Abstract
The COVID-19 outbreak has rapidly progressed worldwide finding the health system, scientists and society unprepared to face a little-known, fast spreading, and extremely deadly virus. Italy is one of the countries hardest hit by the pandemic, resulting in healthcare facilities bearing heavy burdens and severe restrictive measures. Despite efforts to clarify the virus transmission, especially in indoor scenarios, several aspects of SARS-CoV-2 spread are still rudimentary. This study evaluated the contamination of the air and surfaces by SARS-CoV-2 RNA in the COVID-19 isolation ward of a hospital in Milan, Italy. A total of 42 air and surface samples were collected inside five different zones of the ward including contaminated (COVID-19 patients' area), semi-contaminated (undressing room), and clean areas. SARS-CoV-2 RNA detection was performed using real time reverse transcription polymerase chain reaction. Overall, 24.3% of swab samples were positive, but none of these were collected in the clean area. Thus, the positivity rate was higher in contaminated (35.0%) and semi-contaminated (50.0%) areas than in clean areas (0.0%; P<0.05). The most contaminated surfaces were hand sanitizer dispensers (100.0%), medical equipment (50.0%), medical equipment touch screens (50.0%), shelves for medical equipment (40.0%), bedrails (33.3%), and door handles (25.0%). All the air samples collected from the contaminated area, namely the intensive care unit and corridor, were positive while viral RNA was not detected in either semi-contaminated or clean areas. These results showed that environmental contamination did not involve clean areas, but the results also support the need for strict disinfection, hand hygiene and protective measures for healthcare workers as well as the need for airborne isolation precautions.
Keywords: COVID-19, Coronavirus, Outbreak, Airborne transmission, Environmental contamination, Infection control
Graphical abstract
1. Introduction
The novel coronavirus, named Severe Acute Respiratory Syndrome Coravirus 2 (SARS-CoV-2), has rapidly progressed worldwide, and the impact on health systems, science, and society is unprecedented (Torri and Nollo, 2020). The chronology of COVID-19 infections is as follows: On 31 December 2019 the coronavirus disease was first reported as a cluster of pneumonia cases of unknown etiology by the Wuhan Municipal Health Commission in Wuhan City, Hubei Province China (WHO, 2020a). Subsequently, on 7 January 2020, Chinese authorities confirmed that they had identified a novel virus belonging to the same family of coronaviruses as Severe Acute Respiratory Syndrome (SARS). Then on 11 February 2020 the World Health Organization (WHO) announced a name for the new coronavirus disease: COVID-19. Due to the global spread of COVID-19 various international concerns declared a State of Emergency as COVID-19 was considered to be the third highest pathogenic human coronavirus that had emerged in the last two decades (WHO, 2020a). On 11 March 2020 the WHO declared the COVID-19 outbreak a global pandemic (WHO, 2020a).
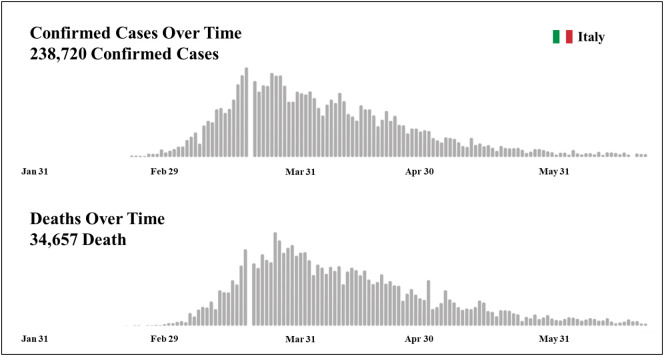
Italy was the first European nation to be affected by COVID-19 with 238,720 confirmed total cases and 34,657 deaths to date (Fig. 1 ; WHO, 2020b). The pandemic broke out and was mainly located in northern Italy with the first Italian COVID-19 patient hospitalized on 21 February 2020 at Codogno Hospital, Lodi (Lombardy-Italy) (Indolfi and Spaccarotella, 2020). In the following days, in Lombardy, there was a rapid increase in the number of cases. The mortality rate in this region alone with a total of 16,579 deaths, is currently greater than that of China (4646 total deaths; Regione Lombardia, 2020; WHO, 2020c). In this context the Italian National Healthcare Service was close to collapse.
Fig. 1.
WHO Italian situation report dated 24 June 2020.
(Adapted from WHO, 2020b.)
