Abstract
A wide spectrum of cardiovascular manifestations has been documented in patients suffering from coronavirus disease-2019 (COVID-19). Usually associated with a poor prognoses, these manifestations include thromboembolic events, acute coronary syndrome, heart failure, and cardiogenic shock. We describe a patient with COVID-19 who presented with subacute myocardial infarction, biventricular thrombi, and bilateral pulmonary emboli. Biventricular thrombi are rare, and their presence raises concern for an underlying prothrombotic condition.
Résumé
Un large panel de manifestations cardiovasculaires a été répertorié chez les patients souffrant de la maladie à coronavirus 2019 (COVID-19). Habituellement associées à un mauvais pronostic, ces manifestations incluent : événements thromboemboliques, syndrome coronarien aigu, insuffisance cardiaque et choc cardiogénique. Nous rapportons le cas d’un patient atteint de COVID-19 qui s'est présenté avec un infarctus du myocarde subaigu, des thrombi biventriculaires et des embolies pulmonaires bilatérales. Les thrombi biventriculaires sont des événements rares, et leur présence suscite des inquiétudes quant à un état prothrombotique sous-jacent.
Patients with COVID-19 have an increased incidence of cardiovascular comorbidities compared with the general population.1 They can present with acute cardiovascular events or exacerbations of pre-existing cardiac conditions. A wide spectrum of cardiovascular manifestations has been documented in patients suffering from COVID-19, such as thromboembolic events, acute coronary syndrome, heart failure, and cardiogenic shock,1 and they are associated with poor prognoses. We describe a patient with COVID-19 who presented with subacute myocardial infarction and bilateral pulmonary emboli associated with biventricular thrombi.
Case
A 63-year-old woman, active smoker, with a known medical history of emphysema presented with a 2-week history of worsening dyspnea, nonproductive cough, and chills. She had chest pain for 24 hours, which resolved the day before admission. Because of the delayed presentation and the resolution of her chest pain, she was managed conservatively with aspirin, clopidogrel, and enoxaparin. A few hours later, she went into cardiac arrest, with an underlying rhythm of monomorphic ventricular tachycardia. After successful cardiorespiratory resuscitation, she was transferred to our tertiary-care academic centre.
On presentation, the patient was tachypneic, with a respiratory rate of 35 breaths per minute. Her oxygen saturation was 93% on 2 L per minute of oxygen via nasal prongs before cardiac arrest. She was intubated during cardiorespiratory reanimation. Blood pressure and heart rate were within normal range. Physical examination showed jugular-vein distension, bibasilar crackles, and lower extremity edema. Laboratory workup revealed mild lymphopenia of 1.3 × 109/L (normal range: 1.5 to 3.5). Platelet count, coagulation parameters, and fibrinogen were normal. Troponin I (0.937 μg/L; normal value < 0.300), creatinine kinase (457 U/L; normal range: 30 to 185), and lactate (4.2 mmol/L; normal range: 0.6 to 2.4) were elevated. Lupus anticoagulant, anti-β-2-glycoprotein, and anticardiolipin antibodies were negative. Results of polymerase chain reaction (PCR) for severe acute respiratory syndrome-COVID-2 (SARS-CoV-2) was positive.
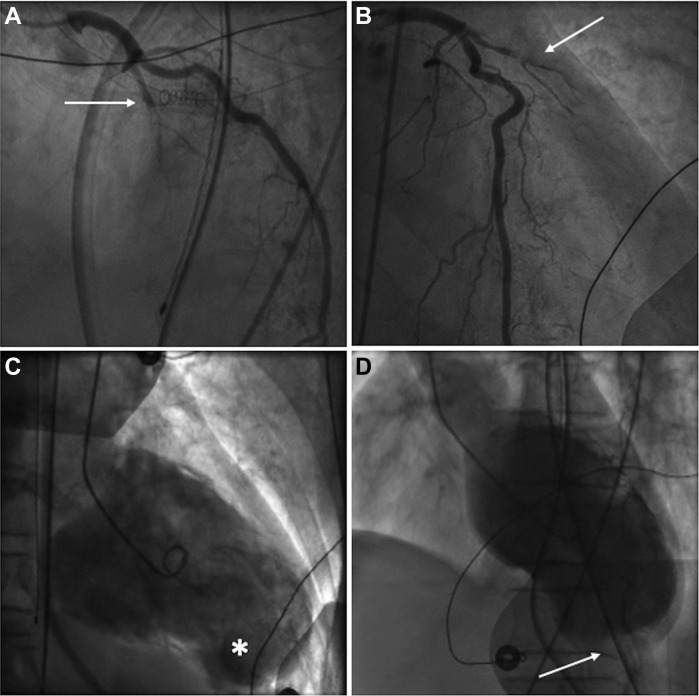
Electrocardiogram revealed sinus rhythm with ST-segment elevation, T-wave inversion, and pathological Q waves in leads V1 to V6, DI, and aVL, consistent with subacute anterolateral ST-elevation myocardial infarction (STEMI). Cardiomegaly with mild interstitial edema was demonstrated on chest radiograph. Coronary angiogram showed 99% stenosis of the proximal left-anterior descending (LAD) coronary artery, with organized thrombi and TIMI-1 blood flow (Fig.1A). The circumflex and right coronary arteries had nonsignificant stenoses. Left ventriculography revealed severe ventricular dysfunction with extended anterolateral akinesis, apical aneurysm, and thrombus (Fig. 1B).
