which may be due to virus induced mucosal damage and/or the dysregulated immune response seen in patients with acute respiratory distress syndrome [3,4].
We report severe COVID-19 pneumonia with multi-triazole-resistant Aspergillus fumigatus co-infection in a patient not known to be immunocompromised to highlight the importance of early diagnosis and detection of triazole resistance.
2. Case
A 66-year-old male presented to the emergency department with a history of progressive shortness of breath (SOB), myalgia, headaches, non-productive cough and fever.
Two days previously, he had contacted his general practitioner (GP) describing a seven-day history of fever and cough. He had returned from the United Kingdom eight days earlier after visiting a relative who was subsequently diagnosed with COVID-19. He was advised to self-isolate and monitor his symptoms. Due to progressive SOB, he re-presented to his GP who referred him to the hospital for further management.
The patient had Type 2 diabetes mellitus, hypertension, hyperlipidaemia and obesity with a body mass index of 35.5 (weight 90kg) with no hospital admissions in the past eight years. His medications included metformin, aspirin, simvastatin and ramipril. He had no history of prior triazole antifungal therapy. He was an ex-smoker but had not been previously diagnosed to have chronic lung disease. He works as a ground maintenance personnel where he is exposed to fungicides daily.
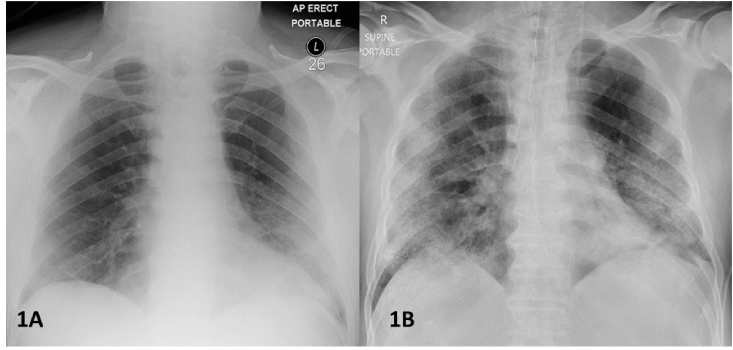
On presentation, a portable chest radiograph was performed which showed unilateral peripheral left basal airspace shadowing (Fig. 1A). In view of the clinical presentation, radiographic findings and the recent exposure history, the patient was admitted under contact and droplet precautions.
Fig. 1.
A. Portable chest radiograph taken on day of admission showing unilateral peripheral left basal airspace shadowing. B. Portable chest radiograph taken on Day 12 of hospitalization (Day 20 of COVID-19 infection). The endotracheal tube and bilateral central lines are in satisfactory position. There has been interval progression of the left peripheral airspace shadowing with additional right upper and lower zone peripheral airspace shadowing with no evidence of cavitation.
On admission (Day 7 of COVID-19 illness), the patient was noted to be pyrexial (temperature of 38.6 °C), tachycardic (pulse rate of 174 beats per minute) with new onset atrial fibrillation, normotensive (BP 117/69 mmHg), tachypnic (24 breaths per minute) with oxygen saturation of 84% on room air. He was commenced on a trial of continuous positive airway pressure with oxygen therapy. Preliminary laboratory results revealed white cell count of 4.7 × 109/L (reference range 4.0–11.0 × 109/L), neutrophil count 3.0 × 109/L (reference range 2.0–7.0 × 109/L), lymphocyte count 0.8 × 109/L (reference range 1.0–3.0 × 109/L) and C-reactive protein of 118 mg/L (reference range < 5.0 mg/L). Nasopharyngeal and oropharyngeal swabs detected SARS-CoV-2 using real-time reverse transcriptase polymerase chain reaction on day 3. He was commenced on azithromycin (500 mg on day 1 and 250 once daily on days 2 and 3) and hydroxychloroquine (200 mg twice daily) orally as per our local protocol at that time.
On day 4 (Day 11 of COVID-19 illness) he developed worsening hypoxic respiratory failure with a Sequential Organ Failure Assessment (SOFA) score of 6 necessitating intensive care unit (ICU) admission for ventilatory support. The patient was proned initially for 15 hours which did not improve his oxygenation. This, combined with significant hemodynamic instability, were contraindications for further proning. His condition continued to deteriorate progressing to multi-organ failure on day 7 (Day 14 of COVID-19 illness) which included acute respiratory failure, acute kidney injury requiring continuous renal replacement therapy and vasopressor support due to septic shock. His SOFA score increased to 12. He had continuing pyrexia and copious amounts of purulent respiratory secretions prompting a repeat septic screen which included culture of blood and endotracheal aspirate (ETA).