Person-to-person transmission routes have previously been described for SARS-CoV-2 with incubation times between two and ten days (Chan et al., 2020). The spread is facilitated through direct personal contact, droplets, hands, or contaminated surfaces (Chan et al., 2020). Laboratory experiments showed that SARS-CoV-2 can remain viably infectious in aerosols for hours and on surfaces for days (van Doremalen et al., 2020). Unfortunately the diffusion of COVID-19 in hospital settings is facilitated by the presence of high viral loads in the respiratory tract of hospitalized infected persons, released in the environment through droplets spread via coughing or sneezing (Rothan and Byrareddy, 2020). In closed and stagnant environments such as hospital wards, droplets can remain suspended for more than 10 min and cover long distances, potentially maintaining their ability to transmit disease (Bourouiba, 2020; Ong et al., 2020; Stadnytskyi et al., 2020). However, knowledge of several aspects of SARS-CoV-2 spread in indoor scenarios is still rudimentary, and, as far as we know, inspections of the SARS-CoV-2 contamination in a European hospital ward have not yet been reported.
The aim of this study was to evaluate the contamination of the air and surfaces by SARS-CoV-2 RNA in the COVID-19 ward of an Italian hospital in order to understand the extent of the viral shedding and then to improve the management of healthcare settings and implement public safety measures.
2. Materials and methods
2.1. Sampling location and methods applied
We conducted the present study on May 12, 2020 inside the COVID-19 ward of the San Carlo Hospital in Milan, Lombardy (Italy). The area under control consisted of the COVID-19 ward, which included an operating room converted into an intensive care unit (ICU) with eight beds (Fig. 2 ). The air conditioning system in the COVID-19 ward consisted of a negative airflow system. Surfaces and objects were wiped down daily with active chlorine (5–10%) disinfectant. On the day of the experimentation the sampling was carried out before the cleaning operations, precisely in the time interval from 8:00 am to 1:00 pm. On the day of the experimentation the temperature and relative humidity (RH) of the COVID-19 ward ranged from 20° to 22 °C and 40 to 60% respectively, while the average outdoor temperature and RH in Milan on that day were 18 °C and 78% respectively.
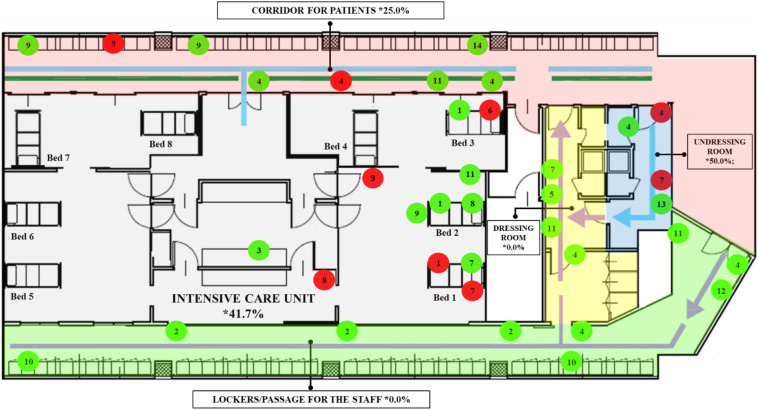
Fig. 2.
Location of sampled surfaces and objects in COVID-19 ward. The areas of the ward are represented by different colours. Color code: Red label = positive samples; Green label = negative samples. Numeric code: 1. Bedrails; 2. Benches; 3. Computer keyboard; 4. Door handles; 5. Gloves box; 6. Hand sanitizer dispenser; 7. Medical equipment; 8. Medical equipment touch screen; 9. Shelf for medical equipment; 10. Staff lockers, 11. Walls; l2. Waste container; 13. Water tap, 14. Window. The indicators and lines in the map represent the dirty/clean paths of the staff and patients.
In this study, swab (n = 37) and air (n = 5) samples were collected from three zones classified as contaminated, semi-contaminated, and clean areas. The contaminated area was a specifically designated area for patients of COVID-19 (corridor for patients and ICU), the clean area was a specifically designated area for non-contaminated items (lockers and passage for the medical staff and a dressing room), and a semi-contaminated area was set up between the contaminated area and the clean area (undressing room). Patients were not allowed to enter the semi-contaminated and clean areas. The different areas within the COVID-19 ward were adjacent but separated by watertight doors with automatic closing system in order to avoid possible contaminations and transmissions. All watertight doors were marked with specific signage that highlighted the area type and the relative prescriptions to limit access to authorized medical staff. There were three patients confirmed with COVID-19 within the ICU. Among these, two were intubated and supported by a respirator (beds no. 1 and 2) while one patient (bed no. 3) was not intubated and without C-PAP nasal mask support (Fig. 2, Fig. 3 ). The ICU patients in beds no. 4, 5, 6, 7, and 8 were dismissed the same day of experimentation. For this reason, no sampling was carried out in the area surrounding these beds as the air and surface samples were taken at the locations where the highest risk of contamination was assumed.