Figure 1.
Coronary angiography shows a proximal left anterior descending artery subtotal stenosis in (A) the right anterior oblique cranial view and (B) in the right anterior oblique caudal view (arrows). Left ventriculography shows the apical aneurysm sac (C) (asterisk), with large left ventricle apical thrombus (D) (arrow).
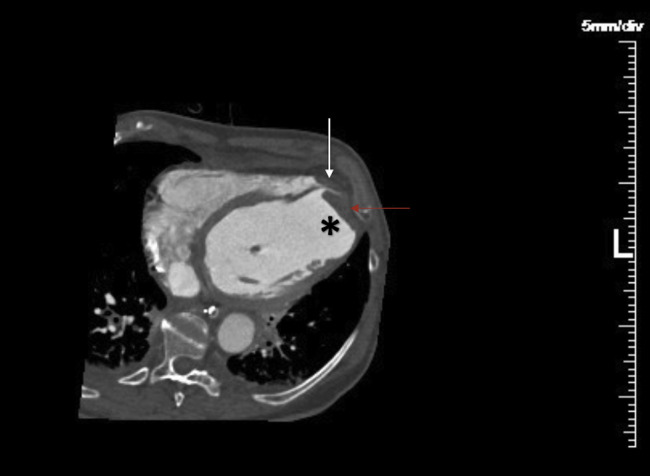
Cardiac tomography, wjich was performed to eliminate a pseudoaneurysm, demonstrated severe systolic dysfunction with a left-ventricular (LV) ejection fraction of 17% and complete akinesis of the LAD artery territory. An apical aneurysm, measuring 5 cm in diameter, and an LV thrombus (LVT) measuring 12-mm in thickness extending over a 6-cm perimeter were found. Unexpectedly, a moderate right ventricular (RV) hypokinesis with a small RV thrombus, measuring 4 mm by 10 mm, and multiple bilateral pulmonary emboli were also noted (Fig. 2 ).
Figure 2.
Cardiac tomography showing an apical aneurysm (asterisk) with apical left-ventricular (red arrow) and right-ventricular thrombus (white arrow).
Given the presence of multiple thrombi in the heart and lungs, therapeutic anticoagulation with intravenous heparin and warfarin was initiated. The patient later deteriorated and required systemic support with vasopressors and inotropic agents. Despite treatment, she died of cardiogenic and pulmonary septic shock.
Discussion
This patient with COVID-19 had an extensive anterior myocardial infarction (MI) with severe systolic dysfunction. The proposed mechanisms for cardiovascular injury in COVID-19 include the release of proinflammatory cytokines, leading to plaque instability, myocardial inflammation, hypercoagulable state, and myocardial depression.1
LVT can occur as a complication of acute MI with ejection fraction below 35% or nonischemic cardiomyopathy.2 It mostly occurs with anterior MI when there is an aneurysm or apical akinesis.2 The damage to the endocardial tissue, the hypercoagulable state, and the loss of regional contractile function led to blood stasis and formation of thrombus.3 With effective reperfusion, the incidence of LVT after MI has decreased in the PCI era from 33% to 10%.3 However, in patients presenting more than 12 hours after the onset of symptoms, a study showed that formation of thrombus was 48% higher than in the early presentation group.4 In contrast, the formation of biventricular thrombi following acute MI, first reported in 1986, is even rarer. The majority of the cases subsequently reported were associated with an underlying prothrombotic condition such as nephrotic syndrome, hypereosinophilic syndrome, heparin-induced thrombocytopenia, antiphospholipid syndrome, and other coagulopathies. The presence of antiphospholipid antibodies has been associated with COVID-19 but was negative in our patient. To our knowledge, this is the first reported case of biventricular thrombi after MI in a patient with COVID-19. Our hypothesis is that the combination of an extensive anterior MI with delayed presentation and COVID-19 caused an important inflammatory cascade that led to formation of thrombi.
Funding Sources
The authors report no funding sources relevant to the contents of this paper.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1326.e11 for disclosure information.
References
- 1.Kang Y., Chen T., Mui D. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020;0:1–10. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meurin P., Brandao Carreira V., Dumaine R. Incidence, diagnostic methods, and evolution of left ventricular thrombus in patients with anterior myocardial infarction and low left ventricular ejection fraction: a prospective multicenter study. Am Heart J. 2015;170:256–262. doi: 10.1016/j.ahj.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Habash F., Vallurupalli S. Challenges in management of left ventricular thrombus. Ther Adv Cardiovasc Dis. 2017;11:203–213. doi: 10.1177/1753944717711139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mir J., Jahangir J.R., Asfandyar Q. Left ventricular thrombus in patients with acute anterior wall myocardial infarction. J Ayub Med Coll Abbottabad. 2014;26:491–495. [PubMed] [Google Scholar]