Empiric antimicrobial therapy for hospital-acquired pneumonia with intravenous (IV) piperacillin-tazobactam (4.5g three times a day) was started. Subsequently, ETA culture grew Klebsiella varicola (susceptible to piperacillin-tazobactam), Aspergillus sp. and Candida albicans. Antifungal therapy with IV liposomal amphotericin B (3 mg per kg once daily) was commenced due to the patients rapidly deteriorating status and uncertainty of the full identification of the mould isolate which may be resistant to triazoles. The Aspergillus sp. was referred for further identification and antifungal susceptibility testing, as were serum samples for detection of 1–3, β-d-glucan (BDG) antigen and galactomannan (GM) antigen and ETA for GM antigen.
His SOFA score on day 11 (Day 18 of COVID-19 illness) increased to 16. Antibiotic therapy was escalated to IV meropenem (1g twice daily) and vancomycin, with dosing based on renal function, due to ongoing pyrexia and deteriorating status. He was not prescribed systemic steroids at any time during his critical illness.
A repeat portable chest radiograph on day 12 (Day 20 of COVID-19 illness) showed progression to additional right upper and lower zone peripheral airspace shadowing with no evidence of cavitation (Fig. 1B). He remained haemodynamically unstable which precluded further radiological investigations. In view of his non-responsive multi-organ failure, and after discussion with his family, the patient's life-support was withdrawn. He died on day 14 of hospitalization (Day 22 of COVID-19 illness).
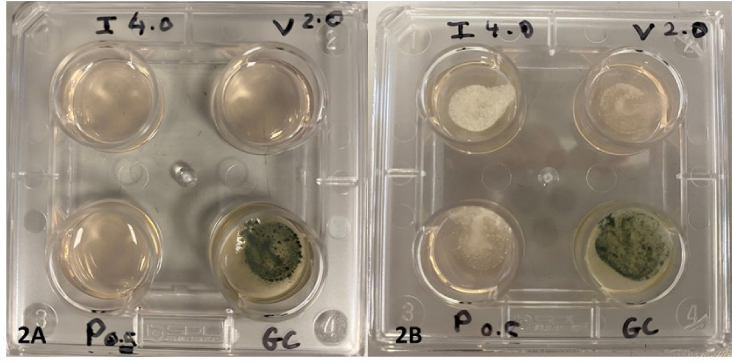
The Aspergillus sp. isolated from the ETA was confirmed to be A. fumigatus. Phenotypic testing utilising a 4-well triazole resistance screen (VIP check™, Mediaproducts BV, The Netherlands) (Fig. 2A and B) as well as determination of minimum inhibitory concentrations (MIC) utilising gradient strips (Liofilchem, Waltham MA, USA) were suggestive of triazole resistance (Table 1) [5]. The MIC to amphotericin was 0.125 mg/L (susceptible ≤ 1 mg/L) [5]. Genotypic testing utilising a commercial assay (Aspergenius™, Pathonostics B.V. The Netherlands) on the ETA detected A. fumigati complex with the TR34L98H resistance mutation in the cyp51A gene, the most common mutation in triazole-resistant A. fumigatus originating from an environmental source. The serum and ETA GM optical density index (ODI) were 1.1 and 5.5 respectively (Platelia™ Aspergillus Ag EIA, WA, USA). Serum BDG was 202 pg per milliliter (Fungitell™ Assay, Associates of Cape Cod, USA). These results (Table 1) were consistent with severe COVID-19 pneumonia and triazole-resistant invasive pulmonary aspergillus co-infection.
Fig. 2.
A and B VIPcheck™ plate, a four well triazole resistance screen plate which contains itraconazole (I) 4 mg per liter, voriconazole (V) 2 mg per liter, posaconazole.
(P) 0.5 mg per liter, and growth control (GC). This is a simple phenotypic test used to screen for triazole resistance due to the common mutations in the cyp51A gene in A. fumigatus from an environmental source. The photos show a susceptible control strain AF293 (2A) where growth is only seen on GC well and the patient's isolate (2B) with growth in all wells consistent with findings seen in A. fumigatus with the cyp51A TR34/L98H mutation.
Table 1.