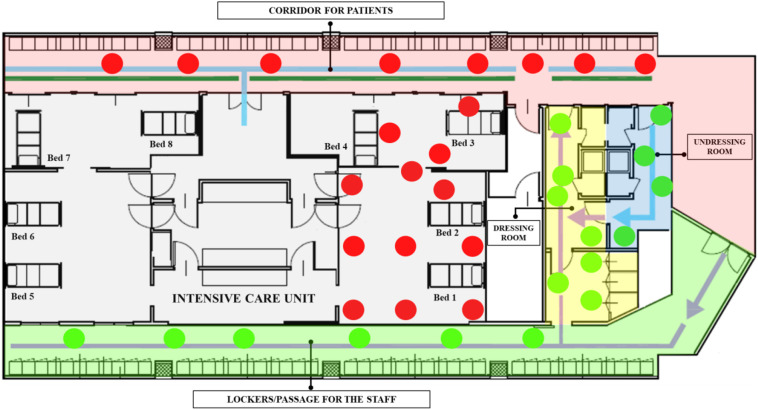
Fig. 3.
Portable Air Sampler position in COVID-19 ward. The areas of the ward are represented by different colours. Color code: Red label = positive samples; Green label = negative samples. The indicators and lines in the map represent the dirty/clean paths of the staff and patients.
The medical and paramedical staff consisted of eight people obligated to wear personal protective equipment (PPE), specifically gloves, aprons, long sleeved gowns, goggles, fluid-repellant surgical masks, face visors, and respirator masks.
Sterile premoistened swabs with a specific virus preservation solution (Biocomma Limited, ShenZhen, PR, China) were used to sample potentially contaminated surfaces and objects such as the bedrails, benches, computer keyboards, door handles, glove boxes, hand sanitizer dispensers, medical equipment, medical equipment touch screens, shelves for medical equipment, staff lockers, walls, waste containers, water taps, and windows (Fig. 2).
The air samples were collected using an MD8 Airport Portable Air Sampler with Gelatine Membrane Filters (Sartorius, Varedo, MB, Italy). One gelatin membrane filter was used for each monitored area. The duration of each aspiration cycle was 40 min with a flow of 50 l/min, for a total volume sampled of 2 m3. The detector was positioned 1.5 m above the floor. The long duration required by the aspiration cycle did not allow repeated sampling in the same area due to operational limitations, but the detector was moved at predefined times to different locations of each area randomly identified (Fig. 3).
2.2. Laboratory analysis: SARS-CoV-2 detection
After air samples and surface swabs were collected, all samples were transferred to the Chemservice – Labanalysis Group (Milan, Italy) laboratory under cool conditions (temperature range between 2 and 8 °C). Laboratory confirmation of the virus was performed using real time reverse transcription polymerase chain reaction (RT-PCR) using the VETfinder “Detection of CoV-19 and SARS and Recovery control in environmental sample” detection kit (Generon s.r.l., San Prospero, Modena, Italy), which is able to detect both SARS-CoV-2 and SARS virus group.
Each reaction contained 20 μl of Ready-to use Mastermixes and 5 μl of RNA sample (final volume 25 μl). Each reaction was run with initial conditions of 55 °C for 10 min (one cycle), 95 °C for 3 min (one cycle), followed by 45 cycles of 95 °C for 15 s, and 58 °C for 30 s, as instructed by the kit supplier. A sample was considered positive when the qRT-PCR Ct value was ≤40.
2.3. Statistical analysis
Descriptive statistics were used to present data as numbers and percentages. The differences in the positive rates between the areas were compared by Fisher exact tests while a z-test was used to compare column proportions. Cramer's V was reported as measure of effect size. Correlation between virus concentration and distance from patients was examined using Spearman rank correlations (ρ). The number of sample locations was calculated based on the area of each room in accordance with the ISO 14644-1. Statistical analyses were performed with SPSS Statistics version 25 (IBM, SPSS Inc., Chicago, IL, USA). Statistical significance occurred when P<0.05.
