Abstract
Background
The number of excess deaths during February–March 2020 in Italy, in comparison with previous years, was considerably higher than the recorded COVID19-related deaths. The present study aimed to explore the association of excess mortality with some indices related to the COVID-19 pandemic and its management.
Methods
Data on all-cause mortality from 20 February–31 March in the years 2015–2020, and demographic, socioeconomic and healthcare organisation data of each Italian region were obtained from the Italian Institute of Statistics. Non-COVID-19-Imputed Excess Mortality (NCIEM) was calculated as the difference between the excess 2020 mortality and reported COVID-19 mortality. The association of NCIEM with the rate of COVID-19 cases, COVID-19 mortality and other potential moderators was assessed using linear regression models.
Results
The nationwide number of excess deaths and COVID-19 deaths was 26,701 and 13,710, respectively, with a difference of 12,991. The NCIEM in different regions showed a direct correlation with COVID-19 mortality (r2 = 0.61, p < 0.001) and total cases (r2 = 0.30, p = 0.012), and an inverse correlation with cases/total tests ratio (r2 = 0.49, p = 0.001). Direct correlations were also found with the proportion of institutionalised elderly, whereas inverse correlations were observed with prevalence of diabetes, cardiovascular mortality and density of general practitioners.
Conclusions
The impact of the COVID-19 pandemic on all-cause mortality was considerably greater than that indicated by official counts of victims. Limited testing capacity and causes of death other than COVID-19 could have contributed to the increase in overall mortality rates.
Keywords: COVID-19, Mortality
Introduction
Italy was the European epicentre of the Coronavirus Disease-19 (COVID-19) pandemic, with the highest number of recorded cases and deaths during and at the end of March 2020 (Anon, 2020a). In some areas of the country, most notably in the Lombardia region, the capacity of hospital care, and particularly of intensive care units, was overwhelmed, possibly contributing to a very high COVID-19-related mortality rate (Remuzzi and Remuzzi, 2020). The Italian Health Ministry (Anon, 2020b) made daily reports on the number of COVID-19 cases and deaths confirmed with microbiological testing (PCR-detected viral mRNA from pharyngeal swabs). Such reports could have considerably underestimated the actual pandemic, due to the relatively high proportion of asymptomatic or oligosymptomatic cases (Anon, 2020c). In addition, limited laboratory capacity could have prevented microbiological testing, even in symptomatic cases. Anecdotical reports have suggested that some patients died of COVID-19 at home, without receiving a confirmed diagnosis and without being included in the official count of COVID-19-related deaths (Lavezzo et al., 2020). In some municipalities of the Province of Bergamo, Lombardia, the number of excess deaths at the Registry Office in March 2020, in comparison with the same months of previous years, was considerably higher than the recorded COVID-19-related deaths (Anon, 2020b). In order to provide some information on the impact of the pandemic on mortality, the Istituto Italiano di Statistica (ISTAT) collected mortality data for the period 20 February–31 March 2020 from Registry Offices of 6866 municipalities (of a total of 7094) throughout Italy. These data, which do not provide any information on the actual cause of death, showed an increase of 27.9% (Anon, 2020c).
The present study aimed to explore the association of excess mortality with some indices related to the pandemic and its management, in order to obtain some insight into possible underlying mechanisms.
Methods
Data on all-cause mortality from 20 February–31 March during 2015–2020 were obtained from ISTAT (Anon, 2020c). Those data included a sample of 6866 (of a total of 7904) Italian municipalities, accounting for a population of 52,452,445 (of a total population of 60,359,546). Data were retrieved daily on cases of COVID-19, COVID-19 deaths (i.e. deaths in individuals with positive test results), and total number of diagnostic tests performed up to 31 March 2020, in each region, from the Health Ministry website (Anon, 2020b). Municipalities included in the ISTAT survey were considered representative of their regions, in order to estimate total regional mortality.
Excess 2020 mortality was defined as the difference between mean 2015–2019 and 2020 mortality rates for the reference period. Non-COVID-19-Imputed Excess Mortality (NCIEM) was calculated as the difference between excess 2020 mortality and reported COVID-19 mortality. The ratio of cases/total tests performed was calculated for each region as an index of aggressiveness of local policies with respect to case finding.
Demographic, socioeconomic and healthcare organisation data from each region were retrieved from ISTAT (Anon, 2020d). The association between NCIEM and the rate of COVID-19 cases, COVID-19 mortality and the other potential moderators listed above, in different regions, was assessed using linear regression models and weighed for total population. Multivariate analyses were also performed with each moderator and adjusting for COVID-19 mortality. Analyses were performed on SPSS (SPSS-Inc., Chicago, IL, USA) 25.0.