Laboratory results.
| Patient's Isolate A. fumigatus |
aClinical breakpoint > Resistant |
|
|---|---|---|
| Antifungal Susceptibility Test | ||
| Minimum inhibitory | ||
| concentration (MIC) mg/L | ||
| Voriconazole | 2.0 | 1 |
| Itraconazole | >32 | 1 |
| Posaconazole | 1.0 | 0.25 |
| Amphotericin B | 0.125 | 1 |
| Fungal antigensb | Patient's results | Cut-off |
| Galactomannan Optical | ||
| Density Index (ODI) | ||
| Serum | 1.1 | ≥ 0.5 |
| Tracheal aspirate | 5.5 | N/A |
| 1,3 β-d-glucan pg/mL | ||
| Serum | 202 | ≥ 80 |
Based on the European Committee on Antimicrobial Susceptibility testing guidelines.
Fungal antigens: Galactomannan cut-off based on Platelia™ Aspergillus Ag EIA and 1–3 β-d-glucan cut-off based on Fungitell™ assay.
3. Discussion
Previously known to cause infections in severely immunocompromised patients, A. fumigatus is now recognised as an emerging pathogen in critical care patients suffering chronic respiratory disorders and as a complication of severe influenza infection [6]. Aspergillus colonisation can rapidly lead to invasive aspergillosis following severe influenza infection due to multiple pathways including structural lung damage coupled with disruption of mucociliary clearance, leukopenia, Th1/Th2 imbalance and diffuse damage to the respiratory mucosa [7]. In addition, severe respiratory viral infections such as influenza have been identified as an independent risk factor for IPA with high mortality [6]. Although our patient did not receive steroids, their use in critical care patients is linked to increased risk of invasive fungal infections [6]. Like other causes of viral pneumonias, SARS-CoV-2 may impair local mucosal and systemic immune defenses [3].
Thirty-four cases of COVID-19 associated invasive aspergillosis (CAPA) have been reported to date [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. These were all patients with severe pneumonia with adult respiratory distress symdome, most of whom had no known history of immunocompromise. These has led to challenges in early diagnosis since most of them do not have the classical risk factors for invasive aspergillosis.
Histopathology and culture from sterile site samples and biopsy remain the gold standard for proven IPA however the definition of probable or “putative” IPA has expanded but remains to be a composite of the host factors, clinical features and mycological evidence [6,18]. Although severe viral pneumonia is not considered a risk factor for IPA or other invasive mould disease according to the European Organisation for Research and Treatment of Cancer/Mycoses Study Group definitions, the structural damage to the lung parenchyma, in addition to the dysregulated immune response, can lead to IPA [6]. Recently a panel of experts proposed a case definition for influenza associated invasive aspergillosis (IAPA) which may be useful to classify COVID-19 patients with invasive aspergillosis. Following this case definition a patient with COVID-19 detected in respiratory sample by PCR, pulmonary infiltrates and a positive serum or bronchoalveolar lavage galactomannan has criteria consistent with probable CAPA [19]. Our patient had radiographic findings consistent with COVID-19 pneumonia. Unfortunately, his clinical status did not allow for computed tomography (CT) to be performed. Other mycological evidence of IPA include culture of Aspergillus sp. from non-sterile sites or detection of fungal antigens such as serum BDG. Our patient had COVID-19 pneumonia, A. fumigatus from a tracheal aspirate with elevated serum BDG and GM and elevated ETA GM, findings consistent with COVID-19 and probable IPA co-infection.
Multi-triazole resistance in A. fumigatus has recently emerged and is linked to the use of triazole-containing compounds as agricultural fungicides or less commonly prolonged triazole use [20]. The former mechanism of resistance typically affects azole näive patients and is characterised by elevated MICs to itraconazole, voriconazole and posaconazole as was found in our patient. This is of serious concern since triazoles are recommended as the first-line and most effective treatment for IPA [21]. Of the 34 cases reported so far, only 7 cases had reported susceptibility results of which one was triazole resistant A. fumigatus [16] similar to our case. Our patient's exposure to fungicides daily in his work has most likely led to exposure and colonisation with triazole-resistant A. fumigatus which was further supported by detection of cyp51A TR34L98H mutation from our patient's ETA, the most prevalent triazole resistance mutation from environmental source [20].