3. Results
A total of 37 swab samples were collected within the five areas and 24.3% of these were positive for viral RNA (Fig. 2). The positivity rate was higher in contaminated (7/20, 35.0%) and semi-contaminated areas (2/4, 50.0%) than in the clean area (0/13, 0.0%; P=0.015; Table 1 ). Cramer's statistic indicated a medium effect for the association between SARS-CoV-2 positivity and area type (V = 0.430; P=0.030). There were no differences between the positivity rate of the corridor for patients (25.0%) and the ICU (41.7%; P=0.642). In these latter two environments, contaminated samples were collected from the surfaces of medical equipment (2/3, 66.7%), touch screens (1/2, 50.0%), shelves (2/5, 40.0%), door handles (1/3, 33.3%), and bedrails (1/3, 33.3%). Positive samples of the undressing room were found on the hand sanitizer dispenser (1/1, 100.0%) and door handles (1/2, 50.0%). The positive rates of swab samples from environmental surfaces of specific sites regardless of area are detailed in Table 2 .
Table 1.
Positive rate of swab samples from environmental surface in different areas and associations between rate of positivity and area type.
| Area type | Area | NO. of tests | NO. of Positive | Rate of Positivity | P value⁎ |
|---|---|---|---|---|---|
| Contaminated | Corridor for patients | 8 | 2 | 25.0% | 0.015 |
| Intensive care unit | 12 | 5 | 41.7% | ||
| Total Contaminated | 20 | 7a | 35.0% | ||
| Semi–contaminated | Undressing room | 4 | 2a | 50.0% | |
| Clean | Locker/passage for medical staff | 9 | 0 | 0.0% | |
| Dressing room | 4 | 0 | 0.0% | ||
| Total Clean | 13 | 0b | 0.0% | ||
| Total | 37 | 9 | 24.3% | – |
Subscript letter denotes a subset of Area type categories whose column proportions do not differ significantly from each other at the 0.05 level (contaminated vs semi-contaminated, contaminated vs clean, semi-contaminated vs Clean; z-test).
P value for association between rate of positivity and area type (contaminated, semi-contaminated or clean; Fisher exact tests).
Table 2.
Positive rate of swab samples according to sampling site.
| Sampling site | NO. of tests | NO. of positive | Rate of positivity | Average virus concentration⁎ |
|---|---|---|---|---|
| Bedrails | 3 | 1 | 33.3% | 21.5 |
| Benches | 3 | 0 | 0.0% | ND |
| Computer keyboard | 1 | 0 | 0.0% | ND |
| Door handles | 8 | 2 | 25.0% | 25.2 |
| Glove box | 1 | 0 | 0.0% | ND |
| Hand sanitizer dispenser | 1 | 1 | 100.0% | 24.0 |
| Medical equipment | 4 | 2 | 50.0% | 22.2 |
| Medical equipment touch screens | 2 | 1 | 50.0% | 22.5 |
| Shelves for medical equipment | 5 | 2 | 40.0% | 23.9 |
| Staff lockers | 2 | 0 | 0.0% | ND |
| Walls | 4 | 0 | 0.0% | ND |
| Waste container | 1 | 0 | 0.0% | ND |
| Water tap | 1 | 0 | 0.0% | ND |
| Window | 1 | 0 | 0.0% | ND |
ND, not determined.
Expressed as Ct-value.
Within the ICU the distance between site samplings and the closest patient was calculated. The Spearman correlation (ρ) between this distance and virus concentration was 0.577 (P=0.308).
Air samples collected from ICU and corridor for patients were positive for viral RNA with mean concentrations of 22.6 and 31.1 Ct value, respectively. Conversely, no SARS-CoV-2 RNA was found in the air of the undressing room, dressing room, and passage/lockers area for staff (Fig. 3).
4. Discussion
To our knowledge, no research has previously been conducted in a European hospital to evaluate the environmental contamination in the air and on surfaces by SARS-CoV-2 RNA.
Overall, nine out of 37 swab samples were positive. Seven of these were collected in the contaminated and two in the semi-contaminated area while no viral RNA was found on the surfaces of the clean area. In particular the positivity rate of the ICU was 41.7%, which is in close agreement with those obtained in the ICUs of Wuhan, China, by Guo et al. (2020) (43.5%) and by Wu et al. (2020) (37.5%).