This work was based on publicly available data and needed no ethical approval.
Results
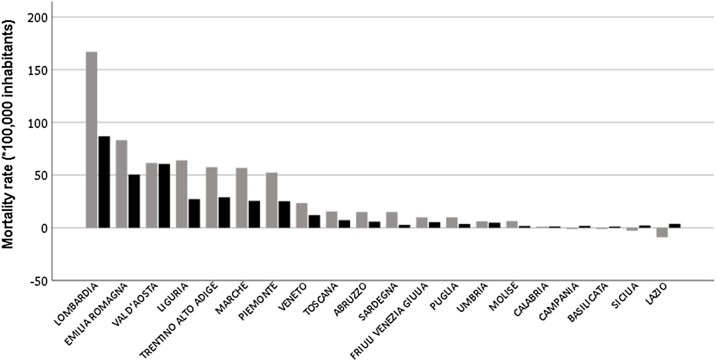
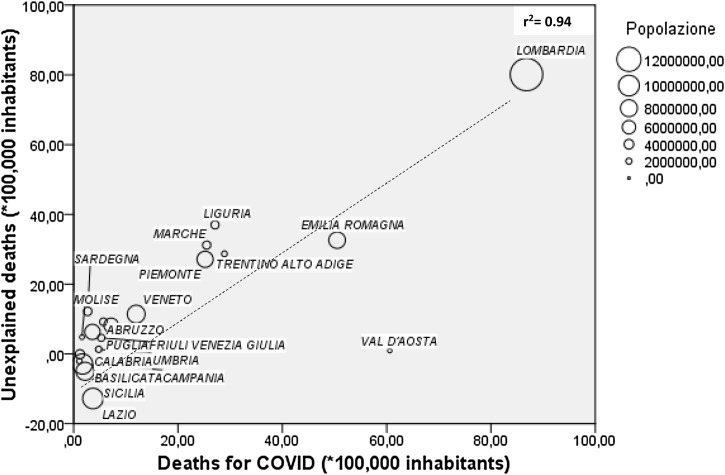
Based on ISTAT data (Anon, 2020c), mortality from 20 February–31 March 2020 was higher than the 2015–2019 average in all regions (Table 1 ). The nationwide number of excess deaths in 2020 can be estimated at 26,701 as compared with 13,710 COVID-19 deaths reported by the Ministry of Health (Anon, 2020a), with a difference of 12,991. Excess 2020 and COVID-19 mortality rates for different regions are summarised in Figure 1. The NCIEM (*100,000 inhabitants) ranged from 80.1 in Lombardia to –12.8 in Lazio, with a national value of 22.2. The NCIEM in different regions showed a significant direct correlation with COVID-19 mortality (Figure 2; r 2 = 0.61, p < 0.001) and total cases (r 2 = 0.30, p = 0.012), and an inverse correlation with cases/total tests ratio (r 2 = 0.49, p = 0.001). Significant direct correlations were also found with mean personal income and the proportion of elderly in nursing homes, whereas inverse correlations were observed with the prevalence of diabetes, 2019 cardiovascular mortality and density of general practitioners (GPs) (Table 2 ). In alternative multivariate analyses, after adjusting for COVID-19 mortality, only prevalence of diabetes retained a statistically significant association with NCIEM (Table 2).
Table 1.
Mortality rates from ISTAT survey (Anon, 2020c) and Ministry of Health data (Anon, 2020b).
| Region | Sample | Total population (n) |
Sample/inhab (%) |
Mortality rate (*100,000 inhab;2015 –2019) |
Mortality rate (*100,000 inhab; 2020) |
Difference in mortality rate (%) |
|---|---|---|---|---|---|---|
| Abruzzo | 1,122,712 | 1,311,580 | 85.6 | 240 | 309 | +9.8 |
| Basilicata | 468,307 | 562,869 | 83.2 | 107 | 135 | –0.9 |
| Calabria | 1,557,705 | 1,947,131 | 80.0 | 624 | 775 | +0.8 |
| Campania | 4,525,320 | 5,801,692 | 78.0 | 875 | 1067 | –1.0 |
| Emilia Romagna | 3,741,501 | 4,459,477 | 83.9 | 960 | 1386 | +35.6 |
| Friuli Venezia Giulia | 1,074,254 | 1,215,220 | 88.4 | 377 | 452 | +7.9 |
| Lazio | 4,309,367 | 5,879,082 | 73.3 | 1657 | 1842 | –7.6 |
| Liguria | 1,358,361 | 1,550,640 | 87.6 | 358 | 405 | +26.9 |
| Lombardia | 9,638,030 | 10,060,574 | 95.8 | 1749 | 3131 | +59.0 |
| Marche | 1,284,278 | 1,525,271 | 84.2 | 210 | 321 | +29.6 |
| Molise | 249,383 | 305,617 | 81.6 | 52 | 81 | +4.5 |
| Piemonte | 4,038,388 | 4,356,406 | 92.7 | 874 | 1192 | +26.9 |
| Puglia | 3,275,620 | 4,029,053 | 81.3 | 622 | 714 | +7.5 |
| Sardegna | 1,421,525 | 1,639,591 | 86.7 | 266 | 358 | +12.4 |
| Sicilia | 3,549,923 | 4,999,891 | 71.0 | 853 | 959 | –2.1 |
| Toscana | 3,129,169 | 3,729,641 | 83.9 | 721 | 842 | +9.5 |
| Trentino Alto Adige | 973,627 | 1,072,276 | 90.8 | 175 | 276 | +34.7 |
| Umbria | 776,173 | 882,015 | 88.0 | 157 | 171 | +3.9 |
| Val d'Aosta | 115,487 | 125,666 | 91.9 | 40 | 48 | +30.7 |
| Veneto | 4,268,093 | 4,905,854 | 87.0 | 1175 | 1336 | +16.4 |
| Italy | 52,452,445 | 60,359,546 | 86.9 | 604.6 | 790.0 | +27.9 |
Inhab, inhabitants.