This case highlights the importance of early evaluation of patients with COVID-19 pneumonia because of the risk of secondary or co-infection with fungal pathogens. Case definitions have been proposed for IAPA [6,19] which can be modified for early recognition of CAPA. The occurrence of multi-triazole resistance in this case emphasises the urgent need for antifungal drug susceptibility testing of Aspergillus isolates using a rapid and simple phenotypic method and/or by detection of Cyp51 gene associated triazole resistance mutations directly on respiratory samples.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interestCOI
T.R.Rogers has received grants and personal fees from Gilead Sciences, personal fees from Pfizer Healthcare Ireland, personal fees from Menarini Pharma outside the submitted work, A.F.Talento has received grant and personal fees from Gilead Sciences and personal fees from Pfizer Healthcare Ireland outside the submitted work. The other authors have no conflict of interests.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N. Estimates of the severity of coronavirus disease 2019 : a model-based analysis. Lancet Infect. Dis. 2020;3099(20):1–9. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin Chuan, Zhou Luoqi, Hu Ziwei, Zhang Shuoqi, Yang Sheng, Yu Tao, Xie Cuihong, Ma Ke, Shang Ke, Wang Wei, Tian Dai-Shi. Dysregulated immune response in patients with COVID-19 in Wuhan China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. Internet. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European committee on antimicrobial susceptibility testing [internet] 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204.pdf [cited 2020 Apr 9]. Available from:
- 6.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. Internet. 2018;6(10):782–792. doi: 10.1016/S2213-2600(18)30274-1. https://linkinghub.elsevier.com/retrieve/pii/S2213260018302741 Available from: [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Vidal C., Barba P., Arnan M., Moreno A., Ruiz-Camps I., Gudiol C. Invasive aspergillosis complicating pandemic influenza A (H1N1) infection in severely immunocompromised patients. Clin. Infect. Dis. 2011;53(6):16–19. doi: 10.1093/cid/cir485. [DOI] [PubMed] [Google Scholar]
- 8.Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;2(20):1–10. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alanio A, Dellière S, Fodil S, Bretagne S. High Prevalence of Putative Invasive Pulmonary Aspergillosis in Critically Ill COVID-19 Patients. :1–5. [DOI] [PMC free article] [PubMed]
- 10.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;(April):528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prattes J., Valentin T., Hoenigl M., Talakic E., Reisinger Pe A.C. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU - a case report. Med. Mycol. Case Rep. 2020 doi: 10.1016/j.mmcr.2020.05.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antinori S., Rech R., Galimberti L., Castelli A., Angeli E., Fossali T. Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: a diagnostic challenge. Trav. Med. Infect. Dis. 2020;(April):101752. doi: 10.1016/j.tmaid.2020.101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaize M., Mayaux J., Nabet C., Lampros A., Marcelin A.-G., Thellier M. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg. Infect. Dis. 2020;26(7) doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19 associated pulmonary aspergillosis. Am. J. Respir. Crit. Care Med. 2020:1–10. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahmer T., Rasch S., Spinner C., Geisler F., Schmid R.M., Huber W. Invasive pulmonary aspergillosis in severe coronavirus disease 2019 pneumonia. Clin. Microbiol. Infect. Internet. 2020;(xxxx) doi: 10.1016/j.cmi.2020.05.032. 2019–20. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer Eelco F.J., Dofferhoff Anton S.M., Hoiting Oscar, Buil J.B., Meis J. 2020. Azole Resistant COVID-19 Associated Pulmonary Aspergillosis in an Immunocompetent Host : a Case Report. (June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutsaert L., Steinfort N., Van Hunsel T., Bomans P., Naesens R., Mertes H. COVID-19-associated invasive pulmonary aspergillosis. Ann. Intensive Care Internet. 2020;10(1):71. doi: 10.1186/s13613-020-00686-4. http://www.ncbi.nlm.nih.gov/pubmed/32488446 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blot S.I., Taccone F.S., Van Den Abeele A.M., Bulpa P., Meersseman W., Brusselaers N. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. Internet. 2012;186(1):56–64. doi: 10.1164/rccm.201111-1978OC. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L365165397 Available from: [DOI] [PubMed] [Google Scholar]
- 19.Verweij Paul E., Rjinders Bart J.A., Brüggemann Roger J.M., Azoulay Ellie, Bassetti Matteo, Blot Stijn, Calandra Thierry, Cornely Oliver. Intensive Care Med Press; 2020. Review of Influenza-Associated Pulmonary Aspergillosis and Proposal for a Case Definition: an Expert Opinion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verweij Paul E., Mellado Emilia, Melchers W. Multiple-triazole resistant aspergillosis. N. Engl. J. Med. 2007;356:1481–1483. doi: 10.1056/NEJMc061720. [DOI] [PubMed] [Google Scholar]
- 21.Patterson T.F., Thompson G.R., Denning D.W., Fishman J.A., Hadley S., Herbrecht R. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016;63(4):e1–60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]