In the present study the correlation between the virus concentration and the distance from patients was also evaluated. The Spearman coefficient suggests that there may be a moderate correlation and that the viral load of the surfaces increases with the patient proximity. However, there was not sufficient evidence to confirm these data as they did not quite achieve significance. The correlation analysis was only carried out on the five positive samples collected within the ICU. As the p-value for a correlation coefficient is affected by the sample size, analyses on a greater number of positive samples could elucidate the relationship between virus concentration and distance from patients. Other aspects should be further investigated. For example, Santarpia et al. (2020) evaluated the association between patients' body temperature and shedding of virus in the environment even if they did not find a statistically significant correlation.
The positivity rate of the corridor for patients was lower but not statistically different than that of the ICU. Moreover, two positive swab samples were obtained from the surfaces of the undressing room. This finding emphasized the criticality of the undressing procedures. Conversely, as mentioned above, no contaminated surfaces by SARS-CoV-2 were found in the area for passage and lockers of medical staff, and in the dressing room. Thus, our findings showed contamination of surfaces in both contaminated and semi-contaminated areas but not in the clean area. This result suggests that the pathway separating semi-contaminated and clean areas as well as the definition of separate dressing and undressing zones are essential.
In particular the PCR-positive samples were obtained from the surface of hand sanitizer dispensers, medical equipment, medical equipment touch screens, shelves for medical equipment, bedrails, and door handles. On the contrary no samples collected from the walls were positive. These results confirmed previous studies in the Wuhan, Nebraska, and South Korea hospitals that reported relatively high positivity rates for the surface of the objects that are frequently touched by medical staff or patients such as computer mice, bedrails, water machine buttons, door handles, telephones, oxygen cylinder valves, personal computers, iPads, cellular phones, reading glasses, and remote controls for televisions (Guo et al., 2020; Ryu et al., 2020; Santarpia et al., 2020; Wu et al., 2020). Ong et al. (2020), analysing some toilet sites in the Singapore hospital, also showed viral RNA contamination of toilet bowl and sink samples.
Although the RT-PCR does not determine the infectivity, the identification of viral RNA in these items indicates the shedding of the virus, and it can be a marker of ineffective cleaning and disinfection (Otter et al., 2016). As previously demonstrated (Wojgani et al., 2012), the door handles are critical points for infection control, but our findings confirm that a special emphasis should be placed on the disinfection of all these surfaces including staff and patients' personal items. Medical and, in particular, electronic equipment needs particular attention as it is possible that their cleaning is intentionally neglected to avoid interference with medical procedures. Fixed use of equipment for each patient and frequent disinfection of all reusable medical equipment could limit the spread of the virus (Wu et al., 2020). Our findings also support the importance of hand hygiene as it could break the cycle deriving from touching contaminated surfaces (Lai et al., 2020). As claimed by the WHO (WHO, 2020d), hand hygiene is an important measure to protect patients, health-care workers, and the environment from contamination. There is still no direct evidence that hand hygiene reduces transmission of SARSCoV-2 (Yang, 2020). However, the fact that SARS-CoV-1 and MERS-CoV virus can survive on surfaces for extended periods (Kampf et al., 2020; Otter et al., 2016) indicates that hand hygiene is a highly defensible measure also in the SARSCoV-2 scenario (Yang, 2020). Moreover, van Doremalen et al. (2020) have recently showed that, under laboratory conditions, SARS-CoV-2 can remain stable on surfaces such as plastic and stainless steel for up to 72 h with a stability similar to that of SARS-CoV-1. Since in the experiment of van Doremalen et al. the virus was artificially nebulized, studies conducted under clinical conditions would be necessary to confirm these data (Peters et al., 2020). Other recent experimental data demonstrated that, in addition to the surface type, the stability of the virus is affected by temperature as the time for inactivation was reduced at 70 °C (Chin et al., 2020). The same authors also proved the effectiveness of some disinfectants such as ethanol, povidone‑iodine, chlorhexidine, and benzalkonium chloride (Chin et al., 2020). Likewise, Ong et al. (2020) showed that the samples collected from COVID-19 patients' rooms after routine cleaning were negative. In this regard detailed guidelines have been provided for cleaning and disinfection procedures in both healthcare and non-healthcare settings from the Centers for Disease Prevention and Control (CDS, 2020; European Centre for Disease Prevention, 2020) and from the WHO (WHO, 2020d). Repeated sampling at several times after the routine cleaning would have provided information on the effectiveness of the disinfection measures and on the persistence of RNA in the environment.