Figure 1.
Excess 2020 (grey bars) and COVID-19 (black bars) mortality rates for different Italian regions.
Figure 2.
Correlation between unexplained and COVID-19 mortality rate (weighted for population) in different Italian regions.
Table 2.
Linear regression analyses exploring association between unexplained mortality rate and other demographic, socioeconomic and healthcare organisation parameters.
| Parameters | Median [quartiles] | Range | Unadjusted |
Adj. for COVID-19 mortality rate |
||
|---|---|---|---|---|---|---|
| Beta | p | Beta | p | |||
| Cases/total test ratio | 7.7 [4.5–11.2] | 2.9–14.8 | –0.70 | 0.001 | –0.13 | 0.09 |
| Mean age (years) | 45 [44–46] | 42–48 | 0.28 | 0.28 | 0.10 | 0.15 |
| Age ≥65 years (%) | 23 [21–25] | 20–30 | 0.28 | 0.24 | 0.10 | 0.13 |
| Urbanisation rate (%) | 25 [17–36] | 0–60 | 0.03 | 0.90 | –0.11 | 0.06 |
| Density (inh./km2) | 162 [104–267] | 39–424 | 0.42 | 0.07 | –0.07 | 0.30 |
| Personal income (1000€/inhab.) | 30 [25–32] | 22–35 | 0.65 | 0.002 | 0.00 | 0.92 |
| Public health expenditure (€/inhab.) | 1848 [1804–2022] | 1730–2113 | 0.20 | 0.41 | 0.00 | 0.91 |
| Intensive care beds (pre-COVID)/mLn*inhab | 81 [74–100] | 23–307 | 0.35 | 0.14 | –0.03 | 0.68 |
| General practitioners/mLn*inhab. | 735 [692–809] | 623–852 | –0.79 | < 0.001 | –0.06 | 0.56 |
| Death for cardiovascular disease (*1000 inhab.) | 31 [30–37] | 27–45 | –0.57 | 0.011 | –0.05 | 0.47 |
| Hypertension prevalence (%) | 31 [26–35] | 23–43 | –0.11 | 0.63 | –0.08 | 0.20 |
| Diabetes prevalence (%) | 5.1 [4.6;5.9] | 3.2–8.2 | –0.60 | 0.005 | –0.15 | 0.028 |
| Elderly in nursing homes (*1000 inhab.) | 2.6 [1.4;5.7] | 0.4–12.8 | 0.72 | < 0.001 | 0.10 | 0.23 |
Abbreviations: inhab.inhabitants; mln.: million. *1000 means ×1000 inhab.
Discussion
Data from the ISTAT survey show that the COVID-19 pandemic is associated with a relevant increase in all-cause mortality rates (Anon, 2020c). This phenomenon has also been observed in other European countries with major COVID-19 outbreaks such as Spain, UK, France, Belgium, Netherlands, and Sweden (Anon, 2020e). Notably, the number of observed deaths was higher than the sum of average deaths in previous years and reported COVID19-related deaths, meaning that a fraction of excess mortality in 2020 cannot explained by official COVID-19 deaths. Excess mortality in European countries participating in the EUROMomo network (Anon, 2020e) is higher than reported COVID-19 mortality (Anon, 2020f), although the difference between the two figures does not appear to be as great as that recorded in Italy.
