Abstract
Recently, in China, in 2019, a new type of disease has arisen caused by a new strain of coronavirus, the SARS-CoV-2 virus, considered extremely worrying due to its high infectivity power and the easy ability to spread geographically. For patients in general, the clinical features resulting from respiratory syndromes can trigger an asymptomatic condition. However, 25 % of patients infected by SARS-CoV-2 can progress to severity. Pregnant women are an unknown field in this complex process, and although they have symptoms similar to non-pregnant women, some points should be considered, such as complications during pregnancy and postpartum. Thus, the aim of this study was to understand the consequences of pregnancy and fetal development, caused by infections by the SARS-CoV, MERS-CoV and SARS-CoV-2 viruses. Among the aforementioned infections, MERS-CoV seems to be the most dangerous for newborns, inducing high blood pressure, pre-eclampsia, pneumonia, acute renal failure, and multiple organ failure in mother. This also causes a higher occurrence of emergency cesarean deliveries and premature births, in addition, some deaths of mothers and fetuses were recorded. Meanwhile, SARS-CoV and SARS-CoV-2 appear to have less severe symptoms. Furthermore, although a study found the ACE2 receptor, used by SARS-CoV-2, widely distributed in specific cell types of the maternal-fetal interface, there is no evidence of vertical transmission for any of the coronaviruses. Thus, the limited reported obstetric cases alert to the need for advanced life support for pregnant women infected with coronaviruses and to the need for further investigation for application in clinical practice.
Keywords: 2019-nCoV, Pregnancy, Pandemic, SARS, Newborns
1. Introduction
Severe Acute Respiratory Syndrome (SARS) appeared in China in 2002 as a contagious disease caused by Coronavirus (CoV) through contact with respiratory droplets and exposure to different fomites. Named SARS-CoV, the virus triggers clinical features such as fever followed by respiratory signs and symptoms that progress rapidly to the asymptomatic infection with atypical pneumonia and respiratory failure [[1], [2], [3], [4], [5]].
Saudi Arabia (in 2014), a new respiratory illness called Middle East Respiratory Syndrome (MERS) also was attributed to CoV infection. MERS-CoV, as well as SARS-CoV, promoted a serious infection that required special care in intensive care units due to its high mortality [4,6,7].
Recently in China, probably in October 2019, a new type of illness caused by a new strain of the coronavirus, the SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) emerged in Wuhan city. The new illness present respiratory symptoms similar to the other two syndromes mentioned above, but the virus is closely related to SARS (82 % similarity to SARS-CoV virus) than to MERS and shows high pathogenicity in humans [[8], [9], [10]].
In contrast to other viral infections, such as Ebola and Influenza, SARS-CoV-2 has high infectious power and easy ability to spread geographically, due to the fact that the infection is asymptomatic in some cases. Although these viruses promote serious complications during pregnancy, few studies have reported the vertical or perinatal transmission of SARS-CoV and MERS-CoV in children born to mothers with the syndrome [11].
New challenges emerged with the recent 2019-nCoV pandemic. Health professionals and organizations worldwide are studying in real-time the high dissemination and lethality promoted by SARS-CoV-2 which overloads the health systems and makes it impossible to treat patients properly [2,11,12]. Pregnant women are an unknown field in this intricate process and so far, we don't have epidemiological data showing significant morbidity and mortality from viral diseases during pregnancy. Thus, the aim of this study was to understand the consequences, in pregnancy and fetal development, caused by SARS-CoV, MERS-CoV, and SARS-CoV-2 infections through studies published in the literature.
2. Methodology
The study used a qualitative methodology with a bibliographic and descriptive approach. The aim was to use integrative and facilitators tools to enable the aggregation of knowledge to readers, with a greater understanding of the identified subject. The electronic search of the literature was performed using the following research sources: Medical Literature Analysis and Retrieval System Online (MEDLINE) and ScienceDirect. The descriptors used to survey the research were: SARS-CoV, MERS-CoV, SARS-CoV-2, 2019-nCoV, COVID-19, pregnancy, pandemic, and newborns.
As inclusion criteria, studies were used that approached each virus and clinical disorders promoted. As the thematic was focused to discover if each illness is able to promote danger in both pregnant and fetus, we also used word association, i.e., SARS + pregnancy, MERS + fetal development, etc. Furthermore, we used articles fully available in the network. For final writing, a total of 145 references were used, which allowed an analytical reading and in-depth interpretation, to identify the answers to the questions elaborated to achieve the proposed goal.
3. Epidemiological overview of infections by SARS-CoV, MERS-CoV, and SARS-CoV-2
The SARS (Severe Acute Respiratory Syndrome) epidemic caused by the SARS-CoV virus, originated in November 2002, through the interspecies transmission of wildlife markets in Guangdong Province, southeastern China. In the months following the primary infection until its end in August 2003, there were 8422 people infected in 26 countries, leading to 916 deaths (10.87 %). These numbers are not on the scale of major epidemics, such as seasonal forms of influenza or 2019-nCoV, but in an era of rapid globalization, the potential for a pandemic was significant [13].
Epidemiological investigations revealed that the spread of SARS-CoV outside China began on February 21, 2003. When 12 people staying at the Metropole Hotel in Hong Kong were infected with the SARS-CoV from an asymptomatic and infected Professor of the Zhongshan University. These 12 people were responsible for spreading the infection to Singapore, Vietnam, Canada, Ireland, and the United States - starting the chain of infection in all these countries. According to WHO estimates, the majority of the more than 8000 probable cases of SARS worldwide originated from this initial contagion [14].
The middle east respiratory syndrome (MERS) became a public health issue in Asian countries initially in 2012. The first recorded death was in a male patient, with renal and respiratory failure caused by the virus [15]. Since 2012, MERS-Cov has spread to many countries in a wide geographic range worldwide, including Lebanon, United States, Italy and Germany [16].
A comparative study of the MERS-CoV full genome sequence showed a higher homology and closeness phylogenetic with bats coronavirus than with coronavirus from other animals [17], leading to believed that MERS-CoV would originate from bats. Curiously, some studies showed that MERS-CoV patients from Saudi Arabia who have jobs involving camels have a higher prevalence of MERS-CoV antibodies than other individuals. These findings, associated with high antibodies levels of MERS-CoV, which also have been detected in camels, showed that these animals can be a source of spread of the infection through human-animal contact [18].
According to the World Health Organization (WHO) in 2012, only 9 cases of MERS-CoV infection were confirmed, including five deaths. The number of cases and deaths became much more significant in 2014, also increasing its spread. From April 2012 to November 2019, 2494 laboratory-confirmed cases and 858 deaths were reported in 27 countries [19,20].
The new coronavirus (SARS-CoV-2) pandemic initially spread from Wuhan, quickly reached all of China, Asia, and finally reached the European, American and African continents [21]. Until the finalization of this manuscript, this new virus is extremely worrying, totaling more than ten millions and four hundred thousand cases worldwide. The USA has the highest number of cases in the world, with more than 2.5 millions. In addition, when comparing graphs (Fig. 1 ) from the first ten weeks of coronavirus spread, it is observed that SARS-CoV-2 has reached 34 times more cases than SARS-CoV. Unfortunately, we don’t have the statistical numbers of the SARS-CoV-2 pandemic due to the current increase in cases. However, until the end of the writing of this manuscript, there were 6.967.910 confirmed cases and 401.368 deaths around the world. Moreover, it is also important to note that both SARS coronaviruses had China as the epicenter [19,22,23].