Our results also brought attention to the undressing procedures and, more generally, to the behavioural measures within the undressing room, which is one of the areas affected by surface contamination. Indications on the dressing and undressing procedures are provided by the Italian Ministry of Health (2020).
All the air samples collected from contaminated areas were positive for viral RNA. Our results are consistent with those of Guo et al. (2020) and Liu et al. (2020) referring to hospitals in Wuhan. Santarpia et al. (2020) demonstrated the positivity of air samples for SARS-CoV-2 in the Nebraska Medical Center as well. Moreover, under laboratory conditions, van Doremalen et al. (2020) showed that SARS-CoV-2 remained viable in aerosols at least for three hours. These findings suggest that the virus could be transported by aerosol processes in the surrounding environment, potentially even in the absence of aerosol-generating procedures (Santarpia et al., 2020). They support the idea that the airborne route has to be considered an important pathway for SARS-CoV-2 contamination as already suggested for SARS-CoV-1 (Booth et al., 2005; Yu et al., 2014). Indeed, several retrospective studies and fluid dynamics simulations concluded that airborne transmission was the main route of SARS-CoV-1 in indoor environments including hospital wards (Li et al., 2005; Yu et al., 2005).
Conversely, Wu et al. (2020) and Cheng et al. (2020b) found no positivity for SARS-CoV-2 in air samples collected in Wuhan No. 7 and Hong Kong Hospitals, respectively. Similar results were obtained in Iran (Faridi et al., 2020) and Singapore (Ong et al., 2020) hospitals. Differences in the sampling and analysis methodology could explain these inconsistencies.
Regardless, a precautionary approach is desirable. The WHO recommends a ventilation rate of at least 288 m3 per hour per person for control of opportunistic airborne transmission (such as SARS and influenza) in health care settings, highlighting the fact that some aerosol-generating procedures (i.e. tracheal intubation, non-invasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, and bronchoscopy) are associated with a significant increase in the risk of disease transmission (WHO, 2009). Moreover, supplementary and precautionary measures should be used including: indoor air purifiers; frequent disinfection of the room; ventilation; avoidance of air recirculation; medical staff training; and appropriate separation between all patients (WHO, 2020d; Zhao et al., 2020). Isolation rooms at negative pressure in segregated areas would be desirable (Fathizadeh et al., 2020; Liu et al., 2020) and, where it is not possible, keep infected patients at distance (at least 1 m according to WHO, 2020b). Moreover, although studies on COVID-19 are not yet fully conclusive (Cheng et al., 2020a), the use of physical barriers such as face masks and FFP or N95 respirators must be recommended for healthcare workers, even when performing aerosol-generating procedures in patients without clinical features (Fathizadeh et al., 2020; Guo et al., 2020; Romano-Bertrand et al., 2020). Several evidences have indeed proven their effectiveness in preventing the spread of respiratory virus infections, including SARS epidemic (Jefferson et al., 2009). Future epidemiological studies will have to confirm the effectiveness of hospital infection control measures also for SARS-CoV-2.
In the present study, all air samples collected within the semi-contaminated and clean areas were negative. The variability in the environmental contamination between areas is of interest as it suggests the effectiveness of the nosocomial airborne isolation precautions. Ventilation systems, physical barriers as well as behavioural management may have contributed to the prevention of the spread of SARS-CoV-2 from contaminated to clean areas.
In this context the home isolation of patients with COVID-19 may not be a good control strategy as the control of airborne transmission is more difficult. Moreover, family members usually do not have adequate protective measures or training to limit viral shedding. Unfortunately, in an emergency context with overly-saturated hospital facilities, as it occurred in Lombardy, it is difficult to find other alternatives.
This research has some limitations. Although RT-PCR is routinely used to detect causative viruses from respiratory secretions (Corman et al., 2020), it is worthwhile to stress that it is a marker of virus shedding, but it does not necessarily indicate the presence of viable virus (Otter et al., 2016). Thus, viral culture should be done to demonstrate viability. Another drawback of our study is that, due to operational limitations during an outbreak, it was only possible to investigate a limited number of rooms. Moreover, repeated sampling would increase knowledge of viral RNA persistence and effectiveness of the cleaning procedures. Indeed, all samples were collected before the disinfection operations. Finally, routine and extended investigations would be indicated to confirm these preliminary results, evaluate the effectiveness of the protective equipment, and monitor the hospital environment.