Considerations on causes underlying this unexplained excess mortality are necessarily speculative, since data on causes of death are unavailable from Registry Office-based reports. Some COVID-19 patients could have died at home or in nursing homes without having received a microbiological diagnosis, thus escaping official records of COVID-19 victims. At the peak of the pandemic, in the second half of March, in several areas of Lombardia and neighbouring regions, healthcare system resources were insufficient for extensive testing of outpatients. In fact, NCIEM appears to be higher in regions with a higher number of recorded COVID-19 cases and deaths. In addition, the cases/total tests ratio, which were assumed as an index of testing capacity, was significantly associated with NCIEM, even though statistical significance was lost after adjusting for recorded COVID-19-related mortality. Notably, NCIEM was higher in regions with a greater proportion of elderly residents in nursing homes, who could have had a lower chance of being properly tested. The lower NCIEM rates in regions with a higher density of GPs points to the relevance of healthcare organisation: a stronger territorial network of healthcare professionals could be more efficient in testing and tracing potential COVID-19 cases, thus reducing under diagnosis.
The association of higher diabetes prevalence with a lower NCIEM rate is more difficult to explain. Diabetes has been associated with worse outcomes of COVID-19 (Huang et al., 2020); since this notion was well known to the medical community, it can be speculated that patients with diabetes and symptoms suggestive of COVID-19 were more accurately tested. It is also possible that the inverse association of diabetes prevalence and NCIEM is spurious, being determined by unavailable confounders. The need to manage the pandemic led to the suspension of other healthcare activities (planned surgery, specialist visits, etc.); the reduction of healthcare for conditions different from COVID-19 could have affected mortality. In addition, individuals affected by other potentially lethal diseases could have refrained from seeking medical care out of fear of being infected in hospitals, emergency rooms and doctors’ offices. Unfortunately, nationwide data on mortality and hospitalisation for causes other than COVID-19 during the pandemic outbreak are not yet available. However, data from the Toscana region show that hospital admissions during March for myocardial infarction, stroke and other severe conditions were reduced by >40% in comparison with previous years (Anon, 2020g).
Available information on mortality during the COVID-19 pandemic is still incomplete. The municipalities included in the ISTAT report (Anon, 2020c) are not necessarily representative of their regions, as assumed for the present analysis; on the other hand, the Ministry of Health does not provide data on recorded COVID-19 deaths split by municipality, only by region. As a consequence, the estimates reported here are intended as preliminary.
Despite these limitations, available data show that the impact of the COVID-19 pandemic on all-cause mortality is considerably greater than that indicated by official counts of victims. Mechanisms underlying this unexplained excess mortality, which could include causes different from COVID-19, deserve further and specific investigation. Many fatal cases of COVID-19 remain undiagnosed because of limited testing capacity; causes of death other than COVID-19 could contribute to the increase in overall mortality rates.
Conflict of interest
Dr Monami reports personal fees from Astra Zeneca, personal fees from Boehringer-Ingelheim, personal fees from Eli-Lilly, personal fees from Novo Nordisk, personal fees from Sanofi, and personal fees from Takeda outside the submitted work. Dr Nreu Besmir is an employer of Novo Nordisk. Dr Mannucci reports grants and personal fees from AstraZeneca, personal fees from Abbott, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Eli Lilly, grants from Genentech, grants from Molteni, personal fees from Merck, grants and personal fees from Novo Nordisk, and personal fees from Sanofi outside the submitted work.
Role of funding
This research was performed as a part of the institutional activity of the unit, with no specific funding. All expenses, including salaries of the investigators, were covered by public research funds assigned to the unit.
Access to data and data analysis
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- https://coronavirus.jhu.edu/map.html. [Accessed 24 April 2020].
- https://github.com/pcm-dpc/COVID-19/blob/master/dati-regioni/dpc-covid19-ita-regioni.csv. [Accessed 24 April 2020].
- https://www.istat.it/it/files//2020/05/Rapporto_Istat_ISS.pdf. [Accessed 24 April 2020].
- http://dati.istat.it/. [Accessed 24 April 2020].
- www.euromomo.eu. [Accessed 24 April 2020].
- https://coronavirus.jhu.edu/map.html. [Accessed 24 April 2020].
- https://www.ars.toscana.it/2-articoli/4360-coronavirus-toscana-e-italia-alla-prova-riapertura-totale-ritorno-a-normalita-mantenendo-alta-attenzione.html?fbclid=IwAR0Dp9AefXUbguCgaylobnh36guwlBXXIrfO9AA88bYusB_zGix5EL5Ojkk. [Accessed on 24 April 2020].
- Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C. Suppression of COVID-19 outbreak in the municipality of Vo, Italy. MedRxiv. 2020;17:20053157. doi: 10.1101/2020.04.17.20053157. [DOI] [Google Scholar]
- Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]