Fig. 1.
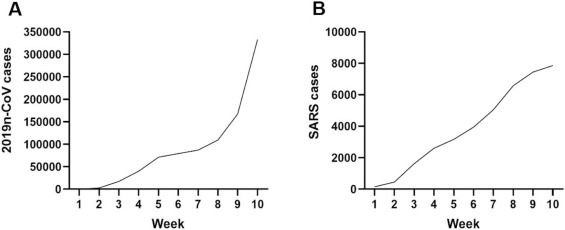
Number of confirmed cases of infected patients by SARS-CoV-2 (A), SARS-CoV (B). Source: WHO, 2002-2010.
4. Characterization of viral particles and their cellular interactions
The SARS-CoV, MERS-CoV and SARS-CoV-2 viruses are part of the coronavirus group, classified in the Coronaviridae family, in the order Nidovirales, known for having the largest RNA genomes. These three viruses have a positive-sense single-stranded RNA genome ((+)ssRNA) ranging in size from 26 to 32 kb. The Coronaviridae family is subdivided based on genotypic and serological characters into four genera: Alfa, Beta, Gama e Deltacoronavirus [24,25]. Currently, all identified coronaviruses that can infect humans belong to the first two genera, being constituted by Alfacoronavírus (AlphaCoVs) hCoV-NL63 and hCoV-229E; and the Betacoronavírus (betaCoVs) HCoV−OC43, HKU1, SARS-CoV, MERS-CoV and SARS-CoV-2 [26,27].
The SARS-CoV genome was the first to be described in 2002, with approximately 29.7 kb in length, followed by MERS-CoV in 2012, with 30.1 kb and the SARS-CoV genome in 2019, with 29.8 kb [[28], [29], [30], [31]], including a variable number (from 6 to 14) of open reading frames (ORFs) [32]. The first ORF represents approximately 67 % of the entire genome, encoding 16 non-structural proteins (nsps), translated by genomic RNA. While the other ORFs encode accessory and structural proteins, translated by 10 subgenomic RNAs [[33], [34], [35], [36], [37]].
The main structural proteins are spike surface glycoprotein (S), small envelope protein (E), membrane protein (M), and nucleocapsid protein (N), whereas S protein plays an essential role in binding to receptors in the host cell, determining host tropism. Structural and accessory proteins have several functions, including induction of apoptosis, interference in the innate immune response, and regulation of cell protein expression [[38], [39], [40], [41]]. The SARS-CoV/SARS-CoV-2 and MERS-CoV spike proteins bind to different host receptors through different receptor-binding domains (RBDs). SARS-CoV and SARS-CoV-2 use the angiotensin-converting enzyme 2 (ACE2) as one of the main receptors and CD209 L as an alternative receptor. While MERS-CoV uses dipeptidyl peptidase 4 (DPP4), also known as CD26 as the primary receptor. Due to this interaction, the enzyme DDP4 began to be widely studied for its association with immune regulation, signal transduction, and apoptosis [42,43].
The comparison of the genomes of SARS-CoV, MERS-CoV, and SARS-Cov-2 showed that they are almost identical. However, protein 8a is present in SARS-CoV and absent in SARS-CoV-2. Another difference is in protein 8b, which has 84 amino acids in SARS-CoV and 121 amino acids in SARS-CoV-2. In addition, in SARS-CoV protein 3b, there are 154 amino acids, while in SARS-CoV-2 there are only 22 amino acids. Further studies need to be carried out in order to characterize how these differences affect functionality and pathogenesis. Although these viruses are in the same clade (Betacoronavirus), MERS-CoV differs in terms of proteins encoded by the pp1ab, pp1a genes, in addition to the E, M, N and accessory proteins 3a, 7a and 8b [8,44,45].
5. Immune response to MERS-CoV, SARS-CoV and SARS-CoV-2 infection
One of the characteristics among SARS-CoV, MERS-CoV, and SARS-CoV-2 in infected patients is severe lymphopenia [[46], [47], [48], [49], [50], [51], [52], [53]]. A notable drop in CD4+ and CD8+ T lymphocytes count occurs early in the course of the disease and is associated with adverse results. In patients infected with SARS-CoV, this lymphocyte involvement is determined by the expression of CD25, CD28, and CD69, in the subsets of CD4+ and CD8+ T cells [54,55]. In MERS-CoV infection, the virus negatively regulates MHC-I, MHC-II, and CD80/86 in antigen-presenting cells (APCs), which results in inhibition of the T lymphocyte response [56]. These events can further impair the function of B and T cells through negative regulation of DPP4 receptors by MERS-CoV [57,58].
The induction of immunosuppression throughout MERS-CoV infection by promoting apoptosis of T lymphocytes was identified as another strategy to manipulate the survival pathways by the host’s immune response [59]. DPP4 is thought to play a significant role in the signaling and activating T cells during the course of MERS-CoV infection [60]. Ying et al. [58] observed that helper CD4+ T cells were more susceptible to MERS-CoV infection. In addition, MERS-CoV can induce T lymphocyte apoptosis by activating intrinsic and extrinsic apoptosis pathways. Interestingly, Mahallawi et al. [61], observed a significant increase in the production of the cytokine IL-17 in patients infected with MERS-CoV. Thus, it is suggested that MERS-CoV infection promotes the induction of Th17 cytokines. These Th17 cytokines have the activity of recruiting neutrophils and monocytes to the site of infection or inflammation and lead to the activation of other cytokine and chemokine cascades, such as IL-1, IL-6, TNF-α, TGF-β, IL-8, and MCP-1 [62].
The immune system is able to produce neutralizing antibodies, which block each virion and impair its entry into host cells. The detection of these antibodies to MERS-CoV in human serum is considered one of the confirmatory diagnosis. These antibodies are potent tools of the adaptive response to MERS-CoV infection. However, serum detection of anti-MERS-CoV antibodies occurs 14–21 days after infection [63,64]. Antibody concentrations increase over time and can last for more than 18 months, and the long-term response is highly dependent on the severity of the infection [65]. The anti-SARS-CoV antibody response can remain detectable for up to 24 months after infection and then gradually decrease until it completely disappears 6 years after infection [66,67].
Several HLA-A*02:01-restricted T lymphocytes that recognize epitopes of SARS-CoV were identified in the peripheral blood mononuclear cells (PBMC) of patients recovered from SARS. The vast majority of these immunogenic epitopes were specific for SARS-CoV S and N proteins [68,69]. Several CD8+ T cell epitopes characterized in the SARS-CoV M protein have also been identified in PBMC of SARS survivors, however, not known about HLA activation [70]. Libraty et al. [71], detected epitopes of CD4+ T cell restricted to HLA-DR in protein S (S729−745, S358−374 and S427–444) in patients recovered of SARS. CD4+ and CD8+ memory T cells were found in a patient who recovered from SARS-CoV infection within four years after the acute phase of the infection. Other observations showed that three epitopes (SSP-1, S978 e S1202) of CD8+ T lymphocytes to HLA-A*02:01-restricted, have been identified for protein S in patients recovered more than one year after infection. Other studies have been showing that virus-specific CD8+ T lymphocytes produced high levels of effector cytokines (IFN-γ and TNF-α) and cytotoxic molecules (perforin and granzyme B) [72].