5. Conclusion
This study provides the first report on the SARS-CoV-2 shedding in the air and on object surfaces in a hospital in northern Italy with important implications for the patients and medical staff protection as well as for the management of hospitals and public health. Indeed, it demonstrated that both air and surfaces within areas designated for patients were contaminated by SARS-CoV-2 RNA. This finding suggests that strict structural and personal protection measures as well as systematic disinfections should be implemented to reduce the risk of infection for healthcare professionals working in these areas. Moreover, the contamination of surfaces within the undressing room highlighted not only the risk of virus spread from the ICUs but also the criticality of the undressing procedures. However, the airborne spread of the viral RNA did not involve the areas where patients do not have access indicating the effectiveness of the physical barriers and staff behavioural precautions. Routine and extensive investigations in health-care settings would be desirable.
CRediT authorship contribution statement
Katia Razzini: Conceptualization, Funding acquisition, Methodology, Supervision, Writing - review & editing. Marta Castrica: Conceptualization, Formal analysis, Software, Visualization, Writing - original draft, Writing - review & editing. Laura Menchetti: Conceptualization, Formal analysis, Software, Visualization, Writing - original draft, Writing - review & editing. Lorenzo Maggi: Conceptualization, Funding acquisition, Investigation, Methodology, Writing - review & editing. Lucia Negroni: Data curation, Validation, Writing - review & editing. Nicola V. Orfeo: Data curation, Validation, Writing - review & editing. Alice Pizzoccheri: Data curation, Validation, Writing - review & editing. Matteo Stocco: Data curation, Validation, Writing - review & editing. Stefano Muttini: Data curation, Validation, Writing - review & editing. Claudia M. Balzaretti: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank the staff of San Carlo, Hospital (Milan, Italy) and the staff of Chemservice, (Novate Milanese, Milan, Italy) and LabAnalysis, Casanova Lonati, (Pavia, Italy) laboratories.
The authors wish to thank Ed Weisman for English revision.
Editor: Damia Barcelo
References
- Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B., Mederski B., White D., Low D.E., McGeer A., Simor A., Vearncombe M., Downey J., Jamieson F.B., Tang P., Plummer F. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA - J. Am. Med. Assoc. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- CDS Guidance for cleaning and disinfecting public spaces, workplaces, businesses, schools, and homes. [WWW document] 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/pdf/Reopening_America_Guidance.pdf URL.
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., Tsoi H.W., Lo S.K.F., Chan K.H., Poon V.K.M., Chan W.M., Ip J.D., Cai J.P., Cheng V.C.C., Chen H., Hui C.K.M., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C.C., Chen J.H.K., Yip C.C.Y., Wong S.-C., Chuang V.W.M., Tsang O.T.Y., Sridhar S., Chan J.F.W., Ho P.-L., Yuen K.-Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020;41:493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C.C., Wong S.-C., Chan V.W.-M., So S.Y.-C., Chen J.H.-K., Yip C.C.-Y., Chan K.-H., Chu H., Chung T.W.-H., Sridhar S., To K.K.-W., Chan J.F.-W., Hung I.F.-N., Ho P.-L., Yuen K.-Y. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19) Infect. Control Hosp. Epidemiol. 2020:1–32. doi: 10.1017/ice.2020.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020 doi: 10.1016/s2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., Van Der Veer B., Van Den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D., Holbrook M., Gamble A., Williamson B., Tamin A., Harcourt J., Thornburg N., Gerber S., Lloyd-Smith J., de Wit E., Munster V. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention . 2020. Disinfection of Environments in Healthcare and Non-healthcare Settings Potentially Contaminated With SARS-CoV-2. [Google Scholar]
- Faridi S., Niazi S., Sadeghi K., Naddafi K., Yavarian J., Shamsipour M., Jandaghi N.Z.S., Sadeghniiat K., Nabizadeh R., Yunesian M., Momeniha F., Mokamel A., Hassanvand M.S., MokhtariAzad T. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathizadeh H., Maroufi P., Momen-Heravi M., Dao S., Köse Ş., Ganbarov K., Pagliano P., Esposito S., Kafil H.S. Protection and disinfection policies against SARS-CoV-2 (COVID-19) Le Infez. Med. 2020;28:185–191. [PubMed] [Google Scholar]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., Li X., Li L., Li C., Cui Y., Fu R.-B., Dong Y.-Z., Chi X.-Y., Zhang M.-Y., Liu K., Cao C., Liu B., Zhang K., Gao Y.-W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indolfi C., Spaccarotella C. The outbreak of COVID-19 in Italy: fighting the pandemic. JACC Case Reports. 2020 doi: 10.1016/j.jaccas.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italian Ministry of Health Disposition of the Italian Minister of Health n. 5443. New indications and clarifications [WWW document] 2020. http://2.flcgil.stgy.it/files/pdf/20200328/circolare-ministeriale-5443-del-22-febbraio-2020-indicazioni-chiarimenti-ministero-della-salute-covid-19.pdf URL.