In patients recovered from SARS, specific memory CD4+ T lymphocytes for HLA-DR08 and HLA-DR15 restricted epitopes of the SARS-CoV S protein have been identified [71]. Virus-specific CD4+ T lymphocytes mainly exhibited a central memory phenotype (CD45RA− CCR7+ CD62L−), while CD8+ memory T lymphocytes were identified as memory effector cells (CD45RA+ CCR7− CD62L−) [70,73,74]. These findings suggest that there is an activity of specific memory T lymphocytes in long-term protection against SARS-CoV infections. In addition, the response of memory B lymphocytes to SARS-CoV decrease significantly after 1–2 years of infection [75].
The immunological findings to date are too preliminary to draw definitive conclusions regarding to SARS-CoV-2 infection. Recent studies have shown inconclusive pathways promoted by the virus in the immune system. Xu et al. [52] showed that the number of CD4+ and CD8+ T cells in the peripheral blood of patiets infected with SARS-CoV-2 is significantly reduced. Zhou et al. [43], investigating 99 cases, also showed a reduction in total lymphocytes (35 %) and an increase of serum IL-6 (52 %) and C-Reactive Protein (84 %) and several studies point to the importance of the "cytokine storm" leading to the 2019-nCoV severity. A recent publication points to the increase of the humoral response in patients who needed intensive care unit (ICU) care due to SARS-CoV-2, such as IL-2, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1A, TNF-α [76]. In addition, the number of CD4+ and CD8+ T cells in the peripheral blood of SARS-CoV-2-infected patients is reduced, but have excessive activation, demonstrating high proportions of HLA-DR (CD4 3.47 %) and CD38 (CD8 39.4 %) double-positive fractions [77].
6. Clinical aspects in pregnant women infected with MERS-CoV, SARS-CoV and SARS-CoV-2 and the influence on fetal development
For patients in general, the clinical characteristics resulting from contact with MERS-CoV, SARS-CoV, and SARS-CoV-2 can trigger an asymptomatic condition, mild symptoms such as a cold with or without fever. However, symptoms can progress to severe cases, corresponding to 25 % of patients infected with SARS-CoV-2 [23], for acute respiratory distress, pneumonia, gastrointestinal problems, liver damage, septic shock, and multiple organ failure, which can lead to death [52,78,79]. Especially in severe cases, SARS-CoV-2 may present multifocal nodular and ground-glass opacities surrounding the pulmonary lobes [80] in which there is an increase in the parenchyma attenuation coefficient, with preservation of bronchovascular marks [81]. In addition, regarding infected pregnant women, some points should be considered, such as symptomatic changes, vertical transmission to the fetus, and classification in risk groups.
As it is a new strain, there is little information about SARS-CoV-2 in pregnant women. In addition, infections by MERS-CoV and SARS-CoV in this profile of patients are regionally limited. Thus, external factors such as local habits may contribute to the general picture. The few published obstetric cases point to the need for advanced life support for these pregnant women and for a severely compromised maternal and gestational prognosis [[82], [83], [84]].
In the city of Wuhan-China, nine pregnant patients, gestational age between 36 to 38 weeks, were diagnosed with SARS-CoV-2. According to Chen et al. [85], the clinical profile of these patients was not severe, none had a history of diseases prior to pregnancy, aged 27–40 years. The most significant symptoms were fever at admission (78 %), postpartum fever (68 %), myalgia (33 %), malaise (22 %), cough (44 %). Moreover, there was one case of pre-eclampsia and one case of abnormal liver function. Regarding laboratory tests, the most relevant data were: leukocyte count (<9.5 × 109 cells/L) (78 %), lymphopenia (<10⁹ cell/L) (56 %), high C-reactive protein (> 10 mg/L) (75 %), confirmation test performed (quantitative SARS-CoV-2 RT-PCR) (100 %). Patients also had a bad pneumatic condition (89 %).
Regarding C-reactive protein, it is present in low concentrations in normal pregnant women. Meanwhile, in acute morbid situations, such as infections, inflammatory processes or tissue destruction, it rises rapidly [86], being observed high values in maternal blood also in conditions such as pre-eclampsia, premature birth or growth restriction intra-uterine [87,88].
Assiri et al. [83] demonstrated that Saudi Arabia accounted for 1308 cases of MERS-CoV between November 2012 and February 2016, of which, five cases were confirmed for pregnant women by the Ministry of Health. Aged between 27–34 years, two of the five patients (40 %) died during the disease. Among the five pregnancies, two (40 %) resulted in perinatal death: one pregnancy resulted in intrauterine fetal death and one newborn died four hours after an emergency cesarean section (C-sec). As symptoms and worsening of the clinical picture, some patients presented: shortness of breath, fever, elevated blood pressure, pre-eclampsia, pneumonia, acute renal failure, acute respiratory distress, and multiple organ failure. Similar results were found by Payne et al. [6], Malik et al. [7], and Alserehi et al. [82]. The pathogenesis of the disease also affects immune cells with reduced activity of NK cells, M1 macrophages, and helper T cells [89].
A group of 12 pregnant women with a confirmed diagnosis for SARS-CoV were analyzed, aged 27–44 years in Hong Kong. All patients had a high fever for two to nine days and the majority had chills, stiffness, malaise, and myalgia. In addition, all developed a status of lymphopenia (<10⁹ cell/L) and lung problems, slightly elevated lactate dehydrogenase (41.6 %), and aminotransferase (33.3 %), and did not present pneumonia. Half of the patients (50 %) required care in the ICU due to the decreased blood flow saturation and part of the group needed mechanical ventilation (33.3 %) [2].
Although pregnant women show symptoms similar to non-pregnant women for Coronavirus, some points should be considered, such as complications during pregnancy and postpartum [[90], [91], [92]]. The risk of viral pneumonia is significantly higher among pregnant women compared to the general population, especially when there is no antiviral therapy [2]. There are no reports for vertical transmission, however, some newborns developed severe retardation in intrauterine growth and life-threatening gastrointestinal complications [3]. As the SARS-CoV-2 appears to have similar pathogenicity to SARS-CoV and MERS-CoV, pregnant women may be at high risk to develop serious infections.
In two cases of SARS-CoV in pregnant patients in the United States, none had a severe outcome. Both patients had healthy babies. Five SARS-related infections, in China, occurred during the second and third trimesters. Moreover, one of those cases, a twins pregnancy, showed that both children died, while the remaining cases did not have SARS-CoV infection [2,93].
In the United States, in 2003, a study showed that two out of eight people with confirmed severe disease (SARS-CoV), were pregnant women. The newborn, from a mother who had positive tests for SARS-CoV, did not develop the disease, abnormality, or congenital malformation. In this investigation, serum, blood samples in the cord and placenta from a patient were used at the time of the child-birth for diagnosis. Breast milk samples on days 12 and 30 postpartum were also negative for SARS-CoV. Blood, stool, and nasopharyngeal swab samples and umbilical cord blood samples did not register the presence of the virus. Stool samples from newborns collected on days 12 and 30 after delivery were also negative for viral RNA. Likewise, RT-PCRs and viral cultures of cord blood and placental tissues were all negative for SARS-CoV. The amniotic fluid obtained from the patients was also negative for SARS-CoV. No viral inclusion body or particle was detected in the samples from childbirth [94].
Robertson et al. [12], reported that SARS-CoV antibodies were detected in the breast milk of an infected patient, with 19 weeks of gestation. However, in this case, no viruses were detected in breast milk. Until now, few cases have been studied to define the risks and provide guidance for the treatment of pregnant women infected with SARS-CoV [94].
An epidemiological investigation was carried out among survivors of the MERS-CoV outbreak in Jordan, in which a fetus in the second-trimester had an acute respiratory illness attributed to MERS-CoV. It was the first occurrence of stillbirth during a MERS-CoV infection and their consequences may help the surveillance and management of pregnant women in contexts of respiratory failure [6].
Only a few reports have been published about pregnant women with an infectious respiratory virus, such as MERS-CoV. A laboratory-confirmed case was reported during a MERS outbreak in the Republic of Korea in a woman in the 35 gestational weeks. She recovered and delivered a healthy baby by emergency cesarean section [95].
Another case of MERS coronavirus infection in a pregnant woman described in South Korea resulted in maternal recovery. This patient had suspected placental detachment, requiring a cesarean section. However, the neonate showed a negative result to MERS-CoV. In addition, a report from the Kingdom of Saudi Arabia showed a 32-week pregnant woman with MERS-CoV in need of ventilatory support and intensive care, who eventually recovered and gave birth to a healthy baby [82,83,[96], [97], [98]].
In a review of 11 pregnant women infected with MERS-CoV, ten had adverse results, six neonates required admission to the intensive care unit and three died. Two newborns were born prematurely due to severe maternal respiratory failure [99,100].
To date, there are two published cases on the obstetric and perinatal aspects of SARS-CoV-2. The first reports on the maternal and perinatal outcomes of patients infected with SARS-CoV-2 were a retrospective assessment of nine women who had their pregnancies resolved in Wuhan-China (previously mentioned). As for perinatal results, it should be noted that there was no fetal death, neonatal death, or neonatal asphyxia. Four patients had premature labor but under 36 gestational weeks. Two of the four premature newborns had a birth weight of less than 2500 g. All nine neonates had Apgar scores of 1 min above 8 and Apgar scores of 5 min above 9. This study related that no cases of vertical virus transmission were detected [85].
The second report, also from China, reports the neonatal prognosis of 10 children born from nine women (one case of twins). The onset of symptoms occurred before delivery in four cases and in two, the symptoms appeared on the day of delivery. In three of them, the clinical picture was manifested after delivery. In addition, in seven of them, the delivery was by cesarean section, apparently none due to SARS-CoV-2. The maternal prognosis was considered good, with the recovery of all of them. The perinatal prognosis was reasonable, although there was no child with an Apgar score of 5 min less than 8. The rate of preterm births was high and one of them died due to digestive bleeding complications. The authors note that in this series of cases, once again, there was no vertical transmission, but the small number of cases does not allow this conclusion to be imperative [101].
In summary, the study conducted by Li et al. [77] revealed that the ACE2 receptor used by SARS-CoV-2 was widely distributed in specific cell types of the maternal-fetal interface cells and fetal organs. Regarding maternal-fetal interface, ACE2 was found highly expressed in these cells, including stromal cells, perivascular cells of decidua and cytotrophoblast, and syncytiotrophoblasts in the placenta. In addition, ACE2 was also expressed in specific cell types of human fetal heart, liver, and lung, but not in the kidneys. Although previous clinical studies have not observed vertical transmission of SARS-CoV-2 among limited cases, this phenomenon still needs to be investigated more carefully in clinical practice. Corroborating this study, the study conducted by Schwartz [102] showed that all neonatal samples tested, including in some cases placentas, were negative for SARS-CoV-2 by RT-PCR. Thus, at this point in the global pandemic of Covid-19, there is no evidence that the SARS-CoV-2 virus undergoes intrauterine or transplacental transmission from infected pregnant women to their fetuses.
A case of neonatal SARS-CoV-2 infection in China with pharyngeal swabs was reported with a positive RT-PCR test 36 h after birth. However, it has not been confirmed yet if the case is a mother-to-child vertical transmission [103]. In addition, two cases of SARS-CoV-2 were reported during the third trimester of pregnancy, in which mothers and newborns had excellent results and the virus has not been identified in all conception products and in the newborns. This reinforces once again that there is evidence of a low risk of intrauterine infection by vertical transmission of SARS-CoV-2 [104].
During the Hong Kong outbreak, a cohort study of five babies born alive to pregnant women with SARS-CoV was conducted. A systematic search through perinatal examinations for transmission of the SARS-associated coronavirus, including RT-PCR assays, viral cultures, and paired serological titers. The virus was not detected in any of the babies' samples.. In addition, none of the babies developed clinical, radiological, hematological, or biochemical evidence suggestive of SARS [105]. Likewise, as there is no evidence that also SARS-CoV-2 can be transmitted transplacentally from the mother to the baby the treatment strategy for children with Covid-19 is based on the adult experience. It is also important to highlight that the disease condition of most children is mild and, to date, few deaths in the pediatric age group have been reported [106].
7. Treatment strategies
Studies have reported that, among other alternatives, the spread of MERS-CoV and SARS-CoV can be inhibited by the use of protease inhibitors, corticosteroids, interferon (IFN) therapy, and repurposing of the clinically established drug. Although the population does not have a specific treatment or vaccine for these diseases, it can be controlled with a series of preventive measures and a drug treatment regime [107,108] which may result in possible therapeutic treatment option for the novel SARS-CoV-2 [77]. The treatment of pregnant women for coronavirus is even more complicated, considering the contraindications for the use of some drugs mentioned bellow.
The process for finding new ways to use medicine with pre-existing functions is known as a repurposing of drugs, this is further stimulated considering the costs and time spent developing and regulating a new drug. Examples of repurposing drugs that somehow demonstrated effectiveness against MERS-CoV and/or SARS-CoV infections are: Ribavirin [109], Nitazoxanide [110], Remdesivir [111] and Lopinavir, although the efficacy and safety of some drugs remain controversial [108,112].
Ribavirin is an approved drug developed to treat HCV and especially children hospitalized for the treatment of the respiratory syncytial virus (RSV). According to Kim et al. [113] in vivo studies performed with combinations of lopinavir/ribavirin (LPV/r), Ribavirin and interferon resulted in the cure of a 64-year-old MERS patient from Korea after 6 days of treatment. In addition, the combination of LPV/r and interferon was also included in a study for a randomized control in Saudi Arabia to MERS patients [114].
Lopinavir combined with ribavirin also presents a synergistic effect being able to inhibit the cytopathic effect of SARS-CoV in vivo and reduce the death rate by about 20%–30% on in vitro and in vivo experiments [[115], [116], [117]]. A molecular modeling study for SARS-CoV-2 was recently carried out to assess the binding effect between Lopinavir, Ritonavir, and two viral proteases, CEP_C30 and PLVL. This study showed that these anti-HIV drugs can bind and induce changes in conformations mainly in CEP_C30 [118]. Even with the promising results to MERS-CoV and SARS-CoV, the use of high doses of ribavirin is worrying, since it can trigger side effects such as anemia [119].
In addition, ribavirin is contraindicated during pregnancy, due to its potential teratogenicity demonstrated in many animal models studied [120]. Controversially, several evidences demonstrate that the teratogenicity effect of ribavirin associated with humans is not significant, as observed when the Ribavirin Pregnancy Registry was established in 2003 [121]. However, more information on possible placental interference caused by ribavirin is needed to ensure its use.
Chloroquine (CQ) and hydroxychloroquine (HCQ) are used to treat mainly malaria and rheumatic diseases, respectively and have a very similar chemical structure [122]. CQ showed to be able to inhibit MERS-CoV in vitro in a dose-dependent manner. [123,124]. In addition, to MERS-CoV, no clinical data are available on the success of chloroquine to patients [125].