- Jefferson T., Del Mar C., Dooley L., Ferroni E., Al-Ansary L.A., Bawazeer G.A., Van Driel M.L., Foxlee R., Rivetti A. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2009;339:792. doi: 10.1136/bmj.b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T.H.T., Tang E.W.H., Fung K.S.C., Li K.K.W. Reply to “does hand hygiene reduce SARS-CoV-2 transmission?”. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:1135. doi: 10.1007/s00417-020-04653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang X., Yu I.T.S., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- Liu Y.Y., Ning Z., Chen Y., Guo M., Liu Y.Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Ho K., Kan H., Fu Q., Lan K. 2020. Aerodynamic Characteristics and RNA Concentration of SARS-CoV-2 Aerosol in Wuhan Hospitals During COVID-19 Outbreak. bioRxiv 2020.03.08.982637. [DOI] [Google Scholar]
- Ong S., Tan Y., Chia P., Lee T., Ng O., Wong M., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAM. 2020;323 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Parneix P., Otter J., Pittet D. Putting some context to the aerosolization debate around SARS-CoV-2. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regione Lombardia Covid-19 [WWW document] 2020. https://experience.arcgis.com/experience/0a5dfcc103d0468bbb6b14e713ec1e30/ URL.
- Romano-Bertrand S., Aho-Glele L.-S., Grandbastien B., Gehanno J.-F., Lepelletier D. Sustainability of SARS-CoV-2 in aerosols: should we worry about airborne transmission? J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B.-H., Cho Y., Cho O.-H., Hong S.I., Kim S., Lee S. Environmental contamination of SARS-CoV-2 during the COVID-19 outbreak in South Korea. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W., Crown K.K., Brett-Major D., Schnaubelt E., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. 2020. Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center. medRxiv 2020.03.23.20039446. [DOI] [Google Scholar]
- Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri E., Nollo G. Public health decision-making in the real world: four points to reshape it after COVID-19. Disaster Med. Public Health Prep. 2020 doi: 10.1017/dmp.2020.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO Press; Geneva, Switzerland: 2009. Natural Ventilation for Infection Control in Health-care Settings. [PubMed] [Google Scholar]
- WHO WHO timeline - COVID-19 [WWW document] 2020. https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19 URL. (accessed 5.18.20)
- WHO covid19.who.italy [WWW document] 2020. https://covid19.who.int/region/euro/country/it URL. (accessed 6.23.20)
- WHO, 2020c. cov19.who.china [WWW Document]. URL https://covid19.who.int/region/wpro/country/cn (accessed 6.23.20).
- WHO . 2020. Coronavirus Disease (COVID-19) Technical Guidance: Infection Prevention and Control during Health Care When COVID-19 Is Suspected: Interim Guidance. [Google Scholar]
- Wojgani H., Kehsa C., Cloutman-Green E., Gray C., Gant V., Klein N. Hospital door handle design and their contamination with Bacteria: a real life observational study. Are we pulling against closed doors? PLoS One. 2012;7 doi: 10.1371/journal.pone.0040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Wang Y., Jin X., Tian J., Liu J., Mao Y. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Does hand hygiene reduce SARS-CoV-2 transmission? Graefes Arch. Clin. Exp. Ophthalmol. 2020 doi: 10.1007/s00417-020-04652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T.S., Wong T.W., Chiu Y.L., Lee N., Li Y. Temporal-spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin. Infect. Dis. 2005;40:1237–1243. doi: 10.1086/428735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T.S., Qiu H., Tse L.A., Wong T.W. Severe acute respiratory syndrome beyond amoy gardens: completing the incomplete legacy. Clin. Infect. Dis. 2014;58:683–686. doi: 10.1093/cid/cit797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Liu Y., Chen C. Air purifiers: a supplementary measure to remove airborne SARS-CoV-2. Build. Environ. 2020;177 doi: 10.1016/j.buildenv.2020.106918. [DOI] [PMC free article] [PubMed] [Google Scholar]