In a recent in vitro study with Vero E6 cells, CQ was effective to inhibit the SARS-CoV-2 infection [123,124]. In addition 70 % of a patient group treated with HQ combined with Azithromycin had no detectable viral load after 6 days of treatment [126]. However, is important to show that this study is an open-label non-randomized clinical trial and the authors didn’t show the inclusion criteria and the triage of patients to ensure patient safety [127]. In this case, the use of chloroquine is still controversial and needs more clinical trials.
In Brazil, due to the lack of specific medications, the Ministry of Health has authorized the use of CQ or HCQ as an adjunct therapy in the treatment of severe forms of COVID-19 in hospitalized patients [128]. Even CQ being considered as an option to SARS-CoV-2 treatment, due to its low cost, easy accessibility and previous studies of its effects in the malaria treatment [103], the capacity to be ototoxic and retinotoxic in the fetal phase for individuals exposed to chloroquine raises concerns about its use [129,130]. In contrast, many studies suggest that there is no increase in congenital malformations associated with the use of HCQ. Evidences also support the compatibility of HCQ with breastfeeding [131] and it is also important to note that for rheumatological diseases it is allowed and recommended the HCQ use during pregnancy [132].
IFN therapy is commonly used when a specific drug or vaccine is not available to treat some virus infection. The type 1 IFN is usually known as a participant to the mammalian host defense against virus infection [133]. The use of recombinant IFN inducers or IFN alone have been appointed as a treatment option for MERS, supported by the fact that coronaviruses are able to suppress IFN, making it necessary to increase this response [134,135]. A recent study in vitro with Vero E6 cells has reported that after pretreatment with IFN-I, a decrease in viral protein and SARS-CoV-2 replication was observed [136].
Currently, IFN-a therapy is classified as category C during pregnancy, as it appears to be linked to abortive effect in animal models, such as the rhesus monkey [137], the drug also interferes in the fertility degree, and the menstrual cycle that normalize after discontinuing treatment. In addition, serum estradiol and progesterone levels were decreased in women treated with IFN [138]. However, IFN-a is used in pregnant women with thrombocytopenia [139] and also was used as an alternative to BCR-GLA-positive leukemia treatment in pregnant women, with no evidence of increases to the rate of congenital anomalies or spontaneous abortions was found [140].
Lectins are a heterogeneous group of proteins capable of reversibly and specific binding to sugars [141]. The Mannose-binding lectin (MBL) participates strongly in the innate immune system before specific antibody responses. Studies report that MBL by binding to SARS glycoprotein S is able to inhibit the viral activity [142]. In addition, a study with serum from 569 SARS patients and 1188 control, showed that the deficient levels of MBL were observed more frequently in infected individuals than in control patients, therefore MBL deficiency can be a factor of susceptibility to infection [143].
The spike glycoprotein from SARS-CoV is a promising drug target. In this regard, a lectin called Griffithsin derived from red algae is known to be able to bind several viral glycoproteins, including SARS-CoV spike glycoprotein and HIV glycoprotein. Griffithsin was used in a preventive evaluated test to HIV, as gel and enema. However, to reach a preventive treatment to SARS-CoV-2, the delivery systems of spike inhibitors needs to be reevaluated [118,144]. No record was found regarding the effects of Griffithsin on pregnant women.
It is important to note that veterinary control of coronavirus is crucial for managing human epidemics, since some, the virus can be responsible for zoonoses [18]. In addition, a recent study using camels demonstrated effectiveness in administering vaccines with Modified Vaccinia Ankara (MVA), capable of expressing the MERS-CoV S protein, resulting in the presence of neutralizing antibodies (NAbs) and decreased levels of infectious viruses in treated camels. However, further studies are needed to ensure the efficiency and safety of this vaccine in humans [145].
8. Conclusion
Published obstetric cases reported the need for advanced life support for pregnant women infected with the coronavirus. Symptoms such as fever, chills, stiffness, myalgia, malaise, and cough, as well as laboratory data such as neutrophilia, lymphopenia, increased C-reactive protein, lactic dehydrogenase and aminotransferase were found at admission. This inflammatory status can promote conditions such as pre-eclampsia, premature birth, or growth restriction intra-uterine. In fact, the fever, high blood pressure, pre-eclampsia, pneumonia, acute respiratory distress, and multiple organ failure, have been reported in the postpartum period, especially in pregnant women infected by MERS-CoV, which seems to be the most dangerous virus for newborns.
Different studies affirmed that some pathophysiological changes were observed in the placentas of CoV-positive mothers. However, the vertical transmission for SARS-CoV and MERS-CoV viruses has not been detected, and few cases showed that the pregnancy resulted in intrauterine fetal death, congenital malformation (due to prematurity) or newborn death after emergency cesarean section. We believe that the coronaviruses infection on pregnant can promote multiple disorders, such as shortness of breath, fever, elevated blood pressure, preeclampsia, pneumonia, and multiple organ failure, which can affect the fetus's health. In relation to the few studies on SARS-CoV-2, as it is a new strain, it is still too early to draw conclusions.
References
- 1.Berger A., Drosten C., Doerr H.W., Stürmer M., Preiser W. Severe acute respiratory syndrome (SARS)—paradigm of an emerging viral infection. J Clin Virol. 2004;29(1):13–22. doi: 10.1016/j.jcv.2003.09.011. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng W.F., Wong S.F., Lam A., Mark Y.F., Yao H., Lee K.C. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology. 2016;38(3):210–218. doi: 10.1080/00313020600696280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne D.C., Iblan I., Alqasrawi S., Nsour M.A., Rha B., Tohme R.A. Stillbirth during infection with Middle East respiratory syndrome coronavirus. J Infect Dis. 2014;209:1870–1872. doi: 10.1093/infdis/jiu068. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik A., El Masry K.M., Ravi M., Sayed F. Middle east respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates. Emerg Infect Dis. 2013;2016(22):515–517. doi: 10.3201/eid2203.151049. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan Jfw, Kok Kh, Zhu Z., Chu H., To Kkw, Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mardani M., Pourkaveh B.A. Controversial Debate: Vertical transmission of COVID-19 in pregnancy. Arch Clin Infect Dis. 2020;15(1):e102286. doi: 10.5812/archcid.102286. [DOI] [Google Scholar]
- 11.Vasylyeva O. Pregnancy and COVID-19, a brief review. Int J Integr Pediatr Environ Med. 2020;5(1):8–13. doi: 10.36013/ijipem.v5i1.71. [DOI] [Google Scholar]
- 12.Robertson C.A., Lowther S.A., Birch T., Tan C., Sorhage F., Stockman L. SARS and pregnancy: a case report. Emerging Infect Dis. 2004;10(2):345. doi: 10.3201/eid1002.030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . 2003. SARS (Severe acute respiratory syndrome)https://www.who.int/ith/diseases/sars/en/ Available from: [Google Scholar]
- 14.WHO . 2003. SARS outbreak contained worldwide.https://www.who.int/mediacentre/news/releases/2003/pr56/en/ Available from: [Google Scholar]
- 15.Muller M.A., Meyer B., Corman V.M., Masri M.A., Turkestani A., Ritz D. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15(6):629. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PubMed] [Google Scholar]
- 16.Meo S.A., Alhowikan A.M., Al-Khlaiwi T., Meio I.M., Halepoto D.M., Iqbal M. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 17.Kandeel M. Bioinformatics analysis of the recent MERS-coV with special reference to the virus-encoded spike protein. Mol Enzymol Drug Targets. 2014;1(1):1–10. doi: 10.21767/2572-5475.10001. [DOI] [Google Scholar]
- 18.Choi J., Kim Mg, Oh Yk, Kim Yb. Progress of Middle East respiratory syndrome coronavirus vaccines: a patent review. Expert opinion on therapeutic patentes. 2017;27(6):721–731. doi: 10.1080/13543776.2017.1281248. [DOI] [PubMed] [Google Scholar]
- 19.WHO . 2019. Middle East respiratory syndrome coronavirus (MERS-CoV)https://www.who.int/emergencies/mers-cov/en/ Available from: [Google Scholar]
- 20.WHO . 2019. Middle East respiratory syndrome coronavirus (MERS-CoV)https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov) Available from: [Google Scholar]
- 21.Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis. 2004;8(4):223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipsitch M., Cohen T., Cooper B., Robins J.M., Ma S., James L. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300(5627):1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) 2020. 2005. Statement on the meeting of the International health regulations.https://www.who.int/news-room/detail/23-01-2020-statement-on-the-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) Available from: [Google Scholar]
- 24.Adams M.J., Carstens E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2012) Arch Virol. 2012;157(7):1411–1422. doi: 10.1007/s00705-013-1688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz . Ninth report of the international committee on taxonomy of viruses. 2012. Virus taxonomy; pp. 486–487. https://doi.org/978-0-12-384684-6. [Google Scholar]
- 26.Bermingham A., Chandi Ma, Brown Cs, Aarons E., Tong C., Langrish C. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Eurosurveillance. 2012;17(40):20290. PMID: 23078800. [PubMed] [Google Scholar]
- 27.Zaki A.M., Boheemen S., Bestebroer T.M., Adme Osterhays, Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 28.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S.N. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 29.Gorbalenya A.E., Enjuanes L., Ziebuhr J., Snijder E.J. Nidovirales: evolving the largest RNA virus genome. Virus Res. 2006;117:17–37. doi: 10.1016/j.virusres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu B., Ge X., Wang L.F., Shi Z. Bat origin of human coronaviruses. Virology jornal. 2015;12(1):221. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhardwaj K., Guarino L., Kao C.C. The severe acute respiratory syndrome coronavirus Nsp15 protein is an endoribonuclease that prefers manganese as a cofactor. J Virol. 2004;78:12218–12224. doi: 10.1128/JVI.78.22.12218-12224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decroly E., Debarnot C., Ferron F., Bouvet M., Coutard B., Imbert I. Crystal structure and functional analysis of the SARS-coronavirus RNA cap 2′-O-methyltransferase nsp10/nsp16 complex. PLoS Pathog. 2011;7(5):e1002059. doi: 10.1371/journal.ppat.1002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sevajol M., Decroly E., Canard B., Imbert I. Insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res. 2014;194:90–99. doi: 10.1016/j.virusres.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehmann K.C., Gulyaeva A., Zevenhoven-Dobbe J.C., Janssen G.M.C., Ruben M., Overkleeft H.S. Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase-containing protein of all nidoviruses. Nucleic Acids Res. 2015;43(17):8416–8434. doi: 10.1093/nar/gkv838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadler K., Rappuoli R. SARS: understanding the virus and development of rational therapy. Curr Mol Med. 2005;5(7):677–697. doi: 10.2174/156652405774641124. [DOI] [PubMed] [Google Scholar]
- 39.Li K., Wohlford-Lenane C.L., Channappanavar R., Parkvv J.E., Earnest J.T., Bair T.B. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc Natl Acad Sci. 2017;114(15):E3119–E3128. doi: 10.1073/pnas.1619109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Z., Zhang Z., Chen W., Cai Z., Ge X., Zhu H. Predicting the receptor-binding domain usage of the coronavirus based on kmer frequency on spike protein. Infect Genet Evol. 2018;61:183–184. doi: 10.1016/j.meegid.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng Zhou, Yang K.L., Wang Z.G., Hu B., Zhang L., Zhang W. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv. 2020;01(22):914952. doi: 10.1101/2020.01.22.914952. [DOI] [Google Scholar]
- 44.Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L.M. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng H., Yang L.T., Wang L.Y., Li J., Huang J., Lu Z.Q. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology. 2006;351(2):466–475. doi: 10.1016/j.virol.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 47.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 48.Panesar N.S. Lymphopenia in SARS. Lancet. 2003;361:1985. doi: 10.1016/S0140-6736(03)13557-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ajlan A.M., Ahyad R.A., Jamjoom L.G., Alharthy A., Madani T.A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. Am J Roentgenol. 2014;203(4):782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 50.Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko J.H., Park G.E., Lee J.Y., Lee J.Y., Cho S.Y., Ha Y.E. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect. 2016;73(5):468–475. doi: 10.1016/j.jinf.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver damage during highly pathogenic human coronavirus infections. Liver Int. 2020;00:1–7. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected by SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;00:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 54.Yu X.Y., Zhang Y.C., Han C.W., Wang P., Xue X.J., Cong Y.L. Change of T lymphocyte and its activated subsets in SARS patients. Acta Academiae Medicinae Sinicae. 2003;25(5):542–546. [PubMed] [Google Scholar]
- 55.Cai C., Zeng X., Ou A.H., Huang Y., Zhang X. Study on T cell subsets and their activated molecules from the convalescent SARS patients during two follow-up surveys. Chinese J Cell Mol Immunol. 2004;20(3):322–324. [PubMed] [Google Scholar]
- 56.Josset L., Menachery V.D., Grajinski L.E., Agnihothram S., Sova P., Carter V.S. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. MBio. 2013;4(3):e00165–13. doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu H., Zhou J., Wong B.H.Y., Li C., Cheng Z.S., Lin X. Productive repliC.aTion of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;454:197–205. doi: 10.1016/j.virol.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ying T., Li W., Dimitrov D.S. Discovery of T-cell infection and apoptosis by Middle East respiratory syndrome coronavirus. J Infect Dis. 2016;213(6):877–879. doi: 10.1093/infdis/jiv381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishii T., Ohnuma K., Murakami A., Takasawa N., Kobayashi S., Dan N.H. CD26-mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc Natl Acad Sci. 2001;98(21):12138–12143. doi: 10.1073/pnas.211439098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manni M.L., Robinson K.M., Alcorn J.F. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev Respir Med. 2014;8(1):25–42. doi: 10.1586/17476348.2014.854167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin W., Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2(1):1–5. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park W.B., RAPM Perera, Choe P.G., Lau E.H.Y., Choi S.J., Chun J.Y. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerging Infect Dis. 2015;21(12):2186. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corman V.M., Albarrak A.M., Omrani A.S., Albarrak A.M., Farah M.E., Almasri M. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62(4):477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alshukairi A.N., Khalid I.K., Ahmed W.A., Dada A.M., Bayumi D.T., Málica L.S. Antibody response and disease severity in healthcare worker MERS survivors. Emerging Infect Dis. 2016;22(6):1113. doi: 10.3201/eid2206.160010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu W., Fontanet A., Zhang P.H., Zhan L., Xin Z.T., Baril L. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006;193(6):792–795. doi: 10.1086/500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang F., Quan Y., Xin Z.T., Wrammert J., Ma M.J., Lv H. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186(12):7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y.D., Sin W.Y.F., Xu G.B., Yang H.H., Wong T.Y., Pang X.W. T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike protein elicit a specific T-cell immune response in patients who recover from SARS. J Virol. 2004;78(11):5612–5618. doi: 10.1128/JVI.78.11.5612-5618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu X., Gao X.M. Immunological responses against SARS-coronavirus infection in humans. Cell Mol Immunol. 2004;1(2):119–122. [PubMed] [Google Scholar]
- 70.Yang L.T., Peng H., Zhu Z.L., Li G., Huang Z.T., Zhao Z.X. Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin Immunol. 2006;120(2):171–178. doi: 10.1016/j.clim.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Libraty D.H., O’Neil K.M., Baker L.M., Acosta L.P., Olveda R.M. Human CD4+ memory T-lymphocyte responses to SARS coronavirus infection. Virology. 2007;368(2):317–321. doi: 10.1016/j.virol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen H., Hou J., Jiang X., Ma S., Meng M., Wang B. Response of memory CD8+ T cells to severe acute respiratory syndrome (SARS) coronavirus in recovered SARS patients and healthy individuals. J Immunol. 2005;175(1):591–598. doi: 10.4049/jimmunol.175.1.591. [DOI] [PubMed] [Google Scholar]
- 73.Openshaw P.J.M. What does the peripheral blood tell you in SARS? Clin Exp Immunol. 2004;136(1):11–12. doi: 10.1111/j.1365-2249.2004.02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang L., Peng H., Zhu Z., Li G., Huang Z., Zhao Z. Persistent memory CD4+ and CD8+ T-cell responses in recovered severe acute respiratory syndrome (SARS) patients to SARS coronavirus M antigen. J Gen Virol. 2007;88(10):2740. doi: 10.1099/vir.0.82839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin J.E., Louder M.K., Holman L.A., Gordon I.J., Enama M.E., Larkin B.D. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26(50):6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Fang. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hui D.S., Azhar E.I., Kim Y.J., Memish Z.A., Oh M.D., Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18(8):e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.European Centre for Disease Prevention and Control (ECDC) 2018. Rapid risk assessment: severe respiratory disease associated with Middle East respiratory syndrome coronavirus (MERS-CoV) 22nd update. [Google Scholar]
- 80.Kong W., Agarwal P.P. Chest imaging apperance of COVID-19 infection. Radiol Cardio Imag. 2020;2(1):e200028. doi: 10.1148/ryct.2020200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santos M.L.O. Ground-glass opacity in diffuse lung diseases: high-resolution computed tomography-pathology correlation. Radiol Bras. 2003;36(6) doi: 10.1590/S0100-39842003000600003. [DOI] [Google Scholar]
- 82.Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East Respiratory Syndrome coronavirus (MERS‐CoV) on pregnancy and perinatal outcome. BMC Infect Dis. 2016;16:105. doi: 10.1186/s12879-016-1437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Assiri A., Abedi G.R., Masri M.A., Saeed A.B., Gerber S.I., Watson J.T. Middle East Respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin Infect Dis. 2016;63(7):951–953. doi: 10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.CDC, Centers for Disease Control and Prevention Update: severe respiratory illness associated with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) worldwide, 2012-2013. MMWR Morb Mortal Wkly Rep. 2013;62(23):480–483. [PMC free article] [PubMed] [Google Scholar]
- 85.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;12 doi: 10.1016/S0140-6736(20)30360-3. 809-8157–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabral A.C.V., Lazaro J.F., Vitral Z.N.R. Maternal serum level of C-reactive protein in gestations complicated by preeclampsia. Rev Bras Ginecol Obstet. 2002;24(1) doi: 10.1590/S0100-72032002000100002. [DOI] [Google Scholar]
- 87.Hvilsom G.B., Thorsen P., Jeune B., Bakketeig L.S. C-reactive protein: a serological marker for preterm delivery? Acta Obstet Gynecol Scand. 2002;81:424–429. doi: 10.1034/j.1600-0412.2002.810509.x. [DOI] [PubMed] [Google Scholar]
- 88.Tjoa M.L., Vugt J.M.V., Go A.T., Blankenstein M.A., Oudejans C.B.M., Wijk J. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59:29–37. doi: 10.1016/S0165-0378(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 89.Robinson D.P., Klein S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee C.H., Huang N., Chang H.J., Hsu Y.J., Wang M.C., Chou Y.J. The immediate effects of the severe acute respiratory syndrome (SARS) epidemic on childbirth in Taiwan. BMC Public Health. 2005;5(1) doi: 10.1186/1471-2458-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee D.T.S., Sahota D., Leung T.N., Yip A.S.K., Lee F.F.Y., Chung T.K.H. Psychological responses of pregnant women to an infectious outbreak: a case-control study of the 2003 SARS outbreak in Hong Kong. J Psychosom Res. 2006;61(5):707–713. doi: 10.1016/j.jpsychores.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lam C.M., Wong S.F., Leung T.N., Chow K.M., Yu WC Wong T.Y., Lai S.T. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. Bjog Int J Obstet Gynaecol. 2004;111(8):771–774. doi: 10.1111/j.1471-0528.2004.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J.P., Wang Y.H., Chen L.N., Zhang R., Xie Y.F. Clinical analysis of pregnancy in second and third trimesters complicated severe acute respiratory syndrome. Zhonghua Fu Chan Ke Za Zhi. 2003;38(8):516–520. [PubMed] [Google Scholar]
- 94.Stockman L.J., Lowther S.A., Coy K., Saw J., Parashar U.D. SARS during pregnancy, United States. Emerg Infect Dis. 2004;10(9):1689–1690. doi: 10.3201/eid1009.040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park M.H., Kim H.R., Choi D.H., Sung J.H., Kim J.H. Emergency cesarean section in an epidemic of the middle east respiratory syndrome: a case report. Korean J Anesthesiol. 2016;69(3):287. doi: 10.4097/kjae.2016.69.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Racicot K., Kwon J.-Y., Also P., Silasi M., Mor G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol. 2014;72:107–116. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jeong S.Y., Sung S.I., Sung J.H., Ahn S.Y., Kang E.S., Chang Y.S. MERS-CoV Infection in a pregnant woman in Korea. J Korean Med Sci. 2017;32(10):1717–1720. doi: 10.3346/jkms.2017.32.10.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Racicot K., Mor G. Risks associated with viral infections during pregnancy. J Clin Invest. 2017;127(5):1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52(3):501. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Favre G., Pomar L., Musso D., Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet. 2020;395:10224. doi: 10.1016/S0140-6736(20)30311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0901-SA. 0: null. [DOI] [PubMed] [Google Scholar]
- 103.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan C., Lei D., Frang C., Li C., Wang M., Liu Y. Perinatal Transmission of COVID-19 Associated SARS-CoV-2: Should We Worry? Clin Infect Dis. 2020;226 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shek C.C., Ng P.C., Fung G.P.G., Cheng F.W.T., Chan P.K.S., Peiris M. Infants born to mothers with severe acute respiratory syndrome. Pediatrics. 2003;112(4) doi: 10.1542/peds.112.4.e254. e254-e254. [DOI] [PubMed] [Google Scholar]
- 106.Lu Q., Shi Y. Coronavirus disease (COVID‐19) and neonate: what neonatologist need to know. J Med Virol. 2020;92:564–567. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mustafa S., Balkhy H., Gabere M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): a review. J Infect Public Health. 2018;11(1):9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus–A possible reference for coronavirus Disease‐19 treatment option. J Med Virol. 2020;92:556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Qasim E.A. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin Infect Dis. 2019;70(9):1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Momattin H., Al-Ali A.Y., Al-Tawfiq J.A. A Systematic Review of therapeutic agents for the treatment of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Travel Med Infect Dis. 2019;30:9–18. doi: 10.1016/j.tmaid.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mulangu S., Davey R.T., Mbaya O.T., Proschan M., Mukadi D., Manzo M.L. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim S.H., Chang S.Y., Sung M., Park J.H., Bin K.H., Lee H. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Rev Infect Dis. 2016;63(3):363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arabi Y.M., Asiri A.Y., Assiri A.M., Jokhdar H.A.A., Alothman A., Balkhy H.H. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21(1):1–8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Momattin H., Zumla A., Memosh Z.A., Al-Tawfiq J.A. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV)–possible lessons from a systematic review of SARS-CoV therapy. Int J Infect Dis. 2013;17(10):e792–e798. doi: 10.1016/j.ijid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang C.K., Jeyachandran S., Hu N.J., Liu C.L., Lin S.Y., Wang Y.S. Structure-based virtual screening and experimental validation of the discovery of inhibitors targeted towards the human coronavirus nucleocapsid protein. Mol Biosyst. 2016;12(1):59–66. doi: 10.1039/C5MB00582E. [DOI] [PubMed] [Google Scholar]
- 117.Dayer M.R., Taleb-Gassabi S., Dayer M.R.S., Lopinavir A Potent Drug against Coronavirus Infection: Insight from Molecular Docking Study. Arch Clin Infect Dis. 2017;12(4) doi: 10.5812/archcid.13823. [DOI] [Google Scholar]
- 118.Lin S., Shen R., He J., Li X., Guo X. Molecular Modeling Evaluation of the Binding Effect of Ritonavir, Lopinavir and Darunavir to Severe Acute Respiratory Syndrome Coronavirus 2 Proteases. bioRxiv. 2020;01(31):929695. doi: 10.1101/2020.01.31.929695. [DOI] [Google Scholar]
- 119.Dieterich, DT. Method of treating anemia caused by ribavirin treatment of hepatitis C using erythropoietin alpha. U.S. Patent 2019; 10: 434-143.
- 120.Nyström K., Waldenstrom J., Lagging M. Ribavirin: pharmacology, multiple modes of action and possible future perspectives. Future Virol. 2019;14(3):153–160. doi: 10.2217/fvl-2018-0166. [DOI] [Google Scholar]
- 121.Sinclair S.M., Jones J.K., Miler R.K., Greene M.F., Kwo P.Y., Maddrey W.C. The Ribavirin Pregnancy Registry: an interim analysis of potential teratogenicity at the mid-point of enrollment. Drug Saf. 2017;40(12):1205–1218. doi: 10.1007/s40264-017-0597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Merino A.C., DLFI S.áez, Molina C.Z., Suárez P.D., Mestre G.B., Sanchez I.J. Hydroxychloroquine, a potentially lethal drug. Med Intensiva. 2017;41(4):257. doi: 10.1016/j.medin.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 123.Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., Nieuwkoop S., Bestebroer T.M. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cong Y., Hart B.J., Gross R., Zhou H., Frieman M., Bollinger L. MERS-CoV pathogenesis and antiviral efficacy of licensed drugs in human monocyte-derived antigen-presenting cells. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mo Y., Fisher D. A review of treatment modalities for Middle East respiratory syndrome. J Antimicrob Chemother. 2016;71(12):3340–3350. doi: 10.1093/jac/dkw338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. J Pre-proof. 2020;20:0924. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.2020. International society of antimicrobial chemotherapy.https://www.isac.world/news-and-publications/official-isac-statement April 3th Available on. [Google Scholar]
- 128.De Souza Oliveira E., De Morais Acln. COVID-19: a pandemic that alert to the population. Inter Am J Med Health. 2020;3:1–7. [Google Scholar]
- 129.Pauque L., Magnard P. Retinal degeneration in two children following preventive antimalarial treatment of the mother during pregnancy. Bull Soc Ophtalmol Fr. 1969;69:466–467. [PubMed] [Google Scholar]
- 130.Kaplan Y.C., Ozsarfati J., Nickel C., Koren G. Reproductive outcomes following hydroxychloroquine use for autoimmune diseases: a systematic review and metaanalysis. Br J Clin Pharmacol. 2016;81(5):835–848. doi: 10.1111/bcp.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaplan Y.C., Koren G. Use of hydroxychloroquine during pregnancy and breastfeeding: an update for the recent coronavirus pandemic (COVID-19) Motherisk Int J. 2020 [Google Scholar]
- 132.Sperber K., Hom C., Chao C.P., Shapiro D., Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9. doi: 10.1186/1546-0096-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Al-Tawfiq J.A., Memish Z.A. Update on therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV) Expert Rev Anti Infect Ther. 2017;15(3):269–275. doi: 10.1080/14787210.2017.1271712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Channappanavar R., Fehr A.R., Zheng J., Wohljord-Lenane C., Abrahante J.E., Mack M. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129(9) doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Habib A.M.G., Ali M.A.E., Zouaoui B.R., Taha M.A.H., Mohammed B.S., Saquib N. Clinical outcomes among hospital patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. BMC Infect Dis. 2019;19(1):1–6. doi: 10.1186/s12879-019-4555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lokugamage K.G., Hage A., Schindewolf C., Rajsbaum R., Menachery V. SARS-CoV-2 sensitive to type I interferon pretreatment. bioRxiv. 2020;03(07):982264. doi: 10.1101/2020.03.07.982264. [DOI] [Google Scholar]
- 137.Yazdani B.P., Matok I., Garcia B.F., Koren G. A systematic review of the fetal safety of interferon alpha. Reprod Toxicol. 2012;33:265–268. doi: 10.1016/j.reprotox.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 138.Spera A.M., Eldin T.K., Tosone G., Orlando R. Antiviral therapy for hepatitis C: Has anything changed for pregnant/lactating women? World J Hepatol. 2016;8(12):557. doi: 10.4254/wjh.v8.i12.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peck‐Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37(6):778–793. doi: 10.1111/liv.13317. [DOI] [PubMed] [Google Scholar]
- 140.Balsat M., Etienne M., Elhamri M., Hayette S., Salles G., Thomas X. Successful pregnancies in patients with BCR‐ABL‐positive leukemias treated with interferon‐alpha therapy during the tyrosine kinase inhibitors era. Eur J Haematol. 2018;101(6):774–780. doi: 10.1111/ejh.13167. [DOI] [PubMed] [Google Scholar]
- 141.Da Silva J.D.F., Silva S.P., Silva S.P.M., Vieira A.M., Araújo L.C.C., Lima T.A. Portulaca elatior root contains a trehalose-binding lectin with antibacterial and antifungal activities. Int J Biol Macromol. 2019;126:291–297. doi: 10.1016/j.ijbiomac.2018.12.188. [DOI] [PubMed] [Google Scholar]
- 142.Zhou Y., Lu K., Pfefferle S., Bertram S., Glowacka I., Drosten C. A single asparagine-linked glycosylation site of the Severe Acute Respiratory Syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J Virology. 2010;84:8753–8764. doi: 10.1128/JVI.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ip W.K.E., Chan K.H., Law H.K.W., Tso G.H.W., Kong E.K.P., Wong W.H.S. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Haagmans B.L., Van Den Brand J.M., Raj V.S., Volz A., Wohksein P., Smits S.L. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351(6268):77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]


